Abstract
Biologically active kinin peptides are released from precursor kininogens by kallikreins. Kinins act on kinin receptors to mediate diverse biological functions including smooth muscle contraction, inflammation, pain and mitogenicity. All components of the kallikrein-kinin system exist in human male genital secretions suggesting that these molecules participate in physiological and pathophysiological genitourinary function. The objective of this study was to assess the consequences of kinin action on prostate cells.
Primary cultures of prostate secretory epithelial (PE) and prostate fibromuscular stromal (PS) cells were established from human prostate tissue. Transcripts encoding both the human B1 and B2 bradykinin receptor subtypes were detected in human prostate transition-zone tissue and in cultured cells by RT–PCR. In receptor binding assays, the B1 subtype predominated on PE cell membranes and the B2 subtype predominated on PS cell membranes. In PS cells, but not in PE cells, BK induced significant inositol phosphate accumulation and [3H]-thymidine uptake. These responses were mediated through the B2 receptor subtype.
The use of signal transduction inhibitors indicated that mitogenic activation by BK occurred through both protein kinase C (PKC) and protein tyrosine kinase dependent mechanisms. PMA (phorbol 12-myristate 13-acetate) produced maximal [3H]-thymidine uptake by PS cells, resulted in cell elongation and caused the α-actin fibres present in PS smooth muscle cells to became organized into parallel arrays along the length of the elongated cells.
In summary, the prostate contains a functional kallikrein-kinin system, which could be significant in physiological and pathophysiological prostate function.
Keywords: Prostate, kinin, bradykinin, kallidin, kinin-receptor, G-protein coupled receptor, protein kinase C, tyrosine kinase
Introduction
All components of the kallikrein-kinin system are present in human male genital secretions: (i) kininogens, the precursors of the kinins; (ii) kallikrein, as the proteolytic enzyme which releases biologically active kinins from kininogens; (iii) the kinins themselves and (iv) kininases (angiotensin converting enzyme) which inactivate kinins by proteolytic degradation (Schill & Miska, 1992). Bradykinin (BK) and lys-BK [kallidin (KD)] represent the circulating and tissue kinin forms generated, respectively, by the actions of plasma and tissue kallikreins. Two types of human kinin receptors have been cloned, B1 and B2. BK and KD preferentially stimulate the B2 receptor subtype whereas the proteolytically degraded forms [des-Arg9]BK and [des-Arg10]KD preferentially stimulate the B1 receptor subtype (Hall, 1992; Regoli & Barabe, 1980). The kinin receptors belong to the G-protein coupled receptor superfamily which span the cell membrane seven times and which couple on the cytoplasmic side of the membrane to heterotrimeric G-proteins (Neer, 1995). Multiple G-proteins exist which can be grouped according to the intracellular response elicited (Neer, 1995). BK has been variously shown to activate phospholipases A2, C and D, adenylate cyclase, guanylate cyclase and to mobilize calcium (Hall, 1992; 1997), suggesting that kinin receptors might interact with multiple G-proteins. Consistent with this, BK has been shown to exert numerous biological effects including vascular smooth muscle contraction (Correa & Graeff, 1975), stimulation of electrolyte fluxes (Manning et al., 1982), the generation of inflammation (Proud & Kaplan, 1988), and pain (Steranka et al., 1988) and mitogenicity (Goldstein & Wall, 1984).
There is evidence to suggest that the kinins might have a role in the pathophysiology of prostate diseases such as benign prostatic hyperplasia (BPH). BPH describes a benign proliferation of stromal and epithelial elements of the prostate gland, however it is fibromuscular stromal proliferation that is associated with the bothersome urinary symptoms that accompany BPH (Shapiro et al., 1992; Bartsch et al., 1979). Reduction of fibromuscular stromal mass and tone are clinically important objectives in the treatment of BPH. BK has been shown to elicit prostatic smooth muscle contraction (Steidle et al., 1990; Watts & Cohen, 1991). Prostate specific antigen (PSA) is a serine protease with a high degree of sequence homology to tissue kallikrein. Fichtner et al. (1996) demonstrated that PSA could release a kinin-like substance from seminal plasma which induced bladder smooth muscle contraction. Furthermore, kinin receptor antagonists inhibited this contractile response. The objective of this study was to examine signal transduction mechanisms activated by kinins in human prostate derived cells in order to provide insight into the potential involvement of kinins in prostate disease.
Methods
Tissue isolation and cell culture
Human prostate tissue was obtained from male patients undergoing prostatectomy for BPH or prostate cancer with Institutional Board of Research Associates approval at NYU Medical Center. Under aseptic conditions in a positive flow tissue culture hood, histopathologically confirmed benign tissue was dissected away from a portion of the gland. For RNA isolation, tissue was snap frozen in liquid nitrogen. Prostate secretory epithelial (PE) and prostate fibromuscular stromal (PS) cells were cultured from mixed explants of prostate tissue as described (Walden et al., 1998).
Measurement of bradykinin and kallidin
The conditioned medium was removed from sub-confluent cultured PE or PS cell monolayers after 24 h. The number of cells in the monolayers was determined by trypsinizing and counting in a Coulter model ZM counter. Peptides were extracted from the conditioned medium samples using the general protocol supplied with radio-immune assay (RIA) kits (Peninsula Laboratories, Sunnyvale, CA, U.S.A.). Briefly, the conditioned media were acidified by adding an equal volume of 1% (v v−1) trifluoroacetic acid (TFA) and applied to equilibrated 200 mg C18 columns (Bakerbond ‘spe'; J.T. Baker, NJ, U.S.A.). The columns were washed in 1% (v v−1) TFA and the peptides were eluted using 60% (v v−1) acetonitrile in 1% (v v−1) TFA. The eluate was evaporated to dryness using a centrifugal concentrator (Savant, Farmingdale, NY, U.S.A.). Recovery from these columns was determined by spiking culture medium with known amounts of BK or KD. For the range of concentrations of kinins present in our tissue and media samples, recovery from the columns was 83±4.4% (n=8). The presence of kinins in the samples was determined using RIA kit RIK-7051 from Peninsula Laboratories (Sunnyvale, CA, U.S.A.). The antisera included with RIK-7051 had a reported cross-reactivity of 100% with both BK and KD. Inter-assay and intra-assay variabilities were ⩽8.2% and ⩽4.9%, respectively.
Receptor binding assays
Kinin receptor binding assays were performed essentially as described (Manning et al., 1986). Briefly, membranes were isolated from PE and PS cells by sonication in ice-cold 25 mM TES, pH 6.8, 1 mM 1, 10 phenanthroline, followed by two rounds of centrifugation at 50,000×g. Incubations were performed for 45 min at 25°C in a volume of 1 ml of assay buffer (TES 25 mM, pH 6.8, 1, 10 phenanthroline 1 mM, bacitracin 140 μg ml−1 teprotide 1 μM, DTT 1 mM and 0.1% BSA) containing [3H]-BK 1 nM or [des-Arg10], [3,4-3H]KD and 50 μg membrane protein (as determined using a colorimetric assay [Biorad, Hercules, CA, U.S.A.)]. Receptor subtype determinations were performed in competition experiments which included the B1 selective agonist [des-Arg10]KD or the B2 selective antagonist Hoe 140. Three separate competitive experiments were performed for each of the competitors. Data were analysed by iterative curve fitting to a one- or two-site binding model (Cheng & Prusoff, 1973). Linear transformation of the binding data was conducted as described (Scatchard, 1949).
Measurement of phosphoinositide hydrolysis
Agonist stimulated phosphoinositide hydrolysis in prostate-derived cells was performed as described (Walden et al., 1998). Briefly, cells were plated in 12-well dishes and at 80% confluence the growth medium was replaced with either regular growth medium (PE cells) or Minimal Essential Medium supplemented with 0.5% (v v−1) FCS (PS cells) that had been supplemented with 5 μCi ml−1[3H]-inositol. The cell monolayers were incubated at 37°C overnight. The medium was then changed to serum-free Ham's F12 containing 10 mM lithium chloride and the indicated concentration of agonist for a further 90 min. Phosphoinositides and inositol phosphates were extracted and analysed.
Thymidine uptake assays
For measurement of thymidine uptake, cell monolayers in 12-well plates were incubated for 72 h in 99.5% RPMI 1640, 0.5% (v v−1) FCS. At this time the compounds BK, FCS, PMA, 4α-PMA, Ro 31-8220, genistein or herbimycin A were added to the medium, either alone or in combination, to the final concentrations indicated under the appropriate section in Results. When used in combination with agonists, the inhibitors were added 30 min prior to agonist addition. Following a 23 h incubation with agonists or agonists/antagonists, 5 μCi / ml−1 of methyl-[3H]-thymidine was added to the medium and the incubation at 37°C continued for 1 h. At this point cells were 80–90% confluent. Thymidine incorporation into cellular DNA was determined as described (Simonson & Herman, 1993).
Immunofluorescence microscopy
For immunofluorescence microscopy, cells were plated into 2-well Lab-Tek® Chamber Slides™ (Nunc, Naperville, IL, U.S.A.). At 80% confluence the cell culture medium was replaced with 99.5% RPMI 1640, 0.5% (v v−1) FCS for 24 h and then BK, FCS, PMA or 4α-PMA were added to the medium to the final concentrations indicated under the appropriate section in Results. Following a 12 h incubation with these reagents, the cell monolayers were rinsed briefly in PBS and fixed in methanol at −20°C. The slide-mounted specimens were then rehydrated in PBS, smooth muscle α-actin fibres were visualized by indirect immunofluorescence microscopy using a mouse monoclonal anti-human smooth muscle α-actin antibody (DAKO Corporation, Carpinteria, CA, U.S.A.) and a fluorescein isothiocyanate (FITC) conjugated rabbit anti mouse IgG secondary antibody (Sigma, St. Louis, MO, U.S.A.).
RNA Isolation and RT–PCR
Total RNA was isolated from cell cultures and tissue samples by acid guanidine thiocyanate phenol chloroform extraction (Chomczynski & Sacchi, 1987). Total RNA (1 μg) was reverse transcribed into cDNA using random primers and Superscript reverse transcriptase (Gibco BRL, Grand Island, NY, U.S.A.). An aliquot (10%) of the reverse transcription reaction was used for PCR. Primer pairs specific for the human kinin receptor subtypes B1 and B2 (Table 1) were designed using the GCG ‘Prime' software package. In all cases the quality of cDNA was confirmed by amplification with human β-actin primers (Table 1). Unless otherwise stated in the text, PCR conditions were 35 cycles of 94°C for 30 s, 53°C for 30 s and 72°C for 1 min using a Perkin Elmer 9600 thermal cycler. By measuring radioactive label incorporation into the correctly sized products after various numbers of PCR cycles, we established that amplification for each primer pair was exponential under the conditions used in this manuscript. PCR products were resolved by electrophoresis in a 5% (v v−1) polyacrylamide, 1×TBE (0.09 M Tris, 0.09 M boric acid, 2 mM EDTA) gel. Authenticity of the PCR products was verified by nucleotide sequencing following isolation of the PCR product from an agarose gel using QiaEx II (Qiagen, Chatsville, CA, U.S.A.) and cloning into the PCR-TRAP vector (GenHunter, Nashville, TN, U.S.A.).
Table 1.
PCR primers used
Materials
Tissue culture reagents were obtained from Sigma (St. Louis, MO, U.S.A.) or Gibco-BRL (Grand Island, NY, U.S.A.). Methyl-[3H]-thymidine, [3H]BK and [des-Arg10],[3,4-3H]KD were obtained from NEN (Boston, MA, U.S.A.). Myo-[3H]-inositol (sp. act., 15 Ci mol−1) was obtained from ARC (Bowling Green, MO, U.S.A.). PMA (Phorbol 12-myristate 13-acetate), 4α-PMA, (4α-phorbol 12-myristate 13-acetate), Ro 31-8220, genistein and herbimycin A were obtained from Research Biochemicals International (Natick, MA, U.S.A.). Bradykinin (BK), [des-Arg10]KD, [des-Arg9Leu8]BK and Hoe 140 were obtained from Peninsula Laboratories (Sunnyvale, CA, U.S.A.). BK is Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg. KD is [Lys0]BK. Hoe 140 is D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]BK, where Hyp is hydroxyproline, Thi is L-3-(2-thienyl)-alanine, Tic is D-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid and Oic is (3S,7S)-9-(3α,7α)-octahydroindol-2-carboxylic acid.
Results
Kinin and kinin receptor expression by prostate derived cells
The single human kininogen gene is transcribed into two different mRNAs by alternate splicing. The two kininogen mRNAs encode high- and low- molecular weight kininogens. Plasma and tissue kallikreins release BK and KD respectively from high- and low-molecular weight kininogen precursors. Using an antiserum which did not cross-react with kininogens, we measured immunoreactive kinin (BK+KD) levels in prostate tissue and in the 24 h conditioned medium of cultured prostate epithelial (PE) and stromal (PS) cells by RIA. Using this assay we determined that immunoreactive kinin was present in benign prostate transition zone tissue at levels of 2.8±0.2 pg mg−1 wet weight (n=8). Immunoreactive kinins were secreted by PS cells into the conditioned medium at rates of 4.9±0.3 pg ml−1 per 106 cells in 24 h. In contrast, immunoreactive kinin levels in the conditioned medium of PE cells were below the limits of detection by RIA (<0.05 pg ml−1 per 106 cells in 24 h). Based on these data we cannot rule out the possibility that PE and/or PS cells secrete kininogens or ACE, however these results suggest that PS cells, but not PE cells have the capacity to generate steady state levels of biologically active kinins.
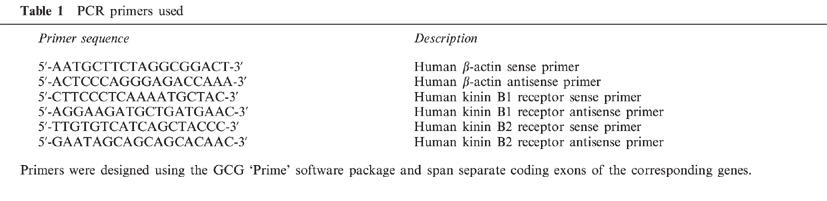
The kinin receptor subtypes expressed by prostate tissue and prostate-derived cultured cells were examined by RT–PCR and by receptor binding assays. Specific amplification products of 327 and 208 bp derived from the human kinin receptor B1 and B2 subtype mRNAs, respectively, were detected using total RNA isolated from benign prostate transition zone prostate tissue and from cultured PS cells (Figure 1). Using PE cell RNA, the B1 product was the predominant product detected. The B2 PCR product was barely detectable in PE cells and may represent cross-contamination by PS cells; by analysis of cell-type specific markers (see Methods) we determined the cross-contamination of cell types to be less than 3% (data not shown).
Figure 1.
Expression of kinin receptor B1 and B2 mRNAs in human prostate derived cell lines and tissue. Total RNA (1 μg) isolated cultured prostate-derived PS cells (lanes 1 and 5) PE cells (lanes 2 and 6) and from prostate tissue (lanes 3 and 7) was reverse transcribed into cDNA and used as a template for PCR amplification with primer pairs specific for human kinin receptor B1 mRNA (lanes 1–4) and B2 mRNA (lanes 5–8). PCR products were resolved by electrophoresis in a 5% acrylamide, 1×TBE gel and stained with ethidium bromide. Correctly sized PCR products of 327 and 208 bp were observed for the B1 and B2 receptor subtype mRNAs, respectively. The authenticity of the PCR products was confirmed by nucleotide sequencing. Lanes 4 and 8 represent sham RT–PCR reactions performed with no reverse transcriptase. M; molecular weight standards.
To determine if the RT–PCR data reflected receptor protein expression, saturation and competition binding experiments were performed using PE and PS cells membranes. Scatchard analysis (Scatchard, 1949) of the saturation binding data indicated a single high affinity binding site present on both PE cell membranes and PS cell membranes. Competition binding experiments revealed a rank order of potency of [des-Arg10]KD>BK>Hoe 140 for PE cell membranes and a rank order of potency of Hoe 140>BK>[des-Arg10]KD for PS cell membranes. Bmax and Kd values obtained using the B1-selective agonist [des-Arg10]KD for PE cell membranes were 55 fmol mg protein−1 and 0.3 nM respectively. The corresponding data for PS cell membranes obtained using BK were 86 fmol mg protein−1 and 0.4 nM respectively. These results suggest that the B1 receptor predominates on PE cell membranes in agreement with the RT–PCR data. The binding studies suggest that the B2 receptor predominates on PS cell membranes. Thus, the B1 receptor transcripts detected in PS cells by RT–PCR do not appear to significantly contribute to the kinin receptor pool. The presence of kinins in prostate tissue extracts and in the conditioned medium of PS cells, and the existence of stromal and epithelial kinin receptors suggest potential autocrine and paracrine mechanisms of action. The agonist effects of kinins on prostate cells were examined further.
Membrane phospholipid hydrolysis by fibromuscular stromal cells in response BK
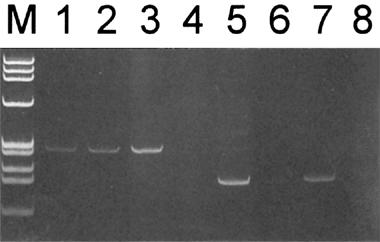
The kinin receptors belong to the seven-transmembrane domain G-protein coupled receptor superfamily. G-protein coupled receptors for ligands which cause contraction such as the kinins often couple to Gq upon agonist binding, resulting in the activation of phospholipase Cβ (PLCβ), leading to membrane phosphatidylinositol (4,5)-bisphosphate (PtdInsP2) breakdown and the production of two intracellular second messengers, diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate (InsP3) (Berridge & Irvine, 1989; Hall, 1992). BK did not stimulate membrane PtdInsP2 breakdown in PE cells (data not shown). In contrast, exposure of PS cells to BK elicited a potent breakdown of membrane PtdlnsP2; BK at a concentration of 10−8 M to 5×10−7 M resulted in maximal InsP3 accumulation and >90% reduction of radioactivity in the PtdlnsP2 pool during a 30 min incubation. The dose response accumulation of lnsP3 in response to BK is shown in Figure 2A. The human B1-kinin receptor specific agonist [des-Arg10]KD had no effect on lnsP3 accumulation by PS cells (Figure 2A). In contrast, InsP3 accumulation in PS cells in response to BK was blocked by the B2 kinin receptor specific antagonist Hoe 140 (Figure 2B). These findings are consistent with our demonstration that the B2 receptor was the predominant subtype present in PS cells.
Figure 2.
BK Induces inositol phosphate accumulation in PS cells. (A) PS cell monolayers were labeled with [3H]-inositol for 16 h and then stimulated in serum free medium containing vehicle, BK or [des-Arg10]KD for 90 min. Incorporation of [3H] into inositol phosphates was determined. Data are represented as the mean fold stimulation over vehicle control±s.e.mean for four independent experiments performed in sextuplicate. *P<0.05 compared to vehicle alone. (B) PS cell monolayers were labelled with 3H-inositol for 16 h and then stimulated in serum free medium containing vehicle, BK (10−8 M), BK (10−8 M) plus [des-Arg9Leu8]BK (5×10−8 M) or BK (10−8 M) plus Hoe 140 (5×10−8 M) for 90 min. Incorporation of [3H] into inositol phosphates was determined. Data are represented as the mean fold stimulation over control±s.e.mean for four independent experiments performed in sextuplicate. *P<0.001 compared to vehicle alone.
BK induces mitogenic signalling in fibromuscular stromal cells
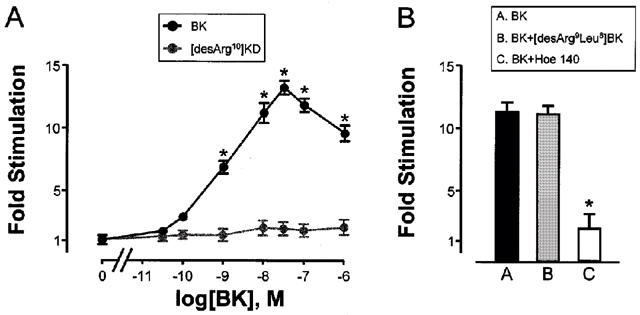
In addition to the short term biological effects, membrane phospholipid derived second messengers can also induce long term biological effects. The binding of InsP3 to specific receptors on the endoplasmic (sarcoplasmic) reticulum releases stored calcium (Berridge & Irvine, 1989). Calcium and DAG are activators of protein kinase C (PKC) which can initiate a signalling cascade culminating in the activation and nuclear translocation of mitogen activated protein kinase (MAPK) with concomitant affects on gene transcription and cell proliferation (Berridge & Irvine, 1989). We examined the effects of BK on mitogenic signalling in prostate cells. BK had no effect on [3H]-thymidine uptake by quiescent PE cells (data not shown). In a dose dependent manner, BK stimulated [3H]-thymidine uptake into DNA by quiescent PS cells (Figure 3A). As expected, [des-Arg10]KD had no effect on [3H]-thymidine uptake by PS cells (Figure 3A). Also as expected, [3H]-thymidine uptake by PS cells in response to BK was blocked by B2 antagonist Hoe 140 (Figure 3B).
Figure 3.
BK Induces [3H]-thymidine uptake by PS cells. (A) Cultured prostate-derived PS cell monolayers were incubated in RPMI 1640, 0.5% (v v−1) FCS for 72 h. Cells were then exposed to vehicle, BK or [des-Arg10]KD for 23 h and pulsed for 1 h with [3H]-thymidine. Incorporation of label into DNA was determined. Data are represented as the mean fold stimulation over vehicle control±s.e.mean for six independent experiments performed in sextuplicate. *P<0.05 compared to vehicle alone. (B) Cultured prostate-derived PS cell monolayers were incubated in RPMI 1640, 0.5% (v v−1) FCS for 72 h. Cells were then exposed to vehicle, BK (10−8 M), BK (10−8 M) plus [des-Arg9Leu8]BK (5×10−8 M) or BK (10−8 M) plus Hoe 140 (5×10−8 M) for 23 h and pulsed for 1 h with [3H]-thymidine. Incorporation of label into DNA was determined. Data are the mean±s.e.mean for four independent experiments performed in sextuplicate. *P<0.001 compared to vehicle alone.
Effects of signal transduction inhibitors on BK-induced mitogenic signalling in PS cells
PKC is a central component of the G-protein coupled receptor signalling pathways activated by contractile ligands. However, it is also known that tyrosine kinases can be activated by a cross-talk between the signalling pathways of G-protein coupled receptors and tyrosine kinase coupled receptors associated with growth factors such as EGF. We used specific signal transduction inhibitors to determine whether PKC-dependent pathways or protein tyrosine kinase (PTK) dependent pathways, or both contribute to mitogenic signal transduction by BK in PS cells. Initially, pilot dose response experiments that involved exposing PS cells to a constant concentration of BK (10−8 M) and various concentrations of PKC or PTK inhibitors. The maximal effective concentration of each inhibitor (obtained from the plateau region of the dose response curves) was used in subsequent experiments. One PKC selective inhibitor, Ro 31-8220 (Dieter & Fitzke, 1991) and two PTK selective inhibitors, herbimycin A (Uehara et al., 1986) and genistein (Akiyama et al., 1987) were used in these experiments. Statistically significant increases in [3H]-thymidine incorporation were observed when BK (10−8 M) was added to PS cultures in the presence of either Ro 31-8220 (1 μM), herbimycin A (6 μg ml−1) or genistein (1 μM) (Figure 4). When Ro 31-8220 and herbimycin A were used in combination, BK was unable to increase nuclear [3H]-thymidine incorporation (Figure 4). The results indicate that mitogenic signalling by BK occurs by both PKC and PTK dependent mechanisms.
Figure 4.
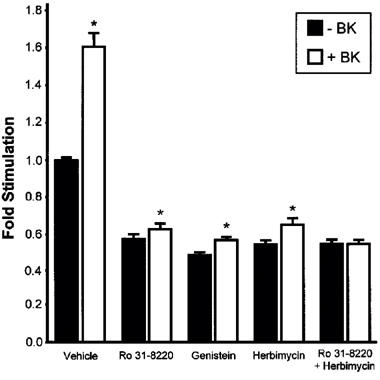
Effects of signal transduction inhibitors on BK induced [3H]-thymidine uptake by PS cells. Cultured prostate-derived PS cell monolayers were incubated in RPMI 1640, 0.5% (v v−1) FCS for 72 h. Cells were then exposed to vehicle, Ro 31-8220 (1 μM), genistein (6 μg ml−1), herbimycin A (1 μM) or both Ro 31-8220 (1 μM) and herbimycin A (1 μM) in the absence or presence of BK (10−8 M) for 23 h and pulsed for 1 h with [3H]-thymidine. Incorporation of label into DNA was determined. Data are represented as the mean fold stimulation over vehicle control±s.e.mean for four independent experiments performed in sextuplicate. *P<0.05 compared to −BK.
Effects of phorbol esters on mitogenic signalling and cell morphology
Because of the involvement of PKC in mitogenic signalling by BK, we tested the effects of PKC activating phorbol esters on [3H]-thymidine incorporation into DNA in quiescent PS cells (Figure 5). Incorporation of [3H]-thymidine into DNA the presence of the inactive isomer 4α-PMA (10 nM) was not different from the vehicle control (Figure 5A). In contrast, PKC activating PMA (10 nM) resulted in a highly significant incorporation of [3H]-thymidine into PS cell DNA. When BK was added to 4α-PMA treated PS cell, [3H]-thymidine incorporation into DNA was not different from the cells treated with BK alone. Addition of BK to PMA treated cells increased [3H]-thymidine incorporation above levels seen with PMA alone, however the increase was not significant.
Figure 5.
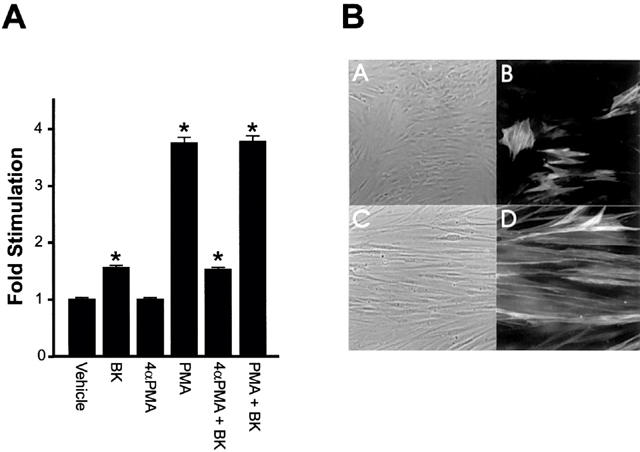
Effects of PKC activation on PS cells [3H]-thymidine uptake and morphology. (A) Cultured prostate-derived PS cell monolayers were incubated in RPMI 1640, 0.5% (v v−1) FCS for 72 h. Cells were then exposed to vehicle, BK, 4α-PMA (10 nM), PMA (10 nM), 4α-PMA (10 nM) plus BK (10−8 M) or PMA plus BK (10−8 M) for 23 h and pulsed for 1 h with [3H]-thymidine. Incorporation of label into DNA was determined. Data are represented as the mean fold stimulation over vehicle control±s.e.mean for four independent experiments performed in sextuplicate. *P<0.05 compared to vehicle alone. (B) PS cell monolayers in Lab-Tek Chamber Slides were incubated in RPMI 1640, 0.5% (v v−1) FCS for 72 h at which point the various compounds were added and the incubation continued for a further 12 h. Cells were then fixed in methanol and incubated with an anti-smooth muscles α-actin primary antibody and a rhodamine labelled secondary antibody. The labelled cells were observed by phase-contrast (A and C) and immunofluorescence (B and D) optics. Images show cells exposed to 10 nM 4α-PMA (A and B) or 10 nM PMA (C and D).
One noticeable aspect of PS cells treated with PMA was their characteristic altered morphology. Cells treated for 12 h with 10 nM PMA were long and slender (Figure 5B, panel C) compared to control cells treated with 4α-PMA (Figure 5B, panel A) or vehicle (data not shown). The PMA treated cells were highly ordered and arranged in parallel arrays with the long axis of the cells facing the same direction and intracellular smooth muscle α-actin fibres were aligned along the long axis of the cell (Figure 5B, panel D). Furthermore, the proportion of cells staining for smooth muscle α-actin increased following PMA treatment. Under normal culture conditions, approximately 20% of the cells stain positively for smooth muscle α-actin (Figure 5B, panel B). PMA treatment caused a 2–3 fold increase in the proportion of the cells that stained positively for smooth muscle α-actin (Figure 5B, panel D). Thus, stimulation of PKC can result in either an increased proliferation of smooth muscle cells, or an increased differentiation of cells from a non-smooth muscle α-actin expressing phenotype to a smooth muscle α-actin expressing phenotype.
Discussion
Kinins generated by the actions of PSA, or other kallikreins, on seminal plasma kininogens (Fichtner et al., 1996) may have a functional role along the genitourinary tract (Schill & Miska, 1992). We have shown that kinins are detectable in human prostate tissue and in the conditioned medium of human-prostate derived stromal cells in culture, suggesting that seminal plasma kininogens are at least in part prostate derived. We have also demonstrated kinin receptor expression in prostate tissue and prostate-derived cell lines. The B2 kinin receptor, which displays greatest sensitivity to BK/KD, predominates on cultured prostatic stromal cell membranes. Kinins mediate a potent contraction of prostatic smooth muscle (Steidle et al., 1990; Watts & Cohen, 1991). Second messengers produced as part of the contractile response are potentially mitogenic (Berridge & Irvine, 1989). Our data has shown that BK can activate PKC-dependent and PTK-dependent mitogenic signal transduction pathways in prostate-derived fibromuscular stromal cells. These findings indicate that kinins can activate multiple signal transduction mechanisms in the prostate that may have important implications in the pathophysiology of prostate diseases such as BPH and prostatitis. In BPH patients with histological acute and chronic-active prostatitis there appears to be a loss of integrity of epithelial tight junctions resulting in large increases in serum levels of PSA (Hasui et al., 1994). This leakage phenomenon could also provide access of seminal plasma kinins to normally segregated prostate tissue. Therefore the mitogenic actions of BK might be significant in terms of BPH which, symptomatically, is associated with an excess stromal proliferation (Shapiro et al., 1992; Bartsch et al., 1979) or in inflammatory diseases such as prostatitis. Kinins are modest in their growth promoting abilities compared to the classical growth factors such as EGF and FGF. The doubling time of cell growth in BPH has been estimated to be 20 years (Berry et al., 1984) and growth factors involved in the underlying etiology of this disease would be expected to have modest mitogenic effects. Furthermore kinin production may be altered during certain pathological conditions in the prostate. For example, it is known that kallikrein production by fibroblasts increases in response to inflammatory mediators (Takano et al., 1995). It has also been shown that tissue kallikrein and B2 kinin receptors are also expressed in prostate cancers (Clements & Mukhtar, 1997). The available data therefore indicates that a functional kallikrein-kinin system is present in both normal and pathological prostate tissue. The activation of multiple signal transduction mechanisms by kinins in the prostate suggests that the kallikrein-kinin system could be significant in physiological and pathophysiological prostate function.
The stromal compartment of the human prostate is composed of smooth muscle cells (predominant component), undifferentiated fibroblasts, connective tissue, nerves and vessels (lymphatic and blood). Stromal (PS) cells that are routinely cultured from human prostate comprise smooth muscle cells in various stages of dedifferentiation, such that only a proportion of the cells that stain positively for the smooth muscle differentiation marker, α-actin (Figure 5B). Our results have shown that activation of PKC in prostatic stromal cells results in both increased mitogenicity and increased expression of smooth muscle α-actin. Activation of PKC by liganded kinin receptors or other G-protein coupled receptors that activate PLCβ may not only contribute to the growth of prostatic stromal cells, but also to the terminal differentiation of these cells. Our findings demonstrate that it may be possible to obtain pure (or purer) cultures of differentiated prostatic stromal smooth muscle cells amenable to further study. Cultured prostatic stromal cells may therefore represent a valuable model to study certain aspects of the growth and differentiation of the stromal compartment of the human prostate. Thus, PS cells may provide valuable insights in the study of proliferative disorders of the prostate such as the biogenesis of stromal nodules in BPH.
Abbreviations
- 4α-PMA
4α-phorbol 12-myristate 13-acetate
- ACE
(angiotensin converting enzyme)
- B1/B2
receptors (human kinin receptor subtypes)
- BK
(bradykinin)
- BPH
(benign prostatic hyperplasia)
- DAG
(diacylglycerol)
- EGF
(epidermal growth factor)
- FCS
(foetal calf serum)
- FGF
(fibroblast growth factor)
- KD
(kallidin)
- InsP3
(inositol (1,4,5)-trisphosphate)
- PCR
(polymerase chain reaction)
- PE cells
(prostate secretory epithelial cells)
- PKC
(protein kinase C)
- PLCβ
(phospholipase Cβ)
- PMA
(phorbol 12-myristate 13-acetate)
- PS cells
(prostate fibromuscular stromal cells)
- PSA
(prostate specific antigen)
- PtdInsP2
(phosphatidylinositol (4,5)-bisphosphate)
- PTK
(protein tyrosine kinase)
- RIA
(radioimmune assay)
- RT–PCR
(reverse transcription PCR)
- TES
(trimethylaminoethanesulphonic acid)
References
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- BARTSCH G., MULLER H.R., OBERHOLZER M., ROHR H.P. Light microscopic stereological analysis of the normal human prostate and of benign prostatic hyperplasia. J. Urol. 1979;122:487–491. doi: 10.1016/s0022-5347(17)56476-9. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., IRVINE R.F. Inositol phosphate and cell signaling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- BERRY S.J., COFFEY D.S., WALSH P.C., EWING L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (150) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3109. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CORREA F.M., GRAEFF F.G. Central site of the hypertensive action of bradykinin. J. Pharmacol. Exp. Ther. 1975;192:670–676. [PubMed] [Google Scholar]
- CLEMENTS J., MUKHTAR A. Tissue kallikrein and the bradykinin B2 receptor are expressed in endometrial and prostate cancers. Immunopharmacology. 1997;36:217–220. doi: 10.1016/s0162-3109(97)00024-6. [DOI] [PubMed] [Google Scholar]
- DIETER P., FITZKE E. RO 31-8220 and RO 31-7549 show improved selectivity for protein kinase C over staurosporine in macrophages. Biochem. Biophys. Res. Commun. 1991;181:396–401. doi: 10.1016/s0006-291x(05)81432-9. [DOI] [PubMed] [Google Scholar]
- FICHTNER J., GRAVES H.C., THATCHER K., YEMOTO C., SHORTLIFFE L.M. Prostate specific antigen releases a kinin-like substance on proteolysis of seminal vesicle fluid that stimulates smooth muscle contraction. J. Urol. 1996;155:738–742. [PubMed] [Google Scholar]
- GOLDSTEIN R.H., WALL M. Activation of protein formation and cell division by bradykinin and des-Arg9-bradykinin. J. Biol. Chem. 1984;259:9263–9268. [PubMed] [Google Scholar]
- HALL J.M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol. Ther. 1992;56:131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- HALL J.M. Bradykinin receptors. Gen. Pharmacol. 1997;28:1–6. doi: 10.1016/s0306-3623(96)00174-7. [DOI] [PubMed] [Google Scholar]
- HASUI Y., MARUTSUKA K., ASADA Y., IDE H., NISHI S., OSADA Y. Relationship between serum prostate specific antigen and histological prostatitis in patients with benign prostatic hyperplasia. Prostate. 1994;25:91–96. doi: 10.1002/pros.2990250206. [DOI] [PubMed] [Google Scholar]
- MANNING D.C., SNYDER S.H., KACHUR J.F., MILLER R.J., FIELD M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982;299:256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- MANNING D.C., VAVREK R.J., STEWART J.M., SNYDER S.H. Two bradykinin binding sites with picomolar affinities. J. Pharmacol. Exp. Ther. 1986;237:504–512. [PubMed] [Google Scholar]
- NEER E.J. Heterotrimeric G-proteins: Organizers of Transmembrane Signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- PROUD D., KAPLAN A.P. Kinin formation: mechanisms and role in inflammatory disorders. Annu. Rev. Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABE J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- SCATCHARD G. The attractions of proteins for small molecules and ions. Ann. NY Acad. Sci. 1949;51:660–672. [Google Scholar]
- SCHILL W.B., MISKA W. Possible effects of the kallikrein-kinin system on male reproductive functions. Andrologia. 1992;24:69–75. doi: 10.1111/j.1439-0272.1992.tb02613.x. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E., HARTANTO V., LEPOR H. The response to alpha blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate. 1992;21:297–307. doi: 10.1002/pros.2990210406. [DOI] [PubMed] [Google Scholar]
- SIMONSON M.S., HERMAN W.H. Protein kinase C and protein tyrosine kinase activity contribute to mitogenic signaling by endothelin-1. Cross-talk between G protein-coupled receptors and pp60c-src. J. Biol. Chem. 1993;268:9347–9357. [PubMed] [Google Scholar]
- STEIDLE C.P., COHEN M.L., NEUBAUER B.L. Bradykinin-induced contractions of canine prostate and bladder: effect of angiotensin-converting enzyme inhibition. J. Urol. 1990;144:390–392. doi: 10.1016/s0022-5347(17)39467-3. [DOI] [PubMed] [Google Scholar]
- STERANKA L.R., MANNING D.C., DEHAAS C.J., FERKANY J.W., BOROSKY S.A., CONNOR J.R., VAVREK R.J., STEWART J.M., SNYDER S.H. Bradykinin is a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO M., YOKOYAMA K., YAYAMA K., OKAMOTO H. Murine fibroblasts synthesize and secrete kininogen in response to cyclic-AMP, prostaglandin E2 and tumor necrosis factor. Biochim. Biophys. Acta. 1995;1265:189–195. doi: 10.1016/0167-4889(94)00218-4. [DOI] [PubMed] [Google Scholar]
- UEHARA Y., HORI M., TAKEUCHI T., UMEZAWA H. Pheotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies innactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol. Cell. Biol. 1986;6:2198–2206. doi: 10.1128/mcb.6.6.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDEN P.D., ITTMANN M., MONACO M.E., LEPOR H. Endothelin-1 production and agonist activities in cultured prostate-derived cells: Implications for regulation of endothelin bioactivity and bioavailability in prostatic hyperplasia. Prostate. 1998;34:241–250. doi: 10.1002/(sici)1097-0045(19980301)34:4<241::aid-pros1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- WATTS S.W., COHEN M.L. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides. 1991;12:1057–1162. doi: 10.1016/0196-9781(91)90060-3. [DOI] [PubMed] [Google Scholar]


