Abstract
The effects of oral propylthiouracil (PTU) treatment on vascular nitric oxide (NO) production were studied in the rat aorta.
Rats were fed a standard low fat diet with or without 0.1% PTU, for 2 or 4 weeks, or for 2 weeks with additional thyroxine injections. Concentration response curves were then constructed to phenylephrine (PE) in both endothelium-intact and denuded aortic rings from these animals and after incubation with 0.1 mM L-NGnitroarginine (L-NOARG). In addition, expression of nitric oxide synthase (NOS) was analysed in sections of aorta from PTU-treated and control rats using rabbit polyclonal antibodies to both inducible NOS (iNOS) and endothelial NOS (eNOS).
Oral PTU treatment resulted in a significant reduction in both the maximum response (control, 0.53±0.02; 2 week PTU, 0.20±0.07; 4 week PTU, 0.07±0.02 g mg−1) and vessel sensitivity (EC50 values: control, 9.10×10−8±0.67; 2 week PTU, 7.45×10−7±1.15; 4 week PTU, 9.73×10−7±0.45 M) to PE in endothelium-intact vessel rings, as compared to controls (P<0.05). Both endothelial removal and incubation with L-NOARG restored the maximum response after 2, but not 4 weeks, although, in general, vessel sensitivity was not altered by either treatment. Vessels from PTU-treated rats given thyroxine injections showed no significant differences between any of the dose response curve parameters. Immunohistochemical analysis suggested that labelling for eNOS may be increased after PTU treatment as compared to control animals, whereas iNOS antibody immunoreactivity was not different between the two groups.
These results suggest that the hyporesponsiveness to PE observed after oral PTU treatment is, in part, due to enhanced nitric oxide (NO) production by the endothelium, and demonstrate for the first time that thyroid hormones may play a role in the regulation of eNOS activity in the rat aorta.
Keywords: Propylthiouracil, nitric oxide, endothelium, rat aorta
Introduction
Propylthiouracil (PTU) is used routinely in the treatment of hyperthyroidism, a common endocrine condition, which may result from various disorders of the thyroid gland, the most prevalent of which is Graves disease (Klein et al., 1994). In addition to the excessive thyroid hormone concentrations associated with this condition, Graves disease has also been found to result in increased stimulation of the adrenergic nervous system. Conversely, PTU-induced hypothyroidism causes decreased responsiveness to adrenoceptor agonists. It has been well established that both cardiac β-adrenoceptor density and responsiveness to agonists which exert their actions via these receptors are decreased in hypothyroidism (Nakashima & Hagino, 1972; Hawthorn et al., 1988), findings which account for the marked bradycardia observed in this condition (Klein & Ojamaa, 1992). In addition, vascular responsiveness to phenylephrine (PE) and other α-adrenoceptor agonists has been found to be decreased in hypothyroidism (Rahmani et al., 1987; Brown et al., 1994a), although the correlation between this disease state and α-adrenoceptor density has not been well established. The inhibitory effects of PTU on PE-induced contractions, which are observed in the resulting hypothyroid state, were found to be restored by thyroxine replacement therapy (Rahmani et al., 1987; Brown et al., 1994b), which suggests that the actions of PTU are dependent on induction of the hypothyroid state and are not due to a direct action of the drug or to other unrelated factors.
In the rat aorta, basal production of nitric oxide (NO) by the endothelium has been found to modulate the responses to α-adrenoceptor agonists, such as PE (Bullock et al., 1986; Frew et al., 1993). However, few studies have examined the effects of oral PTU treatment on NO production. PTU-induced hypothyroidism has been shown to cause marked downregulation of NO synthase (NOS) gene expression in the rat hypothalamus, an effect which was reversed by dietary supplementation with thyroxine (Ueta et al., 1995). In addition, all three NOS isoforms have been found to be expressed in the thyroid gland and to be involved in the increased vascularity associated with this organ in hyperthyroidism (Colin et al., 1995). However, the actions of PTU on vascular NOS activity have not been investigated.
The aim of this study was, therefore, to determine whether the vascular L-arginine-NO pathway is involved in the vascular hyporesponsiveness to α-adrenoceptor agonists which has been shown to occur in PTU-induced hypothyroidism.
The findings of these experiments have been reported to the British Pharmacological Society (Grieve et al., 1998a).
Methods
Preparation of PTU-supplemented diet
A standard low fat powdered diet (88.5% cereal products, 8.5% proteins, 0.5% soya oil energy source, 2.5% vitamins and minerals) was mixed with 0.1% 6-n-propyl-2-thiouracil for 1 min and stored at 4°C until required. Only small quantities (500 g) were made up at any one time to ensure that the animals were receiving freshly prepared diet.
Feeding protocol
Male Wistar rats (initial weights: control diet, 140–160 g; PTU-treated, 290–310 g) were used throughout this study. They were housed under constant climatic conditions (temperature 21–22°C, relative air humidity 50±5%, constant 12 h daylength) with free access to water and a standard low fat diet or the same diet supplemented with PTU, for a period of 2 or 4 weeks. Animal weights and food consumption were recorded daily and fresh food and water provided. The PTU-supplemented diet caused a significant inhibitory effect on animal growth rate, when compared to the control diet (0.76±0.14 vs 7.27±0.62 g day−1, n= 4; P<0.05, unpaired Student's t-test). However, food consumption was found to be not significantly different between rats fed the PTU-supplemented and control diets (32.0±1.0 vs 35.3±1.6 g day−1, n=4). As oral PTU treatment caused a marked inhibition of growth rate, rats of differing initial weights were used in order that the control and PTU-treated animals would be approximately the same size at time of death. A baseline blood sample was taken from the tail vein at time 0 and a terminal blood sample taken by cardiac puncture after 2 or 4 weeks. Blood samples were allowed to clot for 30 min before being centrifuged at 13,000 r.p.m. for 10 min. The serum was harvested and thyroxine concentrations were determined as described below.
Thyroxine replacement in PTU-treated animals
Replacement doses of thyroxine were given to PTU-treated rats following the methods of Lee et al. (1990). Animals were fed a standard low fat diet supplemented with 0.1% PTU for 2 weeks. For the final 4 days, thyroxine replacement injections (50 ng g−1 body weight s.c.) were given. Serum thyroxine concentrations were then measured to establish whether the injected dose had restored the hormone to control levels.
Measurement of serum thyroxine concentration
Serum thyroxine concentrations were determined using a commercially available radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, U.S.A.) based on the method of Britton et al. (1975). Total [125I]-thyroxine reagent (1.0 ml) was added to 25 μl serum in an antibody-coated plastic tube and mixed before being incubated in a water bath at 37°C for 1 h. All visible moisture was then removed from the tubes by inverting and draining on absorbant filter paper for 30 min. The tubes were then assayed for radioactivity in a gamma counter (Packard Cobra 5005) and values corrected for non-specific binding. Thyroxine standards (0–300 nM), prepared from stock solutions were assayed in parallel with the serum samples and the thyroxine concentration was calculated from the standard curve obtained.
Isolated vessel studies
Rats (final weight 300–350 g) fed the standard low fat control diet or the same diet supplemented with 0.1% PTU for 2 weeks or 4 weeks, or for 2 weeks with additional thyroxine injections, were killed with sodium pentobarbitone (200 mg kg−1 body weight i.p.). The entire aorta was then rapidly removed, cleared of any surrounding connective tissue and thoracic portion cut into 3 mm ring segments, taking care not to damage the endothelium. Three vessel segments were taken from each rat, one of which was denuded of endothelium (by gentle rubbing of the luminal surface), and the other two left with an intact endothelium. All rings were placed in organ bath chambers containing 10 ml modified Krebs-Henseleit solution at 37°C (KHS; composition (mM): NaCl 118, KCl 4.57, CaCl2 1.27, KH2PO4 1.19, MgSO4 1.19, NaHCO3 25, glucose 5.55) and prepared for isometric tension recording as previously described (Grieve et al., 1998b). The vessel segments were held under 1 g resting tension and allowed to equilibrate for 1 h, before they were contracted by exchanging the KHS for a depolarizing KHS, where the NaCl had been replaced isotonically with KCl (118 mM). After 15 min, the depolarising KHS was replaced with KHS and resting tension re-established, 0.1 mM L-NGnitroarginine (L-NOARG) was added to the bathing solution of one of the endothelium-intact rings for 30 min, before cumulative concentration contraction response curves were constructed to PE (1 nM to 10 μM) in all three vessel segments.
NOS localization by immunocytochemistry
Expression of NOS was analysed in sections of aorta from PTU-treated and control rats. In addition, a positive control for inducible NOS (iNOS) was obtained by incubating control vessel sections with 1 mg ml−1 lipopolysaccharide (LPS) in KHS at 37°C for 24 h in a tissue culture incubator prior to processing. Immediately after death (sodium pentobarbitone, 200 mg kg−1 body weight i.p.), segments of aorta (10–20 mm) were excised and cleared of all surrounding connective tissue, mounted in a 10% aqueous solution of polyvinylalcohol embedding medium so that the lumen would remain patent, and snap-chilled in n-hexane (grade low in aromatic hydrocarbons at or below at −70°C), before being stored at −70°C until required. The segment was mounted in embedding medium on a cork board, before transverse sections (10 μm) were cut on a Model OTF cryostat (Bright Instrument Company Limited, Huntingdon, U.K.) and collected onto clean, glass, polylysine-coated microscope slides. The sections were then dried at room temperature under an extractor fan for 1 h, before the slides were placed, face up, in a dark plastic box containing paper tissue which had been saturated with distilled water. The aortic sections were then ringed with a wax marker pen, to prevent spreading of the overlying liquid, and covered with 20 μl of Tris-buffered saline (TBS; control) or with the same volume of rabbit polyclonal antibody to either iNOS or endothelial NOS (eNOS; 2% in TBS). The lid was placed on the box and left at room temperature for 18 h. After this incubation period, the sections were washed by pouring off excess liquid and replacing with 3 changes of 20 μl TBS, each for 5 min. The sections were then incubated with 2% FITC-conjugated anti-rabbit immunoglobulin (20 μl; 80% TBS, 20% rat serum to minimize non-specific binding) at room temperature for 1 h. The slides were then washed with TBS, as described earlier, and covered with fluorescent mounting medium and coverslip before being viewed under a Zeiss LSM-510 confocal microscope at 40× magnification.
Drugs and reagents
L-phenylephrine hydrochloride and L-NGnitroarginine were purchased from Sigma Chemical Co. (Poole, U.K.) and were initially dissolved in deionised water (at 10 mM) before being diluted in 0.9% w v−1 saline. All solutions were freshly prepared on the day of the experiment. Concentrations are expressed as the final concentration of each drug in the organ bath. The standard low fat powdered diet was obtained from Special Diet Services Limited (Witham, U.K.), the 6-n-propyl-2-thiouracil, thyroxine (sodium salt) and lipopolysaccharide (from Salmonella typhimurium) from Sigma Chemical Co. (Poole, U.K.). The tissue embedding medium was purchased from Bright Instrument Company Limited (Huntingdon, U.K.), the fluorescent mounting medium and FITC-conjugated anti-rabbit immunoglobulins from Dako Corporation (Carpintera, U.S.A.) and the rabbit polyclonal antibodies to iNOS and eNOS from Santa Cruz Biotechnology Inc. (Santa Cruz, U.S.A.).
Statistical analysis
Data from organ bath experiments were expressed as increase in tone calculated as g tension developed per mg tissue, and were plotted against log agonist concentration. Cumulative concentration contraction response curves for each vessel segment were fitted to the single site logistic equation:
by a modified Marquart procedure using Multifit® (Day Computing, Cambridge, U.K.) running on a Macintosh 475. E.max represents the maximum response, A is the concentration of drug used and n is the Hill slope of the concentration response curve. The best fit values for EC50, E.max and Hill slope for each vessel segment were used to calculate the means±s.e.mean. Significance limits were calculated using a paired Student's t-test or a one way ANOVA with Dunnett's comparison, where appropriate (P<0.05 was taken to be significant).
Results
Effects of oral PTU treatment on serum thyroxine concentrations
The PTU-supplemented diet resulted in a significant decrease in serum thyroxine after both 2 and 4 weeks (from 37.2±4.7 to ⩽3.2 nM, n=4; P<0.05, one way ANOVA with Dunnett's comparison). However, these values were significantly increased above control levels by injection of a replacement dose of thyroxine (from 37.2±4.7 to 156.8±10.7 nM, n=4; P<0.05, one way ANOVA with Dunnett's comparison).
Effects of oral PTU treatment on contractions to PE
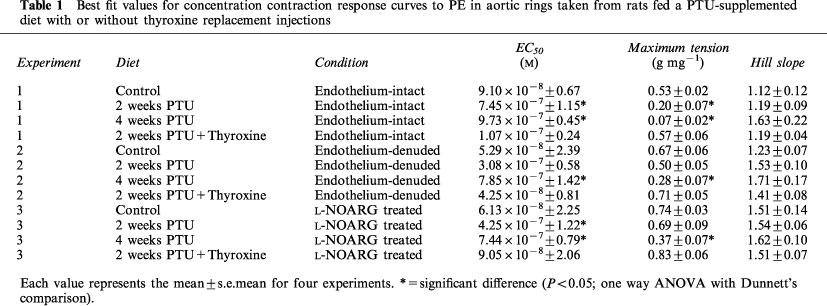
Cumulative concentration contraction response curves to PE of rings taken from animals fed a standard low fat diet or the same diet supplemented with PTU with or without thyroxine replacement injections are shown in Figure 1. The mean best fit dose response curve parameters derived from these curves are presented in Table 1. Oral PTU treatment resulted in a significant reduction in both the magnitude of the maximum response and vessel sensitivity to PE in endothelium-intact vessel rings, as compared to values obtained from control rats. Both endothelial removal and incubation with L-NOARG restored the maximum response after 2, but not after 4 weeks PTU treatment. In general, vessel sensitivity was not altered by endothelial removal or treatment with L-NOARG. The EC50 values were still found to be significantly higher than the respective controls following endothelial denudation after 4 weeks, and following L-NOARG treatment after both 2 and 4 weeks. When thyroxine replacement injections were given together with the PTU-supplemented diet, no significant differences were found between any of the dose response curve parameters of vessels from these animals and those taken from rats fed the standard low fat control diet.
Figure 1.
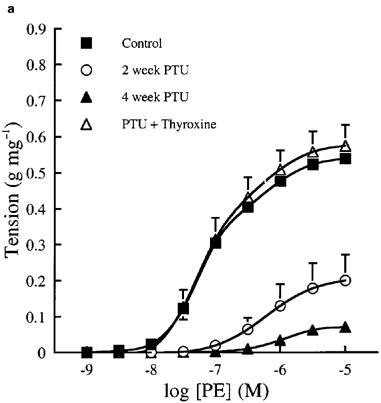
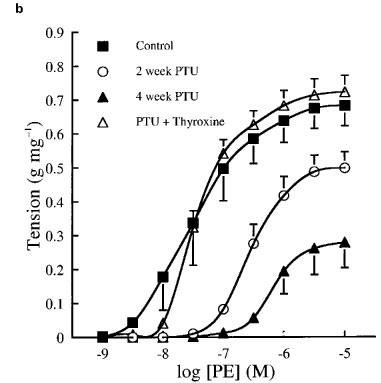
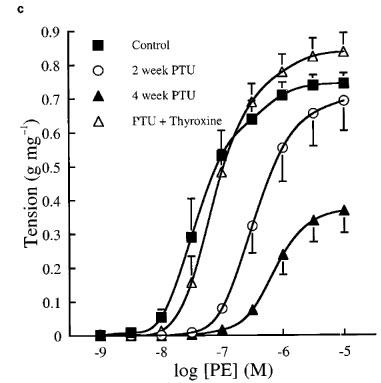
Cumulative concentration contraction response curves to PE in (a) endothelium-intact, (b) endothelium-denuded and (c) endothelium-intact rat aortic rings in the presence of 0.1 mM L-NOARG. The ordinate scale represents contraction expressed as g tension produced per mg of tissue. Each data point represents the mean±s.e.mean; the s.e.means are only included when they exceed the size of the symbol. The line has been drawn by interpolation through these mean values. The best fit values for EC50, maximum tension and Hill slope were derived by a computerized non-linear curve fitting procedure for each vessel segment and these mean values are presented in Table 1.
Table 1.
Best fit values for concentration contraction response curves to PE in aortic rings taken from rats fed a PTU-supplemented diet with or without thyroxine replacement injections
NOS localization by immunocytochemistry in vessels from control and PTU-treated rats
Fluorescent images of sections of rat aorta incubated with TBS and fluorescently labelled with anti-rabbit immunoglobulin are shown in Figure 2a. The observed low-intensity image is due to auto-fluorescence of the collagen fibres, and these did not appear to differ between control and PTU-treated animals. The iNOS antibody immunoreactivity was confirmed by marked increases in iNOS expression evident in vessels pre-incubated with LPS (Figure 2b). Immunolabelling for iNOS in aortic tissue from both control and PTU-treated animals was of a low level and there appeared to be no difference in labelling intensity between the two groups (Figure 2c and d). However, immunolabelling for eNOS showed clear endothelial cell localization, and this appeared to be increased after PTU treatment as compared to control animals (Figure 2e and f).
Figure 2.
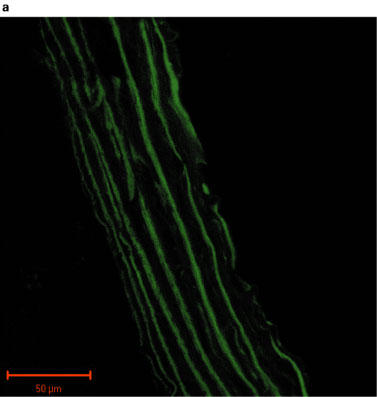
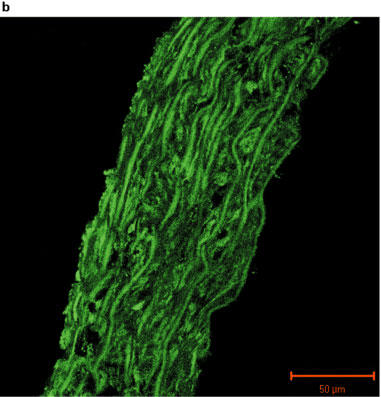
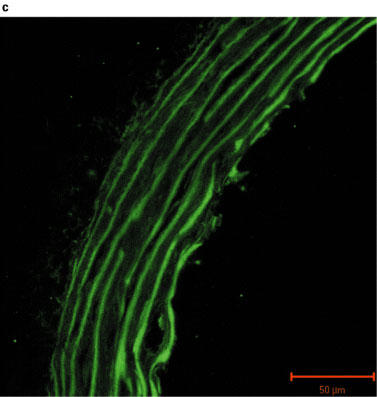
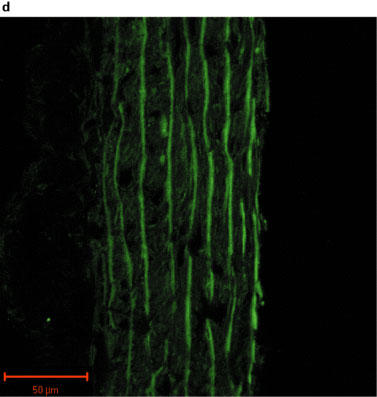
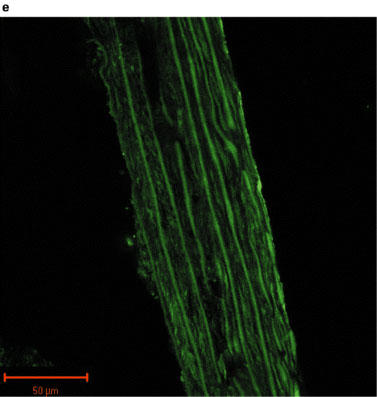
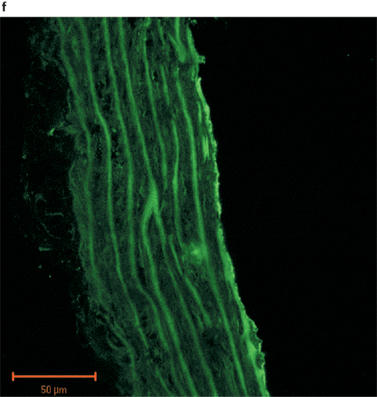
Photographs showing transverse sections of aorta from rats fed a standard low fat control diet or the same diet supplemented with 0.1% PTU for 2 weeks. The vessel segments were frozen in hexane, mounted in embedding medium, and 10 μm sections cut and incubated with TBS or rabbit polyclonal antibodies to iNOS or eNOS before being fluorescently labelled with anti-rabbit immunoglobulin. The above photographs show rat aortic sections which were treated as follows: (a) negative control incubated with TBS alone; (b) iNOS positive control obtained by incubating control vessel section with LPS (1 μg ml−1) for 24 h in tissue culture; (c) control vessel treated with iNOS antibody; (d) PTU-treated vessel incubated with iNOS antibody; (e) control vessel treated with eNOS antibody; (f) PTU-treated vessel incubated with eNOS antibody. All photographs were taken under a Zeiss LSM-510 confocal microscope at 40× magnification with the endothelial surface facing to the right of the section.
Discussion
This study found that oral PTU treatment resulted in a marked hyporesponsiveness to PE in endothelium-intact vessel rings, as compared to values from control animals. The diminished magnitude of these contractile responses were reversed by thyroxine supplementation, endothelial removal and by treatment with exogenous L-NOARG, suggesting that the effects were caused by the PTU-induced hypothyroid state and that they may involve increased endothelial NO production. These findings were supported by our immunohistochemical data which showed an apparent increase in endothelial cell eNOS antibody labelling intensity following dietary treatment, whereas immunoreactivity for iNOS was unchanged by PTU. These results confirm previous findings that PTU-induced hypothyroidism causes marked impairment of the contractile responses seen to PE and other α-adrenoceptor agonists (Rahmani et al., 1987; Brown et al., 1994a), and suggest that this may, in part, be due to enhanced production of NO by the endothelium.
The effectiveness of PTU was confirmed in the present study, as serum thyroxine concentrations were decreased to below the detectable limits of the assay after 2 weeks of this dietary regimen. The inhibition of PE-induced contraction observed after 2 weeks PTU treatment was reversed when thyroxine replacement injections were administered to the animal for the final 4 days. However, radioimmunoassay showed that the dose of hormone used resulted in a significant elevation of serum thyroxine concentrations above control levels, indicating that the animals were actually hyperthyroid for the final few days. This thyroxine replacement regime has previously been used by other workers (Lee et al., 1990) and represents the calculated daily secretion of thyroxine by the rat thyroid gland, although this group did not measure the resulting serum thyroxine concentrations. Nonetheless, these findings indicate that the inhibitory effect of PTU on PE-induced contractions could be reversed by thyroxine treatment alone, suggesting that the observed effects were due to PTU-induced hypothyroidism and not to a direct action of the drug or to other unrelated factors. These results support previous studies which have shown the inhibitory effects of hypothyroidism on the cardiovascular system to be reversed by thyroxine replacement therapy (0.1 mg kg−1 day−1) over both short (2 days) and longer (8 weeks) time periods (Rahmani et al., 1987; Brown et al., 1994b). In addition, previous published data suggests that the hyperthyroid state does not influence vascular responsiveness to PE in any way (Sabio et al., 1994).
After 2 weeks of dietary PTU supplementation, the resulting hyporesponsiveness to PE appeared to be produced, at least in part, by endothelium-derived NO. Removal of the endothelium partially restored maximum contraction to PE and treatment with L-NOARG completely restored the maximum response of these tissues, although vessel sensitivity was still reduced (Figure 1b and c). These findings suggest that the hyporesponsiveness to PE is due to NO production, mainly by the endothelium. The fact that L-NOARG treatment, but not endothelial denudation, restored the maximum response to PE completely was not surprising since L-NOARG treatment is a more specific method of inhibiting NO production by the vessel wall. Indeed, we have shown that 0.1 mM L-NOARG provides maximal inhibition of basal NO production in rat aorta (data not shown), a finding which has been confirmed by other workers (Frew et al., 1993; Mian & Martin, 1995). The decreased sensitivity to PE exhibited by aortic rings from PTU-treated animals might be explained by a reduction in the number of α1-adrenoceptors present on the vascular smooth muscle or a change in their coupling efficiency, as this was not affected by either endothelial denudation or incubation with L-NOARG. However, although it has been established that hypothyroidism causes a decrease in cardiac β-adrenoceptor density (Hawthorn et al., 1988) and may modulate the numbers of α-adrenoceptors present in cardiac (Zwaveling et al., 1996; Zhang et al., 1997) and adipose tissue (Dicker et al., 1992), there is currently no direct evidence to suggest that this condition has a similar effect on vascular α-adrenoceptor density.
After 4 weeks of PTU treatment, the maximum response to PE tended to be further reduced. Both endothelial removal and L-NOARG treatment markedly increased the maximum response (by 400–500%) to a much greater extent than that observed in control tissues (by 25–40%), but these treatments failed to restore the maximum response to control levels. In addition, vessel sensitivity to PE was further reduced after 4 weeks of PTU treatment and this was not affected by endothelial removal or incubation with L-NOARG. These data suggest that endothelium-derived NO contributes to the vascular hyporesponsiveness observed after 4 weeks of PTU supplementation, but that other endothelium-independent mechanisms must also be involved. Again, changes in α-adrenoceptor numbers or efficiency of coupling would seem to be the most likely potential mechanism responsible for the observed effects.
In the present study, weight-matched control animals were used whereas some previous studies investigating hypothyroidism have used age-matched controls. A weight-matched control group was considered appropriate in this study as oral PTU treatment retards the development of these animals. Furthermore, data on the influence of age on the maximum response to PE was collected, and showed no significant effect over the time course of the study (8 weeks, 0.53±0.02 g mg−1; 10 weeks 0.51±0.08 g mg−1, n=4). Indeed, over the age range of the rats used in the experiments described in the present study (up to approximately 10 weeks), previous published work has shown that the potency of PE increases with age (Takayanagi et al., 1989), suggesting that the present data could actually be a slight underestimate of the effect of PTU on vessel sensitivity to PE, the control rats being 2–4 weeks younger than the PTU-treated animals. Furthermore, as no difference in the maximum response to PE was found between age-matched and weight-matched control animals, none of the apparent effect on the maximum response to PE can be attributed to the age difference between the PTU-treated and control animals.
In addition to studying the effects of oral PTU treatment on PE-induced contractions, another series of experiments was performed to investigate the effect of PTU-induced hypothyroidism on endothelium-dependent relaxations. It was found that PTU supplementation alone did not significantly impair maximum per cent relaxations to carbachol (control, 85.5±1.2; 2 week PTU, 76.6±2.8; 4 week PTU, 77.5±1.7; 2 weeks PTU+thyroxine, 79.0±4.5%, n=4), although it was difficult to measure these relaxation responses accurately due to the low level of pre-contraction developed in response to PE, which thus complicated the interpretation of these results. As the mechanism of PTU-induced hyporesponsiveness to vasoconstrictor agents in the rat aorta appears to be due to enhanced basal production of NO, it proved difficult to produce a degree of pre-contraction which was similar for all treatment groups and against which NO-mediated relaxant responses could be accurately measured. Therefore, the present study concentrated on the effects of oral PTU treatment on PE-induced contractions.
Immunohistochemical analysis of NOS expression was performed on vessels taken from animals fed the PTU-supplemented diet for 2 weeks. The results obtained supported the pharmacological data demonstrating that enhanced eNOS-derived NO production may cause the impaired maximum response to PE in the PTU-treated rats. An apparent increase in eNOS labelling intensity was evident in endothelium following PTU treatment (Figure 2f), whereas immunolabelling for iNOS in the aortic tissue was of a very low level and was unaltered following PTU treatment (Figure 2c and d). This suggests, therefore, that after 2 weeks, the PTU-induced hyporesponsiveness to PE may indeed be due to increased eNOS, but not iNOS-derived NO release, predominantly by the endothelium. Thus, the immunohistochemical data support the conclusions drawn from the pharmacological experiments described in this study.
As mentioned earlier, there have been few studies which have investigated the effects of PTU on NO production. Previously, the effects of PTU-induced hypothyroidism on NOS expression have only been studied in the rat hypothalamus and thyroid gland (Colin et al., 1995; Ueta et al., 1995). The present study is, to our knowledge, the first to investigate the actions of PTU on vascular NOS expression. The findings described in this study support those of previous workers showing that oral PTU treatment causes vascular hyporesponsiveness to PE, but, for the first time, indicate that this hyporesponsiveness does not appear to involve increased iNOS expression, but may be due, at least in part, to eNOS-derived NO release from the endothelium. These results, therefore, suggest that thyroid hormones may play a role in the regulation of eNOS activity in the rat aorta.
From the results of the present study, it is not possible to determine whether the observed effects of hypothyroidism were the direct result of a reduction in circulating concentrations of thyroid hormones or mediated by other changes in circulating mediators occurring as a result of the hypothyroid state. For example, hypothyroidism induces changes in the metabolism and excretion of cortisol (Taniyama et al., 1993) and in the responsiveness of the hypothalamic pituitary axis to stimuli mediating the release of growth hormone (Edwards et al., 1989). Further studies, using cultured endothelial cells, are necessary in order to determine whether thyroid hormones have a direct effect on the regulation of eNOS synthesis and activity or produce their effects indirectly via the release of other mediators.
It is possible that under normal conditions, thyroid hormones, either directly or indirectly cause a tonic inhibition of eNOS synthesis, which contributes to the basal tone of the artery. In hypothyroidism, this inhibitory mechanism may be removed, thus increasing the expression of eNOS by the endothelium. The finding that oral PTU treatment appeared to increase eNOS expression was somewhat unexpected as there is a well documented association between hypothyroidism and hypertension (Giannattasio et al., 1997). However, we chose to study the effects of oral PTU treatment on the aorta, which is a conductance vessel. The mechanism of control of eNOS expression may differ in resistance vessels, which are involved in the regulation of blood pressure. The effects of hypothyroidism on endothelium-dependent mediators of vascular tone in these vessels is, therefore, an area which warrants further study.
Another consideration is that as hypertension is a more chronic effect of hypothyroidism, the increased expression of eNOS observed in the present study may represent an effect of hypothyroidism which precedes the development of hypertension. As the signalling pathways which regulate eNOS activation are poorly understood (Forstermann et al., 1998), the findings of the present study may be important in the understanding of the processes involved in the control of this enzyme. eNOS does have important physiological functions which are relevant to large conductance vessels such as inhibition of platelet adhesion (Radomski et al., 1987a) and aggregation (Radomski et al., 1987b) and inhibition of leucocyte adhesion (Lefer & Ma, 1993) and smooth muscle cell proliferation (Taguchi et al., 1993; Mooradian et al., 1995). Further work is necessary to establish whether thyroid hormones are indeed involved in the control of eNOS and to investigate the mechanisms of this regulation.
In conclusion, this study found that oral PTU treatment produced a marked inhibition of contractile responses to PE, which were reversed by thyroxine supplementation and by both endothelial denudation and inhibition of NO production. Immunohistochemical analysis showed that immunoreactivity for iNOS was unaltered, but that eNOS-derived NO production may be increased following oral PTU treatment. Taken together, these findings indicate that PTU-induced hypothyroidism may increase production of NO by the endothelium, suggesting that thyroid hormones play a role in the regulation of eNOS activity in rat aorta.
Acknowledgments
We thank the Medical Research Council for their financial support.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- KHS
Krebs-Henseleit solution
- L-NOARG
L-NG-nitroarginine
- LPS
lipopolysaccharide
- NO
nitric oxide
- NOS
nitric oxide synthase
- PE
phenylephrine
- PTU
propylthiouracil
- TBS
Tris-buffered saline
References
- BRITTON K.E., QUINN V., BROWN B.L., EKINS R.P. A strategy for thyroid hormone tests. BMJ. 1975;3:350–352. doi: 10.1136/bmj.3.5979.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN L., AMOS G., MILLER B. Disease-induced changes in alpha-adrenoceptor-mediated cardiac and vascular responses in rats. Clin. Exp. Pharmacol. Physiol. 1994a;21:721–728. doi: 10.1111/j.1440-1681.1994.tb02575.x. [DOI] [PubMed] [Google Scholar]
- BROWN L., NANKERVIS R., KERR D., SERNIA C. Adrenoceptor-mediated cardiac and vascular responses in hypothyroid rats. Biochem. Pharmacol. 1994b;47:281–288. doi: 10.1016/0006-2952(94)90018-3. [DOI] [PubMed] [Google Scholar]
- BULLOCK G.R., TAYLOR S.G., WESTON A.H. Influence of the vascular endothelium on agonist-induced contractions and relaxations in rat aorta. Br. J. Pharmacol. 1986;89:819–830. doi: 10.1111/j.1476-5381.1986.tb11187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLIN I.M., NAVA E., TOUSSAINT D., MAITER D.M., VAN DENHOVE M-F., LUSCHER T.F., KETELSLEGERS J-M., DENEF J-F., JAMESON J.L. Expression of nitric oxide synthase isoforms in the thyroid gland: evidence for a role of nitric oxide in vascular control during goiter formation. Endocrinology. 1995;136:5283–5290. doi: 10.1210/endo.136.12.7588272. [DOI] [PubMed] [Google Scholar]
- DICKER A., RAASMAJA A., CANNON B., NEDERGAARD J. Increased α1-adrenoceptor density in brown adipose tissue indicates recruitment drive in hypothyroid rats. Am. J. Physiol. 1992;263:E564–E662. doi: 10.1152/ajpendo.1992.263.4.E654. [DOI] [PubMed] [Google Scholar]
- EDWARDS C.A., DIEGUEZ C., SCANLON M.F. Effects of hypothyroidism, tri-iodothyronine and glucocorticoids on growth hormone responses to growth hormone-releasing hormone and His-D-Trp-Ala-Trp-D-Phe-Lys-NH2. J. Endocrinol. 1989;121:31–36. doi: 10.1677/joe.0.1210031. [DOI] [PubMed] [Google Scholar]
- FORSTERMANN U., BOISSEL J-P., KLEINERT H. Expressional control of the constitutive isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- FREW J.D., PAISLEY K., MARTIN W. Selective inhibition of basal but not agonist-stimulated activity of nitric oxide in rat aorta by NG-monomethyl-L-arginine. Br. J. Pharmacol. 1993;110:1003–1008. doi: 10.1111/j.1476-5381.1993.tb13913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANNATTASIO C., RIVOLTA M.R., FAILLA M., MANGONI A.A., STELLA M.L., MANCIA G. Large and medium sized artery abnormalities in untreated and treated hypothyroidism. Eur. Heart J. 1997;18:1492–1498. doi: 10.1093/oxfordjournals.eurheartj.a015477. [DOI] [PubMed] [Google Scholar]
- GRIEVE D.J., AVELLA M.A., BOTHAM K.M., ELLIOTT J. Evidence that endothelium-derived nitric oxide production is increased following oral propylthiouracil treatment in the rat. Br. J. Pharmacol. 1998a;125:97P. doi: 10.1038/sj.bjp.0702501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIEVE D.J., AVELLA M.A., BOTHAM K.M., ELLIOTT J. Effects of chylomicrons and chylomicron remnants on endothelium-dependent relaxation of rat aorta. Eur. J. Pharmacol. 1998b;348:181–190. doi: 10.1016/s0014-2999(98)00142-3. [DOI] [PubMed] [Google Scholar]
- HAWTHORN M.H., GENGO P., WEI X.Y., RUTLEDGE A., MORAN J.F., GALLANT S., TRIGGLE D.J. Effect of thyroid status on beta-adrenoceptors and calcium channels in rat cardiac and vascular tissue. Naunyn Schmiedebergs Arch. Pharmacol. 1988;337:539–544. doi: 10.1007/BF00182728. [DOI] [PubMed] [Google Scholar]
- KLEIN I., BECKER D.V., LEVEY G.S. Treatment of hyperthyroid disease. Ann. Intern. Med. 1994;121:281–288. doi: 10.7326/0003-4819-121-4-199408150-00010. [DOI] [PubMed] [Google Scholar]
- KLEIN I., OJAMAA K. Cardiovascular manifestations of endocrine disease. J. Clin. Endocrinol. Metab. 1992;75:339–342. doi: 10.1210/jcem.75.2.1639931. [DOI] [PubMed] [Google Scholar]
- LEE J.T., LEBENTHAL E., LEE P.C. Thyroidal regulation of rat pancreatic nuclear triiodothyronine receptor during postnatal development. Endocrinology. 1990;126:209–215. doi: 10.1210/endo-126-1-209. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., MA X. Decreased basal nitric oxide release in hypercholesterolemia increases neutrophil adherence to rabbit coronary artery endothelium. Arterioscler. Thromb. 1993;13:771–776. doi: 10.1161/01.atv.13.6.771. [DOI] [PubMed] [Google Scholar]
- MIAN K.B., MARTIN W. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric oxide to destruction by superoxide in rat aorta. Br. J. Pharmacol. 1995;115:993–1000. doi: 10.1111/j.1476-5381.1995.tb15909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORADIAN D.L., HUTSELL T.C., KEEFER L.K. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J. Cardiovasc. Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- NAKASHIMA M., HAGINO Y. Evidence for the existence of α adrenergic receptor in isolated rat atria. Jpn. J. Pharmacol. 1972;22:227–233. doi: 10.1254/jjp.22.227. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987a;92:181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987b;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHMANI M.A., CHEEMA I.R., SEN S., PEOPLES B., RILEY S.R. The effect of hyperthyroidism and hypothyroidism on alpha 1- and α 2-adrenergic responsiveness in rat aortic smooth muscle. Artery. 1987;14:362–383. [PubMed] [Google Scholar]
- SABIO J.M., RODRIGUEZ-MARESCA M., LUNA J.D., DEL RIO C.P., VARGAS F. Vascular reactivity to vasoconstrictors in aorta and renal vasculature of hyperthyroid and hypothyroid rats. Pharmacology. 1994;49:257–264. doi: 10.1159/000139241. [DOI] [PubMed] [Google Scholar]
- TAGUCHI J., ABE J., OKAZAKI H., TAKUWA Y., KUROKAWA K. L-arginine inhibits neointimal formation following balloon injury. Life Sci. 1993;53:PL387–PL392. doi: 10.1016/0024-3205(93)90167-2. [DOI] [PubMed] [Google Scholar]
- TAKAYANAGI I., SHINKAI M., YAMASAWA K. Effects of aging on α1-adrenoceptor mechanisms and the inhibitory effect of diltiazem on noradrenaline maximum response in isolated rat aortic preparation. Can. J. Physiol. Pharmacol. 1989;67:1398–1402. doi: 10.1139/y89-224. [DOI] [PubMed] [Google Scholar]
- TANIYAMA M., HONMA K., BAN Y. Urinary cortisol metabolites in the assessment of peripheral thyroid hormone action: application for diagnosis of resistance to thyroid hormone. Thyroid. 1993;3:229–233. doi: 10.1089/thy.1993.3.229. [DOI] [PubMed] [Google Scholar]
- UETA Y., LEVY A., CHOWDREY H.S., LIGHTMAN S.L. Hypothalamic nitric oxide synthase gene expression is regulated by thyroid hormones. Endocrinology. 1995;136:4182–4187. doi: 10.1210/endo.136.10.7545100. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., XU K., HAN C. Alterations of cardiac α1-adrenoceptor subtypes in hypothyroid rats. Clin. Exp. Pharmacol. Physiol. 1997;24:481–486. doi: 10.1111/j.1440-1681.1997.tb01231.x. [DOI] [PubMed] [Google Scholar]
- ZWAVELING J., BATINK H.D., DE JONG J., WINKLER-PRINS E.A., PFAFFENDORF M., VAN ZWIETEN P.A. Thyroid hormone modulates inotropic responses, α-adrenoceptor density and catecholamine concentrations in the rat heart. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:755–764. doi: 10.1007/BF00166902. [DOI] [PubMed] [Google Scholar]


