Abstract
The effects of carboxy-PTIO and hydroxocobalamin were studied on nitrergic transmission in anococcygeus and retractor penis muscles taken during post mortem examination from young male pigs.
In both muscles under resting conditions, electrical field stimulation (EFS) caused contractions that were sensitive to tetrodotoxin (1 μM) and were greatly inhibited by prazosin (1 μM) and guanethidine (10–30 μM), but were not significantly affected by atropine (1 μM). In the anococcygeus muscle, but not in the retractor penis muscle, guanethidine produced a prolonged contraction.
After tone was raised by guanethidine in the anococcygeus or by phenylephrine (1 μM) in the presence of guanethidine in the retractor penis, EFS caused tetrodotoxin-sensitive relaxations. The EFS-induced relaxations were abolished by the NO synthase inhibitor NG-L-nitro-arginine methyl ester (L-NAME; 100 μM) and its effect was partly overcome by L-arginine (1 mM), indicating it was mediated by nitrergic nerves.
Carboxy-PTIO (0.1–1 mM) had no significant effect in reducing stimulation-induced nitrergic relaxations in either muscle. However, hydroxocobalamin (0.1–1 mM) caused concentration-dependent reductions of nitrergic relaxations in both muscles. Relaxations to exogenous nitric oxide (1 μM) in both muscles were abolished by carboxy-PTIO (0.3 mM) and hydroxocobalamin (0.1 mM).
There were no differences in reactivity to carboxy-PTIO or hydroxocobalamin between anococcygeus and retractor penis muscles from the same species (pig). The finding also confirms earlier observations that the nitrergic transmitter is generally resistant to the NO-scavenger carboxy-PTIO.
Keywords: Anococcygeus (porcine), carboxy-PTIO, hydroxocobalamin, nitric oxide, nitrergic transmission, retractor penis muscle (porcine)
Introduction
Most of the research which led to the initial recognition of nitrergic transmission at autonomic neuroeffector junctions was carried out with the rat anoccygeus and bovine retractor penis muscles, and these were the first tissues in which peripheral nitrergic transmission was demonstrated (for reviews, see: Gillespie et al., 1990; Martin & Gillespie, 1991; Rand & Li, 1995a,1995b).
Although there is indisputable evidence for nitrergic transmission in both muscles, a number of differences have been reported in their responsiveness to various agents. The first difference that was noted was that the nitric oxide synthase (NOS) inhibitor NG-monomethyl-L-arginine (L-NMMA; methylarginine) readily inhibited nitrergic transmission in rat anococcygeus muscle (Li & Rand, 1989; Gillespie et al., 1989), but did not in the bovine retractor penis muscle (Gillespie et al., 1989). In fact L-NMMA, like L-arginine, restored nitrergic relaxations of the bovine retractor penis that had been blocked by the more potent NOS inhibitor NG-nitro-L-arginine (Martin et al., 1993). More recently, it has been reported that the NO-sequestering substance hydroxocobalamin produces concentration-dependent reductions in nitrergic nerve stimulation-induced relaxations in the bovine retractor penis muscle (Paisley & Martin, 1996), but did not reduce nitrergic transmission in rat anococcygeus muscles, at least in concentrations that blocked responses to exogenous nitric oxide (Rajanayagam et al., 1993; Li & Rand, 1993). Furthermore, the NO scavenger carboxy-PTIO did not inhibit nitrergic relaxations in rat and mouse anococcygeus muscles (Rand & Li, 1995c; Lilley & Gibson, 1996) but blocked them in the bovine retractor penis muscle (Paisley & Martin, 1996).
The fact of these discrepancies raises the question as to whether they are due to species or tissue differences. It seems unlikely that there is a tissue difference since the retractor penis is contiguous with the anococcygeus muscles (Klinge & Sjöstrand, 1974; Martin & Gillespie, 1991). The two bands of the anococcygeus muscles unite in a bar on the ventral aspect of the terminal portion of the colon and then project forward as the single retractor penis muscle (or pair of muscles in some species) to their attachment to the cartilaginous shaft of the penis. The muscle is absent in primates and rabbits and is vestigial in rats. Both the anococcygeus and the retractor penis muscles have a noradrenergic sympathetic motor innervation and a NANC parasympathetic inhibitory innervation (Sjöstrand & Klinge, 1995). However, anatomical facts about the two muscles do not provide adequate grounds for surmise about the nature of their neurotransmitter mechanisms.
We had the opportunity to obtain anococcygeus and retractor penis muscles from freshly killed pigs that were undergoing post mortem examination in the course of another series of experiments (see Methods). Therefore, we set out to test in them the agents that had different effects between the rat anococcygeus muscle and the bovine retractor penis. The preliminary results of the findings reported here have been communicated to the Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (Li et al., 1996).
Methods
Young male pigs (40–50 kg) which acted as controls in a study on vaccine production were stunned and bled. During post mortem examination, the anococcygeus and retractor penis muscles were removed, transported to the laboratory in cold oxygenated physiological salt solution (PSS), and longitudinal portions of muscle strips 2–3 mm wide and 1 cm long of each were set up in organ baths at a resting tension of 1 g as described previously for the rat anococcygeus muscle (Gillespie 1972; Li & Rand, 1989). The Royal Melbourne Institute of Technology animal ethics committee approved these additional experiments.
Responses were recorded as changes in isometric tension in response to electrical field stimulation of intrinsic nerves and exogenously applied NO in aqueous solution. The effects of NO-related agents were studied after the tone of the muscle was raised (see Results). Nitrergic relaxations were evoked by electrical field stimulation (EFS) with 1 ms pulses of supramaximal voltage at 2 Hz for 10 s at 2 min intervals applied through a pair of platinum wire electrodes at either side of the muscle. Parallel experiments without addition of drugs acting on nitrergic mechanisms were carried out with tissues from the same donor animal as time controls.
The composition of the PSS was as follows (mM): NaCl, 118; KCl, 4.7; NaHCO3, 25; MgSO4, 0.45; KH2PO4, 1.03; CaCl2, 2.5; d-(+)-glucose, 11.1; disodium edetate, 0.067.
The following drugs were used: L-arginine, atropine sulphate, guanethidine sulphate, hydroxocobalamin hydrochloride, NG-nitro-L-arginine methyl ester (L-NAME), L-phenylephrine hydrochloride, tetrodotoxin (TTX) (Sigma Chemical Co., U.S.A.); carboxy-PTIO (Sapphire Bioscience, Australia); prazosin hydrochloride (Pfizer, U.S.A.). The NO solution (2 mM) was prepared from NO compressed gas (Commonwealth Industrial Gases, Melbourne, Australia) as previously described (Rajanayagam et al., 1993).
Data are expressed as means and standard errors. The significance of differences between means was determined by Student's t-test or one-way analysis of variance (ANOVA). Values of P<0.05 were considered as significant.
Results
In both anococcygeus and retractor penis muscles under resting conditions, EFS (1–5 Hz, 10 s) produced frequency-dependent contractions which were abolished by tetrodotoxin (1 μM) (data not shown). Contractions induced by EFS at 2 Hz were 5.2±0.6 g (n=7) and 5.6±0.4 g (n=8), respectively, in the anococcygeus and the retractor penis muscles. EFS-induced contractions were not significantly affected by atropine (1 μM, Figure 1A), but were significantly reduced by prazosin (1 μM, Figure 1B). The noradrenergic nerve blocking agent guanethidine (10–30 μM) blocked EFS-induced contractile responses in both muscles and increased the tone in the anococcygeus but not in the retractor penis muscle (Figure 1).
Figure 1.
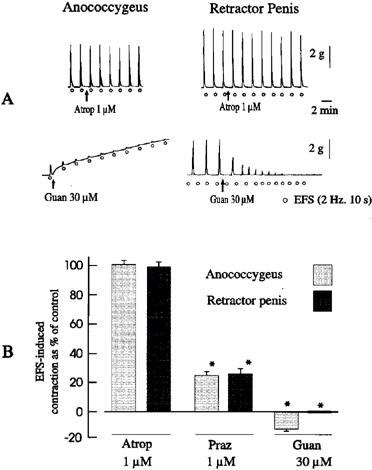
(A) Tracings illustrating the effects of atropine and guanethidine on field stimulation-induced contractile responses of the pig anococcygeus and retractor penis muscle. (B) Mean data for the effects of atropine, guanethidine and prazosin on field stimulation-induced contractions of the pig anococcygeus and retractor penis muscles. Columns represent means and I-bars indicate the s.e.mean of 4–5 experiments. *Indicates a significant difference (P<0.05, Student's t-test) from the corresponding control group.
In anococcygeus muscles, guanethidine alone raised the tone and relaxant responses to EFS were revealed. In retractor penis muscles, the tone was raised by phenylephrine (1 μM) so that relaxations could be manifested. In both muscles, the EFS-induced relaxations were abolished by tetrodotoxin (1 μM, Figure 2B) and by the NO synthase inhibitor L-NAME (100 μM; Figure 2). The effects of L-NAME were partly overcome by L-arginine (1 mM; Figure 2B).
Figure 2.
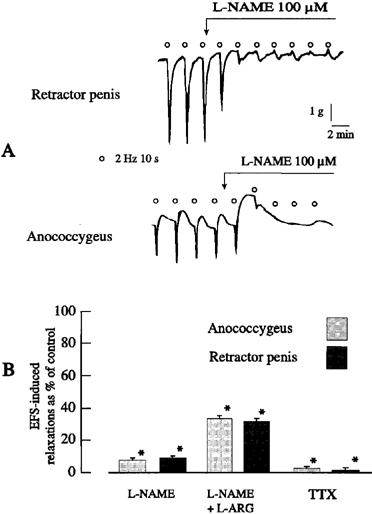
(A) Tracings illustrating the effects of L-NAME on field stimulation-induced relaxant responses of the pig anococcygeus and retractor penis muscles. (B) Mean data for the effects of L-NAME (100 μM), L-NAME plus L-arginine (1 mM) and tetrodotoxin (1 μM) on field stimulation-induced relaxations of the pig anococcygeus and retractor penis muscles muscle. Columns represent means and I-bars indicate the s.e.mean of 4–5 experiments. *Indicates a significant difference (P<0.05, Student's t-test) from the corresponding control group.
Hydroxocobalamin (0.1–1 mM) caused concentration-dependent reductions of nitrergic relaxations in both muscles (Figure 3). Carboxy-PTIO (0.01–1 mM) had no significant effect on stimulation-induced nitrergic relaxations in either muscle (Figure 4). In both muscles, relaxations in response to exogenous NO (1 μM) were very similar in shape and amplitude as those to electrical stimulation. They were reduced or abolished by carboxy-PTIO (0.1 and 0.3 mM) or hydroxocobalamin (30 and 100 μM). The responses to exogenous NO in the presence of 0.1 and 0.3 mM carboxy-PTIO were 19.3±4.4% (n=4) and 0.2±0.3% (n=4), respectively, of the control response (P<0.01, Student's t-test), and 59.7±4.3% (n=4) and 4.5±0.6% (n=4) of the control response (P<0.01, Student's t-test), respectively, in the presence of 30 and 100 μM hydroxocobalamin.
Figure 3.
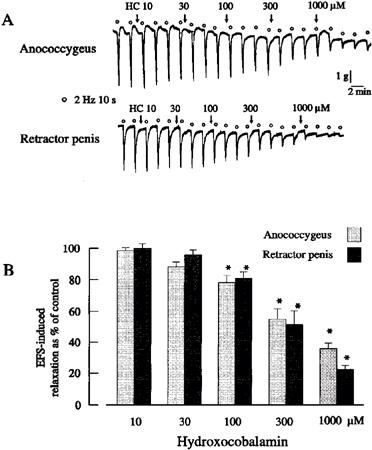
(A) Tracings illustrating the effects of hydroxocobalamin on field stimulation-induced relaxations of the porcine anococcygeus and retractor penis muscles. (B) Mean data for effects of hydroxocobalamin on field stimulation-induced relaxations of the pig anococcygeus and retractor penis muscles. Columns represent means and I-bars indicate the s.e.mean of 4–5 experiments. *Indicates a significant difference (P<0.05, ANOVA followed by the Bonferroni t-test) from the corresponding control group.
Figure 4.
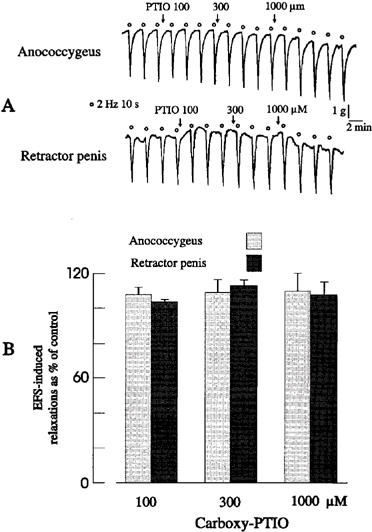
(A) Tracings illustrating the effects of carboxy-PTIO on field stimulation-induced relaxations of the porcine anococcygeus and retractor penis muscles. (B) Mean data for the effects of carboxy-PTIO on field stimulation-induced relaxations of the pig anococcygeus and retractor penis muscles muscle. Columns represent means and I-bars indicate the s.e.mean of 4–5 experiments.
Discussion
The innervations of the anococcygeus muscle and retractor penis muscle have been described by a number of workers in several species. In general, there is a dense network of typical noradrenergic fibres and a less prominent network of nitrergic fibres (nNOS immunoreactive and NADPH-diaphorase positive) (Klinge et al., 1970; Gillespie & Lüllmann-Rauch, 1974; Gibbins & Haller, 1979; Byrne & Muir, 1984; Sheng et al., 1992; Brave et al., 1993; Dail et al., 1990, 1993). The finding that the contractile responses to EFS in both anococcygeus and retractor penis muscles of young pigs were greatly inhibited or blocked by the α1-adrenoceptor antagonist prazosin and by tetrodotoxin indicate that these responses are largely noradrenergic in origin. Similarly, the findings that EFS-induced relaxations in both tissues are blocked by the NOS inhibitor L-NAME, and the effect of L-NAME was partly reversed by L-arginine, indicates that these relaxations are mediated by nitrergic nerves, as already established in these tissues from other species (see Introduction). We are not aware of any previous studies on EFS-induced responses in pig anococcygeus muscle; however, an earlier study by Ambache & Killick (1978) reported a species difference in EFS-induced contractile responses of retractor penis muscles from pig and sheep compared with horse and dog. In their study, a portion of the EFS-induced contraction in adult pig retractor penis muscle was resistant to phentolamine. It seems that a non-adrenergic component may also be involved in EFS-induced contractions in the immature pigs used in the present study, as a small portion of the EFS-induced contractions was prozosin resistant. However, the nature of the non-adrenergic component is not clear. Nevertheless, the main difference in contractile responses we observed is that guanethidine increased the tone in the anococcygeus muscle but not in the retractor penis muscle; this effect may be related to the density of the excitatory nerve fibres in these tissues.
Although nitrergic transmission is absolutely dependent on the functional integrity of nitric oxide synthase, it has been found that responses to exogenous NO and nitrergic nerve stimulation are differentially affected by certain agents such as superoxide generators and NO scavengers including carboxy-PTIO (Rand & Li, 1995a,1995b). These agents generally block the responses to exogenous NO but have little effect on responses to nitrergic nerve stimulation (Hobbs et al, 1991; Rand & Li, 1995c; Lefebvre, 1996).
Carboxy-PTIO is a NO scavenger that inactivates free radical NO (Akaike et al., 1993). It has been shown that carboxy-PTIO inhibits responses to exogenous NO in the rat and mouse anococcygeus muscles but not those induced by nitrergic nerve stimulation (Rand & Li, 1995c; Lilley & Gibson, 1996). However, in the bovine retractor penis, nitrergic responses were inhibited by carboxy-PTIO (Paisley & Martin, 1996). In the present study, carboxy-PTIO in concentrations that blocked responses to exogenous NO failed to inhibit EFS-induced nitrergic relaxations in either the anococcygeus muscle or the retractor penis muscle of the pig, indicating that the differences between rat anococcygeus and bovine retractor penis muscles are likely to be due to a species difference.
Recent debate on the mechanism of nitrergic transmission has offered several explanations for the contrasting actions of certain superoxide generators and NO-scavengers on authentic NO and nitrergic transmission. These include the protection of NO by endogenous superoxide dismutase from inactivation by superoxide (Martin et al., 1994) and the protection of NO by antioxidant agents from inactivation by some NO-scavengers (Lilley & Gibson, 1997), as well as the hypothesis that the nitrergic transmitter may not be free radical NO (Rand & Li, 1995a). Since the effect of carboxy-PTIO is not affected by SOD inhibitors (Rand & Li, 1995c), the involvement of superoxide is unlikely to be involved. On the other hand, the release of antioxidant ascorbate from the nitrergically innervated tissues upon electrical field stimulation (Lilley & Gibson, 1997) may explain the inability of carboxy-PTIO to block he responses to exogenous NO. However it is not clear whether a sufficient amount of ascorbate is released from nitrergic nerves to interact with carboxy-PTIO. Further experiments are necessary to explore the possible mechanism of nitrergic transmission that is resistant to carboxy-PTIO in pig anococcygeus and retractor penis muscles.
Hydroxocobalamin interacts with NO to form an unidentified NO-adduct (Rochelle et al., 1995). Hydroxocobalamin blocked responses to exogenous NO in concentrations that only slightly inhibited or had no effect on responses to nitrergic nerve stimulation in the rat anococcygeus muscle (Li & Rand, 1993; La et al., 1996) and other nitrergically-innervated tissues such as rat gastric fundus (Jenkinson et al., 1995; Lefebvre, 1996) and dog ileocolonic junction (De Man et al., 1995), but it inhibited responses to nitrergic nerve stimulation in the mouse anococcygeus muscle (Lilley & Gibson, 1996) and the bovine retractor penis (Paisley & Martin, 1996) as well as in the guinea-pig basilar artery (Jiang et al., 1997). In the present study, hydroxocobalamin produced concentration-dependent inhibitions of responses to nitrergic nerve stimulation in both the anococcygeus muscle and retractor penis muscle. Explanations for the difference between tissues in regard to actions of hydroxocobalamin may include differences in experimental conditions (La et al., 1997) and may involve tissue factors, although inhibition of superoxide dismutase did not affect the actions of hydroxocobalamin (Lefebvre, 1996; Paisley & Martin, 1996).
In conclusion, the results reveal that there are no differences in reactivity to carboxy-PTIO or hydroxocobalamin between anococcygeus and retractor penis muscles from the same species (pig), and indicate that the difference between rat anococcygeus and bovine retractor penis muscles is due to a species difference. Furthermore, the observations are in accord with the view that the nitrergic transmitter is generally resistant to the NO scavenger carboxy-PTIO, indicating either that the transmitter is protected by one or more endogenous substances or that it may not be free radical NO.
Acknowledgments
This work was supported by a grant from the National Health & Medical Research Council and the Smoking and Health Research Foundation of Australia. We are grateful to Dr N. Gerraty, Department of Applied Biology and Technology, RMIT, for assess to the tissues of young pigs used as controls in his study on a vaccine.
Abbreviations
- ANOVA
one-way analysis of variance
- EFS
electrical field stimulation
- L-NAME
NG-L-nitro-arginine methyl ester
- NANC
non-adrenergic non-cholinergic
- L-NMMA
NG-monomethyl-L-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- PSS
physiological salt solution
- TTX
tetrodotoxin
References
- AKAIKE T., YOSHIDA M., MIYAMOTO Y., SATO K., KOHNO M., SASAMOTO K., MIYAZAKI K., UEDA S., MAEDA H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/NO• through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- AMBACHE N., KILLICK S.W. Species differences in postganglionic motor transmission to the retractor penis muscle. Br. J. Pharmacol. 1978;63:25–34. doi: 10.1111/j.1476-5381.1978.tb07770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVE S.R., TUCKER J.F., GIBSON A., BISHOP A.E., RIVEROS-MORENO V., MONCADA S., POLAK J.M. Localisation of nitric oxide synthase within non-adrenergic, non-cholinergic nerves in the mouse anococcygeus. Neurosci. Lett. 1993;161:93–96. doi: 10.1016/0304-3940(93)90148-e. [DOI] [PubMed] [Google Scholar]
- BYRNE N.G., MUIR T.C. Electrical and mechanical responses of the bovine retractor penis to nerve stimulation and to drugs. J. Auton. Pharmacol. 1984;4:261–271. doi: 10.1111/j.1474-8673.1984.tb00104.x. [DOI] [PubMed] [Google Scholar]
- DAIL W.G., CARRILLO Y., WALTON G. Innervation of the anococcygeus muscle of the rat. Cell Tissue Res. 1990;259:139–146. doi: 10.1007/BF00571438. [DOI] [PubMed] [Google Scholar]
- DAIL W.G., GALLOWAY B., BORDEGARAY J. NADPH diaphorase innervation of the rat anococcygeus and retractor penis muscles. Neurosci. Lett. 1993;17:17–20. doi: 10.1016/0304-3940(93)90906-2. [DOI] [PubMed] [Google Scholar]
- DE MAN J.G., BOECKXSTAENS G.E., DE WINTER B.Y., MOREELS T.G., MISSET M.E., HERMAN A.G., PELCKMANS P.A. Comparison of the pharmacological profile of S-nitrosothiols, nitric oxide and the nitrergic neurotransmitter in the canine ileocolonic junction. Br. J. Pharmacol. 1995;114:1179–1184. doi: 10.1111/j.1476-5381.1995.tb13331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBINS I.L., HALLER C.J. Ultrastructural identification of non-adrenergic, non-cholinergic nerves in the rat anococcygeus muscle. Cell Tissue Res. 1979;200:257–271. doi: 10.1007/BF00236418. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J.S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br. J. Pharmacol. 1972;45:404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J.S., LIU X., MARTIN W. The effects of L-arginine and NG-monomethyl-L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br. J. Pharmacol. 1989;98:1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J.S., LIU X., MARTIN W.The neurotransmitter of the non-adrenergic non-cholinergic inhibitory nerves to smooth muscle of the genital system Nitric Oxide from L-Arginine. A Bioregulatory System 1990Amsterdam: Elsevier; 147–164.ed. S. Moncada. pp [Google Scholar]
- GILLESPIE J.S., LÜLLMANN-RAUCH R. On the ultrastructure of the rat anococcygeus muscle. Cell Tissue Res. 1974;149:91–104. doi: 10.1007/BF00209052. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J., TUCKER J.F., GIBSON A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br. J. Pharmacol. 1991;104:645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON K.M., REID J.J., RAND M.J. Hydroxocobalamin and haemoglobin differentiate between exogenous and neuronal nitric oxide in the rat gastric fundus. Eur. J. Pharmacol. 1995;275:145–152. doi: 10.1016/0014-2999(94)00762-v. [DOI] [PubMed] [Google Scholar]
- JIANG F., LI C.G., RAND M.J. Effect of hydroxocobalamin on vascular responses to the nitrergic transmitter, nitric oxide and EDRF in guinea-pig isolated basilar artery. Eur. J. Pharmacol. 1997;340:181–186. doi: 10.1016/s0014-2999(97)01381-2. [DOI] [PubMed] [Google Scholar]
- KLINGE E., POHTO P., SOLATUNTURI E. Adrenergic innervation and structure of the bull retractor penis muscle. Acta Physiol. Scand. 1970;78:110–116. doi: 10.1111/j.1748-1716.1970.tb04645.x. [DOI] [PubMed] [Google Scholar]
- KLINGE E., SJÖSTRAND N.O. Contraction and relaxation of the retractor penis muscle and the penile artery of the bull. Acta Physiol. Scand. 1974;93 Suppl. 420:1–88. [PubMed] [Google Scholar]
- LA M., LI C.G., RAND M.J. Comparison of the effects of hydroxocobalamin and oxyhaemoglobin on responses to NO, EDRF and the nitrergic transmitter. Br. J. Pharmacol. 1996;117:805–810. doi: 10.1111/j.1476-5381.1996.tb15264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA M., PAISLEY K., MARTIN W., RAND M.J. The effects of hydroxocobalamin on nitrergic transmission in rat anococcygeus and bovine retractor penis muscles: sensitivity to light. Eur. J. Pharmacol. 1997;321:R5–R6. doi: 10.1016/s0014-2999(97)00048-4. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Influence of superoxide dismutase inhibition on the discrimination between NO and the nitrergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 1996;118:2171–2177. doi: 10.1111/j.1476-5381.1996.tb15659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., GERRATY N., RAND M.J. Effects of hydroxocobalamin and carboxy-PTIO on nitrergic transmission in porcine anococcygeus and retractor penis muscles. Proc. Austrl. Physiol. Pharmacol. Soc. 1996;27:167P. doi: 10.1038/sj.bjp.0702496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Effects of hydroxocobalamin and haemoglobin on NO-mediated relaxations in the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1993;20:633–640. doi: 10.1111/j.1440-1681.1993.tb01645.x. [DOI] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus against inhibition of superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidants ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W., GILLESPIE J.S.L-Arginine-derived nitric oxide: the basis of inhibitory transmission in the anococcygeus and retractor penis muscles Novel Peripheral Transmitters 1991Oxford: Pergamon Press; 65–79.ed. Bell, C., pp [Google Scholar]
- MARTIN W., GILLESPIE J.S., GIBSON I.F. Actions and interactions of NG-substituted analogues of L-arginine on NANC neurotransmission in the bovine retractor penis and rat anococcygeus muscles. Br. J. Pharmacol. 1993;108:242–247. doi: 10.1111/j.1476-5381.1993.tb13469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.H.M., PAISLEY K. NANC neurotransmission in the bovine retractor penis is blocked by superoxide anion following inhibition of superoxide dismutase with diethylthiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- PAISLEY K., MARTIN W. Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br. J. Pharmacol. 1996;117:1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJANAYAGAM M.A., LI C.G., RAND M.J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br. J. Pharmacol. 1993;108:3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Ann. Rev. Physiol. 1995a;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G.Nitric oxide in the autonomic and enteric nervous systems Nitric oxide in the nervous system 1995bLondon: Academic Press; 227–279.ed. S.R. Vincent. pp [Google Scholar]
- RAND M.J., LI C.G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDFR. Br. J. Pharmacol. 1995c;116:1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCHELLE L.G., MORANA S.J., KRUSZYNA H., RUSSELL M.A., WILCOX D.E., SMITH R.P. Interactions between hydroxocobalamin and nitric oxide (NO): evidence for a redox reaction between NO and reduced cobalamin and reversible NO binding to oxidized cobalamin. J. Pharmacol. Exp. Ther. 1995;275:48–52. [PubMed] [Google Scholar]
- SHENG H., SCHMIDT H.H., NAKANE M., MITCHELL J.A., POLLOCK J.S., FÖSTERMANN U., MURAD F. Characterization and localization of nitric oxide synthase in non-adrenergic non-cholinergic nerves from bovine retractor penis muscles. Br. J. Pharmacol. 1992;106:768–773. doi: 10.1111/j.1476-5381.1992.tb14411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJÖSTRAND N.O., KLINGE E.Nitric oxide and the neural regulation of the penis Nitric Oxide in the Nervous System 1995London: Academic Press; 281–306.ed. Vincent, S. pp [Google Scholar]


