Abstract
Pitrazepin, 3-(piperazinyl-1)-9H-dibenz(c,f) triazolo(4,5-a)azepin is a piperazine antagonist of GABA in a variety of electrophysiological and in vitro binding studies involving GABA and glycine receptors. In the present study we have investigated the effects of pitrazepin, and the GABAA antagonist bicuculline, on membrane currents elicited by GABA in Xenopus oocytes injected with rat cerebral cortex mRNA or cDNAs encoding α1β2 or α1β2γ2S human GABAA receptor subunits.
The three types of GABAA receptors expressed were reversibly antagonized by bicuculline and pitrazepin in a concentration-dependent manner. GABA dose-current response curves for the three types of receptors were shifted to the right, in a parallel manner, by increasing concentrations of pitrazepin.
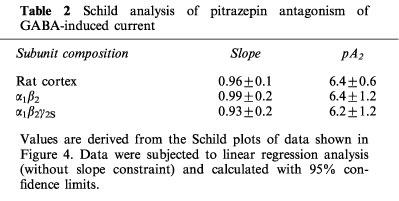
Schild analyses gave pA2 values of 6.42±0.62, n=4, 6.41±1.2, n=5 and 6.21±1.24, n=6, in oocytes expressing rat cerebral cortex, α1β2 or α1β2γ2S human GABAA receptors respectively (values are given as means±s.e.mean), and the Hill coefficients were all close to unity. All this is consistent with the notion that pitrazepin acts as a competitive antagonist of these GABAA receptors; and that their antagonism by pitrazepin is not strongly dependent on the subunit composition of the receptors here studied.
Since pitrazepin has been reported to act also at the benzodiazepine binding site, we studied the effect of the benzodiazepine antagonist Ro 15-1788 (flumazenil) on the inhibition of α1β2γ2S receptors by pitrazepin. Co-application of Ro 15-1788 did not alter the inhibiting effect of pitrazepin. Moreover, pitrazepin did not antagonize the potentiation of GABA-currents by flunitrazepam. All this suggests that pitrazepin does not affect the GABA receptor-chloride channel by interacting with the benzodiazepine receptor site.
Keywords: Benzodiazepine, bicuculline, central nervous system, GABAA receptors, pitrazepin, Xenopus oocytes
Introduction
The N-aryl piperazine derivatives exhibit both antidepressant (amoxapine, mianserine) and antipsychotic (clozapine, clothiapine and metiapine) clinical activities (Squires & Saederup, 1988; 1991); effects which may be partially due to GABAA receptor blockade. On the other hand GABAA receptors are heteromeric complexes made up of four or five subunits that form ligand-gated Cl− channels (Whiting et al., 1995). Receptors made up of different combinations of these subunits have different affinities for GABA, as well as for various allosteric modulators (Levitan et al., 1988; Pritchett et al., 1989a,1989b; Luddens et al., 1990; Puia et al., 1991).
The N-aryl piperazine derivatives fully, or partially, reverse the inhibitory action of GABA on the specific binding of t-butylbicyclophosphorothionate (TBPS) to rat brain membranes in vitro (Squires & Saederup, 1993). Moreover, binding experiments indicate that pitrazepin, the most potent N-aryl piperazine, interacts competitively with muscimol and bicuculline binding sites (Squires & Saederup, 1987). However, unlike bicuculline, pitrazepin inhibits [3H]-flunitrazepam and [3H]-diazepam binding, and interacts with glycine receptors as a potent inhibitor of [3H]-strychnine binding (Gähwiler et al., 1984; Braestrup & Nilsen, 1985). Electrophysiologically, pitrazepin reduces the inhibitory postsynaptic potentials and induces bursting activity in hippocampal and hypothalamic neurons, with a potency greater than that of bicuculline (Gähwiler et al., 1984). Moreover, the bursting activity induced by pitrazepin persists longer than that induced by bicuculline, and it is not affected by the benzodiazepine antagonist flumazenil, questioning the notion derived from binding assays, where pitrazepin antagonized [3H]-flunitrazepam binding.
In view of this, the present study was undertaken to investigate further the effects of pitrazepin on GABA/benzodiazepine receptor complexes heterologously expressed in Xenopus laevis oocytes. For that purpose we have compared the modulation of responses to GABA in oocytes expressing receptors encoded by poly(A)+mRNA isolated from the rat cerebral cortex (containing therefore a variety of GABAA receptor subtypes), or by human GABAA receptor subunit cDNAs: α1β2γ2S representing the most abundant GABA/benzodiazepine sensitive GABAA receptor in the brain (Fritschy et al., 1992; Gao & Fritschy, 1994); and α1β2, for insensitivity to benzodiazepine modulation (Smart et al., 1991; Poulter, et al., 1996).
Methods
Xenopus laevis oocytes
Xenopus laevis frogs were anaesthetized (by immersion in ice-cold water) and stages IV–VI oocytes were manually isolated from the ovary by removing the external layers (epithelium and theca). The surrounding follicular cell envelope was enzymatically removed by collagenase treatment ( SIGMA, type IA) 1 mg ml−1 for 45 min at room temperature in Ringer's solution (in mM: NaCl, 115; KCl, 2; CaCl2, 1.8; HEPES, 5; pH adjusted to 7 with NaOH; see Miledi et al., 1982). Defolliculated oocytes were then stored for 24 h at 16–18°C in Barth's medium (in mM: NaCl, 88; KCl, 1; CaCl2, 0.41; Ca(NO3)2, 0.33; MgSO4, 0.82; NaHCO3, 2.4; HEPES, 5; pH adjusted to 7.4 with NaOH; usually with 0.1 mg ml−1 gentamycin) before injection.
Recombinant plasmids
cDNAs encoding for different GABAA receptor subunits were introduced into the polylinker of plasmids pcDNA3 or pDMD8, so that the human cytomegalovirus (hCMV) promoter-enhancer would drive the constitutive transcription of the cDNAs, once they were injected into the oocyte nucleus. Plasmids were purified by the alkaline lysis method and dissolved at 1 mg ml−1 in Tris-EDTA buffer. Usually 5–15 ng of cDNAs mixture was injected to express GABAA receptors of the desired subunit composition (α1β2 1 : 1; α1β2γ2S2 : 2 : 1).
Preparation of mRNA
Total RNA was extracted from adult rat cerebral cortex using the method of Chomczynski & Sacchi (1987), and poly(A)+mRNA was isolated by chromatography in oligo(dT)-cellulose. Oocytes were injected with 50–100 ng of poly(A)+mRNA dissolved in water and were kept in Barth's medium until used for electrophysiological experiments.
Electrophysiology
Membrane currents were recorded 2–6 days after injection, using a conventional two-microelectrodes voltage-clamp technique (Miledi, 1982), after placing the oocyte in normal frog Ringer's solution. The membrane potential was held at −60 mV, and all drugs were diluted in Ringer's and applied to the oocytes by bath superfusion at 7–15 ml min−1 (bath volume 100 μl). When various drugs were applied to the same oocyte, the control response was allowed to recover to 80–100% before the next drug application. Current-voltage relationships were obtained by applying voltage pulses (3 s in 20 mV steps from −140 to +20 mV) before and during drug applications, and plotting their difference. Dose-response curve data were fitted to the equation:
where y is the agonist response as a percentage of the maximum response, nH is the apparent Hill slope and EC50 is the concentration producing half maximal response. Results are plotted as the means±s.e.mean.
Drugs
All bulk chemicals were purchased from Fisher. (+)-bicuculline, GABA, HEPES, flunitrazepam and flumazenil, from Sigma; and pitrazepin from Sandoz Ltd.
Results
Effects of flunitrazepam, pentobarbitone, zinc and bicuculline
Oocytes expressing receptors encoded by either rat cerebral cortex mRNA, human α1β2 or α1β2γ2S subunits, all responded to GABA with an inward membrane current, due mainly to a flux of chloride ions (c.f. Gundersen et al., 1984). The inset in Figure 1 shows typical responses to GABA in an oocyte expressing rat brain cortex mRNA. At low concentrations, GABA induced a steady inward current, whilst the current evoked by higher concentrations declined with time, even though the drug was continuously applied. Therefore, low concentrations of GABA ≈percnt;EC10 (10 μM for rat cortex and α1β2γ2S receptors and 2 μM for α1β2 receptors) were routinely used to reduce time-dependent desensitization of the receptors. The sigmoidal GABA dose-current response curves (Figure 1) for the rat cortex, α1β2γ2S and α1β2 human receptors, yielded EC50 values of 48±1.3 μM, n=5, 37±1.2 μM, n=7, and of 8.8±1.2 μM n=8, respectively. The Hill coefficients were 1.5 for both rat cortex and α1β2γ2S receptors, whereas α1β2 receptors gave a Hill coefficient of 1.1. These values are similar to those previously reported for oocytes injected with mRNA from rat cerebral cortex (Parker et al., 1986; Polenzani et al., 1991) and oocytes expressing rat GABAA receptors containing α1β2 or α1β2γ2S subunits (Sigel et al., 1990).
Figure 1.
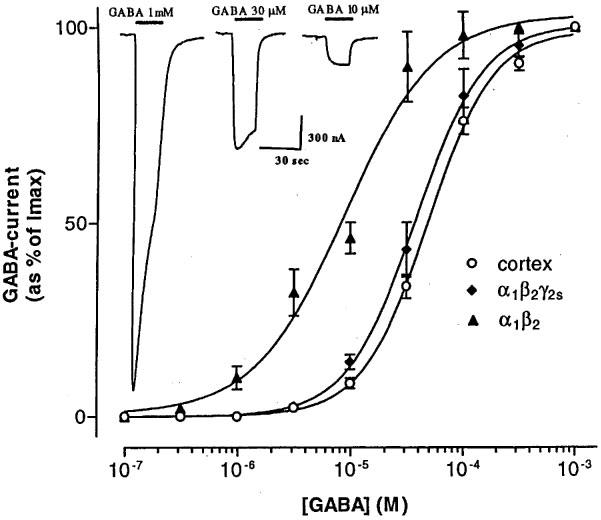
Dose-response relations for GABA-currents evoked by different types of GABAA receptors expressed by rat cerebral cortex mRNA or human cloned α1β2 and α1β2γ2S subunits. Responses were normalized and fitted with the Hill relation. Each point shows the mean±s.e.mean for 5–8 oocytes. Inset: Sample currents elicited in an oocyte injected with rat cortex mRNA. In this and subsequent traces the bars above the records indicate the times of drug applications to oocytes clamped at −60 mV; and inward currents are denoted by downward deflections of the traces.
To characterize further the three types of GABAA receptors expressed, we studied the effects of different modulators. For example, flunitrazepam (0.3 μM) potentiated the amplitude of the GABA-current elicited by activation of rat cortex or α1β2γ2S receptors; but had little or no effect on the GABA-current of oocytes expressing α1β2 receptors. The positive allosteric modulator pentobarbitone (30 μM) potentiated the GABA-currents (c.f. Gundersen et al., 1984; Parker et al., 1986) elicited by all three types of receptors, but its efficacy depended on the receptor subunit composition. In respect to the control (GABA-alone) the potentiation was: rat cortex, 424±45%, n=5, α1β2γ2S, 345±14%, n=6, and α1β2, 197±35%, n=5. At 1 μM, zinc selectively blocked the GABA-current elicited by α1β2 receptors (Smart et al., 1991), whereas it had little or no effect in oocytes expressing α1β2γ2S or cerebral cortex receptors. This suggests that when the three subunits are co-injected they preferentially form α1β2γ2S receptors.
It is well known that bicuculline blocks cerebral cortex GABAA receptors, but fails to block GABAC receptors (Polenzani et al., 1991; Woodward et al., 1993). However, the effects of bicuculline on recombinant α1β2 and α1β2γ2S receptors expressed in Xenopus oocytes had not been well characterized. Therefore, we examined the effects of bicuculline on the three types of receptors. In all cases bicuculline blocked the receptors in a dose-dependent way (Figure 2A). IC50 values for bicuculline acting on rat cortex, α1β2, or α1β2γ2S receptors were 1.8±0.5 μM, n=4, 1.4±0.7 μM, n=4, and 1.4±0.1 μM, n=4, respectively, and the corresponding Hill coefficients were 1.2±0.1, 1.0±0.1 and 1.0±0.1.
Figure 2.
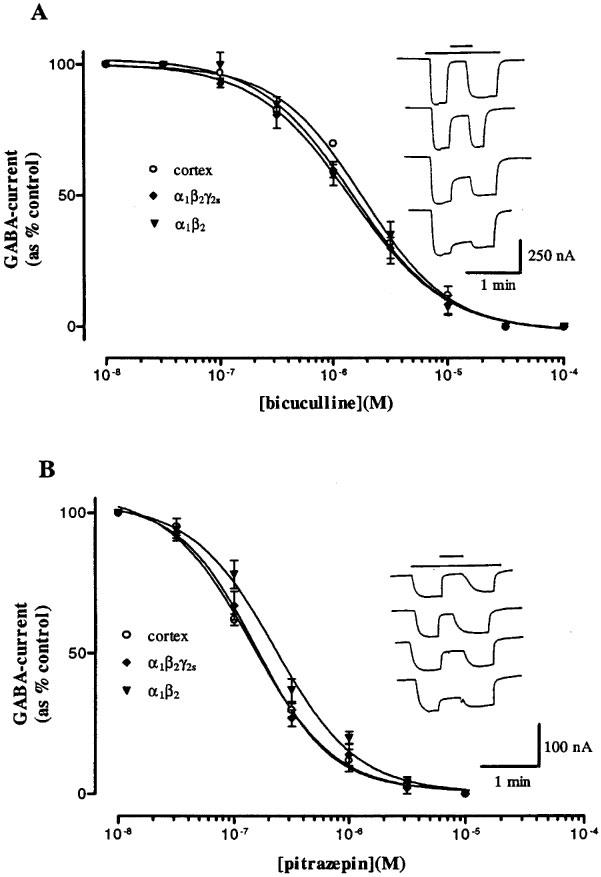
Antagonism of GABA-currents by bicuculline or pitrazepin in oocytes expressing rat cerebral cortex or human α1β2 or α1β2γ2S GABAA receptors. The currents were normalized to the amplitudes of the currents obtained with GABA alone and fitted with the Hill relation. (A) Effect of bicuculline. Each point shows the mean±s.e.mean for 5–7 oocytes. Inset: Sample traces of inhibition by bicuculline (upper bar, from top to bottom: 30, 10, 3 and 1 μM), on IGABA (10 μM, lower bar), in an oocyte expressing α1β2γ2S GABAA receptors. (B) Effect of pitrazepin. The concentration of GABA was 10 μM for rat cortex and α1β2γ2s and 2 μM for α1β2 human GABAA receptors. Inset: Sample traces of inhibition by pitrazepin (upper bar, from top to bottom: 3, 1, 0.3 and 0.1 μM), on IGABA (10 μM, lower bar) in an oocyte expressing α1β2γ2S receptors.
Effects of pitrazepin on GABAA receptors
Pitrazepin (1 μM) inhibited almost completely the GABA-currents evoked by the three types of receptors. Like bicuculline, pitrazepin decreased the GABA-current in a concentration-dependent manner (Figure 2B). Using GABA concentrations that elicited 10–20% of the maximal currents, the dose-inhibition curves gave an IC50 of 138±2.4 nM, n=5, 294±11 nM, n=5, and 148±10 nM, n=5, for rat cortex, α1β2 and α1β2γ2S receptors respectively, and the corresponding Hill coefficients were 1.3±0.1, 1.1±0.1 and 1.4±0.1. Pitrazepin alone, up to 100 μM, did not elicit detectable membrane currents.
For the three types of receptors the GABA current-voltage relationships (e.g. Figure 3) showed an outward rectification at potentials more negative than about −50 mV, and a reversal potential between −10 and −30 mV. Pitrazepin reduced significantly the GABA-currents at all membrane potentials tested; and the rectification and reversal potential of the GABA-currents remained practically unchanged. Although in the case of Figure 3 the inhibition by pitrazepin appears to show some voltage-dependence, the average of seven oocytes expressing α1β2γ2S receptor indicates that, in the range −140 to −20 mV, the inhibition is not voltage-dependent.
Figure 3.
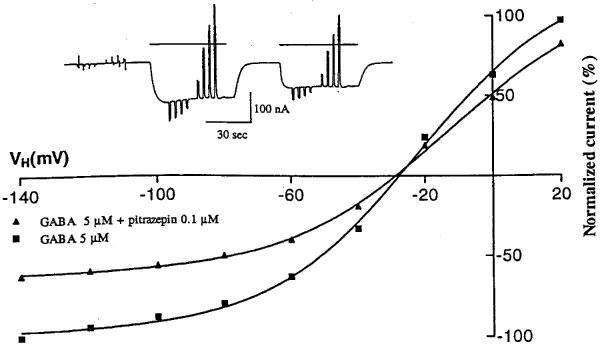
Current-voltage relations for currents evoked by GABA alone, or together with pitrazepin, in an oocyte expressing α1β2γ2S GABAA receptors. The responses were normalized to the current obtained with GABA alone at −140 mV. Each point represents the peak current elicited by GABA (5 μM) at the potentials indicated. Voltage steps (−140 mV to +20 mV, in 20 mV steps) were applied to the oocyte (held at −60 mV) during application of GABA alone or together with pitrazepin (0.1 μM) as illustrated in the inset.
GABA dose-current response curves, obtained in the presence of different concentrations of pitrazepin, showed a parallel shift to the right for the three types of receptors, and the maximum currents obtained in the presence of pitrazepin were not significantly different from those elicited by GABA alone (Figure 4). The curves for α1β2 receptors showed the smallest shift. Concentrations of GABA eliciting 50% of maximal response, in the absence or presence of pitrazepin, were used to obtain the dose ratio (DR) for each pitrazepin concentration. For each receptor type, IC50 values and Hill coefficients of dose-response relationships were significantly increased by pitrazepin (Table 1); and Schild plots obtained without slope constraint for the three types of receptors gave slopes close to 1 and comparable pA2 values (Table 2).
Figure 4.
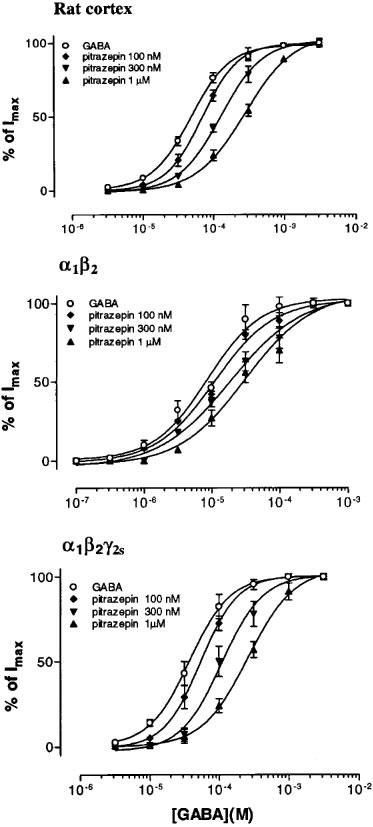
Antagonism of GABA-currents by pitrazepin. Normalized GABA dose-response curves obtained, for the three types of receptors, in the absence or in the presence of pitrazepin. Data fitted to the logistic equation used to determine IC50 and Hill coefficients (n=5).
Table 1.
Analysis of the concentration-response curves for GABA in the absence or presence of pitrazepin in oocytes injected with rat cortex or human GABAA receptor cDNAs
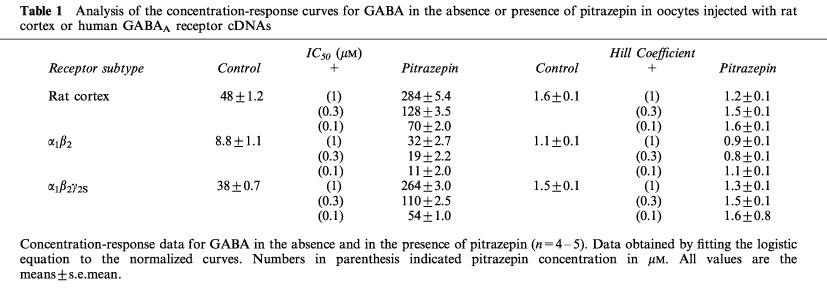
Table 2.
Schild analysis of pitrazepin antagonism of GABA-induced current
Pitrazepin, flunitrazepam and flumazenil interaction
In order to see if the binding sites for a benzodiazepine and for pitrazepin are different, we compared the effect of flumazenil, a potent benzodiazepine antagonist, on GABA currents elicited by GABA plus flunitrazepam, or by GABA plus pitrazepin. For that purpose we used oocytes expressing α1β2γ2S receptors, which displayed the greatest pitrazepin effect. For example, in the oocyte used to illustrate Figure 5, pitrazepin (0.1 μM) decreased the GABA-current to 40% whilst flunitrazepam (0.3 μM) increased it 230%. Flumazenil (0.1 μM) alone did not alter the resting membrane current and did not alter the inhibitory effect of pitrazepin. However, flumazenil abolished the potentiating action of flunitrazepam.
Figure 5.
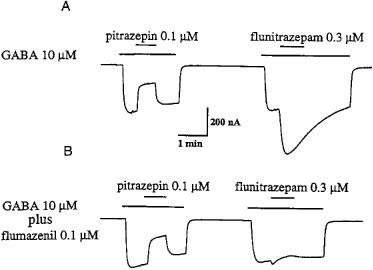
Pitrazepin does not interact with the benzodiazepine site of α1β2γ2S receptors. (A) Pitrazepin inhibits the current induced by GABA whereas flunitrazepam potentiates it. (B) Co-application of GABA plus flumazenil did not affect the inhibition by pitrazepin, but strongly antagonized the flunitrazepam potentiating effect.
To investigate the possibility that pitrazepin acts as a benzodiazepine antagonist we studied the effect of flunitrazepam on GABA currents elicited by 6 μM GABA alone or by 10 μM GABA plus 0.1 μM pitrazepin, at these concentrations the currents elicited in oocytes expressing α1β2γ2S receptors were similar in amplitude. As illustrated in Figure 6, flunitrazepam (0.3 μM) caused the same potentiation in both experimental conditions.
Figure 6.
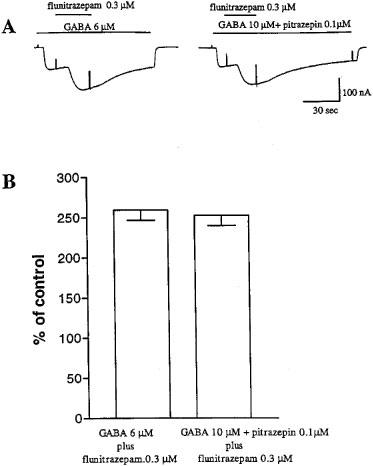
Failure of pitrazepin to antagonize the potentiating effect of flunitrazepam. (A) In an oocyte expressing α1β2γ2S GABAA receptors GABA 6 μM (left) activated inward Cl−-currents of comparable amplitude elicited by GABA 10 μM in the presence of 0.1 μM pitrazepin (right). Co-application of flunitrazepam 0.3 μM in both experimental condition determined a similar potentiation of Cl−-currents. (B) Potentiation of GABA-currents by flunitrazepam. Each column shows the mean±s.e.mean of current amplitude from six oocytes.
Discussion
As far as we know this is the first report on the effects of pitrazepin on the currents evoked by activation of rat cortex and human α1β2 and α1β2γ2S GABAA receptors expressed in Xenopus oocytes. These receptors display properties very similar to those of native receptors present in the vertebrate central nervous system. Such properties include the dose-response relationship (Tyndale et al., 1995), the time-dependent inactivation (Poulter et al., 1996) and a reversal potential similar to the chloride equilibrium potential (Kusano et al., 1982; Miledi et al., 1982). The EC50 values obtained here for rat cerebral cortex and for α1β2 or α1β2γ2S human GABAA receptors are consistent with those found in previous studies, demonstrating that functional GABAA-receptors are formed also in the absence of the γ2-subunit (McKernan and Whiting, 1996). GABAA receptors, studied electrophysiologically in neurons or after injection of poly(A)+ mRNA from rat or chick brain into Xenopus oocytes, exhibited Hill coefficients of 1.4–2.0, indicating a positive cooperativity (Choi & Fischbach, 1981; Miledi et al., 1982; Hattori et al., 1984; Bormann & Clapham, 1985; Smart et al., 1987; Bormann, 1989). The observation that the Hill coefficient of α1β2 receptors was 1.1 might be due to increased desensitization of these GABA receptors (Poulter et al., 1996). However, this interpretation is not likely since a relatively fast perfusion was used, and also because, using the same experimental conditions, other types of GABAA receptors (native or α1β2γ2S) showed Hill coefficients of 1.5. Therefore, our findings confirm that recombinant GABAA receptors can fail to show effects attributed to cooperativity of GABA binding (Burt & Kamatchi, 1991). Similar results have been reported for transfected L929 cells, where α1β2 receptors displayed a low Hill coefficient (nH=1.1) compared with that of α1β2γ2S receptors (nH=1.7) (Angelotti et al., 1993).
The lack of effect of flunitrazepam (1 μM) on GABA-currents in oocytes expressing the α1β2 receptor is in accord with the observation that the γ2-subunit is required for the formation of benzodiazepine sites on the receptors (e.g. Gunther et al., 1995). Also consistent with previous data are the facts that GABA-currents were inhibited by bicuculline in a concentration-dependent manner (Simmonds, 1982), and that bicuculline acts as a competitive inhibitor with a Hill coefficient of approximately 1 (Krishek et al., 1996; Ueno et al., 1997). The similar IC50 values obtained here for the three types of receptors suggest that their affinity for the antagonist is not strongly dependent on the subunit composition of the GABAA receptor. Moreover, although 30 μM pentobarbitone enhanced GABA-currents in native as well as in α1β2γ2S or α1β2 GABAA receptors, the potentiation was significantly greater for native and α1β2γ2S GABAA receptors. This suggests a role for the γ subunit in the modulatory effects of GABA-current by barbiturates.
The principal finding of this study is that pitrazepin blocks the currents elicited by GABA in Xenopus oocytes expressing rat cortex or cloned α1β2 and α1β2γ2S human GABAA receptors with comparable efficacies. Like bicuculline, pitrazepin, appears to act as a competitive inhibitor, shifting the dose-response curve to the right without depressing the maximum response. Furthermore, Schild analysis of curves in the presence of pitrazepin gave a slope of approximately 1 for each type of receptor, suggesting a monomolecular action of pitrazepin on GABAA receptor sites.
The inhibition of GABA induced currents by pitrazepin was fully and rapidly reversible, again similar to the inhibition by bicuculline. Thus, our results agree with previous observations reporting that pitrazepin antagonizes GABA-responses in rat brain hippocampal slices (Gähwiler et al., 1984; Kemp et al., 1986). The IC50 values for bicuculline and pitrazepin show that pitrazepin was ten times more potent than bicuculline in inhibiting the GABA-current; and the current-voltage relations indicate that pitrazepin does not appreciably alter the voltage-dependence and selectivity of the GABAA-gated channel. Furthermore, the pA2 values for inhibition of GABA-currents by pitrazepin determined in this study are similar to those derived using CA1 population spikes in a rat hippocampal slice preparation; which gave a slope of 1 and a pA2 of 6.69 (Kemp et al., 1986). The similarity of pA2 values for pitrazepin action on oocytes expressing rat cortex mRNA (therefore containing an heterogeneous population of GABAA receptors) and in oocytes expressing α1β2 or α1β2γ2S human GABAA receptors, suggests strongly that the effect of pitrazepin on GABA-currents is relatively independent of the subunit composition of GABAA receptors; at least for those studied here.
Interestingly, even though in binding studies pitrazepin displaced [3H]-flunitrazepam, in electrophysiological studies flumazenil did not alter the bursting activity induced by pitrazepin on rat hippocampal neurons (Gähwiler et al., 1984). Because of this, the authors discarded the possibility that the bursting induced by pitrazepin was due to its interaction with benzodiazepine sites. Our results on α1β2γ2S receptors are in agreement with that notion, because flumazenil had practically no effect on the inhibition of GABA-currents by pitrazepin, whereas their potentiation by flunitrazepam was abolished. Furthermore, in our experimental conditions, where endogenous GABA or a GABA-reuptake system are absent, we found that pitrazepin did not alter the potentiation of GABA-currents by flunitrazepam. All of this suggest that pitrazepin does not have a positive or negative functional effect on the benzodiazepine site.
In short, our results provide further evidence that pitrazepin, similar to bicuculline, acts directly on the GABA binding site as a competitive antagonist, and that antagonistic action is not dependent upon the presence of the γ2S subunit. Moreover, our results failed to disclose functional evidence of an interaction of pitrazepin with the GABA receptor benzodiazepine sites. The reason for the discrepancy between the binding and functional studies still remains to be determined.
Acknowledgments
We are grateful to Dr Q.T. Nyugen for computer program (Nguyen & Miledi, 1995) and to Dr Paul J. Whiting for generously providing the GABAA receptor subunit cDNAs. This material is based upon work supported by the National Science Foundation under Grant No IBN 9604499 and the Whitehall Foundation Inc.
Abbreviations
- TBPS
t-butylbicyclophosphorothionate
- Pitrazepin
3-(piperazinyl-1)-9H-dibenz(c,f) triazolo(4,5-a)azepin
References
- ANGELOTTI T.P., UHLER M.D., MACDONALD R.L. Assembly of GABAA receptor subunits: Analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J. Neurosci. 1993;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMANN J. Chloride channels in the mammalian central nervous system. Acta Physiol. Scand. 1989. pp. 582–620. [PubMed]
- BORMANN J., CLAPHAM D.E. γ-aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAESTRUP C., NILSEN M. Interaction of pitrazepin with the GABA/benzodiazepine receptor complex and with glycine receptors. Eur. J. Pharm. 1985;118:115–121. doi: 10.1016/0014-2999(85)90669-7. [DOI] [PubMed] [Google Scholar]
- BURT D.R., KAMATCHI G.L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- CHOI D.W., FISCHBACH G.D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J. Neurophysiol. 1981;45:605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- FRITSCHY J.M., BENKE D., MERTENS S., OERTEL W.H., BACHI T, MOHLER H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÄHWILER B.H., MAURER R., WUTHRICH H.J. Pitrazepin, a novel GABAA antagonist. Neurosci. Lett. 1984;45:311–316. doi: 10.1016/0304-3940(84)90244-1. [DOI] [PubMed] [Google Scholar]
- GAO B., FRITSCHY J.M. Selective allocation of GABAA receptors containing α1 subunits to neurochemically distinct subpopulation of rat hippocampal interneurons. Eur. J. Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- GUNDERSEN C.B., MILEDI R., PARKER I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. Nature. 1984;308:421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- GUNTHER U., BENSON J., BENKE D., FRITSCHY J.M., REYES G., KNOFLACH F., CRESTANI F., AGUZZI A., ARIGONI M., LANG Y. Benzodiazepine-insensitive mice generated by targeted disruption of the γ2 subunit gene of γ-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTORI K., AKAIKE N, , OOMURA Y., KURAOKA S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am. J. Physiol. 1984;246:259–265. doi: 10.1152/ajpcell.1984.246.3.C259. [DOI] [PubMed] [Google Scholar]
- KEMP J.A., MARSHALL G.R., WOODRUFF G.N. Quantitative evaluation of the potencies of GABA-receptor agonist and antagonist using the rat hippocampal slice preparation. Br. J. Pharmacol. 1986;87:677–684. doi: 10.1111/j.1476-5381.1986.tb14585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHEK B.J., MOSS S.J., SMART T.G. A functional comparison of the antagonists bicuculline and picrotoxin at recombinant GABAA receptor. Neuropharmacol. 1996;35:1289–1298. doi: 10.1016/s0028-3908(96)00089-5. [DOI] [PubMed] [Google Scholar]
- KUSANO K., MILEDI R., STINNAKRE J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J. Physiol. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITAN E.S., SCHOFIELD P.R., BURT D.R., RHEE L.M., WISDEN W., KOHLER M., FUJITA N., RODRIGUEZ H.F., STEPHENSON A., DARLINSON M.G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988;335:76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- LUDDENS H., PRITCHETT D.B., KOHLER M., KILLISCH I., KEIMANEN K., MONYER H., SPRENGEL R., SEEBURG P.H. Cerebellar GABAA receptor selective for a behavioral alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- MCKERNAN R.M., WHITING P.J. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- MILEDI R. A calcium-dependent transient outward current in Xenopus leavis oocytes. Proc. Roy. Soc. Lond. B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- MILEDI R., PARKER I., SUMIKAWA K. Synthesis of chick brain GABA receptors by frog oocytes. Proc. Roy. Soc. Lond. B. 1982;216:509–515. doi: 10.1098/rspb.1982.0089. [DOI] [PubMed] [Google Scholar]
- NGUYEN Q.T., MILEDI R. A Windows software package to record from voltage-clamped Xenopus oocytes. J. Neurosci. Meth. 1995;61:213–219. doi: 10.1016/0165-0270(95)00047-x. [DOI] [PubMed] [Google Scholar]
- PARKER I., GUNDERSEN C.B., MILEDI R. Actions of pentobarbital on rat brain receptors expressed in Xenopus oocytes. J. Neurosci. 1986;6:2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLENZANI L., WOODWARD R.M., MILEDI R. Expression of mammalian γ-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Roy. Soc. Lond. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULTER M.O., DOMINGUEZ-PERROT C., FELTZ P. GABAA receptor desensitization: The role of the γ2 subunit and its physiological significance. J. Physiol. 1996;497:145–159. doi: 10.1113/jphysiol.1996.sp021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHET D.B., SONTHEIMER H., SHIVERS B.D., YMER S., KETTENMANN H., SCHOFIELD P.R., SEEBURG P.H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989a;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- PRITCHETT D.B., LUDDENS H., SEEBURG P.H. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989b;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- PUIA G., VICINI S., SEEBURG P.H., COSTA E. Influence of recombinant γ-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of γ-aminobutyric acid-gated Cl−-currents. Mol. Pharm. 1991;39:691–696. [PubMed] [Google Scholar]
- SIGEL E., BAUR R, , TRUBE G., MOEHLER H., MALHERBE P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- SIMMONDS M.A. Classification of some GABA antagonists with regard to site of action and potency in slices of rat cuneate nucleus. Eur. J. Pharmacol. 1982;80:347–358. doi: 10.1016/0014-2999(82)90080-2. [DOI] [PubMed] [Google Scholar]
- SMART T.G., HOUAMED K.M., VAN RENTERGHEM C., CONSTANTI A. mRNA-directed synthesis and insertion of functional amino acid receptors in Xenopus laevis oocytes. Biochem. Soci. Transc. 1987;15:117–122. doi: 10.1042/bst0150117. [DOI] [PubMed] [Google Scholar]
- SMART T.G., MOSS S.J, , XIE X., HUGANIR R.L. GABAA receptors are differentially sensitive to zinc: dependence on subunits composition. Br. J. Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUIRES R.F., SAEDERUP E. GABAA receptor blockers reverse the inhibitory effect of GABA on brain-specific [35S]TBPS binding. Brain Res. 1987;414:357–364. doi: 10.1016/0006-8993(87)90017-5. [DOI] [PubMed] [Google Scholar]
- SQUIRES R.F., SAEDERUP E. Antidepressants and metabolites that block GABAA receptors coupled to 35S-t-butylbicyclophosphorothionate binding sites in rat brain. Brain Res. 1988;441:15–22. doi: 10.1016/0006-8993(88)91378-9. [DOI] [PubMed] [Google Scholar]
- SQUIRES R.F., SAEDERUP E. A rewiew of evidence for GABA-ergic predominance/glutamatergic deficit as a common etiological factor in both schizophrenia and affective psychoses: more support for a continuum hypothesis of ‘functional' psychosis. Neurochem. Res. 1991;16:1099–1111. doi: 10.1007/BF00966587. [DOI] [PubMed] [Google Scholar]
- SQUIRES R.F., SAEDERUP E. Mono N-aril ethylenediamide and piperazine derivates are GABA(A) receptor blockers: implication for psychiatry. Neurochem. Res. 1993;18:787–793. doi: 10.1007/BF00966774. [DOI] [PubMed] [Google Scholar]
- TYNDALE R.F., OLSEN R.W., TOBIN A.J.In Handbook of receptors and channels 1995CRC, Boca ReJen, FL; 265–290.ed. North, R.A. [Google Scholar]
- UENO S., BRACAMONTES J., ZORUMSKI C, , WEISS D.S., STEINBACH J.H. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITING P.J., MACKERMAN R.M., WAFFORD K.A. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int. Rev. Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- WOODWARD R.M., POLENZANI L., MILEDI R. Characterization of bicuculline/baclofen-insensitive (ρ-like) γ-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of γ-aminobutyric acidA and γ-aminobutyric acidB receptor agonists and antagonists. Mol. Pharmacol. 1993;43:609–625. [PubMed] [Google Scholar]


