Abstract
Features of glomerulonephritis are expression of the inducible form of NO synthase (iNOS) as well as expression of the secretory group IIA-phospholipase A2 (sPLA2) in mesangial cells. Interleukin 1β (IL-1β) induces both enzymes with a similar time course resulting in an increase in nitrite production and sPLA2-IIA activity. In this study we investigated the relationship between the formation of NO and sPLA2-IIA induction in rat renal mesangial cells.
Incubation of mesangial cells with the NO-donor, spermine-NONOate, for 24 h induced sPLA2-IIA mRNA expression and activity, whereas S-nitroso glutathione alone had only a small stimulatory effect. Stimulation of cells with IL-1β caused a marked increase in sPLA2-IIA mRNA and activity that were potentiated 3 fold by both NO donors.
Coincubation of cells with IL-1β and the NOS inhibitor, L-NG monomethylarginine (L-NMMA), caused a dose-dependent inhibition of cytokine-induced sPLA2-IIA mRNA expression and activity.
sPLA2-IIA activity was not stimulated by 8-bromo-cyclic GMP indicating that NO-induced sPLA2-IIA induction is independent of cyclic GMP-mediated signal transduction.
These data show that NO contributes to the expression by cytokines of sPLA2-IIA and establishes a novel type of interaction between iNOS and sPLA2-IIA in mesangial cells. This cross-talk between inflammatory mediators may help to promote and sustain an inflammatory state in the kidney.
Keywords: cyclic GMP, phospholipase A2, inducible NO synthase, mesangial cells, NO-donors, signal transduction
Introduction
Group IIA phospholipase A2 (sPLA2-IIA) is an enzyme which is secreted from renal mesangial cells and several other cell types in response to proinflammatory cytokines such as interleukin-1β (IL-1β) or tumour necrosis factor-α (TNF-α) (for review see Pfeilschifter, 1995). The secreted enzyme is thought to participate in propagation of chronic inflammatory processes such as rheumatoid arthritis, sepsis and glomerulonephritis (for review see Vadas & Pruzanski, 1986; 1993). Moreover, it has been suggested that sPLA2-IIA may act as an acute phase protein thereby enhancing inflammation and tissue damage (Hack et al., 1997; Pruzanski et al., 1998). The induction of sPLA2-IIA mRNA and protein secretion occurs after cytokine-stimulation with a time course comparable to the gene expression of other proinflammatory enzymes. One such enzyme is the inducible nitric oxide synthase (iNOS). The product of iNOS, NO, has been implicated in a variety of fundamental biological pathways, including neurotransmission, vasodilatation and inflammation (for review, see Bredt & Snyder, 1994). NO exerts its effects on cell function through a variety of interactions, including binding to haem-containing moieties of enzymes such as soluble guanylate cyclase (Moncada & Higgs, 1993). The activation of guanylate cyclase and the resultant formation of cyclic GMP (cGMP) is responsible for several cellular signalling pathways.
Earlier studies have shown that, in rat mesangial cells, inflammatory cytokines such as IL-1β or TNFα induce the expression of iNOS resulting in the production of large amounts of NO (Pfeilschifter & Schwarzenbach 1990; Pfeilschifter et al., 1992). Several proinflammatory effects of NO have been characterized in different cell types as model systems of glomerulonephritis such as generation of highly reactive peroxynitrite or hydroxyl radicals (Baud et al., 1992) or triggering of cell death by apoptosis or by necrosis (Mühl et al., 1996; Sandau et al., 1997). Evidence for the relevance of NO in inflammatory processes in the kidney has also been provided in animal models for certain types of glomerulonephritis (Cattell & Cook, 1993; 1995). The amplification of cytokine-stimulated iNOS expression by NO itself may be the basis for acute and chronic inflammatory processes in the kidney (Mühl & Pfeilschifter, 1995).
This effect of NO on gene transcription also indicates that NO may have the potential to regulate the expression of other inducible genes. In this respect in rat mesangial cells NO was found to be an important mediator of the regulation of cytokine-stimulated prostaglandin synthesis by activating cyclooxygenase-2 (COX-2; Tetsuka et al., 1996). The induction of prostaglandin biosynthesis is dependent on arachidonic acid release by the action of distinct phospholipases A2 (Kramer & Sharp 1997; Hara et al., 1995; Murakami et al., 1998), and in particular the sPLA2-IIA is reported to be important for initiation and propagation of prostaglandin E2 formation in mesangial cells (Pfeilschifter et al., 1993).
An interaction between NO and sPLA2-IIA induction has not yet been addressed. In this study we evaluated the effects of NO on expression and activity of sPLA2-IIA in IL-1β stimulated rat mesangial cells. Our data highlight the existence of an efficient cross-communication between the NO- and eicosanoid-generating signalling pathways.
Methods
Cell culture
Rat renal mesangial cells were cultured as described previously (Pfeilschifter et al., 1984). The cells were grown in RPMI 1640 supplemented with 10% (v v−1) foetal calf serum, penicillin (100 u ml−1), streptomycin (100 μg ml−1) and bovine insulin (0.66 u ml−1). For experiments, cells were transferred to plastic Petri dishes (Greiner, Frickenhausen, Germany) with 3.5 cm or 10 cm diameter and were cultivated to near confluency. Twenty four hours prior to stimulation and during the experiments cells were starved by incubation in Dulbecco's modified essential medium (DMEM) containing 0.1 mg ml−1 fatty acid-free bovine serum albumin (BSA) in the absence of serum.
Nitrite analysis
Nitrite concentration was determined by the Griess reaction (Green et al., 1982). Cell culture supernatants were collected, and 200 μl were added into a 96-well plate (Greiner), and mixed with 20 μl sulphanilamide (dissolved in 1.2 M HCl) and 20 μl N-naphthylethylenediamine dihydrochloride. After 5 min at room temperature the absorbance was measured at 540 nm with an ELISA-reader (Biorad, München, Germany). Nitrite concentrations were calculated using sodium nitrite as standard.
phospholipase A2 assay
phospholipase A2 activity in the supernatant of mesangial cell cultures was determined with [1-14C]-oleate-labelled Escherichia coli as substrate as described previously (Märki & Franson, 1986). Assay mixtures (750 μl) contained Tris/HCl (pH 7.0) 100 mM, CaCl2 1 mM, [1-14C]-oleate-labelled E. coli (≈5000 c.p.m.) and the enzyme-containing supernatants of the cell cultures at a dilution producing 5% substrate hydrolysis. Reaction mixtures were incubated for 1 h at 37°C in a thermomixer. The reaction was stopped by the addition of 50 μl 1 mM EGTA/1 N HCl and 800 μl ethyl acetate. After extraction of the lipids the orgnaic phase was dried in a vacuum concentrator. Thereafter, the lipids were dissolved in 50 μl ethyl acetate and separated by thin layer chromatography on silicagel G 60 plates (Merck, Darmstadt) using the orgnaic phase of ethylacetate/isooctane/acetic acid/water (110/50/20/100 by vol.) as solvent system. The detection and quantification of the separated lipids was performed with a phosphorimager BAS 1500 from Fuji (Raytest, Straubenhardt, Germany). The sPLA2-IIA-activity is defined as image quants counted from the spots corresponding to [1-14C]-oleic acid. In parallel experiments extraction efficiency was determined to be greater than 95%.
Northern-blot analysis
Confluent mesangial cells were cultured in 10 cm diameter culture dishes. After stimulation cells were washed twice with PBS and harvested using a rubber policeman. Total cellular RNA was extracted from the cell pellets using the guanidinium isothiocyanate/phenol/chloroform method (Sambrook et al., 1989). Samples of 10 μg of RNA were separated on 1.4% agarose/formaldehyde gels and transferred to a gene screen membrane. After UV crosslinking and prehybridization for 4 h the filters were hybridized for 16 h at 42°C to a 32P-labelled cDNA insert from sPLA2-IIA (Van Schaik et al., 1993). DNA probes were radioactively labelled with α-32P-dCTP by random priming. Finally, the filters were washed twice with 2×SSC/0.1% SDS for 2×20 min and several times at 65°C with 0.2×SSC/1% SDS. The signal was detected and quantified with a phosphorimager BAS 1500 (see above). To correct for variations in RNA amount the sPLA2-IIA-probe was stripped and the blots were rehybridized to the α-32P-dCTP-labelled cDNA insert for β-actin. The value of each of the RNA samples hybridized to the sPLA2-IIA-probe was divided by that obtained by rehybridization of the blots to the β-actin probe.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA). For multiple comparisons with the same control group, the limit of significance was divided by the number of comparisons according to Bonferroni.
Materials
Recombinant IL-1β was generously supplied by Dr C. Rordorf, Novartis Pharma Inc., Basel, Switzerland. [1-14C]-oleic acid, and [32P]-dCTP (110 TBq/mmol) were from Amersham-Buchler, Frankfurt, Germany. GSNO was kindly provided by Dr U.K. Messmer, Institute of Pharmacology, University of Frankfurt. Spermine-NONOate and L-NG-monomethylarginine (L-NMMA) were obtained from Alexis (Grünberg, Germany). The cDNA clone coding for β-actin was a generous gift of Dr W. Eberhardt, Institute of Pharmacology, University of Frankfurt. Nylon membranes (Gene Screen) were purchased from NEN Life Science (Köln, Germany). All cell culture media and nutrients were from Gibco BRL (Eggenstein, Germany), and all other chemicals used were from either Merck (Darmstadt, Germany), Sigma (Munich, Germany) or Fluka (Deisenhofen, Germany).
Results
NO-donors GSNO and spermine-NO amplify IL-1β stimulated sPLA2-IIA induction
First we investigated whether the NO-donors S-nitroso-glutathione (GSNO) and spermine-NONOate (spermine-NO) have an influence on IL-1β stimulated sPLA2-IIA induction in mesangial cells. GSNO releases NO into the supernatant over a period of 2–3 h. Spermine-NO has a half life of about 45 min in cell culture and releases 2 moles of NO per mol spermine-NONOate, which leads to higher local concentrations of NO compared to GSNO (Feelisch, 1998; Maragos et al., 1991).
The results in Figure 1A show that a 24 h incubation with GSNO alone had no significant stimulatory effect on sPLA2-IIA activity, whereas spermine-NO (Figure 1B) was able to increase the enzyme activity at 200 μM. After a 24 h coincubation with IL-1β both compounds potentiated the sPLA2-IIA activity about two to three times compared to IL-1β alone. NO donors used up to concentrations of 200 μM had no cytotoxic effects during the time course of the experiments as was monitored by trypan blue staining and lactate dehydrogenase release (data not shown).
Figure 1.
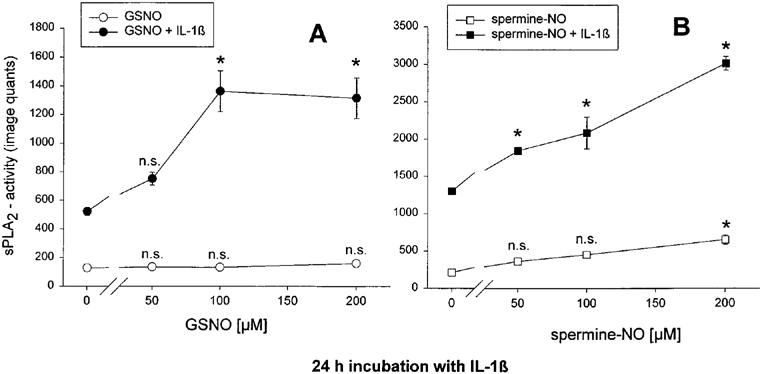
Effect of the NO-donors GSNO (A) and spermine-NO (B) on IL-1β stimulated sPLA2-IIA activity in mesangial cells. Cells were incubated in serum-free DMEM with 0.1 mg ml−1 fatty acid-free BSA for 24 h. Then they were treated for 24 h with IL-1β (2 nM) or vehicle in the absence or presence of different concentrations of the NO-donors as indicated. sPLA2-IIA activity was measured in the cell culture supernatant as described in Methods. Each value represents the means±s.e.mean of three parallel determinations per experiment. The experiment was repeated four times with similar results. Statistical analysis was performed by ANOVA. *P<0.05; n.s., not significant
By performing Northern blot analysis shown in Figure 2, lanes 1–6, we found that corresponding to the enzyme activity the increase in sPLA2-IIA mRNA levels in the presence of spermine-NO alone was more pronounced than in the case of GSNO alone. However, in combination with IL-1β, both NO-donors potentiated the amount of sPLA2-IIA mRNA up to 2.7±0.8 fold (n=5), when compared to IL-1β alone (P<0.05; Student's t-test).
Figure 2.
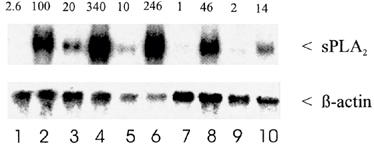
Effect of GSNO, spermine-NO and L-NMMA on sPLA2-IIA mRNA expression in mesangial cells. Cells were treated for 24 h with IL-1β (lane 2) or vehicle (lane 1) in absence or presence of the NO-donors or of different concentrations of L-NMMA. Total cellular RNA was successively hybridized to 32P-labelled sPLA2-IIA and β-actin cDNA probes, and quantification was performed as described in Methods. The numbers at the top of the Northern blot represent the corrected density expressed as the percentage of RNA found in IL-1β stimulated cells. This is a representative Northern blot out of five separate experiments. Lane 1, control; lane 2, IL-1β (2 nM); lane 3, spermine-NO (200 μM); lane 4, spermine-NO (200 μM) plus IL-1β; lane 5, GSNO (200 μM); lane 6, GSNO (200 μM) plus IL-1β; lane 7, L-NMMA (1 mM); lane 8, L-NMMA (1 mM) plus IL-1β; lane 9, L-NMMA (4 mM); lane 10, L-NMMA (4 mM) plus IL-1β
L-NMMA inhibits nitrite formation and sPLA2-IIA induction
In a second approach cells were treated with IL-1β in the presence of L-NMMA, an inhibitor of iNOS activity.
The data in Figure 3 show that with 4 mM L-NMMA, a concentration at which IL-1β stimulated nitrite formation was completely abolished (Figure 3A), the sPLA2-IIA activity was reduced to about 15% of the IL-1β induced activity (Figure 3B). At 1 mM L-NMMA, which reduced the concentration of nitrite in the cell culture medium by 90%, an inhibition of sPLA2-IIA activity of about 50% was obtained.
Figure 3.
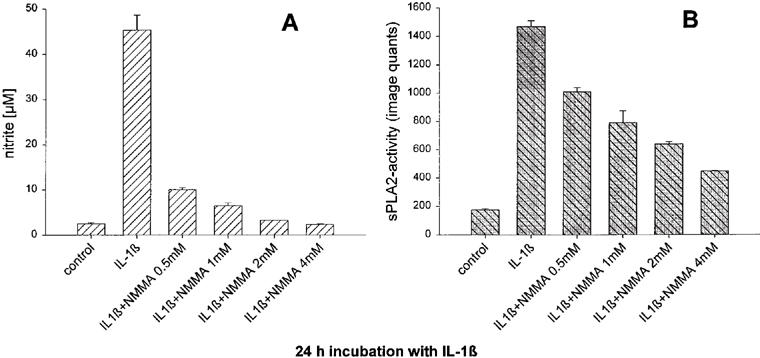
Effect of L-NMMA on IL-1β stimulated nitrite formation (A) and sPLA2-IIA activity (B) in mesangial cells. Cells cultivated for 24 h in serum-free DMEM with 0.1 mg ml−1 BSA were treated for 24 h with IL-1β (2 nM) or vehicle in the absence or presence of different concentrations of L-NMMA as indicated. The concentration of nitrite as well as sPLA2-IIA activity in the cell culture medium were determined as described in Methods. Each value represents the means±s.e.mean of three parallel determinations per experiment. The experiment was repeated four times with similar results.
Under these conditions we performed Northern blot analysis (Figure 2, lanes 7–10). Similar to its effect on the enzyme activity L-NMMA dose-dependently reduced the sPLA2-IIA mRNA levels by 50% at 1 mM (lane 8) and 86% of IL-1β at 4 mM (lane 10). In four separate experiment with L-NMMA-concentrations between 3 and 5 mM the reduction of IL-1β-induced mRNA levels varied between 53 and 86%. L-NMMA alone had no effect on basal sPLA2-IIA-mRNA amounts (lanes 7 and 9). The results show that under conditions of complete inhibition of nitrite formation, the induction of sPLA2-IIA mRNA is also reduced, although not to the same magnitude.
sPLA2-IIA induction is not changed by 8-bromo-cyclic GMP
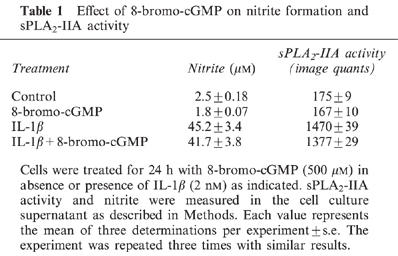
cyclic GMP represents an important second messenger in NO-mediated signalling. Therefore we investigated whether the cell-permeable 8-bromo-cyclic GMP analogue has an effect on IL-1β induced sPLA2-IIA activity. Incubation of mesangial cells for 24 h with 8-bromo-cyclic GMP (500 μM) had no stimulatory effects on either nitrite formation or on sPLA2-IIA activity (Table 1). A 24 h coincubation of mesangial cells with 8-bromo-cyclic GMP and IL-1β did not result in a potentiation of sPLA2-IIA activity, suggesting that the effect of NO on sPLA2-IIA is independent of cGMP-mediated signalling pathways.
Table 1.
Effect of 8-bromo-cGMP on nitrite formation and sPLA2-IIA activity
Discussion
Mesangial cells have been shown to express iNOS and sPLA2-IIA in response to inflammatory cytokines such as IL-1β (for review, see Pfeilschifter, 1994; 1995). A novel finding in our studies is that endogenous NO produced after cytokine stimulation of mesangial cells contributes to the induction of gene expression and activity of sPLA2-IIA. The participation of iNOS activation and NO in sPLA2-IIA gene expression was shown by the results obtained with L-NMMA plus IL-1β where a complete inhibition of nitrite formation resulted in a substantial decrease in sPLA2-IIA. However, the reduction of sPLA2-IIA did not reach basal levels, suggesting that NO is important but not by itself sufficient for sPLA2-IIA gene expression. The cytokine-treatment is necessary for this event and seems to initiate additional NO-independent mechanisms for sPLA2-IIA induction.
Further support for a regulatory role of NO on sPLA2-IIA gene expression was obtained by the use of different NO-donors. NO derived from spermine-NO as well as GSNO stimulated sPLA2-IIA mRNA expression and activity in combination with IL-1β, whereas, when given alone only spermine-NO had a stimulatory effect. The reasons for the discrepancy in the effects on sPLA2-IIA gene expression probably lie in the different capacities of these compound classes to release NO (for review see Feelisch, 1998). The release of 2 moles of NO from spermine-NO occurs with a much faster dissociation rate than the release of 1 mole from GSNO. This likely results in a higher local concentration of NO after spermine-NO-treatment of cells, and it is possible that a certain threshold must be reached in order to achieve induction of sPLA2-IIA gene expression. Thus, the same absolute amounts of NO generated over different periods of time may lead to substantially different rates of NO formation and, consequently, to different effects on cellular responses.
The regulation of gene expression by NO impacts on a variety of proinflammatory mediators. In mesangial cells NO functions in a positive feedback loop that strengthens its own biosynthesis by potentiating iNOS expression (Mühl & Pfeilschifter, 1995). Endogenous NO as well as NO-donors have been implicated in the production of IL-6 and IL-8 in LPS-stimulated whole blood cells, fibroblasts, epithelial cells and hepatoma cells (Remick & Villarete, 1996; Villarete & Remick, 1997) and in a melanoma cell line (Andrew et al., 1995) by affecting regulation at the transcriptional level.
Endogenously formed NO is a potent inducer of COX-2 activity (Salvemini et al., 1993; Swierkosz et al., 1995). NO is hypothesized to interact with the iron-haem centre of the active site of the COX enzymes thereby modulating enzyme activity (Salvemini et al., 1993). In addition, NO stimulates prostaglandin formation in animal models of inflammation (Salvemini et al., 1994; Salvemini, 1997; Sautebin et al., 1995) and in several cell systems such as airway epithelial cells (Watkins et al., 1997), osteoblasts (Kanematsu et al., 1997) and astroglial cells (Mollace et al., 1998). Furthermore, in mesangial cells, NO donors not only stimulate COX-2 activity, but are also potent amplifiers of IL-1β-induced COX-2 mRNA and protein expression (Tetsuka et al., 1996). However, inhibitory effects of NO donors on COX-2 activity and induction, when applied in higher concentrations, have also been described (Swierkosz et al., 1995). Taken together, this evidence indicates that a fine-tuning of NO production seems to be crucial for the ultimate stimulation or inhibition of COX-2 induction.
Prostaglandins as products of COX activity play a major role as mediators of the inflammatory response. The rate-limiting step for prostaglandin biosynthesis is the availability of arachidonic acid as substrate for the COX enzymes. The most important route for arachidonic acid release from phospholipids appears to be by the action of a cytosolic PLA2 (Kramer & Sharp, 1997). However, in mesangial cells the extracellular sPLA2-IIA has been shown to be crucial for potentiation of cPLA2 activity via activation of the c-Raf/MAP-kinase cascade and for sustained prostaglandin E2 formation. This effect was described as cross-talk between sPLA2-IIA and cPLA2 by Huwiler et al. (1997). Here we have shown for the first time that in mesangial cells NO regulated gene expression involves also sPLA2-IIA as an important factor upstream of COX-2 induction and prostaglandin formation.
The mechanisms by which NO modulates sPLA2-IIA gene expression are unknown. The question arises whether the effect of NO on sPLA2-IIA induction is mediated by activation of the soluble guanylate cyclase producing cyclic GMP. We found that the effects of IL-1β on iNOS as well as sPLA2-IIA do not seem to be mediated by this second messenger, because the cell-permeable analogue 8-bromo-cyclic GMP alone neither activated these enzymes nor potentiated the IL-1β induced effects. These results are in agreement with observations reported by Mühl & Pfeilschifter (1995), where dibutyryl-cyclic GMP did also not mimic the amplification of iNOS expression in response to NO donors. Other groups have also found that NO-mediated iNOS- and COX-2-expression is regulated by cyclic GMP-independent mechanisms (Salvemini et al., 1993; Sautebin et al., 1995). In contrast, a cyclic GMP-dependent prostaglandin E2 formation in mesangial cells was described by Tetsuka et al. (1996) and in airway epithelial cells by Watkins et al. (1997). This diversity in the cellular mechanisms might be due to cell- and tissue-specific differences in targets for this second messenger such as the cyclic GMP-dependent protein kinase.
Our results show that in mesangial cells NO has a role as an upstream mediator in the cytokine-induced signalling cascade, which leads to sPLA2-IIA induction, whereby prostaglandin formation might be potentiated. In other words, an inhibition of NO production also is likely to reduce formation of other potent proinflammatory mediators. This novel cross-talk may play a critical role in mediating the amplification of pathological processes in the kidney presenting a therapeutic basis to manipulate the cascade of inflammatory responses.
Acknowledgments
We thank Dr Heiko Mühl and Udo K. Messmer for helpful discussion, Silke Spitzer and Martina Apel for excellent technical assistance and Christiane Rordorf for the generous gift of IL-1β. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 553), by a grant from the Commission of the European Communities (Biomed 2, PL 950979), and by a grant of the Wilhelm Sander Stiftung to Kirsten Scholz and Josef Pfeilschifter.
Abbreviations
- BSA
bovine serum albumine
- cGMP
cyclic GMP
- COX
cyclooxygenase
- DMEM
Dulbecco's modified essential medium
- GSNO
S-nitroso-glutathione
- IL-1β
interleukin-1β
- iNOS
inducible form of NO synthase
- L-NMMA
L-NG-monomethylarginine
- NO
nitric oxide
- spermine-NO
spermine-NONOate
- sPLA2-IIA
secretory phospholipase A2-type IIA
- TNFα
tumour necrosis factor-α
References
- ANDREW P.J., HARANT H., LINDLEY I.J. Nitric oxide regulates IL-8 expression in melanoma cells at the transcriptional level. Biochem. Biophys. Res. Commun. 1995;214:949–956. doi: 10.1006/bbrc.1995.2378. [DOI] [PubMed] [Google Scholar]
- BAUD L., FOUQUERAY B., PHILIPPE C., ARDAILLOU R. Reactive oxygen species as glomerular autacoids. J. Am. Soc. Nephr. 1992;2 10 Suppl:S132–S138. doi: 10.1681/ASN.V210s132. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- CATTELL V., COOK T. Nitric oxide: role in the physiology and pathology of the glomerulus. Exp. Nephrol. 1993;1:265–280. [PubMed] [Google Scholar]
- CATTELL V., COOK T. The nitric oxide pathway in glomerulonephritis. Curr. Opin. Nephrol. Hypertens. 1995;4:359–364. doi: 10.1097/00041552-199507000-00013. [DOI] [PubMed] [Google Scholar]
- FEELISCH M. The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HACK C.E., WOLBINK G.-J., SCHALKWIJK C., SPEIJER H., HERMENS W.T., VAN DEN BOSCH H. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunology Today. 1997;18:111–115. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- HARA S., KUDO I., KOMATANI T., TAKAHASHI K., NAKATANI Y., NATORI Y., OHSHIMA M., INOUE K. Detection and purification of two 14 kDa phospholipase A2 isoforms in rat kidney: their role in eicosanoid synthesis. Biochim. Biophys. Acta. 1995;1257:11–17. doi: 10.1016/0005-2760(95)00011-z. [DOI] [PubMed] [Google Scholar]
- HUWILER A., STAUDT G., KRAMER R.M., PFEILSCHIFTER J. Cross-talk between secretory phospholipase A2 and cytosolic phospholipase A2 in rat renal mesangial cells. Biochim. Biophys. Acta. 1997;1348:257–272. doi: 10.1016/s0005-2760(97)00073-8. [DOI] [PubMed] [Google Scholar]
- KANEMATSU M., IKEDA K., YAMADA Y. Interaction between nitric oxide synthase and cyclooxygenase pathways in osteoblastic MC3T3-E1 cells. J. Bone Miner. Res. 1997;12:1789–1796. doi: 10.1359/jbmr.1997.12.11.1789. [DOI] [PubMed] [Google Scholar]
- KRAMER R.M., SHARP J.D. Structure, function and regulation of Ca2+-sensitive cytosolic phospholipase A2 (cPLA2) FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- MARAGOS C.M., MORLEY D., WINK D.A., DUNAMS T.M., SAAVEDRA J.E., HOFFMAN A., BOVE A.A., ISASAC L., HRABIE J.A., KEEFER L.K. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- MÄRKI F., FRANSON R. Endogenous suppression of neutral-active and calcium-dependent phospholipase A2 in human polymorphonuclear leukocytes. Biochim. Biophys. Acta. 1986;879:149–156. doi: 10.1016/0005-2760(86)90097-4. [DOI] [PubMed] [Google Scholar]
- MOLLACE V., COLASANTI M., MUSCOLI C., LAURO G.M., IANNONE M., ROTIROTI D., NISTICO G. The effect of nitric oxide on cytokine-induced release of PGE2 by human cultured astroglial cells. Br. J. Pharmacol. 1998;124:742–746. doi: 10.1038/sj.bjp.0701852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A. The l-arginine-nitric-oxide pathway. N. Eng. J. Med. 1993;329:2002–2011. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- MÜHL H., PFEILSCHIFTER J. Amplification of nitric oxide synthase expression by nitric oxide in interleukin 1 beta-stimulated rat mesangial cells. J. Clin. Invest. 1995;85:1941–1946. doi: 10.1172/JCI117876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜHL H., SANDAU K., BRÜNE B., BRINER V., PFEILSCHIFTER J. Nitric oxide donors induce apoptosis in glomerular mesangial cells, epithelial cells and endothelial cells. Eur. J. Pharmacol. 1996;317:137–149. doi: 10.1016/s0014-2999(96)00701-7. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., SHIMBARA S., KAMBE T., KUWATA H., WINSTEAD M.V., TISCHFIELD J.A., KUDO I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2s are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- PFEILSCHIFTER J. Mesangial cells orchestrate inflammation in the renal glomerulus. News Physiol. Sci. 1994;9:271–276. [Google Scholar]
- PFEILSCHIFTER J. Molecular approaches to pathophysiology 1995New York: CRC Press; 25–51.Glaser, K.B., Vada P, eds. pp [Google Scholar]
- PFEILSCHIFTER J., KURTZ A., BAUER C. Activation of phospholipase C and prostaglandin synthesis by [arginine]vasopressin in cultures. Biochem. J. 1984;223:855–859. doi: 10.1042/bj2230855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEILSCHIFTER J., ROB P., MÜLSCH A., FANDREY J., VOSBECK K., BUSSE R. Interleukin 1β and tumor necrosis factor α induce a macrophage-type of nitric oxide synthase in rat renal mesangial cells. Eur. J. Biochem. 1992;203:251–255. doi: 10.1111/j.1432-1033.1992.tb19854.x. [DOI] [PubMed] [Google Scholar]
- PFEILSCHIFTER J., SCHALKWIJK C., BRINER V.A., VAN DEN BOSCH H. Cytokine-stimulated secretion of group II phospholipase A2 by rat mesangial cells. J. Clin. Invest. 1993;92:2516–2523. doi: 10.1172/JCI116860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEILSCHIFTER J., SCHWARZENBACH H. Interleukin 1 and tumour necrosis factor stimulate cGMP in rat renal mesangial cells. FEBS Lett. 1990;273:185–187. doi: 10.1016/0014-5793(90)81080-8. [DOI] [PubMed] [Google Scholar]
- PRUZANSKI W., STEFANSKI E., DE BEER F.C., DE BEER M.C., VADAS P., RAVANDI A., KUKSIS A. Lipoproteins are substrates for human secretory group IIA phospholipase A2. Preferential hydrolysis of acute phase HDL. J. Lipid Res. 1998;39:2150–2160. [PubMed] [Google Scholar]
- REMICK D.G., VILLARETE L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J. Leukoc. Biol. 1996;59:471–475. doi: 10.1002/jlb.59.4.471. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D. Regulation of cyclooxygenase enzymes by nitric oxide. Cell. Mol. Life Sci. 1997;53:576–582. doi: 10.1007/s000180050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., SEIBERT K., MASFERRER J.L., MISKO T.P., CURRIE M.G., NEEDLEMAN P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J. Clin. Invest. 1994;93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMBROOK J., FROTSCJ J., MANIATIS T. Cold Spring Harbor Laboratory Press, New York; 1989. Molecular cloning: a Laboratory Manual, Cold Spring Harbor. [Google Scholar]
- SANDAU K., PFEILSCHIFTER J., BRÜNE B. The balance between nitric oxide and superoxide determines apoptotic and necrotic death of rat mesangial cells. J. Immunol. 1997;158:4938–4946. [PubMed] [Google Scholar]
- SAUTEBIN L., IALENTI A., IANARO A., DI ROSA M. Modulation by nitric oxide of prostaglandin biosynthesis in the rat. Br. J. Pharmacol. 1995;114:323–328. doi: 10.1111/j.1476-5381.1995.tb13230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIERKOSZ T.A., MITCHELL J.A., WARNER T.D., BOTTING R.M., VANE J.R. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br. J. Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TETSUKA T., DAPHNA-IKEN D., MILLER B.W., GUAN Z., BAIER L.D., MORRISON A.R. Nitric oxide amplifies interleukin 1-induced cyclooxygenase-2 expression in rat mesangial cells. J. Clin. Invest. 1996;97:2051–2056. doi: 10.1172/JCI118641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VADAS P., PRUZANSKI W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab. Invest. 1986;55:391–404. [PubMed] [Google Scholar]
- VADAS P., PRUZANSKI W. Induction of group II phospholipase A2 expression and pathogenesis of the sepsis syndrome. Circ. Shock. 1993;39:160–167. [PubMed] [Google Scholar]
- VAN SCHAIK R.H.N., VERHOEVEN N.M., NEIJS F.W., AARSMAN A.J., VAN DEN BOSCH H. Cloning of the cDNA coding for 14 kDa group II phospholipase A2 from rat liver. Biochim. Biophys. Acta. 1993;1169:1–11. doi: 10.1016/0005-2760(93)90075-k. [DOI] [PubMed] [Google Scholar]
- VILLARETE L.H., REMICK D.G. Nitric oxide regulation of interleukin-8 gene expression. Shock. 1997;7:29–35. doi: 10.1097/00024382-199701000-00003. [DOI] [PubMed] [Google Scholar]
- WATKINS D.N., GARLEPP M.J., THOMPSON P.J. Regulation of the inducible cyclo-oxygenase pathway in human cultured airway epithelial (A549) cells by nitric oxide. Br. J. Pharmacol. 1997;121:1482–1488. doi: 10.1038/sj.bjp.0701283. [DOI] [PMC free article] [PubMed] [Google Scholar]


