Abstract
We have used a reversible permeabilization method to facilitate the entry of Giα1, 2 and 3 G-protein subunit mRNA antisense or mismatch oligonucleotides into intact tissue, to investigate the G-protein α-subunit coupling of α2-adrenoceptors, neuropeptide Y (NPY) Y1, and A1 adenosine receptors in preparations of the epididymis of the guinea-pig.
The α2-adrenoceptor agonist, xylazine, elicited concentration dependent contractions from preparations of phenylephrine (3 μM)-stimulated epididymis (pEC50 value 6.52±0.39, maximum response 236±41 mg force). Compared to respective mismatch controls the incubation of preparations with Giα2, but not with Giα1 or Giα3 mRNA antisense oligonucleotides (30 μM) reduced the maximal xylazine-potentiation of phenylephrine (3 μM)-stimulated contractility (to 51±12% of Giα2 mismatch control). The oligonucleotide incubations had no effect upon the pEC50 values of xylazine.
The A1 adenosine receptor agonist, cyclopentyladenosine (CPA) elicited concentration dependent contractions from preparations of phenylephrine (3 μM)-stimulated epididymis (pEC50 value 7.66±0.57, maximum response 208±54 mg force). Incubation of preparations of epididymis with Giα1, but neither Giα2 nor Giα3 antisense oligonucleotides reduced the maximal CPA-potentiation of phenylephrine (3 μM)-stimulated contractions (to 55±17% of Giα1 mismatch control), pEC50 values were not affected.
The incubation of preparations with Giα2 antisense mRNA oligonucleotides reduced the maximal NPY-potentiation of phenylephrine (3 μM)-stimulated contractions (to 62±15% of Giα mismatch control). Compared with Giα2 mismatch controls, the incubation of preparations with Giα1 and Giα3 oligonucleotides also reduced the NPY-potentiation of phenylephrine (3 μM)-stimulated contractions.
These studies indicate that, in the guinea-pig epididymis, α2-adrenoceptors and A1 adenosine receptors preferentially couple to effectors through Giα2 and Giα1 subunits respectively. In contrast NPY receptors may elicit effects through either Giα1, 2 or 3 subunits.
Keywords: Antisense oligonucleotides, G-proteins, α2-adrenoceptor, neuropeptide Y (NPY) Y1 receptor, A1 adenosine receptor
Introduction
A1 Adenosine receptors, α2-adrenoceptors and neuropeptide Y (NPY) Y1 receptors, are all members of the G-protein coupled receptor superfamily. In the epididymis of the guinea-pig agonists at the three receptor classes all potentiate contractile responses to the α1-adrenoceptor agonist, phenylephrine, at concentrations which do not directly elicit contractions (Haynes & Hill, 1996; Haynes et al., 1997; 1998a,1998b). The epididymal A1 adenosine receptor and α2-adrenoceptor-mediated responses are both sensitive to pertussis toxin (Haynes & Hill, 1996; Haynes et al., 1998b); indicating coupling through heterotrimeric Gi/o proteins. These G-proteins consist of α-, β- and γ- subunits and upon agonist binding to receptor, GDP is exchanged for GTP on the Gα-subunit and both it and the βγ-subunit are set adrift to interact with effectors (see Clapham, 1996). At present there are at least three classes of the Giα subunit, Giα1, Giα2 and Giα3 and many possible combinations of β- and γ-subunits.
In the early part of this decade several studies demonstrated mRNA-specific effects of antisense oligonucleotides. These studies showed antisense oligonucleotides inhibiting β-adrenergic receptor kinase and protein kinase A activity in human epidermoid carcinoma cells (Shih & Malbon, 1994), smooth muscle cell proliferation and migration (Biro et al., 1993) and smooth muscle cell contractility (Bitar and Zhu, 1993). Other studies have shown that antisense oligonucleotides inhibit G-protein subunit formation in cultured cells (see Kalkbrenner et al., 1996). In contrast to cultured cell studies there are relatively few studies showing antisense oligonucleotide effects upon contractility in vitro.
Although α2-adrenoceptor, A1 adenosine receptor and NPY Y1 receptor activation all potentiate contractile responses to phenylephrine in the epididymis of the guinea-pig, there are subtle differences in these effects. Thus the α2-adrenoceptor, but not A1 adenosine receptor-mediated effects are attenuated by the incubation of preparations with the L-type Ca2+ channel blocker, nifedipine. In addition the α2-adrenoceptor and NPY Y1 receptors, but not the A1 adenosine receptor are significantly coupled to adenylate cyclase (Haynes & Hill, 1996; Haynes et al., 1998b). Recently Lesh et al. (1995) have developed a technique to reversibly permeabilize intact sheets of smooth muscle to facilitate the entry of antisense oligonucleotides. Thus we now use the reversible permeabilization technique of Lesh et al. (1995) to introduce Giα-subunit mRNA antisense oligonucleotides into intact tissues and investigate the possibility that the epididymal α2-adrenoceptor, A1 adenosine and NPY Y1 receptor effects are mediated through the activation of distinct Gα-protein subunits.
Methods
Animals
Male Duncan-Hartley guinea-pigs (650–1000 g) were housed in open runs (20°C) with a 12 h light dark cycle. Food consisted of BeKay pellets with green vegetables and water ad libitum. On the day of use animals were killed by cervical dislocation and both vasa deferentia and testis were removed.
Tissue preparation and reversible permeabilization
The cauda epididymis was unravelled (with the aid of a N.U.M.S. HM 1 binocular dissecting microscope) and placed into modified Krebs solution (of composition, mM: NaCl 118; KCl 4.7; MgSO4 0.45; K2HPO4 2.5; NaHCO3 25; CaCl2 1.9; glucose 11). Preparations of epididymis, each approximately one cm long were used either fresh, reversibly permeabilized (Lesh et al., 1995), or reversibly permeabilized and incubated for 48 or 72 h.
The reversible permeabilization method is as follows: preparations were incubated in buffer I (containing mM: EGTA 10, KCl 120, ATP 5, MgCl2 2, n-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid (TES) 20; at 2°C and pH 6.8, for 20 min), transferred to buffer II (containing mM: KCl 120, ATP 5, MgCl2 2, TES 20; and also antisense oligonucleotides 10 or 30 μM, at 2°C and pH 6.8, for 90 min) then to buffer III (containing mM: KCl 120, ATP 5, MgCl2 10, TES 20; and also antisense oligonucleotides 10 or 30 μM, at 2°C and pH 6.8, for 30 min). Tissues were placed into buffer IV (containing mM: NaCl 140, KCl 5, MgCl2 10, glucose 5.6, 3-[N-morpholino]propanesulfonic acid (MOPS) 2; and also antisense oligonucleotides 10 or 30 μM, at 22°C and pH 7.1) for 30 min then CaCl2 was added (at 15 min intervals) to give final concentrations of 2, 19, 190 and 1900 μM). Preparations were then incubated for 48 or 72 h in Krebs solution: DMEM (Dulbeccos modified Eagles medium containing, L-glutamine, 2 mM; 1 : 1) at 35°C and gassed with carbogen (O2:CO2, 95 : 5).
Following the 48 or 72 h equilibration preparations were attached, with a silk thread (1/0), to a tissue holder and equilibrated in modified Krebs solution (gassed with carbogen), at 36°C. The upper end of each preparation was connected to a Grass FTO3 force-displacement transducer via another silk thread. Preparations of epididymis were suspended at 0.30 g resting force and allowed at least 60 min to equilibrate. Recordings of contractile force were made using Graff FTO3 isometric transducers coupled to a Grass (model 79D) chart recorder. To minimize possible heterogeneity of the responses along the length of epididymis sections of tissue were randomly assigned within each experimental paradigm.
In preliminary studies (reversible permeabilization and incubation – but no antisense oligonucleotides), we found that responses to phenylephrine diminished with increasing length of incubation (a 72 h incubation almost completely abolished responses to phenylephrine, 10 μM). As a consequence we chose to undertake initial antisense oligonucleotide studies using a 48 h incubation.
Effect of agonist upon threshold responses to phenylephrine
We have used a methodology similar to that used in earlier studies (Haynes & Hill, 1996; Haynes et al., 1997; 1998a,1998b) such that the α2-adrenoceptor agonist, xylazine, the neurotransmitter, neuropeptide Y, and the A1 adenosine receptor agonist, CPA were all used to potentiate responses to low concentrations of the α1-adrenoceptor agonist, phenylephrine. Briefly, preparations of epididymis were set up for contractility studies, as described above. Prior to the addition of phenylephrine, one concentration of ATP (100 μM) or KCl (60 mM) was added to each preparation to ensure tissue viability. Following the washout of ATP or KCl preparations were allowed a further 15 min equilibration time. Phenylephrine (100 nM–10 μM) was added to each preparation to establish a small (threshold) contraction (generally 1 or 3 μM). Responses to the repeated addition of threshold concentrations of phenylephrine did not significantly change over time. Preparations were then washed and left for 13 min. The A1 adenosine receptor agonist, CPA, the neuropeptide Y receptor agonist, NPY, or the α2-adrenoceptor agonist, xylazine, were added 90 s prior to the next addition of phenylephrine. This protocol was repeated up to six times (with increasing concentrations of xylazine, NPY or CPA), each preparation receiving only one of these agonists or vehicle. Changes in the response to threshold concentrations of phenylephrine are shown as net increase in (mg) force.
Western blot analysis
Fresh or antisense oligonucleotide incubated tissues were homogenized (20 strokes in a dounce homogeniser) in (100 μl) ice-cold extract buffer (of composition, mM: Tris-HCl, 20; EGTA, 10; EDTA, 1; dithiothreitol, 1; leupeptin, 0.1; benzamidine, 1; phenylmethyl-sulphonylfluoride, 0.1) containing Triton X-100 (1%; v v−1) and soybean trypsin inhibitor (1 μg/ml) at pH 7.4. Membrane preparations were centrifuged (36,000×g for 15 min at 4°C) and the protein concentration determined by the method of Bradford (1976), and adjusted to (1 mg ml−1).
Protein samples were then heated to 95°C in SDS/PAGE sample buffer (0.5 M Tris-HCl, 10% v v−1 glycerol), 2% (w v−1) sodium dodecyl sulphate, 5% 2-mercaptoethanol, 0.05% (v v−1) bromophenol blue, pH 6.8). Protein samples (20 μg) were separated by SDS/PAGE (12% acrylamide gel) using the Bio-Rad Mini-Protean II system. Proteins were transferred to nitrocellulose membranes prior to the addition of blocking buffer (5% w v−1 low fat dried milk) in phosphate buffered saline, PBS, of composition (mM: 154 NaCl, 1.1 KH2PO4, 3.22 Na2HPO4) containing Tween 20 (0.1% v v−1) for 14 h at 4°C. Membranes were then incubated with primary anti-Giα1, α1/2 or α3 protein mouse antibodies (Transduction Laboratories, distributed by Affiniti Research Products Ltd, Exeter, U.K.) for 2 h at room temperature in blocking buffer. Blots were washed three times in washing buffer (PBS/0.1% (v v−1) Tween 20) and then incubated with secondary antibody (horseradish-peroxidase conjugated goat anti-mouse IgG, Fc specific, affinity isolated antibody, Sigma), in blocking buffer, for 1 h at room temperature. Excess antibody was removed with washing buffer before developing the blots using the Enhanced Chemiluminescence detection system (Amersham). Developed film was analysed with a BioRad GS690 imaging densitometer and Profile analyst II software (BioRad).
Statistics
Estimates of −log molar (p) EC50 or pIC50 were generated using a four-parameter logistic curve fitting and graphics program PRISM v2.0 (GraphPad Software Inc., San Diego, CA, U.S.A.). Comparison between concentration-response curves were determined by the use of an iterative curve fitting program, FLEXIFIT (see Guardabasso et al., 1988), significant changes were determined with an F-test. One-way ANOVA or the Student's t-test was used to determine changes between data points. In all cases P<0.05 was taken as the level of significance.
Drugs
Neuropeptide Y (NPY) was obtained from Calbiochem-Novabiochem Ltd, U.K. It was dissolved in 0.01 M acetic acid in H2O and stored at −20°C. On the day of use aliquots of peptide were diluted with modified Krebs buffer containing 0.1% bovine serum albumin. N6-cyclpentyladenosine (CPA) was obtained from Research Biochemicals (Natick, U.S.A.). All other drugs were obtained from the Sigma Chemical Co., U.K. CPA was dissolved in dimethyl sulfoxide and made up to volume in Krebs solution. Other drugs were made up in Krebs solution. Giα1, 2 and 3 antisense and mismatch oligonucleotides (10mer) were eventually supplied by TCS biologicals (Biognostik, GmbH), sequences are available upon request.
Results
Effects of phenylephrine and KCl
As in previous studies phenylephrine elicited concentration-dependent contractions of preparations of the epididymis of the guinea-pig (Haynes & Hill, 1996). Compared to fresh tissues, reversible permeabilization and incubation (48 h) reduced responses to both KCl (60 mM) and to phenylephrine (1 μM and 30 μM), see Table 1. The effects of reversible permeabilization only and reversible permeabilization with a 48 h incubation upon response to xylazine in phenylephrine (1 μM)-stimulated preparations are shown in Figure 1. Given the reduction in response to phenylephrine, seen following reversible permeabilization and 48 h incubation, we increased the concentration of phenylephrine used to elicit threshold responses (to 3 μM).
Table 1.
Effects of reversible permeabilization and 48 h incubation of preparations of epididymis upon response to KCl and to phenylephrine
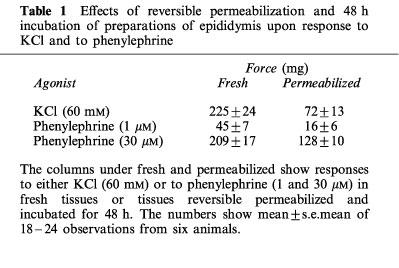
Figure 1.
Effects of reversible permeabilization (RP) and RP with incubation (48 h) upon xylazine concentration-response curves. Preparations were permeabilized or were both permeabilized and incubated (48 h) prior to the construction of xylazine concentration-response curves upon phenylephrine (3 μM)-stimulated preparations of epididymis. Bars represent s.e.mean (some omitted for clarity) of six experiments.
Effects of oligonucleotide incubation
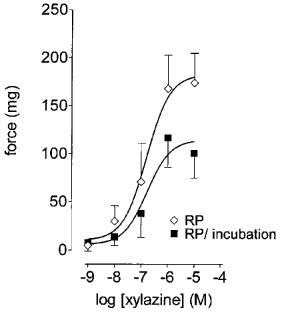
As has been shown previously, xylazine and CPA potentiate phenylephrine contractions, but do not elicit contractions in the absence of phenylephrine (Haynes & Hill, 1996; 1998a,1998b). For initial experiments, we tried a concentration of 10 μM Giα2 subunit mRNA antisense or mismatch oligonucleotides. Under these conditions the mean maximal potentiation of responses to phenylephrine by xylazine, but not by CPA, was significantly (P<0.05, F-test, d.f.=1, 85) reduced compared to mismatch controls (Figure 2a and b).
Figure 2.
Effects of Giα2 mRNA antisense or mismatch oligonucleotides upon response to xylazine or CPA in threshold (phenylephrine, 3 μM)-stimulated preparations of epididymis. (a) shows the effects of Giα2 antisense (AS) or mismatch (MM) oligonucleotides (10 μM) upon responses to xylazine. (b) shows the effects of Giα2 antisense (AS) or mismatch (MM) oligonucleotides (10 μM) upon responses to CPA. Bars represent s.e.mean (some omitted for clarity) of 9–12 experiments.
Responses to xylazine
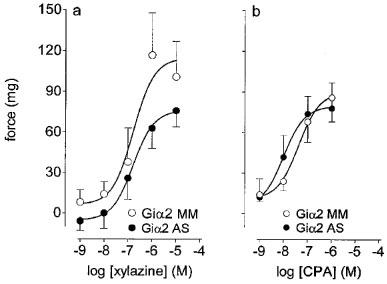
Compared to (Giα1 and Giα3) mismatch controls, the incubation of preparations with Giα1 or Giα3 antisense oligonucleotides (30 μM) had no significant effects upon the xylazine potentiation of phenylephrine-stimulated contractility (Figure 3a and c respectively). Compared to (Giα2) mismatch oligonucleotides (30 μM) the incubation of preparations with Giα2 antisense oligonucleotides (30 μM) significantly (P<0.05, F-test, d.f.=1, 55) reduced the maximal xylazine-potentiation of phenylephrine-stimulated contractility (Figure 3b).
Figure 3.

Effects of antisense mRNA or mismatch oligonucleotides upon responses to xylazine in threshold (phenylephrine, 3 μM)-stimulated preparations of epididymis. (a), (b) and (c) show the respective effects of Giα1, 2 or 3 antisense (AS) or mismatch (MM) oligonucleotides (30 μM) upon responses to xylazine. Bars represent s.e.mean (some omitted for clarity) of 6–12 experiments.
Responses to CPA
Compared with respective mismatch controls the incubation of preparations with Giα1, but not Giα2, Giα3 antisense oligonucleotides (all at 30 μM) significantly (P<0.05, F-test, d.f.=1, 91) inhibited the maximal CPA-potentiation of contractile responses to phenylephrine (Figure 4a, b and c).
Figure 4.
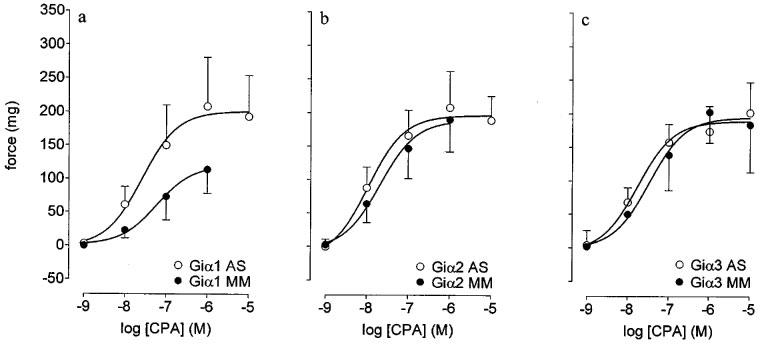
Effects of antisense mRNA or mismatch oligonucleotides upon responses to CPA in threshold (phenylephrine, 3 μM)-stimulated preparations of epididymis. (a), (b) and (c) show the respective effects of Giα1, 2 or 3 antisense (AS) or mismatch (MM) oligonucleotides (30 μM) upon responses to CPA. Bars represent s.e.mean (some omitted for clarity) of six experiments.
Responses to NPY
The incubation of preparations with Giα2 antisense mRNA oligonucleotides significantly (P<0.05, F-test, d.f.=1,55) reduced the maximal NPY-potentiation of responses to phenylephrine (to 62±15% of Giα2 mismatch control; Figure 5). Compared with Giα2 mismatch control the incubation of preparations with Giα1 and Giα3 oligonucleotides also significantly (P<0.05, F-test, d.f.=3,106) reduced the NPY-potentiation of responses to phenylephrine (Figure 5).
Figure 5.
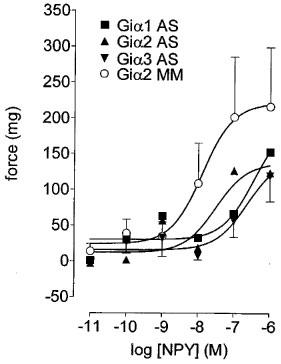
Effects of Giα1, 2 or 3 mRNA antisense (AS) or of Giα2 mismatch (MM) oligonucleotides upon responses to NPY in threshold (phenylephrine, 3 μM)-stimulated preparations of epididymis. Bars represent s.e.mean (some omitted for clarity) of six experiments.
Giα subunit determination
Western blotting confirmed that Giα1, 2 and 3 subunits were present in this preparation (Figure 6). We could not, however, show the abolition of Giα1, 2 and 3 subunit protein bands following respective antisense oligonucleotide incubations in preparations of epididymis (data not shown).
Figure 6.
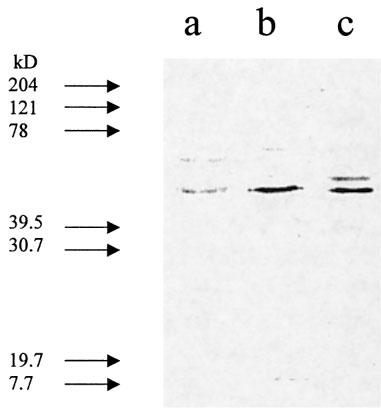
G-protein subunits in membrane fractions of the epididymis of the guinea-pig. Lanes a–c show the relative densities of Giα1, Giα1/2 and Giα3 subunit primary antibody bands in membrane preparations of the cauda epididymis. Each lane shows membrane bands of pooled tissues from six animals. →Indicates molecular weight standard markers.
Discussion
In previous studies we have shown that α2-adrenoceptor, A1 adenosine and NPY receptor agonists act at pre- and post-junctional sites to modulate contractility of the cauda epididymis of the guinea-pig. At the pre-junctional level these agonists inhibit neurotransmitter release (and electrically-evoked contractions), and at a post-junctional level they potentiate response to exogenously applied phenylephrine (Haynes & Hill, 1996; Haynes et al., 1997; 1998a,1998b). That the post-junctional potentiation of contractile responses by Gi/o-coupled receptor agonists is a receptor-selective event rather than a non-selective increase in tissue contractility is evident from the finding that A1 adenosine receptor agonists do not potentiate responses to ATP (Haynes et al., 1998a). Our earlier studies have also demonstrated distinct differences in the possible mechanisms of action and potentiation of phenylephrine-stimulated contractility. Thus α2-adrenoceptors and NPY Y1 receptors, but not A1 adenosine receptors, are significantly coupled to adenylate cyclase, and the α2-adrenoceptor, but not A1 adenosine receptor responses are attenuated by incubation of tissues with nifedipine (Haynes & Hill, 1996; Haynes et al., 1998b). In this study we show that the α2-adrenoceptors and the A1 adenosine receptors of the guinea-pig epididymis act preferentially through distinct G-protein subunits to elicit contractions.
Initially we incubated preparations of epididymis with Giα2 mRNA antisense or mismatch oligonucleotides (at 10 μM). These experiments indicated a selective action of Giα2 mRNA antisense oligonucleotides upon responses to xylazine. Increasing the concentration of Giα2 mRNA antisense oligonucleotide (to 30 μM) did not, however, substantially increase the effect of the oligonucleotide incubation. One possible explanation for this effect may be that, even after a 48 h post-antisense incubation, there is still enough Giα-subunit protein available to elicit effects. Alternatively it is possible that α2-adrenoceptors may elicit effects through the activation of other Giα subunits. We feel that the former explanation is more likely since there were still Giα1, 2 and 3 subunit protein bands present in Western blots, even after the incubation of tissues with Giα1, 2 and 3 subunit mRNA antisense oligonucleotides. Increasing the post-antisense oligonucleotide incubation to 72 h attenuated all contractile activity of preparations of epididymis. At present we feel that this effect may be more likely due to difficulties in keeping preparations of epididymis alive for 72 h, rather than any cytotoxic oligonucleotide effect. Given these findings we settled for a 30 μM Giα subunit mRNA antisense oligonucleotide or mismatch incubation with a 48 h, post-antisense oligonucleotide incubation.
One of the problems associated with antisense oligonucleotide studies has been the use of appropriate controls (for reviews see Wahlestedt, 1994; Kalkbrenner et al., 1996). The advantage of the present study, however, is that we are using agonists acting at three distinct receptor types. Thus we are able to report that Giα1, but not Giα2 or 3, mRNA antisense oligonucleotide or mismatch oligonucleotide incubation had no effect upon responses to xylazine, but significantly reduced responses to CPA. In contrast, responses to xylazine were sensitive to the Giα2 mRNA antisense oligonucleotide incubation but insensitive to Giα1 or Giα3 mRNA antisense or mismatch oligonucleotide incubations. These findings clearly indicate a Giα mRNA subunit-selective effect, rather than a non-specific loss of tissue contractility. In contrast with the A1 adenosine receptor and α2-adrenoceptor-mediated responses, epididymal Y1 receptors are sensitive to incubation with Giα1, 2 and 3 antisense oligonucleotides. At present our explanation for this finding is that the epididymal Y1 receptors promiscuously couple to all Giα protein subunits.
Although there are ongoing clinical trials using antisense oligonucleotide based therapies (see Akhtar and Agrawal, 1997), there are still relatively few studies showing antisense oligonucleotides effects in vitro. The purpose of this study was to use antisense oligonucleotides in an effort to discriminate the Giα subunit specificity of epididymal α2-adrenoceptor, A1 adenosine and NPY Y1 receptors. The results of our functional studies lead us to conclude that the α2-adrenoceptors and A1 adenosine receptors of the epididymis of the guinea-pig are predominantly coupled through Giα2 and Giα1 subunits respectively. In contrast NPY Y1 receptors may couple to either Giα1, 2 or 3 subunits. We conclude that the α2-adrenoceptors and A1 adenosine receptors of the cauda epididymis of the guinea-pig selectively couple through distinct G-protein α-subunits to elicit contractile responses of preparations. The evidence presented in this study may be consistent with our earlier findings (Haynes & Hill, 1996; Haynes et al., 1998b) that the α2-adrenoceptors, but not the A1 adenosine receptors of the cauda epididymis potentiate contractility through a mechanism involving the inhibition of adenylate cyclase.
Acknowledgments
This work was funded through the MRC (Grant reference G9408538).
Abbreviations
- CPA
cyclopentyladenosine
- DMEM
Dulbeccos modified Eagles medium
- MOPS
3-[N-morpholino]propanesulfonic acid
- NPY
neuropeptide Y
- TES
N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid
References
- AKHTAR S., AGRAWAL S. In vivo studies with antisense oligonucleotides. Trends Pharmacol. Sci. 1997;18:12–18. doi: 10.1016/s0165-6147(96)01002-4. [DOI] [PubMed] [Google Scholar]
- BIRO S., FU Y.M., YU Z.X., EPSTEIN S.E. Inhibitory effects of antisense oligodeoxynucleotides targeting c-myc mRNA on smooth muscle cell proliferation and migration. PNAS U.S.A. 1993;90:654–658. doi: 10.1073/pnas.90.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITAR K.N., ZHU X.X. Expression of bombesin-receptor subtypes and their differential regulation of colonic smooth muscle contraction. Gastroenterol. 1993;105:1672–1680. doi: 10.1016/0016-5085(93)91062-m. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytic Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E. The G-Protein nanomachine. Nature. 1996;379:297–299. doi: 10.1038/379297a0. [DOI] [PubMed] [Google Scholar]
- GUARDABASSO V., MUNSON P.J., RODBARD D. A versatile method for simultaneous analysis of families of curves. FASEB J. 1988;2:209–215. doi: 10.1096/fasebj.2.3.3350235. [DOI] [PubMed] [Google Scholar]
- HAYNES J.M., ALEXANDER S.P., HILL S.J. A1 and A2 adenosine receptor modulation of electrically-evoked contractions in the bisected vas deferens cauda epididymis of the guinea-pig. Br. J. Pharmacol. 1998a;124:964–970. doi: 10.1038/sj.bjp.0701909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES J.M., ALEXANDER S.P., HILL S.J. A1 and A2 adenosine receptor modulation of contractility in the cauda epididymis of the guinea-pig. Br. J. Pharmacol. 1998b;125:570–576. doi: 10.1038/sj.bjp.0702095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES J.M., HILL S.J. α-Adrenoceptor mediated responses of the cauda epididymis of the guinea-pig. Br. J. Pharmacol. 1996;119:1203–1210. doi: 10.1111/j.1476-5381.1996.tb16023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES J.M., HILL S.J., SELBIE L.A. Neuropeptide Y (NPY) and peptide YY (PYY) effects in the epididymis of the guinea-pig: evidence of a pre-junctional PYY-selective receptor. Br. J. Pharmacol. 1997;122:1530–1537. doi: 10.1038/sj.bjp.0701544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALKBRENNER F., DIPPEL E., WITTIG B., SCHULTZ G. Specificity of interaction between receptor and G protein: use of antisense techniques to relate G-protein subunits to function. Biochim. Biophys. Acta. 1996;1314:125–139. doi: 10.1016/s0167-4889(96)00072-9. [DOI] [PubMed] [Google Scholar]
- LESH R.E., SOMLYO A.P., OWENS G.K, SOMLYO A.V. A novel technique for the intracellular introduction of antisense oligonucleotides into intact smooth muscle. Circ. Res. 1995;77:220–230. doi: 10.1161/01.res.77.2.220. [DOI] [PubMed] [Google Scholar]
- SHIH M., MALBON C.C. Oligodeoxynucleotides antisense to mRNA encoding protein kinase A, protein kinase C, and beta-adrenergic receptor kinase reveal distinctive cell-type-specific roles in agonist-induced desensitization. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12193–12197. doi: 10.1073/pnas.91.25.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHLESTEDT C. Antisense oligonucleotide strategies in neuropharmacology. Trends Pharmacol. Sci. 1994;15:42–46. doi: 10.1016/0165-6147(94)90107-4. [DOI] [PubMed] [Google Scholar]


