Abstract
Various prostaglandin agonists representing various classes of receptor subtypes were evaluated for their ability to stimulate adenylyl cyclase via the endogenous DP receptor in embryonic bovine tracheal (EBTr) cells. Two antagonists were used to block the agonist-induced cyclic AMP production.
ZK118182 (EC50=16±4 nM), RS-93520 (EC50=23±4 nM), SQ27986 (EC50=33±9 nM), ZK110841 (EC50=33±5 nM), BW245C (EC50=59±19 nM) and PGD2 (EC50=101±10 nM) (n=4–70) were the most potent agonists. Whilst most compounds were full agonists (Emax=100% relative to PGD2), BW245C was significantly more efficacious than PGD2 (Emax=121±3%; P<0.001) and RS-93520 appeared to be a partial agonist (Emax=64±9%; P<0.001).
Agonists from the EP (e.g. enprostil; misoprostol; butaprost), FP (e.g. cloprostenol; fluprostenol; PHXA85), IP (iloprost; PGI2) and TP (U46619) prostanoid receptor classes were weak agonists or inactive in the EBTr cell assay system.
The DP-receptor antagonist, BWA868C, showed a competitive antagonist profile with pA2 values of 8.00±0.02 and 8.14±0.13 in Schild analyses with two structurally different agonists, BW245C and ZK118182, respectively (n=3). AH6809, another purported DP-receptor antagonist, weakly inhibited PGD2- and ZK118182-induced cyclic AMP production (Kis=808±193 nM and 782±178 nM, respectively).
The current studies have characterized the DP receptor positively coupled to adenylyl cyclase in EBTr cells using a wide range of agonist and antagonist prostaglandins. These data support the utility of the EBTr cell line as a useful tool for the evaluation of DP receptor agonists and antagonists and for profiling other classes of prostaglandins.
Keywords: Prostaglandin, DP receptor, EBTr cells, BW245C, BWA868C, AH6809, ZK118182, RS93520, cyclic AMP, adenylyl cyclase
Introduction
The classification for prostanoid receptors that bind the endogenous prostaglandins, PGD2, PGE2, PGF2α, PGI2 and thromboxane A2 are designated DP, EP (with subtypes EP1, EP2, EP3 and EP4), FP, IP, and TP, respectively (Kennedy et al., 1982; Coleman et al., 1984; 1994; Alexander & Peters, 1998). These membrane-bound receptors belong to the superfamily of G-protein-coupled receptors containing seven membrane spanning regions (Narumiya, 1994). EP1, FP, and TP receptors are coupled to phospholipase C via either Gq or Gq/11 which stimulates inositol trisphosphate formation and intracellular Ca2+-mobilization (Coleman et al., 1994; Narumiya, 1994). DP, EP2, EP4 and IP receptors are positively coupled via the Gs protein to adenylyl cyclase which catalyzes the hydrolysis reaction of ATP to the second messenger cyclic AMP (Sugama et al., 1989; Namba et al., 1994; Boie et al., 1995). Although the DP prostanoid receptor has been identified in platelets, smooth muscle cells from various tissues (Coleman et al., 1994), neutrophils (Darius et al., 1994; Wheeldon & Verdey, 1993), and retina (Boie et al., 1995), it has been studied less than other prostanoid receptors due to its low abundance. The DP receptor is thought to be phylogenetically most closely related to the EP2 and IP receptors (Boie et al., 1995). Recently, the DP receptor has been cloned, expressed in transfected cells, and has undergone limited pharmacologic characterization (Hirata et al., 1994; Boie et al., 1995; Kiriyama et al., 1997). As is the case in the EP prostanoid class, there is evidence in the literature to suggest the possibility of subtypes of the DP receptor in human myometrium (Fernandes & Crankshaw, 1995) and canine colonic epithelium (Rangachari et al., 1995), for example.
Agents from the DP receptor class produce a wide variety of biological effects (see Andersen & Ramwell, 1974; Negishi et al., 1993). Prostaglandin D2, for example, causes vascular relaxation (Leff & Giles, 1992; Lydford et al., 1996) and the inhibition of platelet aggregation (Ito et al., 1989; 1990). DP receptor agonists stimulate chloride secretion in canine tracheal epithelial cells (Liu et al., 1996a). In addition, bronchoconstriction is caused by DP receptor activation and this effect appears to be involved in asthmatic complications (Johnston et al., 1995). Agents from this prostanoid receptor class have also been shown to lower intraocular pressure (IOP) (Matsugi et al., 1995; Camras & Alm, 1997). The selective DP receptor antagonist, BWA868C, is known to inhibit adenylyl cyclase in human platelets stimulated by PGD2 (Trist et al., 1989) and a less-selective PG receptor antagonist, AH6809, inhibits the anti-aggregation effect of PGD2 on human platelets (Keery & Lumley, 1988). Some relatively selective DP-receptor agonists include ZK118182 ((5Z,13E)-(9R, 11R, 15S) 0- 9- chloro-15- cyclohexyl -11, 15- dihydroxy- 3-oxo - 16, 17 ,18, 19, 20 - pentanor - 5 ,13 - prostadienoic acid)), ZK110841 ((5Z,13E)-9R,11R,15S)-9b-chlor-15-cyclohexyl-11, 15 -dihydroxy - 16, 17, 18, 19, 20 - pentanor-5, 13 - prostadienoic acid)), SQ27986 ([1S-[1B,2B(5Z),3A(1E,3S),4B]]-7-[3-(3-cyclohexyl-3-hydroxy-1-propenyl)-7-oxabi-cyclo-[2.2.1]hept-2-yl]-5-heptenoic acid)), BW245C (5-(6-carboxyhexyl)-1-(3-cyclohexyl-3-hydroxypropyl-hydantoin)) and RS-93520 ((C3′S,1R,2R, 3S, 6R) -2- C3′-cyclohexyl-3′hydroxyprop-1-ynyl)-3-hydroxybicyclo[4.2.0]oct-7-ylidene)butyrate)) (see Coleman et al., 1994; Alexander & Peters, 1998).
In preliminary studies, bovine embryonic tracheal (EBTr) cells produced cyclic AMP when stimulated with PGD2 (Sugama et al., 1989) and AH6809 antagonized these effects (Ito et al., 1990). However, these constituted limited pharmacological studies on the DP receptor in this cell-line. Therefore, the aims of the present studies were to evaluate several prostaglandin agonists from various receptor classes, as well as to study the effects of the antagonists BWA868C and AH6809, in the EBTr cells using the generation of cyclic AMP as an index of receptor activation in order to better characterize the DP receptor endogenously expressed in these cells.
Methods
Cell culture and cyclic AMP generation
EBTr cells of passages 29–34 were seeded into 48 well culture plates at a density of approximately 4×103 cells per well. The cells were maintained in a humidified atmosphere of 5% CO2 and 95% air and fed semi-weekly with Dulbecco's modified Eagle medium (DMEM) containing 4.5 g/l glucose and supplemented with 2 mM glutamine, 10 mg ml−1 gentamicin, and 10% foetal bovine serum. Upon reaching confluence, the cells were rinsed twice with 0.5 ml of DMEM/F-12. The sample wells were then pre-incubated for 20 min with 0.8 mM ascorbate and 1 mM of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) in DMEM/F-12 at room temperature (RT; 23°C). Time course experiments were conducted to determine the linear range of the DP-receptor-induced cyclic AMP production at 23°C. The agonist stimulus-response was linear up to 80 min under these conditions. The standard incubation protocols for test compounds were as follows: for agonists alone a 15 min incubation was used; when antagonists were tested they were incubated with the cells for a total of 60 min to allow for equilibrium to be established. The latter was based on association/dissociation studies with [3H]-BWA868C binding to DP receptors, where the equilibrium for association was achieved within 60 min at 23°C (unpublished data). Ice-cold 0.1 M acetic acid (150 μl, pH 3.5) was used for the termination of cyclic AMP production and cell lysis. Finally, ice-cold 0.1 M sodium acetate (225 μl, pH 11.5–12.0) was added to neutralize the samples prior to analysis by radioimmunoassay (RIA).
Cyclic AMP RIA techniques have been described previously (Crider et al., 1998; Sharif et al., 1997; 1998). Briefly, experimental sample aliquot, buffer, antibody (100 μl), [125I]- cyclic AMP (100 μl) additions and standard curve preparations were performed manually. Following an overnight incubation at 4°C, bound and free [125I]-cyclic AMP were separated by centrifugation and manual aspiration of the supernatant. For the semi-automated RIA sample manipulation and dilution, cyclic AMP standard curve preparation, antibody and [125I]-cyclic AMP additions were performed by a Biomek 1000® robot (Beckman Instruments, Fullerton, CA U.S.A.). The instrument made dilutions with RIA buffer into a 96 well filtration plate (0.45 μm surfactant-free mixed cellulose). The robot then added [125I]-cyclic AMP (100 μl) to the samples followed by addition of primary antibody (100 μl). The contents were thoroughly mixed by repeated pipetting. Subsequently, an incubation of 16–24 h at 4°C was conducted. A secondary antibody (100 μl) was added by the robot followed by a 20 min room temperature incubation. Bound and free [125I]-cyclic AMP were separated by vacuum filtration. The filters were collected using a Millipore disposable punch tip assembly and manifold. The bound [125I]-cyclic AMP was measured by gamma particle analysis using a Cobra® Auto-Gamma counter (Packard Instruments, Meriden, CT, U.S.A.).
Materials
EBTr cells were purchased from the American Type Culture Collection (ATCC CCL 44, Rockville, MD, U.S.A.). Culture media and supplements were products of Life Technologies (Grand Island, NY, U.S.A.) while FBS was supplied by Hyclone (Logan, UT, U.S.A.). Ascorbic acid and IBMX were obtained from Sigma (St. Louis, MO, U.S.A.). ZK118182 ( ( 5Z,13E) - (9R,11R,15S)0-9-chloro-15-cyclohexyl-11,15-dihydroxy -3 - oxo - 16, 17, 18, 19, 20 - pentanor-5, 13 - prostadienoic acid)) and ZK110841 ((5Z,13E)-9R,11R,15S)-9b-chlor-15-cyclohexyl -11, 15-dihydroxy -16, 17, 18, 19, 20- pentanor -5, 13-prostadienoic acid)) were generous gifts from Schering (Berlin and Bergkamen, Germany); RS-93520 ((C3′S,1R,2R,3S,6R)-2-C3′-cyclohexyl-3′hydroxyprop-1-ynyl)-3-hydroxybicyclo[4.2.0] oct-7-ylidene)butyrate)) was a generous gift from Hoffman LaRoche (Basel, Switzerland). Other prostaglandins such as AH6809 (6 - isopropoxy - 9 - oxoxanthen - 2 - carboxylic acid); SQ27986 ([1S-[1B,2B(5Z),3A(1E,3S),4B]]-7-[3-(3-cyclohexyl-3-hydroxy-1-propenyl) -7-oxabi-cyclo - [2.2.1] hept-2-yl] -5-heptenoic acid); BW245C (5-(6-carboxyhexyl)-1-(3-cyclohexyl-3-hydroxypropyl-hydantoin); enprostil (4,5,6RS)-7[(1R,2R,3R)-3-hydroxy-2-[(E)-(3R)-3-hydroxy-4-phenoxy-1-butenyl]-5-oxocyclopentyl]-4,5-heptadienoic acid); misoprostol, (prost-13-en-1-oic acid, 11,16-dihydroxy-16-methyl-9-oxo-,methyl ester (11a,13E); cloprostenol, (16-m-chlorophenoxy tetranor prostaglandin F2α); fluprostenol, (5Z,13E)-(9S,11R,15S)-9,11,15-trihydroxy-5,13-prostadienoic acid); PHXA85 (15R)-13,14-dihydro-17-phenyl-18,19,20-trinor-PGF2α)); U46619 (9,11-dideoxy-9a,11a-methanoepoxy prostaglandin F2α)) and BWA868C ((±)-3-benzyl-5-(6-carboxyhexyl)-1-(2-cyclohexyl-2-hydroxyethylamino) hydantoin)) were either internally synthesized or were purchased from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). Early studies employed [125I]-cyclic AMP RIA kits supplied by Biomedical Technologies Inc. (Stoughton, MA, U.S.A.). For the semi-automated RIA, we utilized [125I]-cyclic AMP kits purchased from PerSeptive Biosystems (Cambridge, MA, U.S.A.). The 96-well filtration plates (0.45 μm surfactant-free mixed cellulose) were products of Millipore (Bedford, MA, U.S.A.).
Data analysis
Sample counts (CPM) were compared to the standard curve and cyclic AMP production was quantified by linear regression analysis using the Microsoft Excel® software package. The EC50 and IC50 values were generated using either a non-linear iterative curve-fitting program (Michel & Whiting, 1984; Sharif et al., 1991) or the sigmoidal-fit function of the Origin® software packages incorporating the logistics function. The logistical equation for curve fitting was:
x0=EC50 or IC50; p=power; A1=minimal Y value; A2=maximal Y value.
When relative efficacies of various compounds were calculated they were computed as a percentage of the maximal response produced by PGD2 since it was used a standard reference compound in the experiments. When the agonist potency of compounds apparently exceeded 100 μM, the efficacy was listed as that being produced by the highest concentration of the agonist tested, typically this being 100 μM. The pA2 values were derived using the principles described by Arunlakshana & Schild (1959) and as recently utilized for receptor characterization (Sharif & Xu, 1996; Wiernas et al., 1997). When equilibrium inhibition constants (Ki) for AH6809 were calculated the equation of Cheng-Prusoff (1973) was used. A one-way ANOVA and t-test were used to determine significant differences in the efficacies and potencies of the DP receptor agonists.
Results
Survey of prostanoid agonist activities
Initial time-course studies showed that the PGD2-stimulated cyclic AMP production was linear over 10–80 min at 23°C (data not shown). All subsequent experiments were conducted with agonists for 15 min at 23°C. Several different prostaglandin receptor agonists were tested for their potencies and efficacies at the DP receptor in EBTr cells (Table 1 and Figure 1). The most active DP agonists showed the following potencies (EC50 values): ZK118182 (16 nM), RS-93520 (23 nM), SQ27986 (33 nM), ZK110841 (33 nM), BW245C (59 nM) and PGD2 (101 nM) (n=4–70; Table 1). Compounds with activity at EP1 (e.g. sulprostone), EP2 (e.g. butaprost) and EP3 (e.g. enprostil; misoprostol) receptors were weak partial agonists in the EBTr cell-line assay. PGF2α, 11β-PGF2α, and 15-cyclohexyl-PGF2α, while not very potent or efficacious, produced some stimulation of cyclic AMP, whereas the FP-receptor agents such as 17-phenyl-PGF2α, cloprostenol, fluprostenol, and PHXA85 were inactive at concentrations up to 100 μM. In addition, TP (U46619) and IP (iloprost and PGI2) receptor agonists were weak or inactive in this adenylyl cyclase assay system (Table 1).
Table 1.
Potencies and efficacies of various prostaglandins in stimulating cyclc AMP formation in EBTr cells
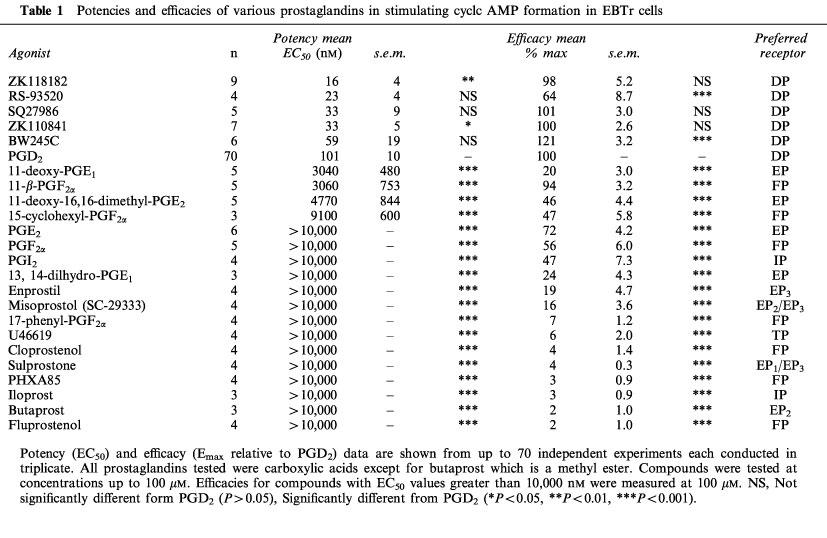
Figure 1.
Representative concentration-response curves for various prostaglandins stimulating cyclic AMP production in the EBTr cells using a 15 min stimulation protocol. Cyclic AMP was measured by a semi-automated RIA. Data points represent means from triplicate samples from a single experiment (vertical lines show s.e.mean). Data from several such experiments are summarized in Table 1. The data for the different prostaglandins were normalized relative to the maximal effects of PGD2. PGD2 was tested as a reference compound in each agonist-response experiment.
Studies with the DP receptor antagonists BWA868C and AH6809
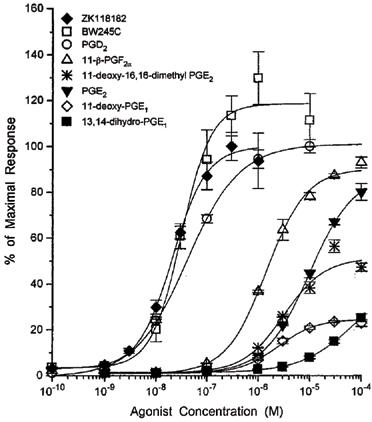
Both BWA868C and AH6809 were evaluated in EBTr cells to characterize their DP antagonist characteristics. Concentration-response curves for two different agonists in the presence or absence of increasing concentrations of BWA868C are shown in Figures 2 and 3. Increasing concentrations of this antagonist produced a rightward shift in the concentration-response curves for BW245C and ZK118182 with no change in the maximal cyclic AMP production. The Schild analyses of these types of data from several experiments are shown in Figures 4 and 5. The apparent pA2 values computed from these studies were: 8.00±0.02 (slope=1.21±0.1; r=0.986) for BW245C and 8.14±0.13 (slope=1.05±0.01; r=0.995) for ZK118182 (n=3 for each agonist). The slopes of the Schild plots for these compounds were not statistically different from unity (P>0.05).
Figure 2.
Concentration-response curves for BW245C stimulating cyclic AMP production in the presence of varying concentrations of the DP-receptor antagonist BWA868C in the EBTr cells. BWA868C was in contact with the cells for a total of 60 min to permit equilibrium. The cyclic AMP produced was measured by a semi-automated RIA. Data points represent means from triplicate samples from a single experiment (vertical lines show s.e.mean). Data from this and other similar experiments were transformed into Schild plots as shown in Figure 4.
Figure 3.
Concentration-response curves for ZK118182 stimulating cyclic AMP production in the presence of the DP-receptor antagonist BWA868C in the EBTr cells. BWA868C was in contact with the cells for a total of 60 min to permit equilibrium. The cyclic AMP was measured by a semi-automated RIA. Data points represent means from triplicate samples from a single experiment (vertical lines show s.e.mean). Data from this and other similar experiments were transformed into Schild plots as shown in Figure 5.
Figure 4.
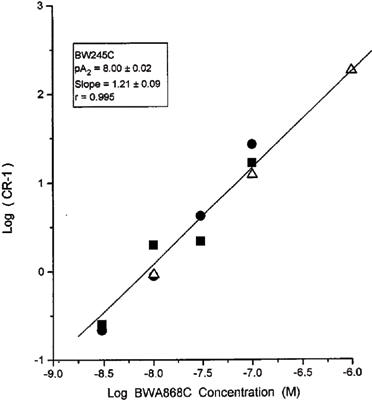
Schild studies with varying concentrations of the DP-receptor antagonist BWA868C versus the agonist BW245C in the EBTr cell cyclic AMP production assay. Analyses of cyclic AMP were performed by a semi-automated RIA. Data points represent means from triplicate samples from individual experiments. Linear regression line through the means of the data points is shown. Three independent experiments were performed, as indicated by the different symbols.
Figure 5.
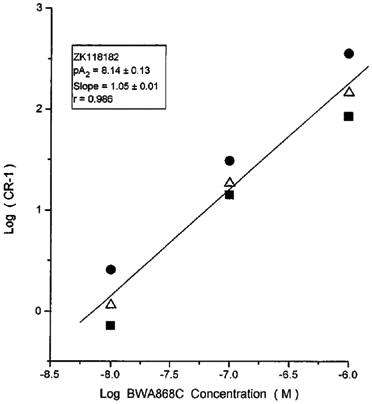
Schild studies with varying concentrations of the DP antagonist BWA868C versus the agonist ZK118182 in the EBTr cell cyclic AMP production assay. The cyclic AMP produced was measured by a semi-automated RIA. Data points represent means from triplicate samples from the individual experiments. Linear regression line through the means of the data points is shown. Three independent experiments were performed, as indicated by the different symbols.
In comparison with BWA868C, AH6809 demonstrated weak antagonism of the cyclic AMP formation stimulated by DP receptor agonists (Figure 6). The equilibrium dissociation constant (Ki) values for AH6809 antagonizing the PGD2 (1 μM)- and ZK118182 (10 nM)-induced cyclic AMP production were 808±193 nM (pKi=6.09; n=3) and 782±178 nM (pKi=6.1; n=4), respectively.
Figure 6.
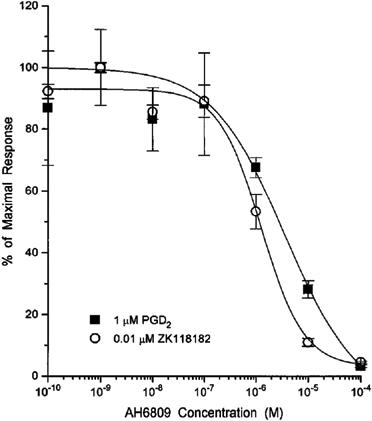
Representative curves for inhibition of DP-agonist-mediated cyclic AMP production by various concentrations of AH6809. The antagonist was in contact with the cells for 60 min to permit equilibrium. The cyclic AMP produced was measured by a semi-automated RIA. Data points represent means from triplicate samples from a single representative experiment (vertical lines show s.e.mean). Composite data from 3–4 similar experiments are described in the Results section.
Discussion
Ito et al. (1990) had provided the first evidence that the EBTr cells contained a DP receptor coupled to cyclic AMP production. In the present studies we have provided a detailed pharmacological characterization of this functional DP receptor using over 20 different agonist prostanoids and two different DP-receptor antagonists, thereby extending the work initiated by Ito et al. (1990).
The data in Table 1 emphasize the selectivity of the prostanoid receptor in EBTr cells for PGD2 and closely related prostaglandins. Numerous compounds of the DP prostanoid class were potent and full agonists which yielded potency values from 16–101 nM for the stimulation of adenylyl cyclase activity while maintaining full efficacy. Our data agreed well with the published results for PGD2 and ZK110841 in the EBTr cell line (Ito et al., 1990). Interestingly, whilst the potency ratio of BW245C and PGD2 in the EBTr cell assay system was approximately two, the ratio observed in human platelets was close to 30 (Giles et al., 1989; 1991). This probably reflected differences in the efficiency and degree of receptor-effector coupling and/or differences in receptor reserves in the two systems.
RS-93520, a DP receptor agent, produced an potency value (23 nM) in the current studies for the stimulation of adenylyl cyclase that was comparable to its potency for the inhibition of human platelet aggregation (38 nM, Alvarez et al., 1991). This compound was unique among the DP-receptor agonists tested in the EBTr cells because it exhibited a partial agonist profile (Emax=64±9%; P<0.001 when compared to PGD2). In contrast, BW245C produced about 121% of the maximal stimulation of adenylyl cyclase activity observed with PGD2 (P<0.001) in the EBTr cells. This phenomenon of increased efficacy of BW245C was also observed in HEK 293 cells expressing the recombinant human DP receptor (Boie et al., 1995) where it was attributed to a subpopulation of endogenous EP receptors. BW245C is known to have activity at EP2 receptors (Giles et al., 1989; Matsugi et al., 1995). However, in our studies since PGE1, PGE2 and a variety of their EP-receptor-analogues were weak stimulators of the cyclase response in the EBTr cells, the apparent higher intrinsic activity of BW245C appear not to be due to stimulation of EP receptors in this cell-line. This phenomenon associated with BW245C and EBTr cell DP-receptor-effector coupling warrants further study in order to better understand the receptor reserve and intrinsic activity properties of this system.
In order to characterize the functional response of the DP receptor in the EBTr cells, prostaglandins from the other receptor classes were evaluated. Several compounds from the EP receptor class exhibited potencies approximately 100 fold less than those for the DP agonists discussed above. Therefore, a means for discriminating prostanoid agonists of different classes was provided by this assay system, with DP-receptor characteristics being the most prominent. In general, EP receptor compounds were partial agonists showing less than 100% of the maximal stimulation achieved with a DP receptor agent. As expected, the EP and IP receptor agonists produced some activity since the DP receptor shows the greatest sequence homology with receptors from this class (Boie et al., 1995). FP agonists showed little or no activity at the DP prostanoid receptor, unlike PGD2 which has considerable efficacy at FP receptors (Giles et al., 1991; Griffin et al., 1997; 1998; Sharif et al., 1998), and also unlike some DP agonists which are relatively potent and efficacious EP2-receptor agonists (Crider et al., 1998). The rank order of potency for stimulating cyclic AMP production in the EBTr cells (ZK110841 = BW245Cthinsp;⩾ PGD2>>PGE2>PGF2α>Iloprost ≈percnt;U46619) is comparable to published data in HEK 293 (EBNA) cells expressing a human cloned DP receptor (Boie et al., 1995; Wright et al., 1998).
Antagonist studies
Antagonist studies with the classic DP-receptor antagonist, BWA868C, were conducted using two different DP receptor agonists, BW245C and ZK118182. BWA868C exhibited competitive antagonist characteristics with both agonists as also observed in several other cells/tissues (Giles et al., 1989; Trist et al., 1989; Senior et al., 1993; Lydford et al., 1996). In our studies, Schild plots (Figures 4 and 5) produced pA2 values of 8.14 for ZK118182 and 8.0 for BW245C when titrated against BWA868C. This showed an agonist independent antagonism that was competitive in nature since the slopes of both lines were not significantly different from unity and maximal efficacy was observed in the presence of the antagonist under these conditions. Similar competitive antagonist effects of BWA868C have been described in human platelets, rabbit jugular vein, and human myometrium (Giles et al., 1989; Trist et al., 1989; Senior et al., 1993). Smooth muscle relaxation experiments with BWA868C in canine dorsal nasal vein, major palatine artery, and saphenous vein produced pKB values of approximately 7.3, 7.6, and 7.1, respectively (Liu et al., 1996b). Adenylyl cyclase stimulation experiments on human platelets with BWA868C versus BW245C produced a pKB estimate of 9.11 (Trist et al., 1989). However, Giles et al. (1989) observed pKB estimates of 9.26 and 8.7 (BW245C versus BWA868C) in platelets and rabbit jugular vein, respectively. Additional data obtained for BWA868C in the current studies (pKi=8.59 and 8.8 versus PGD2 and SQ27986, respectively; data not shown) and the pA2 values mentioned above in our EBTr cell system were thus similar to those data reported for other cells and tissues mentioned above. However, a departure from a simple competitive antagonist profile was observed in platelet aggregation studies with BWA868C and BW245C that produced a Schild slope of 1.25 (Giles et al., 1989). The range of pKB/pA2 values of 7.1–9.3 for BWA868C noted above could have been attributed to varying experimental conditions as well as species differences. However, the possible existence of DP receptor subtypes, as has been proposed in the literature (Fernandes & Crankshaw, 1995; Rangachari et al., 1995), could also explain these results. Taken together, these observations warrant further studies to delineate the possible existence of multiple DP receptor subtypes.
Our studies confirm that AH6809 is a relatively weak antagonist at the DP receptor in EBTr cells just as previously reported by Ito et al. (1990). In our studies, AH6809 produced Ki values of approximately 808 nM (pKi=6.09) and 782 nM (pKi=6.1) when titrated against PGD2 (1 μM) and ZK118182 (10 nM), respectively (see Figure 6 for a representative inhibition plot). AH6809 was shown to possess weak DP receptor antagonists activity in human platelet aggregation assays with a pA2 value of 5.35 when evaluated against PGD2 (Keery & Lumley, 1988). However, while of some pharmacological utility, AH6809 is not a selective agent since it also exhibits antagonist activity at the EP2 receptor (Woodward et al., 1995; Crider et al., 1998). In this respect, BWA868C continues to be the most preferred DP-receptor antagonist of nanomolar affinity and potency.
In conclusion, we have employed a diverse group of prostaglandin agonists with varying potencies and receptor-selectivities to characterize the DP receptor present on EBTr cells coupled positively to adenylyl cyclase and the stimulation cyclic AMP production. The rank orders of prostaglandin potencies clearly indicated DP-receptor pharmacology. This was further borne-out by the use of two known DP-receptor antagonists, BWA868C and AH6809, which exhibited differential potencies as reported for other biological systems in the literature. The data in the current studies support the utility of the EBTr cell line, expressing an endogenous DP receptor, as a useful tool for the evaluation of various prostaglandins for their DP receptor agonist and/or antagonist properties.
Acknowledgments
We would like to thank Dr Tom Dean for his critical review of this manuscript. We appreciate the efforts of the Medicinal Chemistry group in synthesizing some of the key reference compounds.
Abbreviations
- EC50
concentration of agonist required to produce 50% of the maximal response (potency)
- Emax
per cent of the maximal response (intrinsic activity efficacy)
- IC50
concentration of inhibitor required to produce 50% of the maximal inhibition
- Ki
concentration of inhibitor required to produce 50% of the maximal inhibition at equilibrium
- pA2
equilibrium dissociation constant of the antagonist
- RIA
radioimmunoassay
References
- ALEXANDER S.P.H., PETERS J.A.1998 receptor and ion channel nomenclature supplement Trends Pharmacol. Sci. 1998SupplElsevier Salna Ltd; 64–66.Ninth edition. pp [Google Scholar]
- ALVAREZ R., EGLEN R.M., CHANG L.F.K., BRUNO J.J., ARTIS D.R., KLUGE A.F., WHITING R.L. Stimulation of prostaglandin D2 receptors on human platelets by analogs of prostacyclin. Prostaglandins. 1991;42:105–119. doi: 10.1016/0090-6980(91)90070-v. [DOI] [PubMed] [Google Scholar]
- ANDERSEN N.H., RAMWELL P.W. Biological aspects of prostaglandins. Arch. Intern. Med. 1974;133:30–50. [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOIE Y., SAWYER N., SLIPETZ D.M., METTERS K.M., ABRAMOVITZ M. Molecular cloning and characterization of the human prostanoid DP receptor. J. Biol. Chem. 1995;270:18910–18916. doi: 10.1074/jbc.270.32.18910. [DOI] [PubMed] [Google Scholar]
- CAMRAS C.B., ALM A. Initial clinical studies with prostaglandins and their analogues. Survey Ophthal. 1997;41 Suppl. 2:S61–S68. doi: 10.1016/s0039-6257(97)80009-4. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., HUMPHREY P.P.A., KENNEDY I., LUMLEY P. Prostanoid receptors: The development of a working classification. Trends Pharmacol. Sci. 1984;5:303–306. [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. VIII. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- CRIDER J.Y., GRIFFIN B.W., SHARIF N.A. Prostaglandin-stimulated adenylyl cyclase activity via a pharmacologically defined EP2 receptor in human nonpigmented ciliary epithelial cells. J. Ocular Pharmacol. Ther. 1998;14:293–304. doi: 10.1089/jop.1998.14.293. [DOI] [PubMed] [Google Scholar]
- DARIUS H., MICHAEL-HEPP J., THIERAUCH K.-H., FISCH A. Inhibition of human platelets and polymorphonuclear neutrophils by the potent and metabolically stable prostaglandin D2 analog ZK118182. Eur. J. Pharmacol. 1994;258:207–213. doi: 10.1016/0014-2999(94)90482-0. [DOI] [PubMed] [Google Scholar]
- FERNANDES B., CRANKSHAW D. Functional characterization of the prostanoid DP receptor in human myometrium. Eur. J. Pharmacol. 1995;283:73–81. doi: 10.1016/0014-2999(95)00288-v. [DOI] [PubMed] [Google Scholar]
- GILES H., BOLOFO M.L., LYDFORD S.J., MARTIN G.R. Comparative study of the prostanoid receptor profile of 9α11β-PGF2 and prostaglandin D2. Br. J. Pharmacol. 1991;104:541–554. doi: 10.1111/j.1476-5381.1991.tb12465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILES H., LEFF P., BOLOFO M.L., KELLY M.G., ROBERTSON A.D. The classification of prostaglandin DP-receptors in platelets and vasculature using BWA868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN B.W., MAGNINO P.E., PANG I.-H., SHARIF N.A. Pharmacological characterization of an FP prostaglandin receptor on rat vascular smooth muscle cells (A7r5) coupled to phosphoinositide turnover and intracellular calcium mobilization. J. Pharmacol. Expt. Ther. 1998;286:411–418. [PubMed] [Google Scholar]
- GRIFFIN B.W., WILLIAMS G.W., CRIDER J.Y., SHARIF N.A. FP prostaglandin receptors mediating inositol phosphate generation and calcium mobilization in Swiss 3T3 cells: A pharmacological study. J. Pharmacol. Expt. Ther. 1997;281:845–854. [PubMed] [Google Scholar]
- HIRATA M., KAKIZUKA A., AIZAWA M., USHIKUBI F., NARUMIYA S. Molecular characterization of a mouse prostaglandin D receptor and functional expression of the cloned gene. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11192–11196. doi: 10.1073/pnas.91.23.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO S., NARUMIYA S., HAYAISHI O. Prostaglandin D2, a biochemical perspective. Pros. Leuko. Essent. Fatty Acids. 1989;37:219–234. doi: 10.1016/0952-3278(89)90033-1. [DOI] [PubMed] [Google Scholar]
- ITO S., OKUDA E., SUGAMA K., NEGISHI M., HAYAISHI O. Evaluation of ZK110841 and AH6809, an agonist and an antagonist of prostaglandin DP-receptors on human platelets, with a PGD2-responsive cell line from bovine embryonic trachea. Br. J. Pharmacol. 1990;99:13–14. doi: 10.1111/j.1476-5381.1990.tb14645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON S.L., FREEZER N.J., RITTER W., O'TOOLE S., HOWARTH P.H. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur. Respir. J. 1995;8:411–415. doi: 10.1183/09031936.95.08030411. [DOI] [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY I., COLEMAN R.A., HUMPHREY P.P.A., LEVY G.P., LUMLEY P. Studies on the characterization of prostanoid receptors: a proposed classification. Prostaglandins. 1982;24:667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFF P., GILES H. Classification of platelet and vascular prostaglandin D2 (DP) receptors: estimation of affinities and relative efficacies for a series of novel bicyclic ligands (With an appendix on goodness-of-fit analyses) Br. J. Pharmacol. 1992;106:996–1003. doi: 10.1111/j.1476-5381.1992.tb14447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.J., JACKSON D.M., BLACKHAM A., LEFF P. Partial agonist effects of BW A868C, a selective DP receptor antagonist, on Cl− secretion in dog tracheal epithelium. Eur. J. Pharmacol. 1996a;304:117–122. doi: 10.1016/0014-2999(96)00129-x. [DOI] [PubMed] [Google Scholar]
- LIU Y.J., JACKSON D.M., BLACKHAM A. Effects of BW A868C, a selective prostaglandin DP receptor antagonist, in dog isolated vascular preparations. Eur. J. Pharmacol. 1996b;303:187–192. doi: 10.1016/0014-2999(96)00037-4. [DOI] [PubMed] [Google Scholar]
- LYDFORD S.J., MCKECHNIE K.C.W., DOUGALL I.G. Pharmacological studies on prostanoid receptors in the rabbit isolated saphenous vein: a comparison with the rabbit isolated ear artery. Br. J. Pharmacol. 1996;117:13–20. doi: 10.1111/j.1476-5381.1996.tb15148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUGI T., KAGEYAMA M., NISHIMURA K., GILES H., SHIRASAWA E. Selective prostaglandin D2 receptor stimulation elicits ocular hypotensive effects in rabbits and cats. Eur. J. Pharmacol. 1995;275:245–250. doi: 10.1016/0014-2999(94)00788-9. [DOI] [PubMed] [Google Scholar]
- MICHEL A.D., WHITING R.L. Analysis of ligand binding data using a microcomputer. Br. J. Pharmacol. 1984;83:460P. [Google Scholar]
- NAMBA T., OIDA H., SUGIMOTO Y., KAKIZUKA A., NEGISHI M., ICHIKAWA A., NARUMIYA S. cDNA cloning of the mouse prostacyclin receptor. J. Biol. Chem. 1994;269:9986–9992. [PubMed] [Google Scholar]
- NEGISHI M., SUGIMOTO Y., ICHIKAWA A. Prostanoid receptors and their biological actions. Prog. Lipid Res. 1993;32:417–434. doi: 10.1016/0163-7827(93)90017-q. [DOI] [PubMed] [Google Scholar]
- NARUMIYA S.Prostanoid receptors: Structure, function, and distribution Cellular generation, transport, and effects of eicosanoids: biological roles and pharmacological intervention 1994New York: The New York Academy of Sciences; 126–138.ed. Goetzl, E.J., Lewis, R.A. and Rola-Pleszczynski, M. pp [DOI] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., NEGISHI M., SUGAMA K., HATANAKA M., ITO S. Cyclic AMP-mediated inhibition of cell growth by prostaglandin D2 in a fibroblastic cell line (EBTr) Eicosanoids. 1990;3:213–218. [PubMed] [Google Scholar]
- RANGACHARI P.K., BETTI P.-A., PRIOR E.T., ROBERTS L.J., II Effects of a selective DP receptor agonist (BW 245C) and antagonist (BW A868C) on the canine colonic epithelium; an argument for a different DP receptor. J. Pharmacol. Exp. Ther. 1995;275:611–617. [PubMed] [Google Scholar]
- SENIOR J., MARSHALL K., SANGHA R., CLAYTON J.K. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br. J. Pharmacol. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARIF N.A., CRIDER J.Y., GRIFFIN B.W., DAVIS T.L., HOWE W.E. Pharmacological analysis of mast cell mediator and neurotransmitter receptors coupled to adenylate cyclase and phospholipase C on immunocytochemically-defined human conjunctival epithelial cells. J. Ocular Pharmacol. Ther. 1997;13:321–336. doi: 10.1089/jop.1997.13.321. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A., WONG E.H.F., LOURY D., STEFANICH E., EGLEN R.M., MICHEL A.D., WHITING R.L. Characteristics of 5HT3 binding sites in rat cerebral cortex, NG108-15 and NCB-20 neuroblastoma cells using [3H] quipazine and [3H]GR65630 binding. Br. J. Pharmacol. 1991;102:919–925. doi: 10.1111/j.1476-5381.1991.tb12277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARIF N.A., XU S.X. Pharmacological characterization of bradykinin receptors coupled to phosphoinositide turnover in SV40-immortalized human trabecular meshwork cells. Exp. Eye Res. 1996;63:631–637. doi: 10.1006/exer.1996.0157. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A., XU S.X., WILLIAMS G.W., CRIDER J.Y., GRIFFIN B.W., DAVIS T.L. Pharmacology of [3H]prostaglandin E1/[3H] prostaglandin E2 and [3H] prostaglandin F2α binding to EP3 and FP receptor binding sites in bovine corpus luteum: characterization and correlation with functional data. J. Pharmacol. Expt. Ther. 1998;286:1094–1102. [PubMed] [Google Scholar]
- SUGAMA K., TANAKA T., YOKOHAMA H., NEGISHI M., HAYASHI H., ITO S., HAYAISHI O. Stimulation of cAMP formation by prostaglandin D2 in primary cultures of bovine adrenal medullary cells: identification of the responsive population as fibroblasts. Biochim. Biophys. Acta. 1989;1011:75–80. doi: 10.1016/0167-4889(89)90081-5. [DOI] [PubMed] [Google Scholar]
- TRIST D.G., COLLINS B.A., WOOD J., KELLY M.G., ROBERTSON A.D. The antagonism by BWA868C of PGD2 and BW245C activation of human platelet adenylate cyclase. Br. J. Pharmacol. 1989;96:301–306. doi: 10.1111/j.1476-5381.1989.tb11817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELDON A., VERDAY C.J. Characterization of the inhibitory prostanoid receptors on human neutrophils. Br. J. Pharmacol. 1993;108:1051–1054. doi: 10.1111/j.1476-5381.1993.tb13504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIERNAS T.K., GRIFFIN B.W., SHARIF N.A. The expression of functionally-coupled B2-bradykinin receptors in human corneal epithelial cells and their pharmacological characterization with agonists and antagonists. Br. J. Pharmacol. 1997;121:649–656. doi: 10.1038/sj.bjp.0701168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODWARD D.F., PEPPER D.J., BURKEY T.H., REGAN J.W. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH6809), A human EP2 receptor antagonist. Biochem. Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- WRIGHT D.H., METTERS K.M., ABRAMOVITZ M., FORD-HUTCHINSON A.W. Characterization of the recombinant human prostanoid DP receptor and identification of L-644,698, a novel selective DP agonist. Br. J. Pharmacol. 1998;123:1317–1324. doi: 10.1038/sj.bjp.0701708. [DOI] [PMC free article] [PubMed] [Google Scholar]


