Abstract
Ceramide, generated by the hydrolysis of sphingomyelin, mediates the actions of several cytokines such as tumour necrosis factor-α (TNF-α) interferon-γ and interleukin-1β (IL-1β), including their inhibitory effect on tumour proliferation. We have evaluated the role of ceramide in the proliferation of prostate cancer by using the human prostate adenocarcinoma LNCaP cell line.
Treatment of LNCaP cells with neutral or acidic sphingomyelinase or addition of C8- or C2-ceramide, two cell permeable analogues of endogenous ceramide, induced a profound inhibition of cell proliferation. This effect appeared after 24 h, was still present after 72 h of exposure to the drugs and exhibited concentration-dependency (10–200 and 5–200 mU ml−1 for neutral and acidic sphingomyelinase, respectively, and 1–25 μM for C8-ceramide).
The inhibitory effect on cell growth caused by neutral sphingomyelinase and ceramides was rapidly reversible as LNCaP cells rapidly regained their previous proliferation rate following withdrawal of the treatment.
IL-1β produced profound inhibition of LNCaP cell proliferation and caused enhanced ceramide formation.
No clear features of apoptotic cell death were detectable by either oligonucleosome formation, cytofluorimetric analysis or nuclear staining following exposure of LNCaP cells to neutral sphingomyelinase, ceramide or IL-1β. However, clear changes in LNCaP cell cycle distribution were detectable following these treatments. In contrast, treatment with acidic sphingomyelinase or TNF-α induced apoptotic death detectable by flow cytometric analysis and bisbenzimide staining.
In conclusion, our data demonstrate that preferential activation of distinct enzymatic pathways by cytokines may lead to different outcomes in the viability of LNCaP cells.
Keywords: Prostate, neutral sphingomyelinase, acidic sphingomyelinase, IL-1β, TNF-α, apoptosis, cell cycle
Introduction
In the last several years ceramide has emerged as a potential intracellular mediator of various agents that are able to control cell growth, differentiation and survival (Hannun, 1994; Pushkareva et al., 1995). Binding of these factors to their own receptors causes activation of sphingomyelinases that cleave membrane sphingomyelin to generate ceramide which, in turn, affects cell function by modulating a series of intracellular events. In different cellular systems, various factors increase sphingomyelin hydrolysis including several cytokines such as tumour necrosis factor-α (TNF-α; Kim et al., 1991; Kolesnick & Golde, 1994), interferon-γ (IFN-γ; Kim et al., 1991), interleukin-1β (IL-1β; Kolesnick & Golde, 1994), nerve growth factor (NGF; through its low affinity p75NGFR) (Dobrowsky et al., 1994), 1,25-dihydroxy-vitamin D (1,25 (OH2)-D3; Okazaki et al., 1989, 1990), the fas antigen as well as chemotherapeutic agents among others (Hannun, 1994).
Ceramide has been demonstrated to have multiple functional targets, but its main activities include induction of cell differentiation (Okazaki et al., 1990), cell cycle arrest (Jayadev et al., 1995) and apoptosis (Obeid et al., 1993; Jarvis et al., 1994). Because of this, it appears important to delineate the exact role of this potential intracellular growth-suppression regulator under particular conditions such as during the abnormal cell growth that takes place in tumour proliferation. The therapeutic efficacy of cytokines has been demonstrated in several types of human cancer including experimental models of human prostate carcinoma (Sherwood et al., 1990; van Moorselaar et al., 1991). Cytokine treatment has also been shown to modify several prostate carcinoma properties including metastatic potential (Fruehauf et al., 1990). In addition, various cytokines have been reported to exert cytotoxic and anti-proliferative effects in prostate adenocarcinoma cell lines (Fruehauf et al., 1990; Nakajima et al., 1995; Sokoloff et al., 1996; Ritchie et al., 1997) suggesting a direct control of prostate carcinoma cell growth by cytokines. In particular, TNF-α, IFN-γ and interleukin-6 have been reported to induce inhibition of cell growth in both androgen-dependent and androgen-independent adenocarcinoma cell lines (Fruehauf et al., 1990; Nakajima et al., 1995; Ritchie et al., 1997) whereas IL-1β exerts a much greater inhibitory effect on the androgen-dependent LNCaP cells (Ritchie et al., 1997).
The exact mechanism(s) that mediate these actions of cytokines on prostate cells have not yet been clearly elucidated. However, the reported link between cytokines and sphingomyelin hydrolysis in other cellular systems (Kim et al., 1991; Kolesnick & Golde, 1994) suggests that this intracellular pathway may be involved in the control of tumour prostatic cell growth. To investigate this possibility, we have activated this intracellular transducing pathway directly, by exogenous addition of the enzyme sphingomyelinase or ceramide analogues to the human prostate adenocarcinoma LNCaP cell line (Horoszewicz et al., 1983). In parallel, the effect of IL-1β and TNF-α on LNCaP cell growth and viability and the involvement of sphingomyelin hydrolysis in these actions were evaluated.
Methods
LNCaP cell culture
The androgen-sensitive human prostatic adenocarcinoma LNCaP cells (Type Culture Collection, Rockville, MD, U.S.A.) were maintained in RPMI-1640 medium supplemented with FCS 10% (v v−1), penicillin (100 U ml−1) and streptomycin (100 μg ml−1) (all from GIBCO, Grand Island, NY, U.S.A.).
Cell proliferation studies
Evaluation of LNCaP cell growth was carried out by cell counting and [3H]-thymidine incorporation studies. In the first case, cells were plated into 24-well multiwell plates (Falcon, Lincoln Park, NJ, U.S.A.), exposed to various treatments for different lengths of time (24-72 h), then detached with a 0.01% v v−1 trypsin solution (GIBCO) and counted with the aid of a haemocytometer. For MTT assay, LNCaP cells, after various treatments, were incubated with the dye solution (MTT, 0.9 mg ml−1 final concentration, Sigma, St Louis, MO, U.S.A.) for 2 h at 37°C. The solubilization solution containing 20% w v−1 SDS was then added for an additional 1 h and formazan production was evaluated in a plate reader (absorbance=560 nm). The incorporation of [3H]-thymidine into LNCaP cells was assessed by incubating cells with [3H]-methylthymidine (2 μCi ml−1, New England Nuclear, Milan, Italy; sp. act. 20 Ci mmol−1) during the last 6 h of exposure to various treatment. Cells were then precipitated with 1 M HClO4 and the incorporated radioactivity was assessed by scintillation counting.
Evaluation of apoptotic death
Biochemical and morphological approaches were used to detect apoptosis in LNCaP cells exposed to sphingomyelinase or ceramides. Quantitative analysis of DNA fragmentation was performed using a kit for cell death detection enzyme-linked immunosorbent assay (Boehringer Mannheim, Germany) based on the photometric sandwich immunoassay of cytoplasmic histone-associated DNA fragments. Briefly, cells were lysed and the cytosolic fraction was separated by centrifugation at 20,000×g for 10 min. The procedure used to detect histone-associated oligonucleosomes present in the cytosolic fraction was provided with the kit. Briefly, the microtiter plate module was coated with anti-histone antibody prior to addition of the cytoplasmic fraction prepared as above. A peroxidase-linked antibody recognizing DNA was then added and the enzymatic activity was determined photometrically (405 nm) following addition of a specific substrate.
A prediploid DNA component, indicative of apoptotic DNA fragmentation, was detected by cytofluorimetric analysis after staining cells with the nuclear dye propidium iodide (Krishan, 1975). Briefly, following specific treatments, cells were detached from the dish with the aid of a cell scraper and maintained in 70% v v−1 ethanol at −20°C overnight, thus allowing fixation and permeabilization. Cells were then repeatedly washed and incubated with RNAse (100 μg ml−1; Sigma) for 1 h at 37°C to eliminate all RNA present. A final incubation with propidium iodide (50 μg ml−1; Sigma) for 30 min was performed prior to analysis using a Coulter Elite flow cytometer. Cell debris was gated out based on light scatter evaluation and analysis was restricted to cells with either diploid and hypodiploid DNA content.
Microscopic analysis of DNA fragmentation was carried out by labelling cells with the nuclear dye bisbenzimide (Sigma) as described (Copani et al., 1995). LNCaP cells were fixed with methanol:acetic acid (3 : 1, by volume) for 30 min at room temperature (RT) then washed three times in phosphate buffer solution and incubated with 0.4 μg ml−1 bisbenzimide for 30 min at 37°C. After washing in water, cells were viewed for nuclear chromatin morphology in a Leitz fluorescence microscope with a 40× oil-immersion objective.
Cell cycle analysis
The same procedure described for cytofluorometric detemination of apoptosis was used. Following exposure to various treatments, LNCaP cells were detached from the dishes, fixed with ice cold 70% v v−1 ethanol and stored overnight at −20°C. Cells were repeatedly washed and incubated with RNAse and propidium iodide as described above. DNA content and ploidy was assessed and the Multicycle AV software program (Phoenix Flow Systems, San Diego, CA, U.S.A.) was used to analyse cell cycle distribution profiles.
Evaluation of sphingomyelin hydrolysis
LNCaP cells were incubated in the presence of [3H]-serine (Amersham Life Science, Milan, Italy; sp. act. 26 Ci mmol−1) for 72 h prior to exposure to IL-1β or TNF-α. Reaction was stopped by addition of methanol:chloroform:HCl (100 : 100 : 1, v v−1) and a balanced salt solution containing 10 mM EDTA and the aqueous and lipid phases were separated by centrifugation. Glycerophospholipids present in the lipid phase were saponified in methanolic KOH (0.1 M for 1 h at 37°C) prior to resolution of sphingomyelin by sequential one-dimensional TLC as described (Kolesnick, 1987), using chloroform:benzene:ethanol (80 : 40 : 75, v v−1) followed by chloroform:methanol:28% ammonia (65 : 25 : 5, v v−1) as solvents. Plates were analysed using a digital autoradiographer (EG&G Berthold). Ceramide was measured using a diacylglycerol kinase assay (Preiss et al., 1986) with a commercially available kit (Amersham Life Science). Phosphorylated lipids were extracted and run on TLC using chloroform : methanol: : acetic acid (65 : 15 : 5, by volume) as solvents. The ceramide phosphate spots were quantitated by the means of an InstantImager from Packard.
Drugs
Sphingomyelinases (EC 3.1.4.12) from Staphylococcus aureus (pH 7.4) and from human placenta (pH 4.5), both from Sigma, were provided in a solution containing 50% glycerol. D-erythro-sphingosine, N-octanoyl (C8-ceramide), D-erythro-sphingosine, N-acetyl (C2-ceramide) and D-erythro-sphingosine, dihydro-, N-acetyl (C2-dihydroceramide) (all from Calbiochem, La Jolla, CA, U.S.A.) were dissolved in DMSO at an initial concentration of 10 mM and stored at −80°C. Subsequent dilutions were made in aqueous solutions. In preliminary experiments DMSO (at dilutions comparable to those present in ceramide solutions) was tested for its ability to modify LNCaP cell growth. Given that DMSO per se was not effective, a double control (±DMSO) was however used in all experiments in which ceramides were employed. Human recombinant IL-1β was from Calbiochem, whereas hTNF-α was purchased from PeproTech Inc. (Rocky Hill, NJ, U.S.A.).
Statistical analysis
Data are expressed as mean±s.e.mean. Statistical analysis was performed by Student's t-test or, where appropriate, by one-way analysis of variance (ANOVA) followed by Newman-Keuls test for significance. Differences were considered to be statistically significant when P<0.05.
Results
In LNCaP cells, stimulation of the sphingomyelin pathway by addition of bacterial neutral sphingomyelinase or, alternatively, treatment with cell-permeable ceramides, C8- or C2-ceramide, a condition which mimics the activation of the endogenous sphingomyelin cascade, produced a profound inhibition of cell proliferation (Figures 1 and 2). This effect was present after a short-term exposure to the two agents and maximal after a 48–72 h-treatment period when a 50–60% reduction of cell number was observed (Figure 1). The specificity of the effect was confirmed by the inability of C2-dihydroceramide, an inactive analogue of C2-ceramide (Bielawska et al., 1993), to produce any modification of LNCaP cell proliferation (Figure 1). C2- and C8-ceramide caused a concentration-dependent inhibition of LNCaP cell proliferation (Figure 2). Maximal effect was observed at a concentration of 25 μM ceramides, but C8-ceramide exhibited a greater potency, causing a significant inhibitory effect at a concentration of 1 μM (IC30=1.3 vs 6.9 μM for C2-ceramide; Figure 2, upper panel). A concentration-dependent reduction of viable LNCaP cells following a 24 h-exposure to either C8- or C2-ceramide was also measurable with the MTT proliferation assay (64.8±4 and 62.0±3% of control values for C8- and C2-ceramide at 25 μM, respectively). Higher concentrations of C8- and C2-ceramide (50–100 μM) produced a dramatic reduction of LNCaP cell number; however, clear signs of cell injury were evident under these conditions (not shown).
Figure 1.
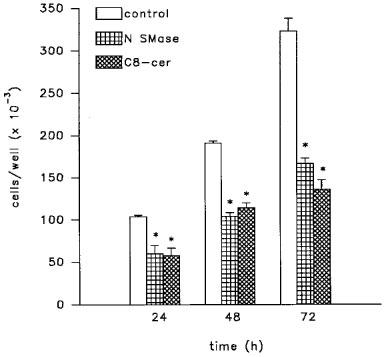
Inhibitory effect of neutral sphingomyelinase (N SMase; 200 mU ml−1) and C8-ceramide (C8-cer; 25 μM) on the proliferation of LNCaP cells. Cells were treated for different lengths of time as indicated. Data are mean±s.e.mean from three independent studies. *P<0.05 vs untreated controls by Student's t-test.
Figure 2.
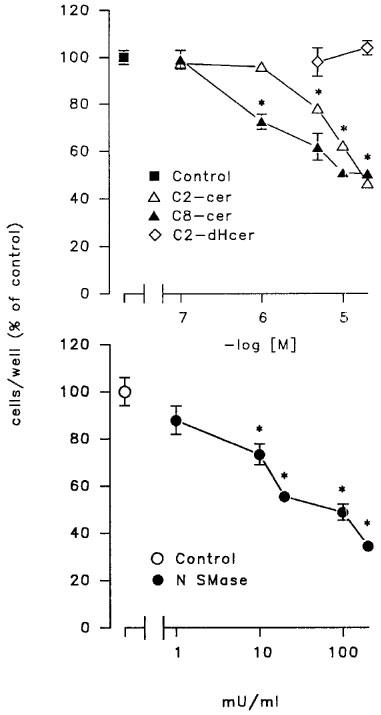
Concentration-response curves for C8- and C2-ceramides and neutral sphingomyelinase on LNCaP cell proliferation. Cells were exposed to each agent for 48 h, detached with a trypsin solution and counted with a hemocytometer. Data are mean±s.e.mean from one experiment run in triplicate representative of 3–4 independent studies. When not visible, the error bar is within the symbol. *P<0.05 by one way ANOVA followed by Newman-Keuls for significance.
Treatment with neutral sphingomyelinase produced a dose-dependent inhibition of LNCaP cell number with an IC30 of 10.1 mU ml−1 (Figure 2, lower panel). This effect was present at 10 mU ml−1 and maximal at 200 mU ml−1 (Figure 2, lower panel). In addition, the effect was rapidly reversible, as shown by the ability of LNCaP cells pre-exposed to neutral sphingomyelinase (200 mU ml−1 for 48 h) or C8-ceramide (10 μM) (Figure 3) to restart proliferating after a 48 h wash-out period.
Figure 3.
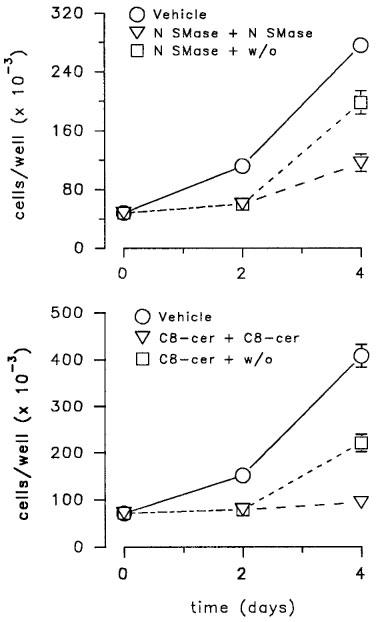
Reversibility of the inhibitory effect of neutral sphingomyelinase (upper panel) and C8-ceramide (lower panel) on LNCaP cell proliferation. Cells were treated at day 0 and day 2 with vehicle, neutral sphingomyelinase (N SMase, 200 mU ml−1) and C8-ceramide (C8-cer, 10 μM) or with N SMase or C8-cer at day 0 and vehicle at day 2. Data (mean±s.e.mean) are from one experiment representative of 3–4.
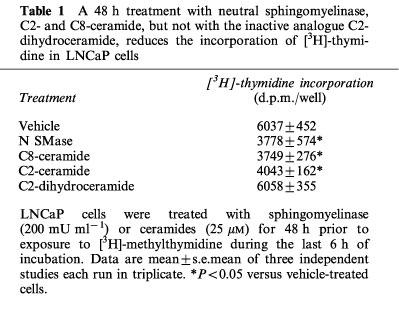
The inhibitory effect of neutral sphingomyelinase and ceramide analogues (with no effect of dihydroceramide) on LNCaP cell number was accompanied by a marked reduction of the proliferation rate as evidenced by [3H]-thymidine incorporation studies (Table 1).
Table 1.
A 48 h treatment with neutral sphingomyelinase, C2- and C8-ceramide, but not with the inactive analogue C2-dihydroceramide, reduces the incorporation of [3H]-thymidine in LNCaP cells
To determine exogenous stimulation of sphingomyelin hydrolysis by ligand-induced receptor activation, LNCaP cells were exposed to IL-1β, a well known activator of the sphingomyelin pathway in other cellular systems (Kolesnick & Golde, 1994; Mathias et al., 1993; Santana et al., 1996). A 30 min treatment with 1 ng ml−1 IL1-β produced a marked reduction of sphingomyelin levels paralleled by enhanced formation of ceramide (Figure 4). Similarly, addition of TNF-α to LNCaP cells reduced sphingomyelin concentrations with a concomitant increase in ceramide production (Figure 4). As expected, the intracellular mechanisms activated by IL-1β in LNCaP cells triggered a functional response. As already reported (Ritchie et al., 1997), a long-term exposure (48 h) of LNCaP cells to increasing concentrations of IL1-β produced a remarkable, concentration-dependent, reduction of cell proliferation as measured by cell number (Figure 5A) and [3H]-thymidine incorporation (Figure 5B). As previously shown (Fruehauf et al., 1990; Nakajima et al., 1995; Ritchie et al., 1997), a comparable inhibition of LNCaP cell proliferation was also present following exposure for 48 h to TNF-α (476±38 and 248±31×103 cells well−1 for control and 20 ng ml−1 TNF-α, respectively).
Figure 4.
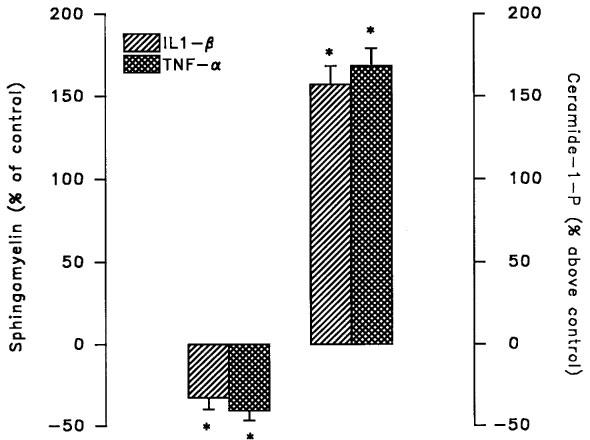
Effect of IL-1β and TNF-α on sphingomyelin hydrolysis in LNCaP cells. Cells preincubated for 72 h with [3H]-serine were exposed to the IL-1β (1 ng ml−1) or TNF-α (20 ng ml−1) for 30 min prior to extraction of lipids and separation of sphingomyelin by sequential monodimensional TLC. Alternatively, lipids were extracted and used in a diacylglycerol kinase assay prior to separation of ceramide-1-phosphate by TLC. Values of control samples were 1266±92 d.p.m. 106 cells−1 for sphingomyelin and 331±24 pmol 106 cells−1 for ceramide-1-phosphate. Values are mean±s.e.mean of three independent experiments. *P<0.05 versus untreated control by Student's t-test.
Figure 5.
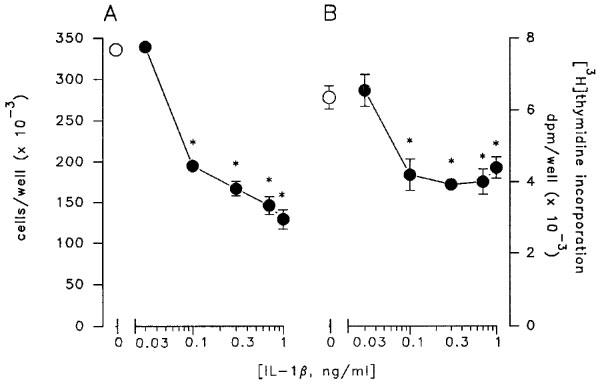
Concentration-response curve of the inhibitory effect of IL-1β on LNCaP cell growth. Cells were exposed for 48 h to increasing concentrations of the cytokine and counted with a haemocytometer (A) or incubated with [3H]-thymidine during the last 6 h of treatment (B). Data are mean±s.e.mean of 3–5 independent studies and were analysed by one-way ANOVA followed by Newman-Keuls t-test (*P<0.05).
To investigate more precisely the effect of ceramide analogues and ceramide-generating agents on LNCaP cell proliferation, cell cycle profiles were analysed by flow cytometry in LNCaP cells exposed for 24 h to C8-ceramide, IL-1β or TNF-α. As shown in Table 2, incubation of cells with 10 μM C8-ceramide produced a significant decrease in the number of cells entering the S phase of the cycle with a resulting 50–60% reduction in the overall percentage of cells in the S-G2/M phases. In contrast, cells exposed to 20 ng ml−1 TNF-α did not exhibit any significant change of cell cycle distribution, despite the appearance of a net hypodiploid population representing about 40% of the total cellular population (Figure 6B).
Table 2.
LNCaP cells exposed for 24 h to C8-ceramide (10 μM), IL-1β (1 ng ml−1) but not TNF-α (20 mg ml−1) modified their cell cycle distribution profiles
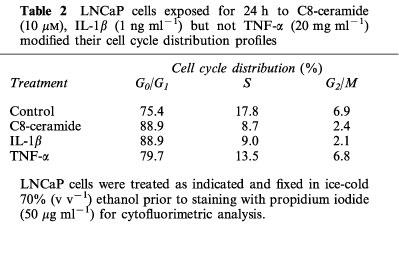
Figure 6.
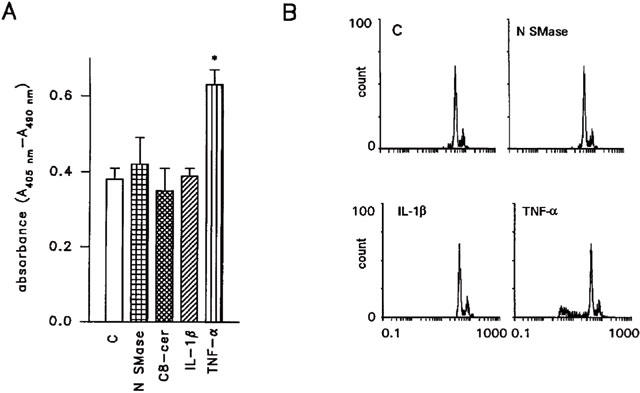
Effect of a 48 h treatment with neutral sphingomyelinase (N SMase, 200 mU ml−1) C8-ceramide (25 μM), IL-1β (1 ng ml−1) and TNF-α (20 ng ml−1) on the induction of apoptotic death in LNCaP cells. (A) shows oligonucleosome formation, as detected by a cell death detection ELISA, in cells exposed to neutral sphingomyelinase, C8-ceramide, IL-1β and TNF-α. The appearance of a cell population with hypodiploid DNA in LNCaP cells treated with TNF-α, but not in cells exposed to neutral sphingomyelinase and IL-1β is shown in (B). In (A) data are from one experiment representative of three. *P<0.05 versus control (Student's t-test). In (B) representative data are shown.
Short- (3, 6 h), intermediate- (12, 24 h) or long-term (48, 72 h) treatment of LNCaP cells with either neutral sphingomyelinase or C8-ceramide (up to 25 μM) was not able to induce apoptotic cell death. As shown in Figure 6 which illustrates representative data from LNCaP cells exposed to neutral sphingomyelinase (200 mU ml−1), C8-ceramide (25 μM) or IL-1β (1 ng ml−1) for 48 h, no characteristic features of apoptosis were evident. Figure 6A shows the lack of enhanced oligonucleosome formation, indicative of nuclear fragmentation in a cellular population undergoing apoptotic death. Accordingly, as shown in Figure 6B, the characteristic appearance of a hypodiploid cell population was missing in cells treated for 48 h with neutral sphingomyelinase (200 mU ml−1) or IL-1β (1 ng ml−1). No induction of apoptotic death was present in cells exposed to the three agents for shorter time points (6–24 h; not shown). In contrast, an increased oligonucleosome formation (Figure 6A) and the appearance of a hypodiploid population (Figure 6B) was evident in LNCaP cells exposed for 48 h to TNF-α (20 ng ml−1). In addition, morphological evaluation of LNCaP cells after staining with the nuclear dye bisbenzimide clearly demonstrated the lack of picnotic and/or fragmented nuclei in neutral sphingomyelinase – (Figure 7B) and IL-1β-treated cultures (Figure 7C) but a characteristic apoptotic picture in TNF-α-treated cells (Figure 7D).
Figure 7.
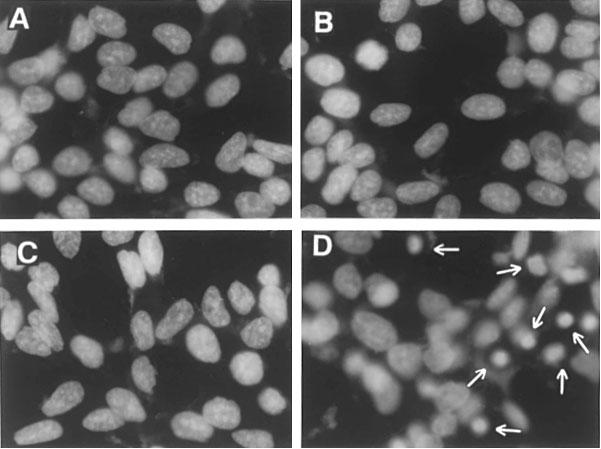
TNF-α, but not neutral sphingomyelinase or IL-1β, induces chromatin condensation in LNCaP cells. Cells were incubated with each agent for 48 h, fixed in methanol/acetic acid, stained with bisbenzimide and visualized by fluorescence microscopy. A=control; B=neutral sphingomyelinase (200 mU ml−1); C=IL-1β (1 ng ml−1); D=TNF-α (20 ng ml−1). Arrowheads indicate condensed nuclei.
In an attempt to correlate TNF-α action on LNCaP cells to specific intracellular targets for this cytokine, we have analysed the effect of acidic sphingomyelinase on LNCaP cell proliferation and death, based on the reported ability of TNF-α to stimulate the activity of this enzyme (Testi, 1996). As shown in Figure 8A, treatment for 48 h with acidic sphingomyelinase concentration-dependently inhibited the number of LNCaP cells. Maximal effect was observed at concentrations of 100–200 mU ml−1 (Figure 8A) whereas higher concentrations (500–1000 mU ml−1) exhibited pronounced cytotoxicity (not shown). The reduction of LNCaP cell number following exposure to acidic sphingomyelinase was accompanied by induction of apoptotic death as shown by appearance of chromatin condensation and fragmentation (Figure 8B) and cytofluorimetric analysis (Figure 8C).
Figure 8.
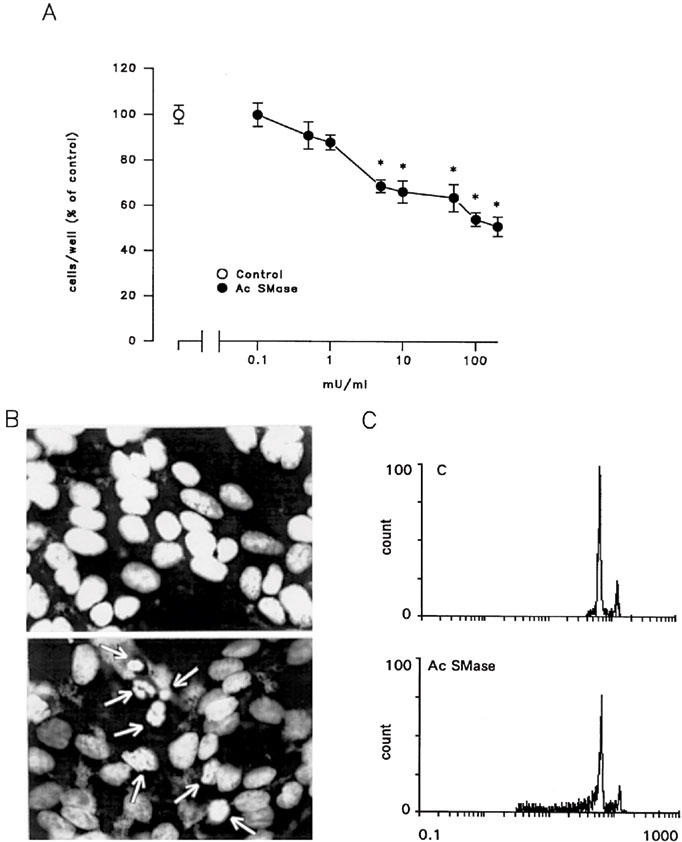
A 48-h treatment with acidic sphingomyelinase (Ac SMase) reduces, in a concentration-dependent manner, LNCaP cell number (A) and induces apoptotic death as shown by nuclear condensation and fragmentation (B, lower photograph) and DNA ploidy profiles at flow cytometric analysis (C). In (B) arrowheads indicate condensed nuclei. In (A) *P<0.05 by one-way ANOVA and Newman-Keuls t-test for significance.
Discussion
Understanding the mechanisms involved in the control of tumour growth is a major goal of basic research in oncology. Prostate adenocarcinoma represents one of the most common malignancies in adult males and LNCaP cells (Horoszewicz et al., 1983) provide a useful in vitro model to study the intracellular mechanisms involved in the control of prostate cancer growth.
Cytokines such as TNF-α, IL-1β and IFN-γ exert cytostatic effects in prostate carcinoma cell lines (Sherwood et al., 1990; Fruehauf et al., 1990; Nakajima et al., 1995; Ritchie et al., 1997), modify several typical markers of prostate carcinoma cells (Fruehauf et al., 1990) and are efficacious in the treatment of prostate cancer xenografts transplanted in nude mice (Sherwood et al., 1990; van Moorselaar et al., 1991). The exact mechanism by which cytokines induce molecular changes in prostate target cells is still under investigation. Several cytokines including TNF-α, IFN-γ and IL-1β are known to activate sphingomyelin hydrolysis generating ceramide, which by its ability to cause cell differentiation, cell-cycle arrest and apoptosis (reviewed in Pushkareva et al., 1995), is recognized as one of the intracellular second messengers that regulate growth suppression.
Addition of cell-permeable analogues of ceramide or sphingomyelinase induced a reduction in LNCaP cell proliferation. A similar inhibitory effect on cell growth has been reported in Molt-4 human leukaemia cells and Wi38 human fibroblasts where treatment with a synthetic ceramide induced almost complete G0/G1 cell cycle arrest (Jayadev et al., 1995). C8-ceramide exhibited a greater potency than C2-ceramide, a phenomenon that may be ascribed to the different physicochemical and pharmacokinetic properties of the two molecules. The specificity of the effect of ceramide was demonstrated by the observation that the immediate precursor of ceramide, dihydroceramide, which lacks the trans double bond at C4-C5 of the sphingoid base backbone and is thus devoid of biological actions (Bielawska et al., 1993), did not produce any significant effect on the number of LNCaP cells. The anti-mitogenic activity exerted by neutral sphingomyelinase and ceramide was also proved by the rapid recovery of cell proliferation upon removal of the two agents.
IL-1β, which significantly activated sphingomyelin hydrolysis in LNCaP cells, greatly reduced the cell proliferation of this cell line without causing apoptotic death. Activation of sphingomyelin hydrolysis does not necessarily initiate an apoptotic response; agents known to activate the sphingomyelin cascade such as IFN-γ and TNF-α have also been reported to reduce the proliferation of oligodendrocyte precursors without modifying cell survival (Agresti et al., 1996). Moreover, ceramide also exhibits anti-apoptotic, protective activity in primary cultures of sympathetic (Ito & Horigome, 1995) and hippocampal (Goodman & Mattson, 1996) neurons and exerts mitogenic effect in quiescent, Swiss 3T3 fibroblasts (Olivera et al., 1992), suggesting that its role in cellular function does not inevitably involve a death program. In LNCaP cells, the inhibitory effect of IL-1β on cell proliferation (Kemick et al., 1996; Ritchie et al., 1997), together with its ability to reduce cell chemotaxis (Ritchie et al., 1997), has been related to the ability of IL-1β to affect the Insulin-like growth factor (IGF)-IGF binding protein-3 system and to counteract the mitogenic effect of IGF (Kemick et al., 1996; Ritchie et al., 1997). Our present data, however, strongly support a direct action of IL-1β on the control of LNCaP cell proliferation through activation of the sphingomyelin cascade. 1,25 (OH2)-D3, which triggers sphingomyelin hydrolysis in different cellular systems (Okazaki et al., 1989, 1990), exerts potent antiproliferative and differentiating activity in LNCaP cells grown in the presence of serum (Skowronski et al., 1993), determining cell cycle arrest in G1 (Blutt et al., 1997).
Recently, Hermann et al. (1997) have reported that in LNCaP cells, C2-ceramide treatment induces apoptosis, as detected by measurement of cells undergoing plasma membrane rupture. Besides the different methodological approach used in that study, low micromolar concentrations of C2-ceramide were able to induce apoptosis in LNCaP cells cultured in the presence of low serum (0.5% FCS), a condition that we have intentionally avoided to eliminate possible activation of endogenous ceramide production by serum deprivation (Jayadev et al., 1995). In addition, in our hands, high concentrations of either C2- or C8-ceramide (above 30 μM) produced marked cytotoxicity with clear signs of cell disruption. Such a destructive phenomenon, at similar ceramide concentrations, has been described previously in other cellular systems (Hebestreit et al., 1998).
In LNCaP cells TNF-α was able to induce apoptotic death and to cause increased accumulation of ceramide. Various studies have identified several components of the pathway involved in this TNF-α effect (reviewed in Testi, 1996; Wertz & Hanley, 1996). In most cell types, apoptosis by TNF-α appears to be mediated by the 55 kDa receptor (TNFR1) belonging to the NGF/TNF ‘death' receptor types (Smith et al., 1994). Intracellular signalling from TNFR1 has recently been more clearly defined and dual activation of sphingomyelin hydrolysis with involvement of two distinct enzymes operating under different topological and regulatory conditions have been described (Wiegmann et al., 1994). Thus, an acidic sphingomyelinase localized in endosomes and caveolae is activated upon interaction of TNF-α with TNFR1 and is mainly responsible for the death program initiated by TNF-α in several cellular systems (Testi, 1996). Accordingly, LNCaP treatment with acidic sphingomyelinase produced a profound reduction in cell number paralleled by clear signs of apoptotic death. Activation of a neutral sphingomyelinase by TNF-α may participate in the induction of cell death but seems primarily involved in the regulation of cell proliferation and survival (see Testi, 1996 for a review). Thus, differential responses to TNF-α may take place in various cell types depending on preferential activation of intracellular signals.
IL-1β which affects solely LNCaP cell proliferation, but does not induce cell death, is likely to involve primarily neutral sphingomyelinase in its action. Indeed, in some cellular systems, IL-1β has been reported to cause activation of acidic sphingomyelinase, but the involvement of this pathway in the responses elicited by IL-1β appears controversial (Cobb et al., 1996; Masamune et al., 1996). In LNCaP cells, IL-1β may act on both neutral and acidic sphingomyelinase, but the resulting generation of ceramide in selected intracellular compartments is not sufficient to induce a death response. In this respect it appears important to note that IL-1β and IFN-γ, both agents that act on sphingomyelin hydrolysis, are able to initiate an apoptotic response when co-applied to LNCaP cells, while being unable to induce cell death when given alone (unpublished observation).
In conclusion, the present results provide evidence to support the involvement of the sphingomyelin cascade in the control of proliferation and viability of prostate adenocarcinoma LNCaP cells. In particular, induction of a neutral sphingomyelinase seems to be not sufficient to accomplish a death program while being adequate to generate a cascade of events allowing cells to modify their proliferation pattern.
Acknowledgments
This work was supported in part by FAR grants from the Universities of Pavia (P.L. Canonico) and Catania (M.A. Sortino). The authors wish to thank Mr Sandro Giacchetto for his helpful technical assistance.
Abbreviations
- 1,25 (OH2)-D3
1,25-dihydroxy-vitamin D
- FCS
foetal calf serum
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- NGF
nerve growth factor
- TNF-α
tumour necrosis factor-α
References
- AGRESTI C.D., URSO D., LEVI G. Reversible inhibitory effect of interferon-γ and tumour necrosis factor-α on oligodendroglial lineage cell proliferation and differentation in vitro. Eur. J. Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- BIELAWSKA A., CRANE H.M., LIOTTA D., OBEID L.M., HANNUN Y.A. Selectivity of ceramide-mediated biology. J. Biol. Chem. 1993;268:26226–26232. [PubMed] [Google Scholar]
- BLUTT S.E., ALLEGRETTO E.A., PIKE J.W., WEIGEL N.L. 1,25-dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of LNCaP prostate cells and cause accumulation of cells in G1. Endocrinology. 1997;138:1491–1497. doi: 10.1210/endo.138.4.5063. [DOI] [PubMed] [Google Scholar]
- COBB R.R., FELTS K.A., PARRY G.C.N., MACKMAN N. D609, a phosphatidylcholine-specific phospholipase C inhibitor, blocks interleukin-1β-induced vascular cell endothelial molecule 1 gene expression in human endothelial cells. Mol. Pharmacol. 1996;49:998–1004. [PubMed] [Google Scholar]
- COPANI A., BRUNO V.M., BARRESI V., BATTAGLIA G., CONDORELLI D.F., NICOLETTI F. Activation of metabotropic glutamate receptors prevents neuronal apoptosis in culture. J. Neurochem. 1995;64:101–108. doi: 10.1046/j.1471-4159.1995.64010101.x. [DOI] [PubMed] [Google Scholar]
- DOBROWSKY R.T., WERNER M.H., CASTELLINO A.M., CHAO M.V., HANNUN Y.A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- FRUEHAUF J.P., MYERS C.E., SINHA B.K. Synergistic activity of suramin with tumor necrosis factor-α and doxorubicin on human prostate cancer cell lines. J. Natl. Cancer Inst. 1990;82:1206–1209. doi: 10.1093/jnci/82.14.1206. [DOI] [PubMed] [Google Scholar]
- GOODMAN Y., MATTSON M.P. Ceramide protects hippocampal neurons against excitotoxic and oxidative insults, and amyloid β-peptide toxicity. J. Neurochem. 1996;66:869–872. doi: 10.1046/j.1471-4159.1996.66020869.x. [DOI] [PubMed] [Google Scholar]
- HANNUN Y.A. The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- HEBESTREIT H., DIBBERT B., BALATI I., BRAUN D., SCHAPOWAL A., BLASER K., SIMON H.U. Disruption of fas receptor signaling by nitric oxide in eosinophils. J. Exp. Med. 1998;187:415–425. doi: 10.1084/jem.187.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANN J.L., MENTER D.G., BEHAM A., VON ESCHENBACH A., MCDONNEL T.J. Regulation of lipid signaling pathways for cell survival and apoptosis by bcl-2 in prostate carcinoma cells. Exp. Cell Res. 1997;24:442–451. doi: 10.1006/excr.1997.3653. [DOI] [PubMed] [Google Scholar]
- HOROSZEWICZ J.S., LEONG S.S., KAWINSKI E., KARR J.P., ROSENTHAL H., CHU T.M., MIRAND E.A., MURPHY G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- ITO A., HORIGOME K. Ceramide prevents neuronal programmed cell death induced by nerve growth factor deprivation. J. Neurochem. 1995;65:463–466. doi: 10.1046/j.1471-4159.1995.65010463.x. [DOI] [PubMed] [Google Scholar]
- JARVIS W.D, , KOLESNICK R.N, , FORNARI F.A, , TRAYLOR R.S, , GEWIRTZ D.A., GRANT S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc. Natl. Acad. Sci. U.S.A. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAYADEV S., LIU B., BIELAWSKA A.E., LEE J.Y., NAZAIRE F., PUSHKAREVA M.Y., OBEID L.M., HANNUN Y.A. Role for ceramide in cell cycle arrest. J. Biol. Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- KEMICK M.S., WANG D., LIN T.Interleukin-1 inhibits the proliferation of the human prostate cancer line LNCaP 1996P1–84.Program of the 10th International Congress of Endocrinology, San Francisco 1996(Abstract)
- KIM M.Y., LINARDIC C., OBEID L., HANNUN Y.A. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor α and γ-interferon. Specific role in cell differentiation. J. Biol. Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- KOLESNICK R.N. 1,2-Diacylglycerols but not phorbol esters stimulate sphingomyelin hydrolysis in GH3 pituitary cells. J. Biol. Chem. 1987;262:16759–16762. [PubMed] [Google Scholar]
- KOLESNICK R., GOLDE D.W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- KRISHAN A. Rapid flow cytometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASAMUNE A., IGARASHI Y., HAKAMORI S. Regulatory role of ceramide in interleukin (IL)-1β-induced E-selectin expression in human umbilical vein endothelial cells. Ceramide enhances IL-1β action, but is not sufficient for E-selectin expression. J. Biol. Chem. 1996;271:9368–9375. doi: 10.1074/jbc.271.16.9368. [DOI] [PubMed] [Google Scholar]
- MATHIAS S., YOUNES A., KAN C.-C., ORLOW I., JOSEPH C., KOLESNICK R.N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1β. Science. 1993;259:519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA Y., DELLIPIZZI A., MALLOUH C., FERRERI N.R. Effects of tumor necrosis factor-α and interferon-γ on the growth of human prostate cancer cell lines. Urol. Res. 1995;23:205–210. doi: 10.1007/BF00393299. [DOI] [PubMed] [Google Scholar]
- OBEID L.M, , LINARDIC C.M., KAROLAK L.A., HANNUN Y.A. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., BELL R.M., HANNUN Y.A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. J. Biol. Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- OKAZAKI T., BIELAWSKA A., BELL R.M., HANNUN Y.A. Role of ceramide as a lipid mediator of 1α, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- OLIVERA A., BUCKLEY N.E., SPIEGEL S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J. Biol. Chem. 1992;267:26121–26127. [PubMed] [Google Scholar]
- PREISS J., LOOMIS C.R., BISHOP W.R., STEIN R., NIEDEL J.E., BELL R.M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J. Biol. Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- PUSHKAREVA M., OBEID L.M., HANNUN Y.A. Ceramide: an endogenous regulator of apoptosis and growth suppression. Immunol. Today. 1995;16:294–297. doi: 10.1016/0167-5699(95)80184-7. [DOI] [PubMed] [Google Scholar]
- RITCHIE C.K., ANDREWS L.R., THOMAS K.G., TINDALL D.J., FITZPATRICK L.A. The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis: implications for the development of metastatic disease. Endocrinology. 1997;138:1145–1150. doi: 10.1210/endo.138.3.4974. [DOI] [PubMed] [Google Scholar]
- SANTANA P., LLANES L., HERNANDEZ I., GONZALES-ROBAYNA I., TABRAUE C., GONZALES-REYES J., QUINTANA J., ESTEVEZ F., RUIZ DE GALARRETA C.M., FANJUL L.F. Interleukin-1β stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology. 1996;137:2480–2489. doi: 10.1210/endo.137.6.8641202. [DOI] [PubMed] [Google Scholar]
- SHERWOOD E.R., FORD T.R., LEE C., KOZLOWSKI J.M. Therapeutic efficacy of recombinant tumor necrosis factor alpha in an experimental model of human prostatic carcinoma. J. Biol. Response Modif. 1990;9:44–52. [PubMed] [Google Scholar]
- SKOWRONSKI R.J., PEEHL D.M., FELDMAN D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132:1952–1960. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- SMITH C.A., FARRAH T., GOODWIN R.G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF M.H., TSO C.-L., KABOO R., TANEJA S., PANG S., DEKERNION J.B., BELLDEGRUN A.S. In vitro modulation of tumor progression-associated properties of hormone refractory prostate carcinoma cell lines by cytokines. Cancer. 1996;77:1862–1872. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1862::AID-CNCR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- TESTI R. Sphingomyelin breakdown and cell fate. Trends Biol. Sci. 1996;21:468–471. doi: 10.1016/s0968-0004(96)10056-6. [DOI] [PubMed] [Google Scholar]
- VAN MOORSELAAR R.J., VAN STRATUM P., BORM G., DEBRUYNE M., SCHALKEN J.A. Differential antiproliferative activities of α- and γ-interferon and tumor necrosis factor alone or in combinations against two prostate cancer xenografts transplanted in nude mice. Prostate. 1991;8:331–344. doi: 10.1002/pros.2990180407. [DOI] [PubMed] [Google Scholar]
- WERTZ I.E., HANLEY M.R. Diverse molecular provocation of programmed cell death. Trends Biol. Sci. 1996;21:359–364. [PubMed] [Google Scholar]
- WIEGMANN K., SCHUTZE S., MACHLEIDT T., WITTE D., KRONKE M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]


