Abstract
The vasomotor and cyclic GMP-elevating activity of YC-1, a novel NO-independent activator of soluble guanylyl cyclase (sGC), was studied in isolated rabbit aortic rings and compared to that of the NO donor compounds sodium nitroprusside (SNP) and NOC 18.
Similarly to SNP and NOC 18, YC-1 (0.3–300 μM) caused a concentration-dependent, endothelium-independent relaxation that was greatly reduced by the sGC inhibitor 1-H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ 10 μM; 59% inhibition of dilation induced by 100 μM YC-1) suggesting the activation of sGC as one mechanism of action.
Preincubation with YC-1 (3 and 30 μM) significantly increased the maximal dilator responses mediated by endogenous NO in aortic rings that was released upon exposure to acetylcholine, and enhanced the dilator response to the exogenous NO-donors, SNP and NOC 18, by almost two orders of magnitude.
Vasoactivity induced by SNP and YC-1 displayed different kinetics as evidenced by a long-lasting inhibition by YC-1 (300 μM) on the phenylephrine (PE)-induced contractile response, which was not fully reversible even after extensive washout (150 min) of YC-1, and was accompanied by a long-lasting elevation of intracellular cyclic GMP content. In contrast, SNP (30 μM) had no effect on the vasoconstrictor potency of PE, and increases in intravascular cyclic GMP levels were readily reversed after washout of this NO donor compound.
Surprisingly, YC-1 not only activated sGC, but also affected cyclic GMP metabolism, as it inhibited both cyclic GMP break down in aortic extracts and the activity of phosphodiesterase isoforms 1–5 in vitro.
In conclusion, YC-1 caused persistent elevation of intravascular cyclic GMP levels in vivo by activating sGC and inhibiting cyclic GMP break down. Thus, YC-1 is a highly effective vasodilator compound with a prolonged duration of action, and mechanisms that are unprecedented for any previously known sGC activator.
Keywords: Nitric oxide, soluble guanylyl cyclase, vasoconstriction, vasodilation, endothelium, nitrates, cyclic GMP, YC-1, phosphodiesterase
Introduction
Endothelium-derived nitric oxide (NO) is an important regulator of vascular functions by controlling blood vessel tone as well as blood cell interactions with the vascular wall (Moncada & Higgs, 1991; Schmidt & Walter, 1994). The action of NO as a vasodilator is mediated by the activation of vascular smooth muscle soluble guanylyl cyclase (sGC; Arnold et al., 1977; Böhme et al., 1978), a signal transduction enzyme that forms the second messenger molecule cyclic GMP. The latter modulates the activity of several cyclic GMP effector proteins that lead to vasorelaxation (Schmidt et al., 1993). Vascular dysfunction may be associated with either ectopic overproduction of NO in the vascular smooth muscle (e.g. in sepsis; Busse & Mülsch, 1990) or with relative NO deficiency. To overcome a pathological state of NO deficiency, different NO donor compounds have been clinically used for over 100 years, e.g. in angina pectoris and coronary heart disease. However, many NO donors carry the disadvantage of causing tolerance and cross tolerance after prolonged usage (Zelis & Mason, 1975) making drug-free intervals necessary. Moreover, NO donor-based therapy carries the potential risk of peroxynitrite formation from the reaction of exogenous NO with tissue-derived superoxide. Peroxynitrite formation in turn may lead to protein S-nitrosylation (Stamler, 1994) and tyrosine nitration (Beckman et al., 1994), and may be of pathophysiological significance in atherosclerosis (Beckman et al., 1994).
Thus, a recent report on a novel NO-independent type of sGC activator, 3-(5′-hydroxymethyl-2′furyl)-1-benzyl-indazol (YC-1; Ko et al., 1994) was met with great interest. This compound is a potent and direct activator of sGC (Friebe et al., 1996; Mülsch et al., 1997) and mimics many of the known functions of NO and NO donors, such as inhibition of platelet aggregation (Wu et al., 1995) and adhesion (Wu et al., 1997) as well as inhibition of vascular smooth muscle contraction (Mülsch et al., 1997) and proliferation (Yu et al., 1995). Moreover, it has been shown that YC-1 and NO activate sGC in vitro in a synergistic manner (Friebe et al., 1996; Mülsch et al., 1997), and YC-1 may potentiate the actions of exogenous as well as endogenous carbon monoxide on sGC (Friebe et al., 1996).
In the present study, the vasomotor activity of YC-1 as well as its effects on vascular cyclic GMP levels were further characterized using isolated vascular ring preparations. These effects were compared to those of classical NO donor-type activation of sGC. Since intracellular cyclic GMP levels are not only determined by the cyclic GMP synthesis, but also by cyclic GMP breakdown, we considered the possibility that YC-1 also modulated the activity of phosphodiesterases.
Methods
Blood vessel preparation and measurement of tension
Isolated rabbit aortic rings were superfused with KHB under isometric conditions, and force development was measured as described previously (Galle et al., 1995b). Rabbits (1.8–2.3 kg) of either sex and mixed strains were used. After i.v. administration of heparin (500 units) and an overdose of sodium pentobarbitone (80 mg kg−1), they were sacrificed by exsanguination. The thoracic aorta was removed and placed in KHB, cleaned of fat and connective tissue, and cut into ring segments (3–4 mm long). The rings were mounted into small organ baths (2 ml) and connected to a strain gauge transducer (Hugo Sachs, Germany) to record changes in isometric tension. The aortic rings were superfused at a constant rate (3 ml min−1) with KHB (37°C, bubbled with a 95% O2 and 5% CO2 gas mixture). In some experiments, the endothelium was removed mechanically by inserting the tip of a pair of forceps into the lumen and rolling the tissue back and forth several times on a paper towel moistened with physiological salt solution. At the beginning of each experiment, rings were stretched to a resting tension of 2.5 g to optimize vasoconstrictor responses and then contracted with PE (1 μM) and, once the contractions had reached a plateau, the endothelial integrity of the preparations (E+) or absence of endothelium (E−) was verified by adding ACh 1 μM to the superfusate. Only arteries with a vasodilator response of >70% inhibition of preconstriction were considered E+. To confirm that the ACh-induced vasodilation was mediated by endothelium-derived nitric oxide, in parallel experiments the inhibitory effect of Nω-nitro-L-arginine on the vasodilator responses to ACh was investigated as described (Galle et al., 1995a). The preparations were then washed and allowed to equilibrate with KHB for 45 min before being contracted a second time with PE.
When a stable vasoconstriction to PE of 3–5 g was reached, concentration-response curves to either ACh (3 nM–3 μM), YC-1 (0.3–300 μM), SNP (1 nM–30 μM), or NOC 18 (1 nM–30 μM) were constructed. The effects of YC-1 on the vasodilator responses to ACh, SNP, and NOC 18 were investigated by pretreating aortic rings with different concentrations of YC-1 (3, 30 and 300 μM) for 60 min, before the respective concentration-response curves were determined, in the course of which (usually about 60 min) YC-1 was still present. In each experiment, three aortic rings obtained from the same animal were studied simultaneously. In experiments involving YC-1, one ring was incubated with an equivalent amount of DMSO to serve as time-matched control. In aortic rings pretreated with the high concentrations of YC-1 (30 and 300 μM), higher concentrations of PE (10–30 μM) were required to induce the same level of preconstriction. Under these conditions, the control rings were exposed to identical PE concentrations as the YC-1-treated aortic rings. The effects of the soluble guanylyl cyclase inhibitor ODQ on the dilator responses elicited by NOC 18 or YC-1 were examined by preincubating the aortic rings with 10 μM ODQ for 10 min prior to the addition of vasodilators during which ODQ was still present. Again, in each experiment, three aortic rings obtained from the same animal were studied simultaneously, with one ring serving as time-matched solvent control for ODQ-treated arteries. Contractile responses elicited by cumulative concentrations of PE were studied in control rings and in rings pretreated for 60 min with either YC-1 (300 μM) or SNP (30 μM).
Determination of intravascular cyclic GMP content
To determine the vascular cyclic GMP content of rabbit aortic rings under experimental conditions, rings were equilibrated for 30 min in KHB, and then for a further 60 min with either YC-1 (300 μM), SNP (30 μM), or an aliquot of the respective buffer (with and without DMSO) serving as a solvent control. Segments were collected either immediately by freezing the segments in liquid nitrogen, or allowed to re-equilibrate in KHB without YC-1 or SNP (for 5, 20 or 60 min), and then snap-frozen. All segments were pulverized in a liquid nitrogen-cooled stainless steel mortar, and then transferred into 300 μl of 0.5 M trichloracetic acid. From this point on, cyclic GMP standards were processed and treated identically to the samples. After a 30 min incubation period on wet ice, the acid extracts were sonicated (Sonifier 250, Branson, Danbury, CT, U.S.A.; output control 3, duty cycle 30%, 20 impulses) and then centrifuged at 4°C for 10 min at 12,000× g to precipitate cell debris and proteins. The pellets were used for total protein determination (Lowry et al., 1951). To remove trichloracetic acid, the supernatants were extracted three times with 5 volumes of water-saturated diethyl ether. Any residual diethyl ether was removed by heating the samples to 90°C for 3–5 min. A 100 μl aliquot of this supernatant fraction was used for cyclic GMP quantitation by radioimmunoassay (Biotrend, Köln, Germany). The amount of cyclic GMP in each blood vessel ring was standardized to fmol cyclic GMP mg−1 protein.
Preparation of rabbit aortic crude supernatant fractions
Five rabbit aortas (about 2.5 g) were frozen in liquid nitrogen and pulverized in a liquid nitrogen-cooled stainless steel mortar. The following steps were performed at 4°C. The rabbit aortic powder was resuspended in 5 ml homogenization buffer containing (in mM) triethanolamine (pH 7.5) 50, EDTA 0.5, glutathione 7, PMSF 0.2, pepstatin A 1 μM and leupeptin 1 μM, and then homogenized in a glass-glass tissue douncer. The homogenate was brought to a final volume of 7.5 ml by adding homogenization buffer and then centrifuged for 10 min at 4,000× g. The crude supernatant fraction (2.5 mg protein ml−1) was aliquoted, frozen in liquid nitrogen and kept at −70°C until further analysis.
cyclic GMP phosphodiesterase (PDE) activity in rabbit aortic extracts
The effect of YC-1 on cyclic GMP break down in rabbit aortic extracts was determined at 37°C in a total volume of 100 μl, containing (in mM): Tris 20, imidazole/HCl (pH 7.5) 20, MgCl2 3, magnesium-acetate 15, calmodulin 250 nM, Ca2Cl 250 μM, rabbit aortic extract 10 μl, and, as a substrate, 15 μM cyclic GMP. YC-1 was added to a final concentration of 100 μM, while in the control experiments only the solvent was included. Reactions were started by adding rabbit aortic extract and stopped after 0, 2, 5, 10, 15, and 30 min by heating to 95°C. The cyclic GMP content was then assayed using a commercially available enzyme-linked immunoassay (Biotrend, Köln, Germany).
Inhibition of PDE isoenzymes by YC-1
PDE activity was determined as described (Thompson et al., 1979) with some modifications (Bauer & Schwabe, 1980). The assay mixture contained (in mM): Tris-HCl (pH 7.4) 50, MgCl2 5, cyclic AMP or cyclic GMP 0.5 μM, [3H] -cyclic AMP or [3H]-cyclic GMP (about 30,000 c.p.m. per assay), the PDE isozyme-specific additions described below, the indicated concentration of YC-1 and an aliquot of the enzyme solution at a final assay volume of 200 μl. A stock solution and appropriate serial dilutions of YC-1 were prepared in DMSO so that the final DMSO concentration in the assays was 0.5 % (v v−1) which itself did not alter PDE activity. After preincubation for 5 min at 37°C, the reaction was started by the addition of substrate (cyclic AMP or cyclic GMP), and the assays were incubated for a further 15 min at 37°C. 50 μl of 0.2 N HCl was then added to stop the reaction and the assays were left on ice for about 10 min. Following incubation with 25 μg 5′-nucleotidase (Crotalus atrox snake venom) for 10 min at 37°C, the assays were loaded on QAE Sephadex A-25 columns (Econo columns, Bio-Rad, 1 ml bed volume). The columns were eluted with 2 ml of 30 mM ammonium formiate (pH 6.0), and the eluate was counted for radioactivity. With the exception of PDE1 (see below) results were corrected for blank values (measured in the presence of denatured protein) which were below 5% of total radioactivity. The amount of cyclic nucleotide hydrolyzed did not exceed 30% of the original substrate concentration ensuring enzyme saturation over the full course of the assay.
PDE1 (Ca2+/calmodulin-dependent) from bovine brain was kindly provided by Dr Gietzen (Ulm, Germany, Gietzen et al., 1982). This isozyme was assayed in the presence of Ca2+ (1 mM) and calmodulin (100 nM) using cyclic GMP as substrate. A blank value measured in the presence of EGTA 1 mM (in the absence of Ca2+/calmodulin) was subtracted from all values. PDE2 (cyclic GMP-stimulated) from rat heart was chromatographically purified as described (Schudt et al., 1991b) and assayed in the presence of cyclic GMP (5 μM) using cyclic AMP as substrate. PDE3 (cyclic GMP-inhibited) and PDE5 (cyclic GMP-specific) were assayed in homogenates from human platelets as described (Schudt et al., 1991b) using cyclic AMP or cyclic GMP as substrates. PDE4 (cyclic AMP-specific) was tested in the cytosol of human PMN homogenates derived from buffy coats as described (Schudt et al., 1991a) using cyclic AMP as substrate. The PDE3-specific inhibitor motapizone (1 μM) was included to suppress PDE3 activity originating from contaminating platelets.
Statistics
Data are presented as means±standard error of the mean (s.e.mean). Vasodilator responses are expressed in percentage of the initial steady state constriction induced by PE. EC50 values were calculated by non-linear regression analysis using the Prism software (GraphPad, San Diego, CA, U.S.A.). Statistical analysis was performed using the SigmaStat 2 statistical software (Jandel GmbH, Erkrath, Germany). Drug effects were compared by two-way ANOVA (one factor being repeated measures of drug concentrations, and the other factor being the particular intervention), followed by all pairwise multiple comparison procedures (Tukey test). Differences were considered significant at an error probability of P<0.05. The n value refers to the number of animals used unless indicated otherwise.
Chemicals
Phenylephrine (PE), 3-(5′-hydroxymethyl-2′furyl)-1-benzyl-indazol (YC-1) and 1-H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) were obtained from Hoechst Marrion Roussel (Frankfurt, Germany); sodium nitroprusside (SNP) and acetylcholine (ACh), from Sigma (Munich, Germany); 1-hydroxy-2-oxo-3,3-bis(3-aminoethyl)-1-triazine (NOC 18), from ICN (Eschwege, Germany). YC-1 and NOC 18 were dissolved in dimethyl sulphoxide (DMSO) and, as all other drugs unless indicated otherwise, further diluted in Krebs-Henseleit buffer (in mM): (KHB, 118.3; NaCl, 4; KCl, 2.5; CaCl2, 1; MgSO4, 1.2; KH2PO4, 24; glucose, 11.1; NaHCO3, 26 μM and EDTA).
Results
YC-1-induced vasodilation is inhibited by ODQ
As shown in Figure 1, cumulative concentrations of YC-1 (0.3–300 μM) produced concentration-dependent vasodilations in endothelium-intact rabbit aortic rings (E+) preconstricted with PE (A), which were similar to those observed with the NO donor NOC 18 (3 nM–30 μM, B). However, YC-1-induced vasorelaxation developed more slowly than that caused by the NO donor compound (not shown). Inhibition of soluble guanylyl cyclase with ODQ (10 μM) resulted in a significant reduction of vasodilator responses to either YC-1 or NOC 18, indicating an sGC-mediated mechanism in both cases.
Figure 1.
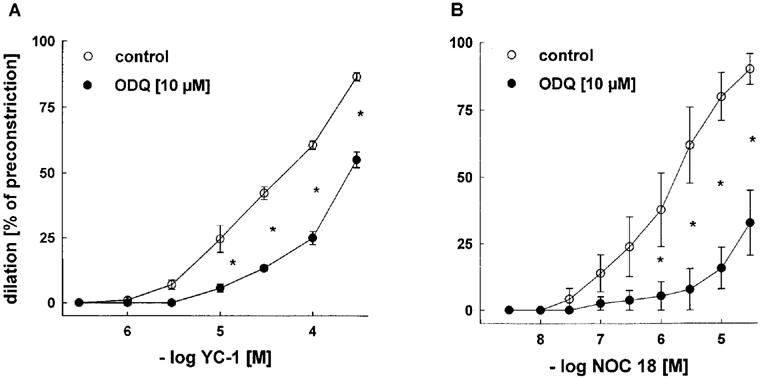
Effects of ODQ (10 μM) on vasodilations induced by cumulative concentrations of YC-1 (A) or NOC 18 (B) in endothelium-intact rabbit aortic rings preconstricted with PE (1 μM). Dilations are expressed as per cent of preconstriction. ODQ significantly inhibited YC-1- or NOC 18 -induced dilations. Data represent mean±s.e.mean; n=7; *indicates P<0.05, Tukeys test for pairwise comparison after two way ANOVA.
YC-1-induced vasodilation in endothelium-intact and in endothelium-denuded rabbit aortic rings
To examine whether the effects of YC-1 are affected by tonic vascular NO release, we investigated the vasodilating effect of YC-1 in rabbit aortic rings with and without endothelium. Cumulative concentrations of YC-1 (1–300 μM) produced concentration-dependent vasodilations both in E+ arteries and endothelium-denuded (E−) aortic rings, indicating that YC-1-induced vasodilation is endothelium-independent (Figure 2). YC-1 was significantly more effective in E+ as compared to E− vessels. It must be noted that the solvent for YC-1, DMSO, also had a small impact on vessel tone at the high concentrations, as shown in Figure 2 (control curves).
Figure 2.
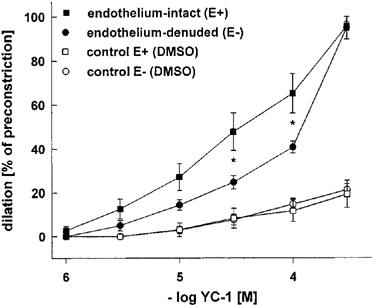
Vasodilations induced by cumulative concentrations of YC-1 in endothelium-intact and endothelium-denuded rabbit aortic rings preconstricted with PE (1 μM). Dilations are expressed as per cent of preconstriction. Control experiments show that high concentrations of DMSO, required to dissolve YC-1, itself caused weak dilations of E+ rings. YC-1 induced dilator responses were significantly different in endothelium-intact and endothelium-denuded aortic rings at the indicated YC-1 concentrations. Data represent mean±s.e.mean; n=7; *indicates P<0.05, Tukeys test for pairwise comparison after two way ANOVA.
YC-1 increased the effectiveness of the ACh-induced vasodilator response
To investigate whether YC-1 has a direct effect on the action of endogenous NO released from the endothelium upon exposure to ACh, we analysed the ACh-induced vasodilator responses in an experimental set-up in which E+ aortic rings were preconstricted with PE and dilated by cumulative concentrations of ACh (3 nM–3 μM) in the presence of different concentrations of YC-1 (3, 30, and 300 μM) (Figure 3). In these experiments, YC-1 pretreatment significantly lowered the sensitivity of the aortic rings to PE (see also below, Figure 6). The higher the YC-1 concentration was, the higher was the concentration of PE required in order to induce preconstrictions that were stable and of similar magnitude (3–5 g) (1 μM PE at 3 μM YC-1, 10 at 30 μM, and 30 at 300 μM). In the control experiments (i.e. in the absence of YC-1), these higher PE concentrations shifted the concentration response curve for ACh to the right (Figure 3). Moreover, the maximal dilator response to ACh, which reached 80% of preconstriction (1 μM PE, Figure 3A), was significantly decreased when PE concentrations were increased (54 and 52% of preconstriction at 10 and 30 μM PE, respectively; Figure 3B and Figure C). In the presence of YC-1 (3 and 30 μM), however, the effectiveness of ACh, i.e. the maximal response to ACh at a given PE concentration, was significantly increased (Figure 3A and Figure B). At very high PE and YC-1 concentrations, no significant effect of YC-1 on the effectiveness of ACh was observed, as the vasodilations in response to ACh showed a great variability under these conditions (Figure 3C), pointing to limitations of the experimental set-up. In contrast, there was no significant change in ACh potency in the presence of YC-1.
Figure 3.
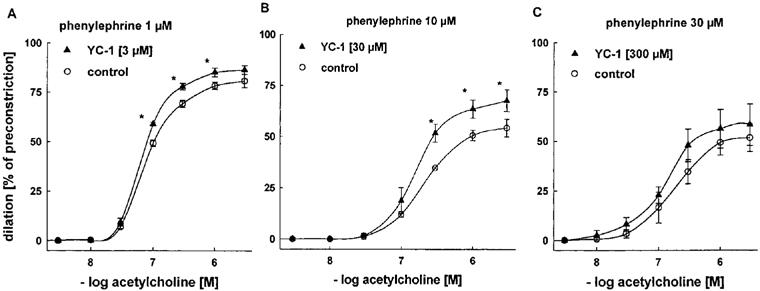
Effects of YC-1 on vasodilations induced by cumulative concentrations of ACh in endothelium-intact rabbit aortic rings preconstricted with PE. Aortic rings pretreated with 30 μM (B) or 300 μM (C) YC-1 required a higher concentration of PE (10 μM and 30 μM, respectively) for a similar level of preconstriction, compared to aortic rings pretreated with 3 μM (A) YC-1 (1 μM PE). Dilations are expressed as per cent of preconstriction. EC50 values for ACh: 0.09±0.03 (1 μM PE), 0.08±0.03 (1 μM PE+3 μM YC-1), 0.27±0.07 (10 μM PE), 0.20±0.06 (10 μM PE+30 μM YC-1), 0.21±0.04 (30 μM PE), and 0.14±0.03 μM (30 μM PE+300 μM YC-1). Data represent mean±s.e.mean; n=8; *indicates P<0.05, Tukeys test for pairwise comparison after two way ANOVA.
Figure 6.
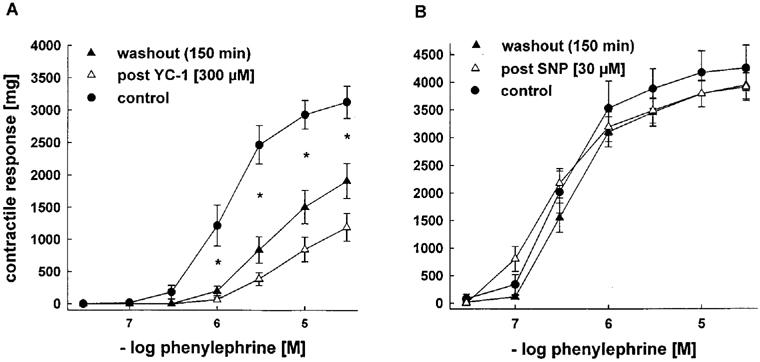
Effects of pretreatment with YC-1 [300 μM, left] or SNP [30 μM, right] on vasoconstrictions induced by cumulative concentrations of PE in endothelium-intact rabbit aortic rings. Contractile responses are expressed in mg. Pretreatment of aortic rings with YC-1 resulted in significantly lower contractile responses to cumulative concentrations of PE compared to untreated control arteries. After 150 min of washout of YC-1, this impairment was only partly reversible. *indicates P<.05 control vs YC-1-treated aortic rings before and after washout, n=7 each, Tukeys test for pairwise comparison after two way ANOVA. In contrast, contractile responses to PE were not significantly altered by pretreatment with SNP.
SNP- and NOC 18-induced vasodilator responses in rabbit aortic rings treated with YC-1 are potentiated
Next we studied the effects of YC-1 on the endothelium-independent vasodilation by exogenous NO donors. This time, E− aortic rings were pretreated with YC-1, and the vasodilator responses to SNP (Figure 4) and NOC 18 (Figure 5) were studied according to a protocol identical to that for ACh-induced vasodilations. Again, significantly higher concentrations of PE were required to induce stable constrictions (3–5 g) in YC-1-pretreated vessels. These increasing PE concentrations (in the absence of YC-1) shifted the concentration-response curves for both NO donors to the right (Figures 4 and Figures 5). Importantly, and in contrast to ACh (Figure 3), both NO-donors were able to induce complete relaxations (100% of preconstriction) at high concentrations. Accordingly, their effectiveness was not improved by YC-1. However, pretreatment with 30 and 300 μM YC-1 markedly increased the potency of SNP, as it shifted the concentration-response curve for SNP to the left (Figure 4; EC50 at 10 μM PE: 0.18±0.01 μM in control rings vs 0.02±0.002 μM in rings treated with 30 μM YC-1; at 30 μM PE: 1.16±0.17 μM in control rings vs 0.03±0.007 μM in rings treated with 300 μM YC-1). Similarly, YC-1 pretreatment increased the potency of NOC 18 (Figure 5; EC50 values at 10 μM PE: 5.67±0.20 μM in control rings vs 0.77±0.05 μM in rings treated with 30 μM YC-1).
Figure 4.
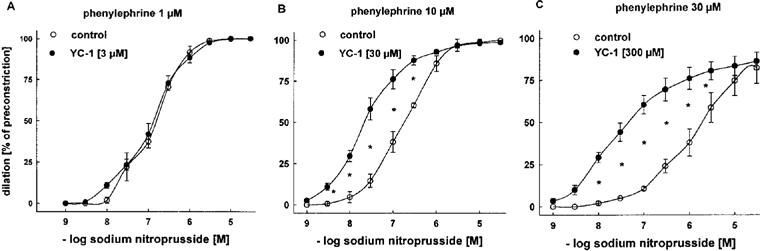
Effects of YC-1 on dilations induced by cumulative concentrations of SNP in endothelium-intact rabbit aortic rings preconstricted with PE. Aortic rings treated with 30 μM (B) and 300 μM (C) YC-1 required a higher concentration of PE (10 and 30 μM), respectively for a similar level of preconstriction, compared to aortic rings treated with 3 μM (A) YC-1 (1 μM PE). Dilations are expressed as per cent of preconstriction. YC-1 at concentrations of 30 μM and 300 μM potentiated SNP-induced dilations. EC50 values for SNP: 0.14±0.02 (1 μM PE), 0.13±0.01 (1 μM PE+3 μM YC-1), 0.18±0.01 (10 μM PE), 0.02±0.002 (10 μM PE+30 μM YC-1), 1.16±0.17 (30 μM PE), and 0.03±0.007 μM (30 μM PE+300 μM YC-1). Data represent mean±s.e.mean; n=8; *indicates P<0.05, Tukeys test for pairwise comparison after two way ANOVA.
Figure 5.
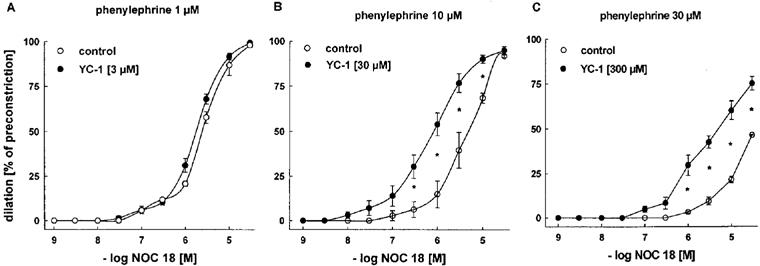
Effects of YC-1 on dilations induced by cumulative concentrations of the NONOate NOC 18 in endothelium-intact rabbit aortic rings preconstricted with PE. Aortic rings treated with 30 μM (B) and 300 μM (C) YC-1 required a higher concentration of PE (10 μM and 30 μM, respectively) for a similar level of preconstriction, compared to aortic rings treated with 3 μM (A) YC-1 (1 μM PE). Dilations are expressed as per cent of preconstriction. YC-1 at concentrations of 30 μM and 300 μM significantly potentiated NOC 18-induced dilations. EC50 values for NOC 18: 3.08±0.42 (1 μM PE), 2.13±0.23 (1 μM PE+3 μM YC-1), 5.67±0.20 (10 μM PE), 0.77±0.05 μM (10 μM PE+30 μM YC-1). No EC50 values could be determined at 30 μM PE and 30 μM PE+300 μM YC-1 (C), as the concentration-response curves were not complete. Data represent mean±s.e.mean; n=8; *indicates P<0.05, Tukeys test for pairwise comparison after two way ANOVA.
Contractile responses to PE in YC-1- and SNP-treated aortic rings
The finding that YC-1 lowered the sensitivity of aortic rings to PE prompted us to study whether this effect is specific for YC-1 or can also be achieved by preincubation with other vasodilators, i.e. SNP. Rabbit aortic rings were therefore preincubated with either YC-1 or SNP at concentrations which elicited a full vasorelaxation in preconstricted arteries (300 or 30 μM, respectively). The contractile response to cumulative concentrations of PE was determined both directly after the preincubation period and after a period of 150 min washout of YC-1 or SNP. As shown in Figure 6A, pretreatment of aortic rings with YC-1 resulted in significant depression of the contractile responses to PE when compared to untreated control arteries. This impairment persisted even after 150 min of repeated washout of YC-1, indicating a long lasting inhibition of contraction by YC-1. In contrast, contractile responses to PE after SNP pretreatment were indistinguishable from untreated controls after 150 min washout (Figure 6B). In fact, the contractile response returned to normal as soon as 5 min after SNP removal.
Vascular content of cyclic GMP in YC-1- and SNP-treated aortic rings
To investigate whether the long lasting effect of YC-1 on contractile responses coincided with elevated cyclic GMP levels, we determined the vascular cyclic GMP content under identical conditions as above, i.e. after YC-1 and SNP treatment. Thereby, aortic rings were preincubated for 60 min with either YC-1 (300 μM) or SNP (30 μM), or the respective solvent as control. Figure 7 shows the intravascular cyclic GMP content of aortic rings immediately after the incubation period, and after a 5, 20, or 60 min washout phase. As expected, SNP-treatment resulted in a moderate increase in intravascular cyclic GMP that rapidly declined during the washout phase. In contrast, YC-1-stimulated aortic rings showed an even more pronounced elevation in intravascular cyclic GMP that declined much slower during the subsequent washout period.
Figure 7.
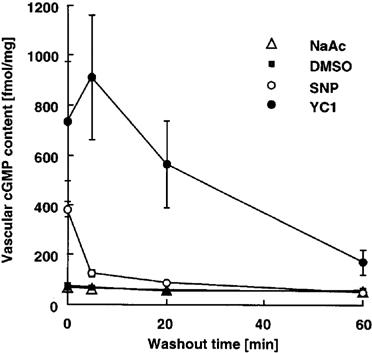
Intravascular cyclic GMP content of endothelium-intact rabbit aortic rings immediately after a 60 min incubation period with YC-1 (300 μM), or with SNP (30 μM), or with an aliquot of the respective buffer as control, and after a 5, 20, or 60 min washout phase. Intravascular cyclic GMP content is expressed in fmol mg−1 protein. n=6. SNP-treatment resulted in a moderate increase in intravascular cyclic GMP that rapidly declined during the washout phase. In contrast, YC-1-stimulated aortic rings showed a strong and prolonged elevation in intravascular cyclic GMP.
YC-1 inhibits vascular cyclic GMP degradation
To investigate whether the strong and persistent increase in intravascular cyclic GMP levels in the presence of YC-1 is at least partially due to inhibition of cyclic GMP break down in addition to activation of sGC, we examined the effect of YC-1 on cyclic GMP levels in rabbit aortic crude supernatant fractions. As shown in Figure 8, exogenous cyclic GMP was rapidly degraded with first order kinetics by rabbit aortic extracts when incubated at 37°C. However, in the presence of YC-1, degradation of cyclic GMP was significantly attenuated. Increases in endogenous cyclic GMP levels in rabbit aortic extracts, induced by activation of endogenous sGC in response to 100 μM YC-1, were below the detection limit of the cyclic GMP assay (20 nM) and therefore neglectable in this experiment. Thus, YC-1 contributes to cyclic GMP elevation by inhibition of cyclic GMP degradation.
Figure 8.
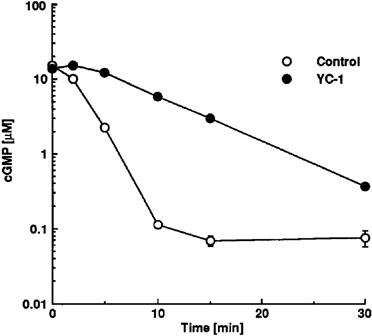
Degradation of cyclic GMP in the presence of rabbit aortic cytosolic extracts in the absence or presence of 100 μM YC-1. cyclic GMP (15 μM) was incubated in the presence of rabbit aortic cytosolic extract at 37°C (see Methods), and cyclic GMP content (expressed in μM) was determined after 0, 2, 5, 10, 15, and 30 min of incubation. For each time point, n=3–6.
YC-1 is an inhibitor of PDE isoenzymes
To investigate whether YC-1 is an inhibitor of PDE activity, we examined the effect of YC-1 on cyclic GMP-degrading activity of calcium/calmodulin-dependent PDE1 (Gietzen et al., 1982) and of cyclic GMP-specific PDE5 (Schudt et al., 1991b). As shown in Figure 9A, YC-1 inhibited cyclic GMP degradation by both PDE isoenzymes. Importantly, YC-1 inhibited PDE activities in the same concentration range as it induced vascular relaxations. Moreover, YC-1 also inhibited cyclic AMP-degrading activity of cyclic GMP-stimulated PDE2 (Schudt et al., 1991b), cyclic GMP-inhibited PDE3 (Schudt et al., 1991b), and cyclic AMP-specific PDE4 (Schudt et al., 1991a) (Figure 9B), with IC50 values of 31.6, 51.3 and 8.5 μM, respectively. In conclusion, YC-1 is not only a potent activator of sGC, but also a novel PDE inhibitor with moderate potency and low isoenzyme specificity.
Figure 9.
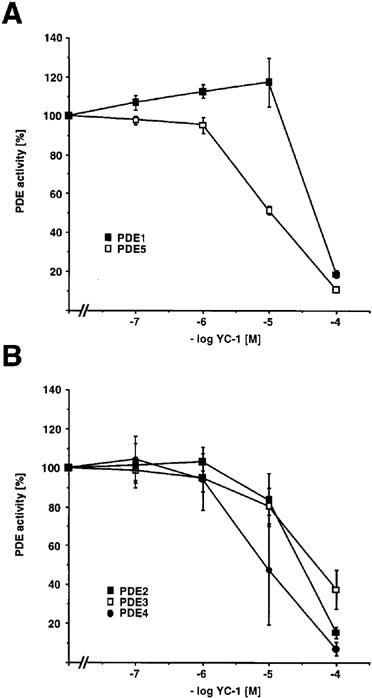
Concentration-response curves for YC-1 to inhibit different PDE isoenzymes in vitro. PDE isozyme preparations and PDE assays were performed as described in Methods. For calcium/calmodulin-stimulated PDE1 and for PDE5, enzyme activities were determined with cyclic GMP as substrate (A). For cyclic GMP-stimulated PDE2, and for PDE3 and PDE4, enzyme activities were determined with cyclic AMP as substrate (B). PDE activities are expressed as percentage of PDE activity of the respective isoenzyme in the absence of YC-1. Data represent mean±s.e.mean, n=3–5.
Discussion
One of the main obstacles in the chronic clinical application of nitrovasodilators or NO donors is their ability to induce tolerance. Such NO-based sGC activators are also affected by conditions where tissue superoxide levels are elevated resulting in direct scavenging of NO (Münzel et al., 1995). YC-1 represents the first compound of a novel class of direct activators of sGC that stimulates sGC without releasing NO. Therefore, its mechanism of action is of great interest both clinically and with respect to the molecular mechanisms of sGC regulation. In the present study, we confirm that YC-1 is an endothelium-independent vasodilator, consistent with its ability to directly activate sGC. However, we report a previously unrecognized long lasting inhibition of phenyl-ephrine-elicited contraction and corresponding increases in intracellular cyclic GMP levels in response to YC-1. This inhibition was partially irreversible as it was not overcome by repeated washouts of YC-1. In contrast, the nitrovasodilator SNP failed to inhibit contractions after its removal from the organ bath and induced only moderate and transient increases in cyclic GMP levels. Moreover, we identify the mechanism of this YC-1 effect as non-specific PDE inhibition by which elevations of intravascular cyclic GMP levels are augmented through concomitant inhibition of cyclic GMP degradation. These results suggest that YC-1 action in vivo is fundamentally different from that of the classical NO donor compounds. YC-1 combines two distinct, but synergistic activities, i.e. sGC activation and PDE inhibition, and therefore represents a novel class of pharmacological compounds (Figure 10). However, this mixed mechanism of action needs to be taken into account in future pharmacological studies using YC-1 as a tool to selectively study the effects of sGC stimulation.
Figure 10.
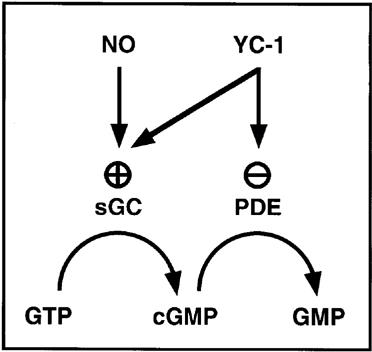
Effects of YC-1 on the regulation of intracellular cyclic GMP levels.
It is evident that activation of sGC is essential for YC-1 actions in vivo, as for example YC-1-induced vasodilation is attenuated by ODQ, an inhibitor of sGC (Garthwaite et al., 1995). In this respect, YC-1 resembles the classical nitrovasodilators. Consequently, YC-1 and NO–induced vasorelaxations are equally sensitive to ODQ. Our finding that YC-1 is a novel PDE inhibitor confirms and extends the results of a recent study that suggested YC-1 inhibited cyclic AMP break down in myocardium (Wegener et al., 1997). YC-1 does not show structural similarity to any known PDE inhibitor; however, its potency is low, ranging between the non-selective PDE inhibitors 3-isobutyl-1-methylxanthine (IBMX) and theophylline (Dent & Rabe, 1996). Low potency, together with its low selectivity, make YC-1, however, an unattractive lead compound for further development of related PDE inhibitors.
We cannot rule out that, in addition to sGC activation and PDE inhibition, there are other mechanisms of action that contribute to the observed differences between YC-1 and NO donor kinetics on the inhibition of PE-induced contraction and vascular cyclic GMP levels. Different modes of diffusion, uptake, or distribution may also partly account for the observed delayed reversibility of YC-1 effects. For example, NO is thought to be rapidly degraded once released from the respective donor compound, whereas YC-1 might persist in the cell in an active state. Alternatively, YC-1 may cause a long-lasting activation of sGC whereas NO-induced activation is only transient. However, Mülsch et al. (1997) have shown that YC-1-induced activation of purified sGC is rapidly reversible.
It has been shown that YC-1 and NO activate purified sGC in vitro in a synergistic manner (Friebe et al., 1996; Mülsch et al., 1997). Here we show that YC-1 increased both the vasodilator response induced by endogenous (ACh-elicited endothelium-derived) NO and exogenous NO (released from the NO donor compounds SNP and NOC 18). However, there was one noteworthy difference between these two interactions. Effects of exogenous NO donors were considerably potentiated in the presence of YC-1 as shown previously (Mülsch et al., 1997), in a similar manner as observed for NO effects on purified sGC. In contrast, YC-1 at low concentrations slightly increased the effectiveness but not the potency of ACh-induced, NO-mediated dilations. These distinct effects of YC-1 on exogenous and endogenous NO confirm that NO donor compounds and ACh do not necessarily induce identical signal transduction processes, and provide a tool to further study the nature and action of endogenous NO. YC-1 will also be useful to characterize pharmacologically the endogenous NO-containing compound also in non-adrenergic-non-cholinergic (NANC) smooth muscle relaxation (Gillespie et al., 1989) and other physiological processes (Schmidt & Walter, 1994).
In conclusion, YC-1 is the first representative of a novel class of sGC activator compounds, that combines the functions of a classical nitrovasodilator with PDE inhibition and leads to a long-lasting cyclic GMP-mediated inhibition of vasoconstriction. It will now be of interest to further elucidate the molecular mechanisms of YC-1 versus NO action upon sGC, in normal and particularly in nitrovasodilator-tolerant blood vessels and conditions coinciding with elevated superoxide tissue levels.
Acknowledgments
We thank Dr Peter Kotsonis for helpful discussions, and Dr Mylinh La for carefully reading the manuscript. The skilful technical assistance of Susanne Clemens-Richter is also gratefully acknowledged. We thank Dr Karl Schönafinger, Hoechst Marion Roussel, for the generous gift of YC-1. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Ga 431/1-4 and SFB/C7) and the Fritz-Thyssen-Stiftung.
Abbreviations
- ACh
acetylcholine
- cyclic GMP
cyclic 3′:5′ guanosine monophosphate
- NOC 18
1-hydroxy-2-oxo-3,3-bis(3-aminoethyl)-1-triazine
- E+
endothelium-intact
- E−
endothelium-denuded
- ODQ
1-H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one
- PDE
phosphodiesterase(s)
- PE
phenylephrine
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- YC-1
3-(5′-hydroxymethyl-2′furyl)-1-benzyl-indazol
References
- ARNOLD W.P., MITTAL C.K., KATSUKI S., MURAD F. Nitric oxide activates guanylate cyclase and increases guanosine 3′,5′-monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER A.C., SCHWABE U. An improved assay or cyclic 3′,5′-nucleotide phosphodiesterase with QAE sephadex A-25. Naunyn-Schmiedeberg's Arch. Pharmaracol. 1980;311:193–198. doi: 10.1007/BF00510259. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., CHEN J., ISCHIROPOULOS H., CROW J.P. Oxidative chemistry of peroxynitrite. Meth. Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., YE Y.Z., ANDERSON P.G., CHEN J., ACCAVITTI M.A., TARPEY M.M., WHITE C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem. Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- BÖHME E., GRAF H., SCHULTZ G. Effects of sodium nitroprusside and other smooth muscle relaxants on cyclic GMP formation in smooth muscle and platelets. Adv. Cyclic Nucleotide Res. 1978;9:131–143. [PubMed] [Google Scholar]
- BUSSE R., MÜLSCH A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Letters. 1990;275:87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- DENT G., RABE K.F.Effects of theophylline and non-selective xanthine derivatives on PDE isoenzymes and cellular function Phosphodiesterase inhibitors 1996London, UK: Academic Press; 41–64.ed Schudt, C., Dent, G. & Rabe, K.F. pp [Google Scholar]
- FRIEBE A., SCHULTZ G., KOESLING D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- GALLE J., BENGEN J., SCHOLLMEYER P., WANNER C. Impairment of endothelium-dependent dilation in rabbit renal arteries by oxidized lipoprotein(a) Circulation. 1995a;92:1582–1589. doi: 10.1161/01.cir.92.6.1582. [DOI] [PubMed] [Google Scholar]
- GALLE J., SCHINI V., STUNTZ P., SCHOLLMEYER P. Interleukin-1β induces the formation of nitric oxide in isolated juxtaglomerular cells: Influence on renin secretion. Nephrol. Dial. Transplant. 1995b;10:191–197. [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C., NIELSEN E., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Molecular Pharmacology. 1995;48:184–188. [PubMed] [Google Scholar]
- GIETZEN K., SADORF I., BADER H. A model for the regulation of the calmodulin-dependent enzymes erythrocyte Ca2+-transport ATPase and brain phosphodiesterase by activators and inhibitors. Biochem. J. 1982;207:541–548. doi: 10.1042/bj2070541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J.S., LIU X., MARTIN W. The effects of L-arginine and Ng-monomethyl L-arginine on the response of the rat anococcygeus to NANC nerve stimulation. Br. J. Pharmacol. 1989;98:1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KO F.N., WU C.C., KUO S.C., LEE F.Y., TENG C.M. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MONCADA S., HIGGS E.A. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur. J. Clin. Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- MÜLSCH A., BAUERSACHS J., SCHÄFER A., STASCH J.-P., KAST R., BUSSE R. Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators. Br. J. Pharmacol. 1997;120:681–689. doi: 10.1038/sj.bjp.0700982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜNZEL T., SAYEGH H., FREEMAN B.A., TARPEY M.M., HARRISON D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., LOHMANN S.L., WALTER U. The nitric oxide and cGMP signal-transduction pathway. Biochem. Biophys. Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., WALTER U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., FORDERKUNZ S., HATZELMANN A., ULRICH V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991a;344:682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., MÜLLER B., UKENA D. Zardaverine as a selective inhibitor of phosphodiesterase isozymes. Biochem. Pharmacol. 1991b;42:153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S. Redox signalling: nitrosylation and related interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- THOMPSON W.J., TERASAKI W.L., EPSTEIN P.M., STRADA S.J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv. Cycl. Nucl. Res. 1979;10:69–92. [PubMed] [Google Scholar]
- WEGENER J.W., GATH I., FÖRSTERMANN U., NAWRATH H. Activation of soluble guanylyl cyclase by YC-1 in aortic smooth muscle but not in ventricular myocardium from rat. Brit. J. Pharmacol. 1997;122:1523–1529. doi: 10.1038/sj.bjp.0701542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C.C., KO F.N., KUO S.C., LEE F.Y., TENG C.M. YC-1 inhibited human platelet aggregatiaon through NO-independent activation of soluble guanylate cyclase. Br. J. Pharmacol. 1995;116:1973–1978. doi: 10.1111/j.1476-5381.1995.tb16400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C.C., KO F.N., TENG C.M. Inhibition of platelet adhesion to collagen by cGMP-elevating agents. Biochem. Biophys. Res. Commun. 1997;231:412–416. doi: 10.1006/bbrc.1996.5998. [DOI] [PubMed] [Google Scholar]
- YU S.M., CHENG Z.J., GUG J.H., LEE F.Y., KUO S.C. Mechanism of antiproliferation caused by YC-1, an im indazole derivative, in cultured rat A10 vascular smooth-muscle cells. Biochem. J. 1995;306:787–792. doi: 10.1042/bj3060787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- ZELIS R., MASON D.T. Isosorbide dinitrate. Effect on the vasodilator response to nitroglycerin. J.A.M.A. 1975;234:166–170. doi: 10.1001/jama.234.2.166. [DOI] [PubMed] [Google Scholar]


