Abstract
Experiments were designed to determine in two arteries (the guinea-pig carotid and the porcine coronary arteries) whether or not the endothelium-derived hyperpolarizing factor (EDHF) can be identified as potassium ions, and to determine whether or not the inwardly rectifying potassium current and the Na+/K+ pump are involved in the hyperpolarization mediated by EDHF.
The membrane potential of vascular smooth muscle cells was recorded with intracellular microelectrodes in the presence of Nω-L-nitro-arginine (L-NA) and indomethacin.
In vascular smooth muscle cells of guinea-pig carotid and porcine coronary arteries, acetylcholine and bradykinin induced endothelium-dependent hyperpolarizations (−18±1 mV, n=39 and −19±1 mV, n=7, respectively). The hyperpolarizations were not affected significantly by ouabain (1 μM), barium chloride (up to 100 μM) or the combination of ouabain plus barium.
In both arteries, increasing extracellular potassium concentration by 5 or 10 mM induced either depolarization or in a very few cases small hyperpolarizations which never exceeded 2 mV.
In isolated smooth muscle cells of the guinea-pig carotid artery, patch-clamp experiments shows that only 20% of the vascular smooth muscle cells expressed inwardly rectifying potassium channels. The current density recorded was low (0.5±0.1 pA pF−1, n=8).
These results indicate that, in two different vascular preparations, barium sensitive-inwardly rectifying potassium conductance and the ouabain sensitive-Na+/K+ pump are not involved in the EDHF-mediated hyperpolarization. Furthermore, potassium did not mimic the effect of EDHF pointing out that potassium and EDHF are not the same entity in those arteries.
Keywords: Potassium, endothelium-derived hyperpolarizing factor, EDHF, endothelium, vascular smooth muscle cells, inwardly rectifying potassium current, Na+/K+ pump, ouabain, barium
Introduction
The endothelium controls local blood flow by releasing different factors including nitric oxide (NO, Furchgott & Zawadzki, 1980), prostacyclin (Moncada & Vane, 1979) and the unidentified endothelium-derived hyperpolarizing factor (EDHF, Félétou & Vanhoutte, 1988). The hyperpolarization of the underlying smooth muscle cells, produced by EDHF, is supposed to involve the opening of potassium channels. The amplitude of the hyperpolarization is inversely related to the extracellular concentration of K+ ions, and it disappears in K+ concentrations higher than 25 mM (Chen & Suzuki, 1989; Nagao & Vanhoutte, 1992; Corriu et al., 1996). Endothelium-dependent hyperpolarizations are associated with an increase in rubidium efflux (Taylor et al., 1988). Inhibitors of calcium-activated potassium channels, such as tetraethylammonium, tetrabutylammonium, apamin or the combination of apamin plus charybdotoxin prevent endothelium-dependent hyperpolarizations (Chen et al., 1991; Nagao & Vanhoutte, 1992; Van de Voorde et al., 1992; Murphy & Brayden, 1995; Garland & Plane, 1996; Corriu et al., 1996, Chataigneau et al., 1998). It has been generally assumed, but not proved, that the target of the potassium channel blockers were on the vascular smooth muscle cells membrane (Zygmunt & Högestätt, 1996; Petersson et al., 1997; Chataigneau et al., 1998). However, calcium-activated potassium channels are also expressed in endothelial cells (Marchenko & Sage, 1996) and based on experiments performed in the hepatic artery of the rat, Edwards et al. (1998) have proposed that these potassium channel blockers could act on the intima and therefore prevent potassium efflux from the endothelial cells. They suggested that potassium ions released by endothelial cells and accumulating in the extracellular space could provoke hyperpolarization of vascular smooth muscle cells by activating the inwardly rectifying potassium conductance and the Na+/K+ pump. Thus, in the hepatic artery of the rat, potassium ions could be EDHF.
The present studies were designed to verify this hypothesis in tissues from two different species in which EDHF responses have been extensively studied: the carotid artery of the guinea-pig and the porcine coronary artery.
Methods
Microelectrode studies
Male Hartley guinea-pigs (250–300 g) were anaesthetized by intraperitoneal administration of pentobarbitone (200 mg kg−1). Male and female Large-White pigs (20–25 kg) were anaesthetized by intramuscular injection of a combination of tilamine plus zolepam (25 mg kg−1). The internal carotid artery of the guinea-pig and the porcine coronary arteries were dissected and cleaned of adherent connective tissues. The carotid arteries of the guinea-pig were pinned to the bottom of an organ chamber adventitia upward. The porcine coronary arteries were slit open and pinned with the intimal surface upward. The tissues were superfused with a thermostated modified Krebs-Ringer bicarbonate solution of the following composition (in mM): NaCl 118.3, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11.1 and EDTA 0.026. In some experiments EDTA was omitted to avoid the chelation of barium. Transmembrane potential was recorded by using glass capillary microelectrodes (tip resistance of 30–90 MΩ) filled with KCl (3 M) and connected to the headstage of a recording amplifier (intra 767, WPI). Successful impalements were signalled by a sudden negative drop in potential from the baseline (zero potential reference) followed by a stable negative potential for at least 3 min. In order to inhibit nitric oxide synthase and cyclooxygenase, all the experiments were performed in the presence of Nω-nitro-L-arginine (L-NA) and indomethacin (Chataigneau et al., 1998).
Patch-clamp studies
The media of guinea-pig carotid artery was dissected from cleaned arteries. The smooth muscle cells were enzymatically dissociated (Quignard et al., 1998). Whole-cell potassium current of freshly isolated vascular smooth muscle cells were recorded at room temperature using the patch-clamp technique (whole cell configuration). In order to record potassium currents, a calcium-free intracellular solution was used with the following composition (in mM): KCl 130, MgCl2 2, adenosine triphospahte (ATP) 3, GTP 0.5, HEPES 25, EGTA 10, glucose 11. The cells were superfused with a solution containing (in mM): NaCl 80, KCl 50, CaCl2 2, MgCl2 1.2, HEPES 10 and glucose 11. Data were recorded with pClamp6 software (Axon Instruments, U.S.A.) through a RK-400 amplifier (Biologic, France).
Drugs
The following drugs were used: acetylcholine, barium, bradykinin, indomethacin, NωL-nitro-arginine, ouabain (Sigma, La Verpillère, France); charybdotoxin, apamin and tetrodotoxin (Latoxan, Rosans, France); 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3oxide (carboxy-PTIO, Alexis Biochem., Paris, France). All drugs were dissolved in water at the exception of indomethacin which was dissolved in deionized water and an equimolar concentration of Na2CO3.
Statistics
Data are shown as mean±s.e.mean; n indicates the number of cells in which membrane potential was recorded. Statistical analysis was performed using Student's t-test for paired or unpaired observations. Differences were considered to be statistically significant when P was less than 0.05.
Results
Guinea-pig carotid artery
Microelectrode experiments
In the presence of L-NA (100 μM) and indomethacin (5 μM), the resting membrane potential of the vascular smooth muscle cells was −53±1 mV (n=50). Acetylcholine (1 μM) induced a long-lasting endothelium-dependent hyperpolarization (−18±1 mV, n=39). The addition of the NO scavenger, carboxy-PTIO (10 μM), did not alter either the resting potential membrane (−52±2 mV, n=16) or the acetylcholine induced hyperpolarization (−19±1 mV, n=16). In the following studies, the experiments were performed in presence of L-NA, indomethacin and carboxy-PTIO.
The addition of barium (50 μM), ouabain (1 μM) or the combination of ouabain plus barium did not affect significantly the resting membrane potential of the vascular smooth muscle cells or the hyperpolarization produced by acetylcholine (1 μM; Figure 1).
Figure 1.
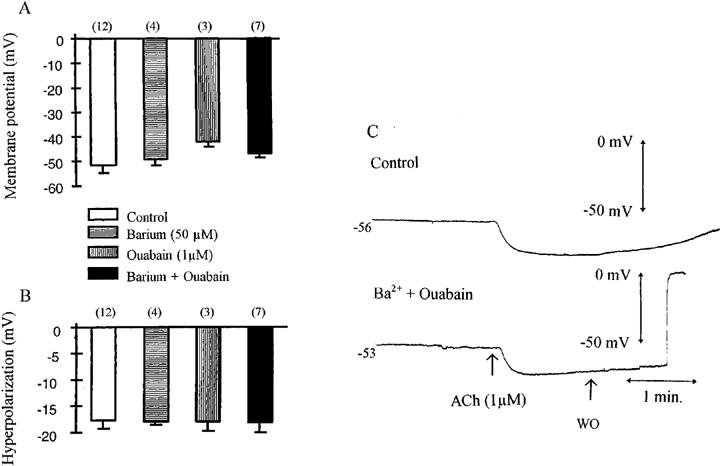
Effects of barium and ouabain on acetylcholine (1 μM)-induced endothelium-dependent hyperpolarization in the guinea-pig isolated carotid artery with endothelium. The experiments were performed in the presence of L-NA (100 μM), indomethacin (5 μM) and carboxy PTIO (10 μM). Effects of the potassium channel blocker barium (50 μM) and the Na+/K+ pump inhibitor ouabain (1 μM) on the membrane potential (A) and on the hyperpolarization elicited by acetylcholine in guinea-pig carotid artery (B). Data are shown as mean±s.e.mean, numbers in brackets indicate the number of experiments. (C) Original traces showing the endothelium-dependent hyperpolarizations elicited by acetylcholine (1 μM) in control condition (upper trace) and in the presence of the combination of barium (50 μM) plus ouabain (1 μM, lower trace).
Increasing the extracellular concentration of potassium ions from the control value of 5.9 to 8.4 mM, did not affect the resting membrane potential. Elevating potassium concentration by 5 mM induced either small depolarization (up to 5 mV) or small hyperpolarization which never exceeded 2 mV, while increasing the potassium concentration by 10 mM induced depolarization (Figure 2). In presence of tetrodotoxin (1 μM), the resting membrane potential was not affected (−53±3 mV, n=9). Increasing the concentration of potassium ions by 2.5, 5 or 10 mM produced only depolarizations. The hyperpolarization induced by acetylcholine (1 μM) was not significantly influenced by the presence of tetrodotoxin (Figure 2).
Figure 2.
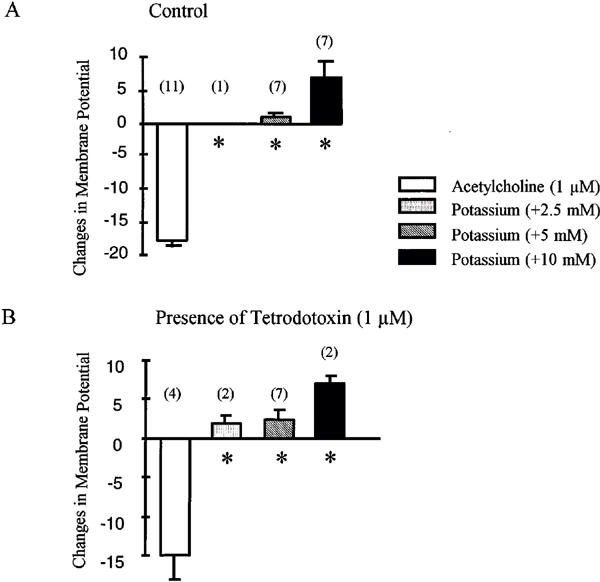
Acetylcholine (1 μM) and addition of potassium (2.5, 5 and 10 mM) induced changes in membrane potential in the guinea-pig isolated carotid artery with endothelium: (A) in the absence or (B) in the presence of tetrodotoxin (1 μM). The initial concentration of potassium was 5.9 mM. The experiments were performed in the presence of L-NA (100 μM), indomethacin (5 μM) and carboxy PTIO (10 μM). Data are shown as mean±s.e.mean, numbers in brackets indicate the number of experiments. The asterisks indicate a statistically significant difference with the response produced by acetylcholine (P<0.05).
In potassium free solution, the membrane potential was −48±4.3 mV (n=6). Increasing the concentration of potassium from 0 to 5 mM induced a transient hyperpolarization which was abolished by the presence of ouabain (1 μM). In potassium free solution, acetylcholine elicited a hyperpolarization which was significantly larger than in control solution (Figure 3).
Figure 3.
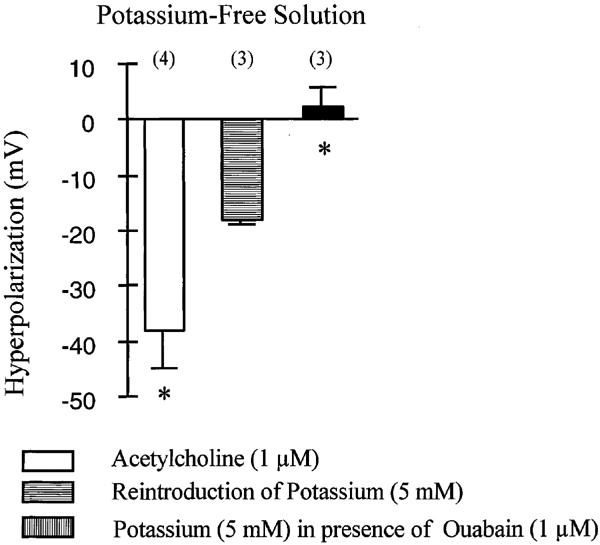
Effect of acetylcholine (1 μM) and the reintroduction of potassium ions (5 mM), in the guinea-pig isolated carotid artery with endothelium, incubated in potassium free solution. The experiments were performed in the presence of L-NA (100 μM), indomethacin (5 μM) and carboxy PTIO (10 μM). Data are shown as mean±s.e.mean, numbers in brackets indicate the number of experiments. The asterisks indicate a statistically significant difference with the response produced by the addition of potassium in the absence of ouabain (P<0.05).
Patch-clamp experiments
With the patch-clamp technique (whole cell configuration), in the presence of 50 mM of potassium in the superfusing solution, the presence of inwardly rectifying potassium currents were investigated in isolated smooth muscle cells of the guinea-pig carotid artery. Cells were maintained at a holding potential of −20 mV and subjected to an instantaneous hyperpolarizing pulse to −150 mV and subsequently to a ramp depolarization from −150 to +30 mV. In eight out of 40 cells, a small inward current could be recorded (0.5±0.1 pA/pF, n=8). The amplitude of this inward current was significantly reduced by lowering the concentration of potassium from 50 to 5 mM (0.1±0.1 pA/pF, n=4) and the potential of reversion was shifted to the left (data not shown). This current was inhibited by barium (50 μM; Figure 4). The average current density, in the 40 cells recorded, was: 0.1±0.2 pA/pF
Figure 4.
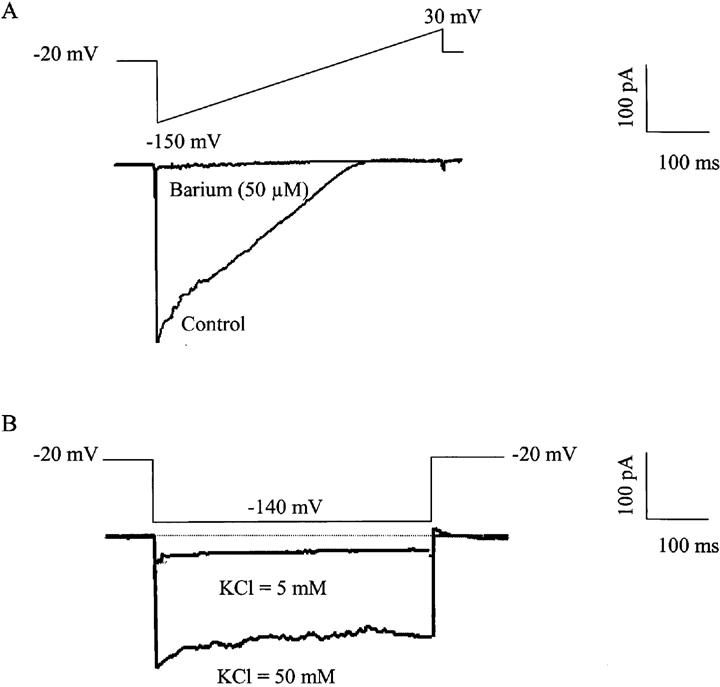
Inwardly rectifying potassium currents in isolated vascular smooth muscle cells of the guinea-pig carotid artery. (A) Effect of barium (50 μM) in a freshly isolated vascular smooth muscle cell of the guinea-pig carotid artery expressing an inwardly rectifying potassium current (ramp depolarization from −150 mV to +30 mV, holding potential: −20 mV). (B) Effect of reducing the potassium concentration from 50 to 5 mM in the amplitude of the inwardly rectifying potassium current in a freshly isolated vascular smooth muscle cell of the guinea-pig carotid artery (holding potential −20 mV, hyperpolarizing pulse to −140 mV).
Porcine coronary artery
Microelectrode experiments
In the presence of L-NA (30 μM) and indomethacin (10 μM), the resting membrane potential of the vascular smooth muscle cells of the porcine isolated coronary artery was −48.4±1.2 mV (n=7). In the presence of ouabain (1 μM), barium (100 μM) or the combination of the two inhibitors (ouabain: 1 μM and barium: 30 μM) the cells were significantly depolarized to −43.2±0.4 mV (n=5), −40.8± 2.4 mV (n=4) and −42.5±0.5 mV (n=4), respectively. Bradykinin (30 nM) induced an endothelium-dependent hyperpolarization of the vascular smooth muscle cells (−18.7±1 mV, n=7) which was not significantly affected by the presence of ouabain, barium or their combination (−21.2±2.1 mV, n=5; −22.5±4.2 mV, n=4 and −24.1± 3.1 mV, n=4; respectively; Figure 5).
Figure 5.
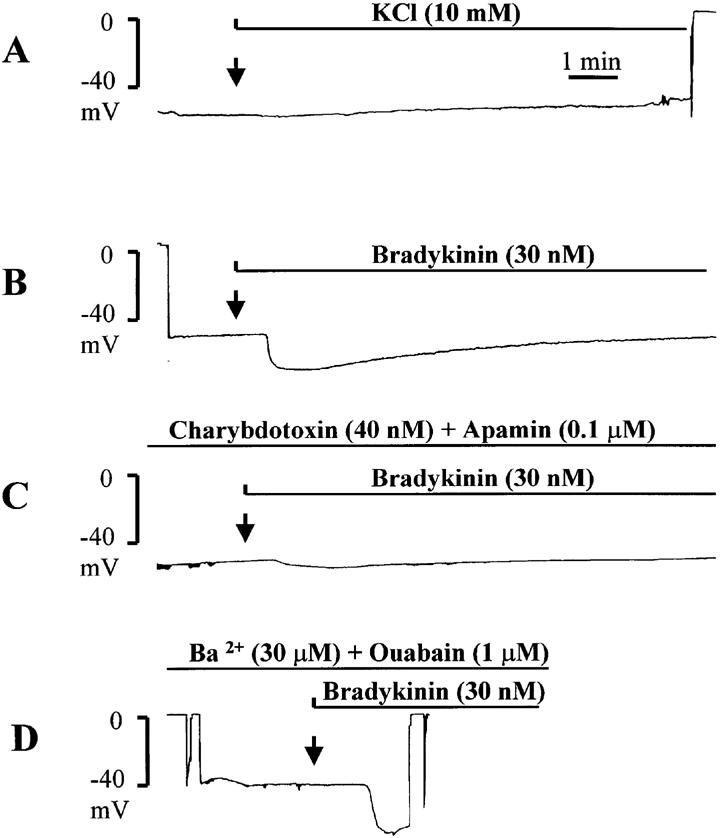
Original traces of intracellular recordings of membrane potential in isolated porcine coronary artery with endothelium showing the effects of the addition of potassium (final concentration: 10 mM) and bradykinin (30 nM). The experiments were performed in the presence of L-NA (30 μM) and indomethacin (10 μM). (A) Addition of potassium. (B) Addition of bradykinin under control conditions. (C) Addition of bradykinin in the presence of charybdotoxin (40 nM) plus apamin (0.1 μM). (D) Addition of bradykinin in the presence of barium (30 μM) plus ouabain (1 μM).
Increasing the extracellular concentration of potassium from 5.9 to 10 mM induced either depolarizations, or in two coronary arteries a transient hyperpolarization (which did not exceed 2 mV) followed by a depolarization (+7.7±0.5 mV, n=6; Figure 5). Increasing the extracellular concentration of potassium from 5.9 to 15 mM induced depolarization (+10.0±2.0 mV, n=2).
The combination of apamin (0.1 μM) plus charybdotoxin (40 nM) significantly inhibited the hyperpolarization produced by bradykinin (−17.8±1.2 mV, n=4 and −5.0±1.3 mV, n=6, in control and in the presence of the toxins, respectively) without affecting the resting membrane potential (−48.1±0.8 mV, n=6; Figure 5).
Discussion
The present study shows that, in contrast to the hepatic artery of the rat, the endothelium-dependent hyperpolarizations, observed in the carotid artery of the guinea-pig and in the porcine coronary artery, are not mimicked by the addition of potassium ions and are unlikely to involve the inwardly rectifying potassium channel and the Na+/K+ pump.
In the rabbit carotid artery, Cohen et al. (1997) have suggested that the acetylcholine-induced hyperpolarization in the presence of different inhibitors of NO synthase is linked to a residual release of NO by the endothelium. However, in the carotid artery of the guinea-pig, in the presence of a high concentration of L-NA and indomethacin, the addition of carboxy PTIO, a potent scavenger of NO (Akaike et al., 1993), did not affect the hyperpolarization produced by acetylcholine, indicating that, in this tissue, EDHF is the factor involved in the response to acetylcholine and not residual NO release (Corriu et al., 1996; Chataigneau et al., 1998).
In certain vascular beds such as the coronary and cerebral arteries of the rat, increasing the extracellular concentration of potassium ions (from 6 to 16 mM) relaxes the blood vessels and hyperpolarizes the smooth muscle cells up to 14 mV (Edwards et al. (1998); McCarron & Halpern, 1990; Knot et al., 1996). However, this phenomenon is usually observed in small but not in large arteries. For instance, potassium induced a hyperpolarization in the small cerebral artery of rat whereas it induced a depolarization in larger cerebral arteries (Edwards et al., 1988). These hyperpolarizations, induced by potassium, are inhibited by low concentrations of barium (<100 μM), a specific inhibitor of inward rectifying potassium conductance (K-ir) at these low concentrations (Nelson & Quayle, 1995). K-ir channels are voltage-dependent potassium channels with an open probability which decreases with depolarization. The open state probability is increased by a modest rise in extracellular potassium concentration (Faraci & Heistad, 1998). The level of expression of K-ir channel in vascular smooth muscle cells is inversely related to the size of the blood vessels, e.g. the expression of K-ir is more preponderant in smaller blood vessels (Quayle et al. 1996). This explains the different effect of potassium in small and large blood vessels (Edwards et al., 1988). A rise in extracellular potassium could also cause hyperpolarization and relaxation of the vascular smooth muscle cells by activating the Na+/K+ pump without involvement of K-ir (Prior et al., 1998).
In the carotid artery of the guinea-pig, barium at a concentration which fully inhibits the inwardly rectifying potassium channel, as observed in the patch clamp experiments, did not affect the hyperpolarization induced by acetylcholine. Experiments involving barium were performed in the absence of EDTA in order to avoid the chelation of the divalent cation. These results are in agreement with the lack of effect of barium on the EDHF-mediated hyperpolarization described in rat mesenteric arteries (White & Hiley, 1997), rabbit mesenteric arteries (Murphy & Brayden, 1995) or guinea-pig coronary arteries (Parkington et al., 1995). A major difference between this study and the results reported in the hepatic artery of the rat (Edwards et al., 1998) is the absence of hyperpolarization in response to the addition of potassium ions and the low level of expression of the inwardly rectifying potassium channels. In the isolated smooth muscle cells of the guinea-pig carotid artery, in order to record the inwardly rectifying potassium channels, intra and extracellular solutions were optimized. The intracellular calcium-free solution in the presence of EGTA and the addition of ATP ruled out the involvement of calcium-activated potassium channels and ATP-sensitive potassium channels, respectively. Furthermore, a holding potential of −20 mV was applied to inactivate voltage gated potassium currents. Under these conditions the current observed had the characteristic of an inward rectifying potassium current (Quayle et al., 1997). The current was slowly inactivating, its amplitude increased with the concentration of extracellular potassium, it showed a strong rectification for outward current, and finally the inward current was inhibited by a low concentration of barium. However, the expression of this conductance in the vascular smooth muscle cells of the guinea-pig carotid artery was minimal. Indeed, even in the presence of a high extracellular potassium concentration (50 mM) in order to amplify the inward potassium current, no or minimal barium-sensitive current could be recorded. By contrast, it has been demonstrated that these cells express large voltage-gated and calcium-activated potassium currents (Quignard et al., 1998). The absence of K-ir conductance has also been described in other vascular bed (Quayle et al., 1997).
In the guinea-pig carotid artery, elevating potassium concentration induced depolarization, and in some instance a small hyperpolarization. The hyperpolarization observed was smaller than 2 mV and this hyperpolarization was not reproducible twice on the same tissue (data not shown) which is in contrast to the hyperpolarization produced by acetylcholine being often larger than 18 mV and reproducible in the same artery (Chataigneau et al., 1998). As the effect of potassium could have been affected by the unwanted alteration in neurotransmitters release by depolarized axons (Lorenz & Vanhoutte, 1975), the same experiments were performed in the presence of tetrodotoxin. In these conditions, potassium only depolarized the guinea-pig carotid artery smooth muscle cells. In the second tissue studied, the porcine coronary artery, potassium produced also depolarization of the vascular smooth muscle cells. The activation of K-ir by potassium ions could be dependent of the passive wall tension of the blood vessel (McPherson & Keily, 1995) and it could be argued that the experimental conditions of the present study were not optimal to observe potassium ions-induced hyperpolarization. Nevertheless, the fact that acetylcholine induced a hyperpolarization whereas potassium could not, indicates that the effect of acetylcholine involves a different mechanism.
In potassium free solution, the reintroduction of potassium induced a transient hyperpolarization which was abolished by the presence of ouabain, confirming that the Na+/K+ pump is activated under these conditions (Haddy, 1983) and that the concentration of ouabain used in the present study was effective. The transient nature of the hyperpolarization is expected as the activation of the pump would decay to a new steady-state level when the intracellular sodium is reduced by the ongoing pump activity. This indicates that even in blood vessels such as the coronary and cerebral arteries of the rat which respond by a sustained hyperpolarization in response to a rise in potassium (Knot et al., 1996), the participation of the Na+/K+ pump is unlikely in the sustained portion. Finally, the external site for potassium on the pump is supposed to be nearly saturated at 5 mM of potassium (half-activation : 1–2 mM, Hexum, 1981).
In potassium free solution, the hyperpolarization induced by acetylcholine was significantly larger than in control solution. The potentiation of the effect of acetylcholine is most likely due to the major increase of the driving force for potassium ions (as predicted from the Nernst equation) and the involvement of Na+/K+ pump is unlikely as ouabain did not affect acetylcholine-induced hyperpolarization. Endothelium-dependent hyperpolarizations sensitive to ouabain have been described in some vascular tissues such as the canine coronary artery (Félétou & Vanhoutte, 1988), the cerebral artery of rabbit (Brayden, 1990) and the porcine coronary artery (Olanrewaju et al., 1997), but the concentration used or the duration of the incubation required to observe the inhibition suggest that ouabain exerted other pharmacological effects than inhibition of the Na+/K+ pump. Indeed, in other studies, ouabain did not affect the hyperpolarization induced by acetylcholine in the ear artery of the rabbit (Suzuki, 1988) and in the canine coronary artery (Chen et al., 1989). However, ouabain induced a depolarization of the cell membrane which was significant in the porcine coronary artery but did not reach significance in the guinea-pig carotid artery, indicating that the pump is involved in the control of the resting membrane potential.
In conclusion, in the two tissues studied in the present work, neither ouabain, barium, nor their combination inhibited the hyperpolarization produced by acetylcholine or bradykinin. In contrast, the combination of the two toxins, charybdotoxin plus apamin, inhibited this hyperpolarization (guinea-pig carotid artery: Corriu et al., 1996; Chataigneau et al., 1998 and pig coronary artery: present studies). Furthermore, potassium ions did not mimic the EDHF responses in terms of amplitude and time course. This indicates that the endothelium-dependent hyperpolarizations produced by acetylcholine or bradykinin are probably not dependent of K-ir or Na+/K+ pump activation. The site of action of charybdotoxin and apamin remains to be determined. In the hepatic artery of the rat, the combination of these two toxins (Zygmunt et al., 1997) as well as the combination of ouabain plus barium inhibited the EDHF-mediated hyperpolarization (Edwards et al., 1998). The origin of the discrepancies between this study and the results obtained by Edwards et al. (1998) in the hepatic artery of the rat is not clear at present but may involve the level of expression of K-ir. Nevertheless, at present, the hypothesis that K+ ion represents EDHF cannot be extended to every vascular bed (Vanhoutte, 1998).
Abbreviations
- ATP
adenosine triphosphate
- carboxy-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3oxide
- EDHF
endothelium-derived hyperpolarizing factor
- K-ir
inward rectifying potassium conductance
- L-NA
Nω-L-nitro-arginine, NO, nitric oxide
References
- AKAIKE T., YOSHIDA M., MIYAMOTO Y., SATO K., KOHNO M., SASAMOTO K., MIYAZAKI K., UEDA S., MAEDA H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor NO through a radical reaction. Biochem. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilatation. Am. J. Physiol. 1990;259:H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- CHATAIGNEAU T., FÉLÉTOU M., DUHAULT J., VANHOUTTE P.M. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br. J. Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., HASHITANI H., SUZUKI H. Endothelium-dependent relaxation and hyperpolarization of canine coronary artery smooth muscle in relation to the electrogenic Na-K pump. Br. J. Pharmacol. 1989;98:950–956. doi: 10.1111/j.1476-5381.1989.tb14625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J. Physiol. (London) 1989;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., YAMAMOTO Y., MIWA K., SUZUKI H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am. J. Physiol. 1991;260:H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., PLANE F., NAJIBI S., HUK I., MALINSKI T., GARLAND C.J. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRIU C., FÉLÉTOU M., CANET E., VANHOUTTE P.M. Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br. J. Pharmacol. 1996;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS F.R., HIRST G.D., SILVERBERG G.D. Inward rectification in rat cerebral arterioles, involvement of potassium ions in autoregulation. J. Physiol. (London) 1988;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- FARACI F.M., HEISTAD D.D. Regulation of the cerebral circulation: role of endothelium and potassium channel. Physiol. Rev. 1998;78:54–75. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- FÉLÉTOU M., VANHOUTTE P.M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br. J. Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F.Relative importance of endothelium-derived hyperpolarizing factor for the relaxation of vascular smooth muscle in different arterial beds Endothelium-derived hyperpolarizing factor 19961Harwood Academic Publishers, Amsterdam; 173–179.ed. Vanhoutte P.M. [Google Scholar]
- HADDY F.J. Potassium effects in arterial smooth muscle mediated by Na+/K+-ATPase. Faseb J. 1983;42:239–245. [PubMed] [Google Scholar]
- HEXUM T.D. Characterization of Na+/K+ ATPase from vascular smooth muscle. General Pharmacol. 1981;12:393–396. doi: 10.1016/0306-3623(81)90098-7. [DOI] [PubMed] [Google Scholar]
- KNOT H.J., ZIMMERMANN P.A., NELSON M.T. Extracellular potassium-induced hyperpolarization and dilatations of rat coronary and cerebral arteries involve inward rectifier potassium channels. J. Physiol. (London) 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORENZ R.R., VANHOUTTE P.M. Inhibition of adrenergic neurotransmission in isolated veins of the dog by potassium ions. J. Physiol. (London) 1975;246:479–500. doi: 10.1113/jphysiol.1975.sp010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Calcium-activated potassium channels in the endothelium-of intact rat aorta. J. Physiol. (London) 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARRON J.G., HALPERN W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am. J. Physiol. 1990;259:H902–H908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- MCPHERSON G.A., KEILY S.G. Electrophysiological properties of the rat middle cerebral artery at different levels of passive wall tension. Clin. Exp. Pharmacol. Physiol. 1995;22:724–731. doi: 10.1111/j.1440-1681.1995.tb01926.x. [DOI] [PubMed] [Google Scholar]
- MONCADA S., VANE J.R. Pharmacology and endogenous roles of prostaglandins, endoperoxides, thromboxane A2 and prostacyclin. Pharmacol. Rev. 1979;30:293–331. [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Apamin-sensitive potassium channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J. Physiol. (London) 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAO T., VANHOUTTE P.M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J. Physiol. (London) 1992;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- OLANREWAJU H.A., HARGITTAI P.T., LIEBERMAN E.M., MUSTAFA S.J. Effect of ouabain on adenosine receptor-mediated hyperpolarization in porcine coronary artery smooth muscle. Eur. J. Pharmacol. 1997;322:185–190. doi: 10.1016/s0014-2999(96)00992-2. [DOI] [PubMed] [Google Scholar]
- PARKINGTON H.C., TONTA M., COLEMAN H., TARE M. Role of membrane potential in endothelium-dependent relaxation of guinea-pig coronary arterial smooth muscle. J. Physiol. 1995;484:469–480. doi: 10.1113/jphysiol.1995.sp020679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSSON J., ZYGMUNT P.M., HÖGESTÄTT E.D. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br. J. Pharmacol. 1997;120:1344–1350. doi: 10.1038/sj.bjp.0701032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR H.M., WEBSTER N., QUINN K., BEECH D.J., YATES M.S. K(+)-induced dilation of a small renal artery: no role for inward rectifier K+ channels. Cardiovasc. Res. 1998;37:780–790. doi: 10.1016/s0008-6363(97)00237-x. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., DART C., STANDEN N.B. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J. Physiol. (London) 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- QUIGNARD J.-F., CHATAIGNEAU T., CORRIU C., DUHAULT J., FÉLÉTOU M., VANHOUTTE P.M.Potassium channels involved in EDHF-induced hyperpolarization of the smooth muscle cells of the isolated guinea-pig carotid artery Endothelium-derived hyperpolarizing factor 19982Harwood Academic Press, Amsterdam, The Netherlands in press; ed. Vanhoutte P.M. [Google Scholar]
- SUZUKI H.A. The electrogenic Na-K pump does not contribute to endothelium-dependent hyperpolarization in the rabbit ear artery. Eur. J. Pharmacol. 1988;156:295–297. doi: 10.1016/0014-2999(88)90337-8. [DOI] [PubMed] [Google Scholar]
- TAYLOR S.G., SOUTHERTON J.S., WESTON A.H., BAKER J.R.J. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br. J. Pharmacol. 1988;94:853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE VOORDE J., VANHEEL B., LEUSEN I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ. Res. 1992;70:1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M. An old-timer makes a come-back. Nature. 1998;396:213–216. doi: 10.1038/24261. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated responses and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., EDWARDS G., WESTON A.H., LARSSON B., HÖGESTÄTT E.D. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat hepatic artery. Br. J. Pharmacol. 1997;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D.Endothelium-dependent hyperpolarization and relaxation in the hepatic artery of the rat Endothelium-derived hyperpolarizing factor 19961Harwood Academic Publishers, Amsterdam; 191–202.ed. Vanhoutte P.M [Google Scholar]


