Abstract
We examined effects of γ-aminobutyric acid (GABA) on vasoconstriction and noradrenaline (NA) release induced by electrical renal nerve stimulation (RNS) in the isolated pump-perfused rat kidney.
RNS (1 and 2 Hz for 2.5 min each, 0.5-ms duration, supramaximal voltage) increased renal perfusion pressure (PP) and renal NA efflux. GABA (3, 10 and 100 μM) attenuated the RNS-induced increases in PP by 10–40% (P<0.01) and NA efflux by 10–30% (P<0.01). GABA did not affect exogenous NA (40 and 60 nM)-induced increases in PP.
The selective GABAB agonist baclofen (3, 10 and 100 μM) also attenuated the RNS-induced increases in PP and NA efflux, whereas the RNS-induced responses were relatively resistant to the selective GABAA agonist muscimol (3, 10 and 100 μM).
The selective GABAB antagonist 2-hydroxysaclofen (50 μM), but not the selective GABAA antagonist bicuculline (50 μM), abolished the inhibitory effects of GABA (10 μM) on the RNS-induced responses.
The selective α2-adrenoceptor antagonist rauwolscine (10 nM) enhanced the RNS-induced responses. GABA (3, 10 and 100 μM) potently attenuated the RNS-induced increases in PP by 40–60% (P<0.01) and NA efflux by 20–50% (P<0.01) in the presence of rauwolscine.
Prazosin (10 and 30 nM) suppressed the RNS-induced increases in PP by about 70–80%. Neither rauwolscine (10 nM) nor GABA (10 μM) suppressed the residual prazosin-resistant PP response.
These results suggest that GABA suppresses sympathetic neurotransmitter release via presynaptic GABAB receptors, and thereby attenuates adrenergically induced vasoconstriction in the rat kidney.
Keywords: γ-aminobutyric acid (GABA), noradrenaline release, GABAB receptor, sympathetic nerves, rat kidney
Introduction
γ-Aminobutyric acid (GABA), one of the major inhibitory neurotransmitters in the central nervous system, has been found in several peripheral tissue (Erdö, 1985). GABA depolarizes myenteric neurons that contain acetylcholine (Cherubini & North, 1984), and increases spontaneous release and inhibits electrical stimulation-evoked release of acetylcholine from these neurons (Taniyama et al., 1983). GABA also suppresses the stimulation-evoked [3H]-noradrenaline (NA) release from the isolated rat atria (Bowry & Hudson, 1979) and goat cerebral artery (Miranda et al., 1989) without affecting basal release. These findings indicate that GABA can modulate peripheral neurotransmission.
The kidney has also been suggested as having a GABA system. It has been demonstrated that the kidney contains significant levels of GABA (Goodyear et al., 1980; 1982) and glutamate decarboxylase that synthesizes GABA from L-glutamic acid (Wu et al., 1978; Erdö, 1985; Erdö & Wolff, 1990). Specific binding sites for GABA have also been confirmed in the kidney (Amenta et al., 1988; Erdö, 1990). Moreover, it has been reported that 4-aminobutyrate aminotransferase that metabolizes GABA exists in tubular cell fractions prepared from the kidney (Goodyer et al., 1980), and that a high-affinity GABA uptake system exists in isolated brush border vesicles isolated from the rat kidney (Goodyer et al., 1985).
The renal sympathetic nervous system participates in the control of vascular tone and urine formation in the kidney (DiBona & Kopp, 1997). NA released from the nerve endings contracts arterioles and evokes tubular reabsorption through stimulation of α1-adrenoceptors. Thus the change in neural NA release is one of the major determinants for renal function. However, little is known of whether GABA is able to influence renal sympathetic nervous system.
The aim of this study is to clarify whether GABA modulates adrenergic neurotransmitter release in the kidney and, if so, which subtype of GABA receptors was responsible for the modulation by GABA. Electrical renal nerve stimulation (RNS) was applied in the isolated pump-perfused rat kidney, and the RNS-induced changes in noradrenaline (NA) efflux and perfusion pressure (PP) were determined before and during infusion of GABA in combination with a GABAA antagonist bicuculline or a GABAB antagonist 2-hydroxysaclofen. The effects of a GABAA agonist muscimol and a GAGAB agonist baclofen on the RNS-induced responses were also examined.
Methods
Preparation
The isolated pump-perfused rat kidney preparation was made according to the methods described by Rump & Majewski, (1987), Snel et al. (1995) and Mi & Jackson, (1995) with slight modifications. Male Wistar rats, weighing 250–350 g, were housed at 21–24°C and maintained on a standard diet and water ad libitum. Rats were anaesthetized with sodium pentobarbital (50 mg kg−1 i.p.), and the kidneys were exposed via a midline incision. The aorta was ligated just above the origin of the left renal artery, and a PE-50 cannula was immediately inserted into the renal artery through the aorta. The kidney was flushed for several minutes by infusion of oxygenated Tyrode's solution (3 ml min−1). The left kidney was carefully separated from surrounding tissue, and the left renal vein and the left ureter were cut. The kidney was then removed from the rat and immediately placed in a water-jacketed chamber, temperature of which was maintained at 37°C with thermostatically controlled water circulator (NTT-1200, EYELA, Tokyo, Japan). The right renal artery was cannulated via the mesenteric artery, and the right kidney was also isolated in a similar manner. The right kidney was placed in another set of the perfusion chamber and treated in the same way as the left kidney. The kidney was perfused with Tyrode's solution (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1.1, NaHCO3 12, NaH2PO4 0.42, D(+)-glucose 5.6, at a constant rate of 5 ml min−1 using a peristaltic pump (MP-3 N-H, EYELA). The Tyrode's solution was gassed with 95% O2 and 5% CO2 and pumped through a warming coil (37°C) fitted with a bubble trap. The perfusate passed through the kidney only once and was not recirculated. Bipolar platinum electrodes were placed around the renal artery to stimulate the renal nerves. One of the side arms of the perfusion system was connected to a pressure transducer (MPU-0.5, Nihon Kohden, Tokyo, Japan) for continuous monitoring of perfusion pressure (PP) with a carrier amplifier (AP-601G, Nihon Kohden) and a linear-writing pen recorder (SR6511, Graphtech Co., Yokohama, Japan). More than 1 h was allowed for stabilization before start of experiments. The kidneys were randomly divided into 12 experimental groups.
Drug infusion
Vehicle and drugs were infused into the perfusate at 0.05 ml min−1 through side arms of the perfusion system using motor-driven infusion pumps (model 100, KD Scientific, U.S.A.). Drug doses shown below are calculated concentrations in the perfusate.
Experimental protocols
Effects of GABA receptor agonists on RNS-induced vasoconstriction and NA efflux
RNS was applied at increasing frequencies of 1 and 2 Hz (duration, 0.5 ms; supramaximal voltage, 30–50 V) for 2.5 min each. The perfusate sample exiting the kidney was collected before starting RNS and during the last 30 s of each RNS for determination of renal NA efflux. A series of RNS and perfusate sampling was performed four times at 20-min intervals: one control period followed by three consecutive drug infusion periods. Infusion of vehicle (Group 1, n=6), GABA (3, 10 and 100 μM; Group 2, n=5), muscimol (3, 10 and 100 μM; Group 3, n=5) or baclofen (3, 10 and 100 μM; Group 4, n=5) were started 15 min before the start of RNS in the second to fourth experimental periods.
Effects of GABA receptor antagonists on RNS-induced vasoconstriction and NA efflux
A series of RNS and perfusate sampling was performed three times in the same manner as in Group 1 except that bicuculline (50 μM; Group 5, n=6) or 2-hydroxysaclofen (50 μM; Group 6, n=6) was infused in the second and third experimental periods and GABA (10 μM) was infused at the third experimental period.
Effects of GABA on RNS-induced vasoconstriction and NA efflux in the presence of rauwolscine
A series of RNS was performed four times in the same manner as in Group 1 except that rauwolscine was infused throughout the four experimental periods, beginning at 15 min before the first set of RNS and sampling. Infusion of vehicle (Group 7, n=5) or GABA (3, 10 and 100 μM; Group 8, n=6) were started 15 min before the start of RNS in the second to fourth experimental periods.
Effects of prazosin and prazosin plus rauwolscine on RNS-induced vasoconstriction
A series of RNS was performed three times in the same manner as in Group 1 except that prazosin (10 nM) was infused at the second experimental periods, and prazosin (30 nM; Group 9, n=5) or rauwolscine (10 nM; Group 10, n=6) was infused at the third experimental period.
Effects of GABA on RNS-induced vasoconstriction in the presence of prazosin
A series of RNS was performed three times in the same manner as in Group 1 except that prazosin (10 nM; Group 11, n=6) was infused in the second and third experimental periods and GABA (10 μM) was infused at the third experimental period.
Effects of GABA on NA-induced vasoconstriction
NA was infused at increasing doses of 40 and 60 nM for 5 min each. A series of infusion of NA was performed three times with 20-min intervals: one control period followed by two consecutive drug infusion periods. Infusion of GABA at 3 and 10 μM (Group 12, n=5) was started 15 min before the start of infusion of NA in the second to third experimental periods, respectively.
Measurement of renal NA efflux
Perfusate samples were transferred into chilled tubes. Catecholamines were extracted from perfusate by the alumina adsorption method, and NA concentration was determined by high-performance liquid chromatography with amperometric detector (LC-4C, Bioanalytical Systems, West Lafayette, IN, U.S.A.), as described previously (Hayashi et al., 1987). The NA efflux was calculated by multiplying the perfusate NA concentration by the perfusion rate.
Drugs
γ-aminobutyric acid (GABA; Wako, Osaka, Japan), muscimol, baclofen, (−)-norepinephrine hydrochloride, prazosin hydrochloride (Sigma Chemicals Co., U.S.A.), rauwolscine hydrochloride (Research Biochemicals International, U.S.A.), (−)-bicuculline methchloride (Research Biochemicals International, U.S.A.) and 2-hydroxysaclofen (Tocris Cookson, U.S.A.) were used. All drugs were dissolved in the Tyrode's solution.
Statistics
All values are expressed as means±s.e.mean. Basal values and the RNS-induced changes in PP and NA efflux in three or four experimental periods were compared using single factor analysis of variance for repeated measures, and Dunnett's test was applied to analyse statistical differences between the values obtained in the first experimental period and those in other experimental periods. Differences at P<0.05 were considered to be statistically significant.
Results
RNS elevated NA efflux (Groups 1–8, Figures 1, 2, 3 and 4) and PP (Groups 1–12, Figures 1, 2, 3, 4 and 5) in a frequency-dependent manner in the first experimental period of each group.
Figure 1.
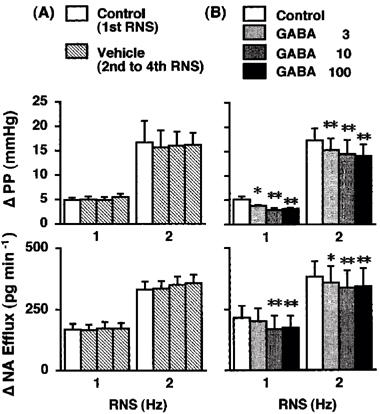
Effects of vehicle (A; Group 1, n=6) and GABA (B; Group 2, n=5) on the renal nerve stimulation (RNS)-induced changes in perfusion pressure (PP) and noradrenaline efflux (NA Efflux) in the isolated pump-perfused rat kidney. Δ, changes from basal values in response to RNS. Vehicle (Tyrode's solution) and GABA (3, 10 and 100 μM) were infused into the perfusate at 0.05 ml min−1. *P<0.05, **P< 0.01 compared with each control value.
Figure 2.
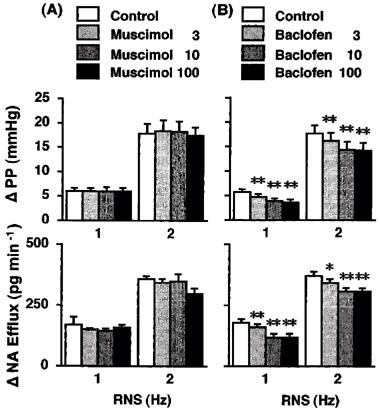
Effects of muscimol (A; Group 3, n=5) and baclofen (B; Group 4, n=5) on the renal nerve-stimulation (RNS)-induced changes in perfusion pressure (PP) and noradrenaline efflux (NA Efflux) in the isolated pump-perfused rat kidney. Δ, changes from basal values in response to RNS. Muscimol (3, 10 and 100 μM) and baclofen (3, 10 and 100 μM) were infused into the perfusate at 0.05 ml min−1. *P<0.05, **P<0.01 compared with each control value.
Figure 3.
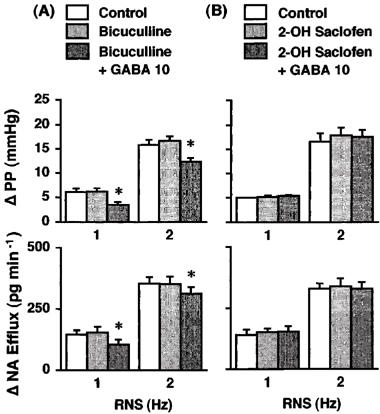
Effects of GABA on the renal nerve stimulation (RNS)-induced changes in perfusion pressure (PP) and noradrenaline efflux (NA Efflux) in the presence of bicuculline (A; Group 5, n=6) or 2-hydroxysaclofen (B; Group 6, n=6) in the isolated pump-perfused rat kidney. Δ, changes from basal values in response to RNS. Bicuculline (50 μM), 2-hydroxysaclofen (50 μM) and GABA (10 μM) were infused into the perfusate at 0.05 ml min−1. *P<0.01 compared with each control value.
Figure 4.
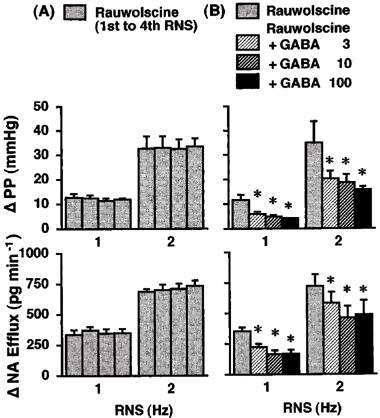
Effects of rauwolscine (A; Group 7, n=5) and rauwolscine plus GABA (B; Group 8, n=6) on the renal nerve stimulation (RNS)-induced changes in perfusion pressure (PP) and noradrenaline efflux (NA Efflux) in the isolated pump-perfused rat kidney. Δ, changes from basal values in response to RNS. Rauwolscine (10 nM) and GABA (3, 10 and 100 μM) were infused into the perfusate at 0.05 ml min−1. *P<0.01 compared with each value obtained before GABA infusion.
Figure 5.
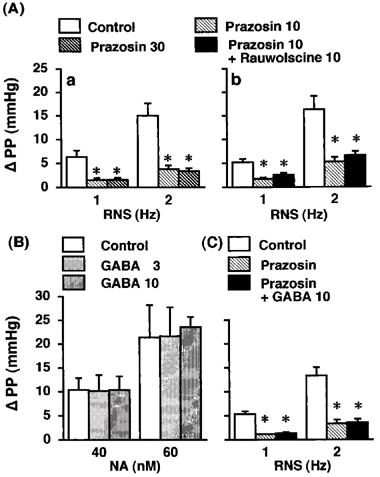
Effects of prazosin (A-a; Group 9, n=5), prazosin plus rauwolscine (A-b; Group 10, n=6), and prazosin plus GABA (C; Group 11, n=6) on the renal nerve stimulation (RNS)-induced changes in perfusion pressure (PP), and effects of GABA (B; Group 12, n=5) on exogenous noradrenaline (NA)-induced changes in PP in the isolated pump-perfused rat kidney. Δ, changes from basal values in response to RNS or exogenous noradrenaline (NA). Prazosin (Group 9, 10 and 30 nM), Groups 10 and 11, 10 nM), rauwolscine (10 nM) and GABA (Group 11, 10 μM; Group 12, 3 and 10 μM) were infused into the perfusate at 0.05 ml min−1. *P<0.01 compared with the control value.
The second, third and fourth application of RNS in the absence of drugs (during the vehicle infusion) produced PP and NA efflux responses by the same degree as those upon the first application of RNS (Group 1, Figure 1).
GABA at 3, 10 and 100 μM attenuated the 1-Hz RNS-induced increase in PP by 23±9% (P<0.05), 38±11% (P<0.01) and 34±10% (P<0.01) and the increase in NA efflux by 10±2%, 30±9% (P<0.01) and 25±7% (P<0.01), respectively, and the 2-Hz RNS-induced increase in PP by 11±2% (P<0.01), 18±5% (P<0.01) and 19±4% (P<0.01) and the increase in NA efflux by 7±2% (P<0.05), 14±5% (P<0.01) and 13±5% (P<0.01), respectively (Group 2, Figure 1).
Baclofen (3, 10 and 100 μM) also attenuated the RNS-induced increases in PP and NA efflux in a similar manner as GABA (Group 4, Figure 2). Muscimol (3, 10 and 100 μM) failed to affect the RNS-induced increases in PP and NA efflux except that the highest dose of muscimol (100 μM) tended to attenuate the NA efflux response to 2-Hz RNS (Group 3, Figure 2).
Neither bicuculline (50 μM; Group 5, Figure 3) nor 2-hydroxysaclofen (50 μM; Group 6, Figure 3) affected the RNS-induced increases in PP and NA efflux. In the presence of the selective GABAA antagonist bicuculline, GABA (10 μM) attenuated the 1-Hz RNS-induced increases in PP and NA efflux by 39±2% (P<0.01) and 24±3% (P<0.01), respectively, and the 2-Hz RNS-induced increases in PP and NA efflux by 21±5% (P<0.01) and 11±1% (P<0.01), respectively (Group 5, Figure 3). On the other hand, GABA failed to attenuate the RNS-induced responses in the presence of the selective GABAB antagonist 2-hydroxysaclofen (Group 6, Figure 3).
Rauwolscine (10 nM; Groups 7–8, Figure 4) enhanced the RNS-induced increases in PP and NA efflux; the responses were about two times higher than those obtained in the control periods of other experimental groups. The second, third and fourth application of RNS in the presence of rauwolscine produced PP and NA efflux responses by the same degree as those upon the first aplication of RNS (Group 7, Figure 4). The inhibitory effects of GABA on the RNS-induced increases in PP and NA efflux were more pronounced in the presence of rauwolscine; GABA at 3, 10 and 100 μM attenuated the 1-Hz RNS-induced increase in PP by 49±4% (P<0.01), 56±3% (P<0.01) and 60±4% (P<0.01) and the increase in NA efflux by 36±5% (P<0.01), 51±7% (P<0.01) and 51±5% (P<0.01), respectively, and the 2-Hz RNS-induced increases in PP by 36±5% (P<0.01), 42±5% (P<0.01) and 46±8% (P<0.01) and the increase in NA efflux by 17±2% (P<0.01, 35±9% (P<0.01) and 33±11% (P<0.01), respectively (Group 8, Figure 4).
Prazosin at 10 and 30 nM inhibited the 1-Hz RNS-induced increase in PP by 73±3% (P<0.01) and 74±3% (P<0.01) and the 2-Hz RNS-induced increases in PP by 74±3% (P<0.01) and 77±4% (P<0.01), respectively (Group 9, Figure 5A-a). In the presence of prazosin (10 nM), rauwolscine (10 nM; Group 10, Figure 5A-b) or GABA (10 μM; Group 11, Figure 5C) did not further suppress the RNS-induced increases in PP.
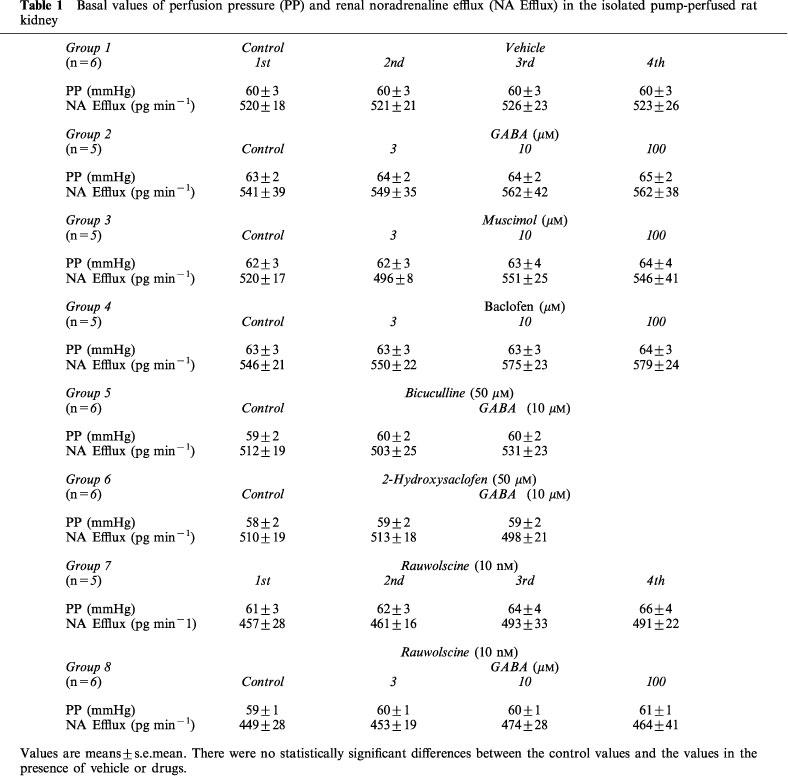
Exogenous NA (40 and 60 nM; Group 12, Figure 5B) elevated PP in a dose-dependent manner. GABA (3 and 10 μM) did not affect the NA-induced increase in PP (Group 12, Figure 5B). Neither the GABA agonists (Table 1), the GABA antagonists (Table 1) nor the α-adrenoceptor antagonists (data not shown) affected basal values of PP and NA efflux.
Table 1.
Basal values of perfusion pressure (PP) and renal noradrenaline efflux (NA Efflux) in the isolated pump-perfused rat kidney
Discussion
It has been suggested that GABA can modulate renal function (Monasterolo et al., 1996), but a role of GABA on the renal sympathetic neurotransmission remains unclear. In the present study, we examined the effects of GABA and GABA receptor agonists and antagonists on the adrenergically induced vasoconstriction and NA release in the isolated perfused rat kidney.
RNS at 1 and 2 Hz elevated renal PP in a frequency-dependent manner, which was suppressed with prazosin by 70–80%. Rauwolscine did not further suppress the RNS-induced increase in PP in the presence of prazosin. Thus, the RNS-induced PP response may be mediated predominately by α1-adrenoceptors. It was important to note that RNS also evoked frequency-dependent increases in renal NA efflux. The RNS-induced PP and NA efflux responses were reproducible when the series of RNS was applied four times in the absence of drugs.
GABA (3 and 10 μM) attenuated both the RNS-induced PP and NA efflux responses. GABA at the highest dose (100 μM) did not further suppress the RNS-induced responses, indicating that the maximal inhibitory effect (about 40 and 30% suppression of the PP and NA efflux responses, respectively) was obtained with 3–10-μM GABA. Exogenous NA (40 and 60 nM) elevated renal PP in a concentration-dependent manner which was also suppressed with prazosin by about 70% (data not shown). Since GABA at all doses failed to affect the increases in PP induced by exogenous NA, GABA is likely not to act on the postsynaptic site of the renal vasculature. Our present results suggest that GABA interferes with neural NA release and thereby attenuates vasoconstriction during activation of sympathetic nervous system in the kidney. It has been reported previously that GABA can suppress peripheral adrenergic neurotransmission in several kinds of vasculature such as the isolated goat cerebral artery (Miranda et al., 1989), rabbit pulmonary artery (Starke & Weitzell, 1980) and rabbit ear artery (Manzini et al., 1985). The present study is the first to demonstrate an inhibitory role of GABA in neurotransmission of the renal vasculature.
The sympathetic neurotransmitter release is controlled by an autoinhibition mechanism mediated by presynaptic α2-adrenoceptors (Langer, 1981). Cancellation of the α2-adrenoceptor-mediated autoinhibition would be expected to reveal an underlying inhibitory effect of GABA more clearly if the autoinhibition predominantly regulates neural NA release in the kidney. In this regard, we also examined the effects of GABA on the RNS-induced responses in the presence of an α2-adrenoceptor antagonist rauwolscine. Rauwolscine enhanced the RNS-induced increases in NA efflux and PP, while addition of GABA caused a marked suppression of the enhanced NA efflux (by 20–50%) and PP responses (by 40–60%). These results suggest that the inhibitory effect of GABA on NA release is blunted by the α2-adrenoceptor-mediated presynaptic autoinhibition mechanism.
Monasterolo et al. (1996) has reported that GABA increased basal values of fractional water, sodium and glucose excretion in the isolated perfused rat kidney. Their findings imply that GABA modulates urine formation by directly acting on the postsynpatic site of the renal tubules, since their experiments were performed in isolated kidneys that have little neural or hormonal input, and they did not apply the nerve stimulation. Because the renal sympathetic nervous system plays a major role in the control of tubular reabsorption (DiBona & Kopp, 1982) as well as of vascular tone, GABA would be expected to influence urine formation through its presynaptic inhibitory action on the renal tubular neurotransmission. Our present study could thus provide further evidence for the modulation by GABA of renal functions, although we did not measure changes in urine formation.
Kwan et al. (1996) have demonstrated that GABA suppresses electrical stimulation-evoked purinergic contraction of the isolated rat vas deferens in the presence of phentolamine. In our present study, prazosin even at the high dose (30 nM) failed to abolish the RNS-induced PP response; about 20–30% of the increases in PP still remained, and addition of rauwolscine failed to suppress this residual PP response. The RNS-induced renal neurotransmitter release and vasoconstriction may thus include non-adrenergic components. However, the residual PP response in the presence of prazosin was also resistant to GABA. It is therefore likely that GABA exclusively acts on the adrenergic component of the RNS-induced vasoconstriction in the kidney.
The actions of GABA are mediated by at least two distinct receptor types, GABAA and GABAB, in many kinds of peripheral tissue as well as in the central nervous system. GABA releases catecholamines from the isolated perfused adrenal medulla via GABAA receptors (González et al., 1992), suggesting that GABAergic mechanisms are involved in the regulation of adrenal medullary function. On the other hand, GABA has been reported to affect sympathetic neurotransmission by acting on presynaptic GABAB receptors in the rabbit pulmonary artery (Starke & Weitzell, 1980), the rabbit ear artery (Manzini et al., 1985) and the bovine ovarian follicle (Kannist et al., 1986). Binding sites for GABAA receptors (Amenta et al., 1988) and GABAB receptors (Erdö, 1990) have been confirmed to exist in the rat kidney. We therefore examined which type of GABA receptors might be responsible for the inhibition of NA release from the renal sympathetic nerve endings.
The selective GABAB agonist baclofen attenuated the RNS-induced increases in PP and NA efflux in a similar manner as GABA. On the other hand, the selective GABAA agonist muscimol did not substantially affect the RNS-induced responses. GABA suppressed the RNS-induced PP and NA efflux responses in the presence of the selective GABAA antagonist bicuculline, but failed to attenuate them in the presence of the selective GABAB antagonist 2-hydroxysaclofen. Together, these results suggest that the presynaptic inhibitory effect of GABA is mediated by GABAB receptors.
However, the highest dose (100 μM) of muscimol did slightly attenuate the NA efflux response to 2-Hz RNS. Muscimol at this dose might stimulate the presynaptic GABAB receptors, but muscimol (100 μM) has been shown to be unable to displace specific [3H] (−)-baclofen binding in the rat kidney (Erdö, 1990). Therefore, we cannot rule out the possibility that GABAA receptors also modulate the renal neurotransmission.
In conclusion, our present study suggests that GABA acts on the presynaptic GABAB receptors to suppress neurotransmitter release, and thereby attenuates renal vasoconstriction during activation of sympathetic nervous system in the rat kidney.
Abbreviations
- PP
perfusion pressure
- RNS
renal nerve stimulation
References
- AMENTA F., CAVALLOTTI C., IACOPINO L., ERDÖ S.L. Autoradiographic localization of the GABAA receptor agonist, 3H-muscimol within rat kidney. Pharmacology. 1988;36:390–395. doi: 10.1159/000138327. [DOI] [PubMed] [Google Scholar]
- BOWERY N.G., HUDSON A.L. α-Aminobutyric acid reduces the evoked release of 3H-noradrenaline from sympathetic nerve terminals. Br. J. Pharmacol. 1979;66:108P. [PMC free article] [PubMed] [Google Scholar]
- CHERUBINI E., NORTH R.A. Action of γ-aminobutyric acid on neurons of guinea-pig myenteric plexus. Br. J. Pharmacol. 1984;82:93–100. doi: 10.1111/j.1476-5381.1984.tb16445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIBONA G.F., KOPP U.C. Neural control of renal function. Physiol. Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- ERDÖ S.L. Peripheral GABAergic mechanisms. Trends Pharmac. Sci. 1985;6:205–208. [Google Scholar]
- ERDÖ S.L. Baclofen binding sites in rat kidney. Eur. J. Pharmacol. 1990;184:305–309. doi: 10.1016/0014-2999(90)90622-d. [DOI] [PubMed] [Google Scholar]
- ERDÖ S.L., WOLFF J.R. γ-Aminobutyric acid outside the mammalian brain. J. Neurochem. 1990;54:363–372. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- GONZÁLEZ M.P., OSET-GASQUE M.J., CASTRO E., BUGEDA J., ARCE C., PARRAMÓN M. Mechanism through which GABAA receptor modulates catecholamine secretion from bovine chromaffin cells. Neuroscience. 1992;47:487–494. doi: 10.1016/0306-4522(92)90263-2. [DOI] [PubMed] [Google Scholar]
- GOODYER P.R., LANCASTER G., VILLEENEUVE M., SCRIVER C.R. The relationship of 4-aminobutyric acid metabolism to aminoniagenesis in renal cortex. Biochem. Biophys. Acta. 1980;633:191–200. doi: 10.1016/0304-4165(80)90405-5. [DOI] [PubMed] [Google Scholar]
- GOODYER P.R., MILLS M., SCRIVER C.R. Properties of gamma-aminobutyric acid synthesis by rat renal cortex. Biochem. Biophys. Acta. 1982;716:348–357. doi: 10.1016/0304-4165(82)90027-7. [DOI] [PubMed] [Google Scholar]
- GOODYER P.R., ROZEN R., SCRIVER C.R. A gamma-aminobutyric acid-specific transport mechanism in mammalian kidney. Biochem. Biophys. Acta. 1985;818:45–52. doi: 10.1016/0005-2736(85)90136-1. [DOI] [PubMed] [Google Scholar]
- HAYASHI Y., HISA H., SATOH S. Role of prostaglandin in norepinephrine and renin release in canine kidney. Am. J. Physiol. 1987;253:F929–F934. doi: 10.1152/ajprenal.1987.253.5.F929. [DOI] [PubMed] [Google Scholar]
- KANNIST P., OWMAN C., SCHMIDT G., WALLES B. Evidence for prejunctional GABAB receptors mediating inhibition of ovarian follicle contraction induced by nerve stimulation. Eur. J. Pharmacol. 1986;122:123–129. doi: 10.1016/0014-2999(86)90167-6. [DOI] [PubMed] [Google Scholar]
- KWAN Y.W., NGAN M.-P., TSANG K.-Y., LEE H., CHU L.-A. Presynaptic modulation by L-glutamate and GABA of sympathetic co-transmission in rat isolated vas deferens. Br. J. Pharmacol. 1996;118:755–761. doi: 10.1111/j.1476-5381.1996.tb15464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER S.Z. Presynaptic regulation of the release of catecholamines. Pharmacol. Rev. 1981;32:337–362. [PubMed] [Google Scholar]
- MANZINI S., MAGGI C.A., MELI A. Inhibitory effect of GABA on sympathetic neurotransmission in rabbit ear artery. Arch. Int. Pharmacody. 1985;273:100–109. [PubMed] [Google Scholar]
- MI S., JACKSON E.K. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J. Pharmacol. Exp. Ther. 1995;273:728–733. [PubMed] [Google Scholar]
- MIRANDA F.J., TORREGROSA G., SALOM J.B., CAMPOS V., ALABADÍ J.A., ALBORCH E. Inhibitory effect of GABA on cerebrovascular sympathetic neurotransmisison. Brain Res. 1989;492:45–52. doi: 10.1016/0006-8993(89)90887-1. [DOI] [PubMed] [Google Scholar]
- MONASTEROLO L.A., TRUMPER L., ELÍAS M.M. Effects of γ-aminobutyric acid agonists on the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1996;279:602–607. [PubMed] [Google Scholar]
- RUMP L.C., MAJEWSKI H. Modulation of norepinephrine release through alpha 1- and alpha 2-adrenoceptors in rat isolated kidney. J. Cardiovasc. Pharmacol. 1987;9:500–507. doi: 10.1097/00005344-198704000-00016. [DOI] [PubMed] [Google Scholar]
- SNEL C.A., MOONS M.M., RUSSEL F.G., MULDER G.J. Disposition of the bromosulfophthalein-glutathione conjugate in the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1995;273:1300–1306. [PubMed] [Google Scholar]
- STARKE K., WEITZELL R. γ-Aminobutyric acid and postganglionic sympathetic transmission in the pulmonary artery of the rabbit. J. Auton. Pharmacol. 1980;1:45–51. doi: 10.1111/j.1474-8673.1980.tb00440.x. [DOI] [PubMed] [Google Scholar]
- TANIYAMA K., KUSONOKI M., SAITO N., TANAKA C. GABA evoked ACh release from isolated guinea-pig ileum. Life Sci. 1983;32:2349–2353. doi: 10.1016/0024-3205(83)90765-8. [DOI] [PubMed] [Google Scholar]
- WU J.-Y., CHUDE J., WEIN J., ROBERTS E. Distribution and tissue specificity of glutamate decarboxylase. J. Neurochem. 1978;30:849–857. doi: 10.1111/j.1471-4159.1978.tb10793.x. [DOI] [PubMed] [Google Scholar]


