Abstract
Selective activation of peripheral benzodiazepine receptors (PBRs) in adrenal cells and brain oligodendrocytes promotes steroidogenesis. Three 2-phenyl-imidazo[1,2-a]pyridine derivatives (CB 34, CB 50 and CB 54) have now been investigated with regard to their selectivity for PBRs and their ability to stimulate central and peripheral steroidogenesis in rats.
The three CB compounds (10−10–10−4 M) potently inhibited the binding of the PBR ligand [3H]-PK 11195 to brain and ovary membranes in vitro, without substantially affecting [3H]-flunitrazepam binding to central benzodiazepine receptors. These compounds (10−7–10−4 M) also had little or no marked effects on GABA-evoked Cl− currents in voltage-clamped Xenopus oocytes expressing human α1β2γ2S GABAA receptors. In addition, they failed to affect ligands binding to GABAB, D1/D2 dopamine, muscarinic acetylcholine, N-methyl-D-aspartic acid and opiate receptors.
Intraperitoneal administration of CB compounds (3–50 mg kg−1) induced a dose-dependent increase in the concentrations of neuroactive steroids in plasma and brain. The brain concentrations of pregnenolone, progesterone, allopregnanolone and allotetrahydrodeoxycorticosterone (THDOC) showed maximal increases in 96±3, 126±14, 110±12 and 70±13% above control, respectively, 30 to 60 min after injection of CB 34 (25 mg kg−1). CB 34 also increased the brain concentrations of neuroactive steroids in adrenalectomized-orchiectomized rats, although to a lesser extent than in sham-operated animals, suggesting that CB compounds stimulate brain steroidogenesis independently of their effects on peripheral tissues.
The increase in brain and plasma neurosteroid content induced by CB 34 was associated with a marked anticonflict effect in the Vogel test. Our results indicate that the three CB compounds tested are specific and potent agonists at peripheral benzodiazepine receptors, and that they stimulate steroidogenesis in both the brain and periphery.
Keywords: 6,8-disubstituted aminopyridines; peripheral benzodiazepine receptor; steroidogenesis; anxiety
Introduction
Certain natural and synthetic steroids such as pregnenolone, progesterone and alphaxalone, rapidly inhibit CNS excitability as a result of a stereoselective and high-affinity interaction with type A receptors for GABA, the major inhibitory neurotransmitter in brain (Majewska, 1992; Lambert et al., 1995). Depending on their concentration, the binding of such neuroactive steroids to GABAA receptors results in either potentiation of GABA-induced Cl− currents or a direct opening of the receptor-operated Cl− channel (Majewska et al., 1986; Harrison et al., 1987; Puia et al., 1990; Hill-Venning et al., 1994; Lambert et al., 1995), which may account for their anxiolytic, anticonvulsant, hypnotic and anaesthetic effects (Holzbauer et al., 1985; Crawley et al., 1986; Mendelson et al., 1987; Belelli et al., 1990; Bitran et al., 1991; Wieland et al., 1991; Kokate et al., 1994; Lambert et al., 1995; Concas et al., 1988).
The plasma and brain concentrations of neuroactive steroids can be increased as a result of selective activation of peripheral benzodiazepine receptors (PBRs) located in the inner mitochrondrial membrane of adrenal cells and brain oligodendrocytes, respectively (Papadopoulos, 1993). Thus, selective PBR ligands stimulate steroidogenesis in CNS and peripheral cells both in vitro and in vivo (Guarnieri et al., 1992; Papadopoulos et al., 1992; Romeo et al., 1992; Korneyev et al., 1993; McCauley et al., 1995). The observation that peripheral and central synthesis of neuroactive steroids can be modulated pharmacologically, together with the marked anxiolytic, anticonvulsant, sedative and hypnotic effects elicited by i.c.v. or i.p. injection of neuroactive steroids, has prompted a search for new and more selective PBR ligands that are able to stimulate brain and peripheral steroidogenesis with great efficacy.
Recently, a new class of compounds, 2-phenyl-imidazo[1,2-a]pyridine derivatives, some of which exhibit high affinity for the PBR, were prepared by reaction of the substituted 2-aminopyridines with bromoketoamides (Trapani et al., 1997). We have now investigated the effects of three of these compounds, termed CB 34, CB 50 and CB 54 (Figure 1), on brain and plasma neurosteroid concentrations in normal and adrenalectomized-orchietomized (ADX-ORX) rats. Using electrophysiological techniques we have also examined their effects on human recombinant GABAA receptors expressed in Xenopus oocytes and additionally determined the effects in a simple behavioural model of conflict-induced stress (the Vogel test).
Figure 1.

Structures of PK 11195 and of 2-phenyl-imidazo[1,2-a]pyridines.
Methods
Animals
Adult male or female Sprague-Dawley CD rats (Charles River, Como, Italy), with body masses of 200–250 g at the beginning of experiments, were maintained under an artificial 12 h light/dark cycle (light on 0800–2000 h) at a constant temperature of 23±2°C and 65% humidity. Food and water were freely available, and the animals were acclimatized for 7–10 days before use. Experiments were performed between 0800 and 1400 h. Adrenalectomy-orchiectomy was performed under chloral hydrate anaesthesia (400 mg per kilogram of body mass, i.p.), after which the animals received isotonic saline as drinking water for 14 days, until the day of experiments. Animal care and handling throughout the experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). The experimental protocol was approved by the Animal Ethical Committee of the University of Cagliari.
Drugs
For in vitro studies, PK 11195 (Sigma, Milan, Italy) and CB compounds were prepared in methyl sulphoxide and serially diluted in the appropriate buffer; control incubations received the same amount of solvent. For in vivo studies, PK 11195, CB compounds and diazepam were dissolved by adding one drop of Tween 80 per 5 ml of physiological saline and were administered intraperitoneally in a volume of 0.3 ml per 100 g of body mass.
Assay of [3H]-PK 11195 binding
After killing, the brain and ovaries were rapidly removed from rats, the cerebral cortex was dissected and both tissues were stored at −80°C until assayed. The tissues were thawed and homogenized in 50 volumes of Dulbecco's phosphate-buffered saline (PBS) (pH 7.4) at 4°C with a Polytron PT 10 (setting 5 for 20 s). The homogenate was centrifuged at 40,000×g for 30 min, and the resulting pellet was resuspended in 50 volumes of PBS and recentrifuged. The new pellet was resuspended in 20 (cerebral cortex) or 150 (ovary) volumes of PBS and used for the assay. [3H]-PK 11195 binding was determined in a final volume of 1000 μl, comprising 100 μl of membranes (0.15–0.20 mg of protein for cerebral cortex, 0.01–0.02 mg for ovary), 100 μl of [3H]-PK 11195 (85.5 Ci mmol−1; New England Nuclear) at a final assay concentration of 1 nM, 5 μl of drug solution or solvent, and 795 μl of PBS (pH 7.4 at 25°C). Incubations were performed at 0°C for 90 min, initiated by addition of membranes and terminated by rapid filtration through glass-fibre filter strips (Whatman GF/B) in a Cell Harvester filtration manifold (Brandel). The filters were each rinsed with two 4-ml volumes of ice-cold PBS, and filter-bound radioactivity was quantified by liquid scintillation spectrometry. Non-specific binding was defined as binding in the presence of 10 mM unlabelled PK 11195.
Assay of [3H]-flunitrazepam binding
Cerebral cortex was homogenized with a Polytron PT 10 in 50 volumes of ice-cold 50 mM Tris-HCl (pH 7.4), and the homogenate was centrifuged twice at 20,000×g for 10 min. The final pellet was reconstituted in 50 volumes of Tris-HCl buffer and used for the binding assay. [3H]-flunitrazepam binding was determined in a final volume of 1000 μl, comprising 400 μl of membrane suspension (0.4–0.5 mg of protein), 400 μl of Tris-HCl buffer, 100 μl of [3H]-flunitrazepam (7.4 Ci mmol−1; New England Nuclear), and 100 μl of drug solution or solvent. Incubations were performed for 60 min at 0°C, and were terminated by rapid filtration through glass-fibre filter strips (Whatman GF/B). The filters were then rinsed with ice-cold. Tris-HCl buffer and filter-bound radioactivity was quantitated by liquid scintillation spectrometry. Nonspecific binding was determined as binding in the presence of 5 μM diazepam, and represented about 10% of total binding.
Isolation of Xenopus oocytes and injection with cDNA
Complementary DNAs encoding the α1, β2, and γ2S subunits of human GABAA receptors were subcloned into the pCDM8 expression vector (Invitrogen, San Diego, CA, U.S.A.) (Hadingham et al, 1993). The cDNAs were purified with the use of a Wizard Miniprep kit (Promega), resuspended in distilled water and stored in portions at −20°C until injection. Stage V and VI oocytes were isolated from sections of Xenopus laevis ovary and exposed to collagenase type IV (Sigma) as described previously. A mixture of the three subunit cDNAs (0.5 ng each in 30 ml of total volume) was injected into the oocyte nucleus with a 10 μl glass micropipette (tip diameter, 10–15 μm), and the oocytes were then perfused at 20°C in modified Barth's solution [in mM: NaCl 88, KCl 1, NaHCO3 2.4, HEPES-NaOH (pH 7.5) 10, MgSO4 0.82 Ca(NO3)2 0.33, CaCl2 0.91] supplemented with sodium pyruvate (2 mM), penicillin (10,000 U l−1), streptomycin (10 U l−1), gentamicin (50 U l−1) and theophylline (0.5 mM). Oocytes were usually incubated for up to 4 days, during which time they were transferred to fresh incubation medium each day.
Electrophysiological recording
Electrophysiological recording from oocytes was initiated 1 day after cDNA injection and was performed as described previously. In brief, oocytes were placed in a rectangular recording chamber (volume, 100 μl) and continuously perifused with modified Barth's saline at a flow rate of 2 ml min−1 at room temperature. They were impaled at the animal pole with two microelectrodes (0.5–5 MΩ) filled with filtered 3 M KCl, and subjected to voltage clamp at −70 mV with an Axoclamp 2-B amplifier (Axon Instruments, Burlingame, CA, U.S.A.). Resting membrane potentials usually ranged between −30 and −50 mV. The oocytes were exposed to GABA in the absence or presence of drugs for 20 s. Intervals of 5 min were allowed between applications of low concentrations of GABA alone, and of at least 10 min when GABA was applied at higher concentrations or with other drugs.
Extraction and assay of steroids
Male rats were killed at the indicated times either by guillotine (for measurement of plasma steroids) or by focussed microwave irradiation (70 W cm−2 for 4 s) to the head (for measurement of brain steroids). This latter procedure results in a virtually instantaneous inactivation of brain enzymes (Mao et al., 1974), thus minimizing postmortem steroid metabolism. Brains were rapidly (<1 min) removed from the skull, and the cerebral cortices were dissected and then frozen at −20°C until steroid extraction. Steroids were extracted and purified as previously described (Barbaccia et al., 1996). Briefly, steroids present in cerebral cortical homogenates [400 mg of protein in 4 ml of PBS (pH 7.0)] were extracted three times with ethyl acetate, and the combined organic phases were dried under vacuum. The resulting residue was dissolved in 5 ml of n-hexame and applied to a SepPak silica cartridge (Waters), and components were eluted with n-hexane and 2-propanol (7:3, v v−1). Steroids were separated and further purified by HPLC on a 5 mm Lichrosorb-diol column (250×4 mm) (Phenomenex) with a discontinuous gradient of 2-propanol (0–30%) in n-hexane. Progesterone, which coelutes with cholesterol, was further purified by washing the corresponding dried HPLC fractions twice with 200 μl of dimethyl sulphoxide and water (400 μl). Progesterone was then extracted from the aqueous phase twice with 1.5 ml volumes of n-hexane. The recovery (70–80%) of steroids through the extraction and purification procedures was monitored by adding a trace amount (6000–8000 c.p.m.; 20–80 Ci mmol−1) of tritiated standard to the brain homogenate. Steroids were quantified by radioimmunoassay as previously described (Purdy et al., 1990; Barbaccia et al., 1994; 1996), with specific antibodies to pregnenolone, progesterone and corticosterone (ICN, Costa Mesa, CA, U.S.A.). Antibodies to allotetrahydrodeoxycorticosterone (THDOC) and to allopregnanolone were generated in rabbits and sheep, and characterized as previously described (Purdy et al., 1990).
Protein concentration was measured by the method of Lowry et al. (1951) with bovine serum albumin as standard.
Blood was collected from the trunk of killed rats into heparinized tubes and centrifuged at 900×g for 20 min at room temperature. The resulting plasma was frozen (−80°C) until assayed for steroids. Steroids were extracted from plasma with 1.5 ml of ethyl acetate.
Vogel's anticonflict test
Groups of 15–20 rats that had been habituated for 4–5 days to the handling manoeuvres that precede the anticonflict test were deprived of water for 24 h before the conflict session. The test was performed as previously described (Corda et al., 1983). Briefly, rats were placed in a clear plexiglass box (20×28×20 cm) with a stainless steel grid floor, and the box was enclosed in a sound-attenuated ventilated chamber (Lafayette Instruments, Lafayette, IN, U.S.A.). Water was provided through a stainless steel drinking tube that extended 1 cm into the box, 3 cm above the floor. The drinking tube and the grid floor were connected to a constant-current shock generator and a ‘drinkometer'. The shock generator delivered one shock (0.4 mA for 0.5 s) for each cumulative period of 15 licks; such a period of cumulative drinking was termed a ‘licking period'. Experiments were performed 25 min after administration of CB 34 or diazepam, rats were habituated to the test box for 5 min; the drinking tube was then inserted and the animals were allowed to lick for three licking periods (training period) before the onset of punishment.
Statistical analysis
Results are presented as means±s.e.mean. All data were analysed by one or two way analysis of variance (ANOVA). The data obtained studying the effect of CB compounds and PK 11195 on steroidogenesis were analysed comparing the effect of all compounds for each steroid. Individual means comparison were made using Newman-Keuls post test.
Results
Affinities of CB compounds for peripheral and central benzodiazepine receptors
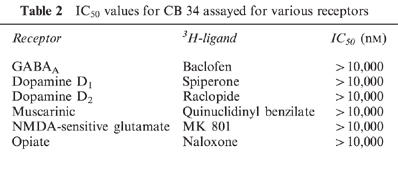
The affinities of the three CB compounds for central and peripheral benzodiazepine receptors were evaluated by measuring their ability to compete with [3H]-flunitrazepam and [3H]-PK 11195 for binding to membrane preparations from the cerebral cortex and ovary. Their effects were compared with those of unlabelled PK 11195, a selective ligand of the PBR (Le Fur et al., 1983). CB 34, CB 50 and CB 54 each showed a high affinity for PBRs in the cerebral cortex and ovary, and their potencies with brain membranes were greater than those with ovary membranes (Table 1). For both tissues, the rank order of potency was CB34>CB54>CB50. In contrast, CB 34 and CB 50 did not show any affinity for central benzodiazepine receptors labelled by [3H]-flunitrazepam; the IC50 values for CB 54 was 4±0.4 μM. In addition, the three imidazopyridines, at concentrations up to 10 mM, failed to displace [3H]-baclofen from GABAB receptors, [3H]-spiperone or [3H]-raclopide from dopamine receptors, [3H]-quinuclidinyl benzilate ([3H]-QNB) from muscarinic Ach receptors, [3H]-MK 801 from N-methyl-D-aspartate-sensitive glutamate receptors or [3H]-naloxone from opiate receptors in rat cortical membranes. The data obtained with CB 34 are presented in Table 2.
Table 1.
Potency of CB compounds in competing with [3H]-PK 11195 or [3H]-flunitrazepam for binding to membrane preparations from rat cerebral cortex or ovary
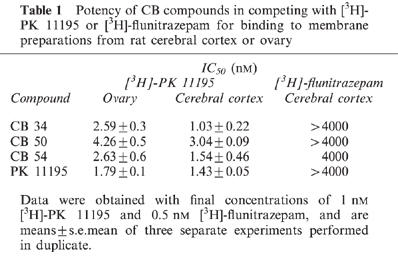
Table 2.
IC50 values for CB 34 assayed for various receptors
Electrophysiological effects of CB compounds at recombinant GABAA receptors
The effects of CB compounds on GABA-evoked Cl− currents in voltage-clamped Xenopus oocytes expressing human α1β2γ2S GABAA receptors were compared with those of PK 11195 and alprazolam, a central benzodiazepine receptor ligand (Figure 2). PK 11195 at concentrations of 0.1–100 μM had no significant effect on GABA-evoked Cl−1 currents. Alprazolam, 0.1–100 μM, significantly increased GABA-evoked currents. At 10 μM, CB 34 and CB 50 had no effect on the GABA response whereas CB 54 (10 and 30 μM) increased the GABA-evoked current by 33±7%. However, at 100 μM, CB 54 had no effect whereas CB 34 and CB 50 inhibited GABA-evoked currents by 20±8 and 29±10%, respectively. These inhibitory effects of CB 34 and CB 50 were insensitive to flumazenil (1 mM), a central benzodiazepine receptor antagonist (data not shown), suggesting that they were not mediated by central benzodiazepine recognition sites.
Figure 2.


Effects of PK 11195, CB compounds and alprazolam on GABA-evoked Cl− currents in Xenopus oocytes expressing human recombinant α1β2γ2S GABAA receptors. (A) Tracings were obtained from a single oocyte expressing human α1β2γ2S GABAA receptors and show the effect of the different CB compounds on GABA-evoked Cl− currents. Drugs were perfused together with GABA at the concentration of 3 μM for 20 s. (B) Data represent the per cent change in the control response to GABA at the EC20 (2–10 μM) induced by various concentrations of each test drug, and are means±s.e.mean for 4–7 different oocytes. aP<0.05, bP<0.01 vs control.
Effects of CB compounds on steroidogenesis
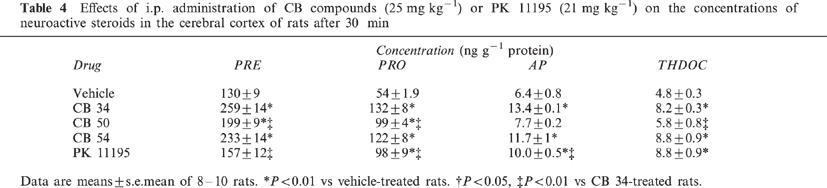
Intraperitoneal administration of CB compounds (25 mg kg−1) or an equimolar dose (21 mg kg−1) of PK 11195 in rats resulted in significant increases in the plasma concentrations of neuroactive steroids measured after 30 min (Table 3). The effects of CB 34 were the most marked, with increases in pregnenolone (F(4,35)=6.69), progesterone (F(4,35)=35.18), allopregnanolone (F(4,40)=23.27) and allotetrahydrodeoxycorticosterone (THDOC) (F(4,45)=29.71) of 85±10, 217±22, 150±15 and 123±10%, respectively. The three CB compounds also markedly increased the amounts of neuroactive steroids in the brain (Table 4). CB 34 increased the levels of pregnenolone (F(4,40)=23.4), progesterone (F(4,35)=21.36), allopregnanolone (F(4,45)=14.17) and allotetrahydrodeoxycorticosterone (THDOC) (F(4,35)=6.81) by 99±10, 144±14, 109±2 and 71±6%, respectively. CB 50 increased the cortical concentrations of pregnenolone (F(4,40)=23.4) and progesterone (F(4,35)=21.36) by 53±6 and 83±7%, respectively, but failed to significantly affect those of allopregnanolone (q=7.054) and THDOC (q=1.415). PK 11195 also increased the amounts of neuroactive steroids in the cerebral cortex (pregnenolone, +21±9% (F(4,40)=23.4); progesterone, +81±16% (F(4,35)=21.36); allopregnanolone, +56±8% (F(4,40)=14.17); THDOC, +83±18% (F(4,35)=6.81). All three CB compounds and PK 11195 increased the plasma concentration of corticosterone (F(34,35)=12.84), with CB 34 being most efficacious (Table 3).
Table 3.
Effects of i.p. administration of CB compounds (25 mg kg−1) or PK 11195 (21 mg kg−1) on plasma concentrations of neuroactive steroids in rats after 30 min
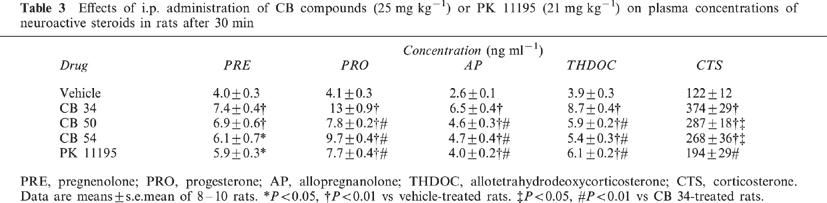
Table 4.
Effects of i.p. administration of CB compounds (25 mg kg−1) or PK 11195 (21 mg kg−1) on the concentrations of neuroactive steroids in the cerebral cortex of rats after 30 min
We next investigated the dose-response relationship and time course for the effects of CB 34 on the concentrations of neuroactive steroids in the cerebral cortex. CB 34 induced significant increases in the cortical concentrations of progesterone (+52%, F(5,54)=15.08), allopregnanolone (+20%, F(5,66)=26.03) and THDOC (+27%, F(5,54)=6.6), but not in that of pregnenolone (+14%, q=0.731), at a dose of 3 mg kg−1 (Figure 3). Higher doses (6 to 50 mg kg−1) induced dose-dependent increases in the brain concentrations of all four neuroactive steroids measured. The effects of CB 34 at a dose of 25 mg kg−1 were also time dependent (Figure 4). The increases in the cortical amounts of pregnenolone (+96%, F(4,55)=27.65), progesterone (+126%, F(4,45)=36.89), allopregnanolone (+110%, F(4,45)=24.69) and THDOC (+70%, F(4,45)=23.01) were maximal 30 or 60 min after drug administration, and thereafter returned to control values. Similar time courses were apparent for the CB 34-induced changes in the concentrations of these steroids in plasma (data not shown).
Figure 3.

Dose-response relations for the effects of CB 34 on the cerebrocortical concentrations of pregnenolone (A), progesterone (B), allopregnanolone (C) and THDOC (D). Rats were killed 30 min after i.p. injection of the indicated doses of CB 34. Data are means±s.e.mean of 10–12 rats. aP<0.05, bP<0.01 vs vehicle-treated animals.
Figure 4.

Time courses of the CB 34-induced changes in the cerebrocortical concentrations of pregnenolone (A), progesterone (B), allopregnanolone (C) and THDOC (D). Rats were killed at the indicated times after injection of CB 34 (25 mg kg−1, i.p.) or vehicle. Vehicle control bar presents the mean of neurosteroids level following injection of vehicle at 15, 30, 60 and 90 min. Data are means±s.e.mean of 10–12 rats. aP<0.01 vs vehicle-treated animals.
We also examined whether PK 11195, which acts as a partial agonist with antagonistic properties at the PBR (Le Fur et al., 1983; Benavides et al., 1984) could antagonise CB 34-induced steroidogenesis. Prior treatment (−10 min) with PK 11195 at a dose (40 mg kg−1) that per se significantly stimulated steroidogenesis by an extent similar to that for a lower dose (25 mg kg−1) of CB 34, prevented the CB 34-induced changes in the brain (Figure 5) and plasma (data not shown) concentrations of neuroactive steroids.
Figure 5.

Effects of pretreatment with PK 11195 on the CB 34-induced increases in the cerebrocortical concentrations of neuroactive steroids. Rats were injected with PK 11195 (40 mg kg−1, i.p.) 10 min before CB 34 (25 mg kg−1, i.p.), and were killed 30 min after CB 34 administration. Data are means±s.e.mean of 8–10 rats and are expressed as a percentage of the corresponding concentrations for vehicle-treated rats. aP<0.05, bP<0.01 vs vehicle-treated animals. cP<0.01 vs CB 34-treated animals.
Effects of CB 34 on brain neurosteroids in ADX-ORX rats
The determine whether the CB 34-induced increases in the cortical concentrations of neuroactive steroids are attributable to increased steroidogenesis in the brain or to an increased supply of steroids from peripheral sources, we examined the effects of CB 34 in ADX-ORX rats (Figure 6). CB 34 (25 mg kg−1) induced significant increases in the concentrations of pregnenolone (+45%) progesterone (+75%) and allopregnanolone (+55%) in the cerebral cortex of ADX-ORX rats after 30 min. However, these effects were substantially reduced relative to those apparent in sham-operated animals. CB 34 had no effect on the plasma concentrations of steroids in ADX-ORX rats (data not shown).
Figure 6.
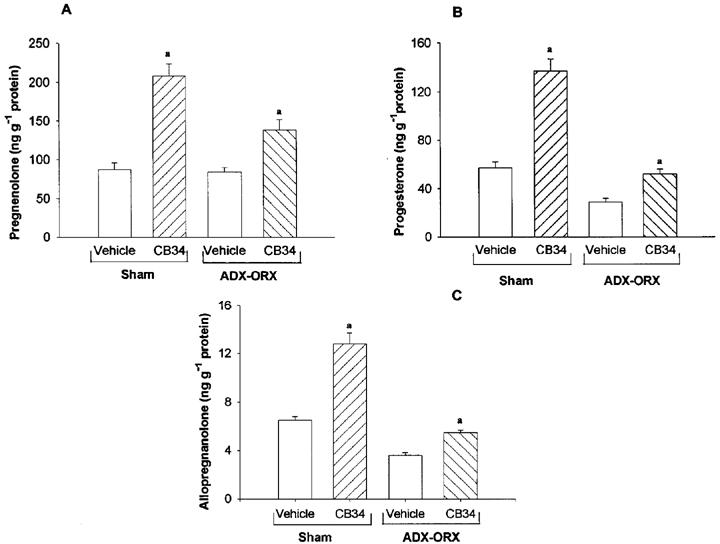
Effects of CB 34 on the concentrations of pregnenolone (A), progesterone (B) and allopregnanolone (C) in the cerebral cortex of ADX-ORX rats. ADX-ORX or sham-operated rats were injected with CB 34 (25 mg kg−1, i.p.) and killed after 30 min. Data are the means±s.e.mean of 8–10 rats. aP<0.01 vs respective vehicle-treated group.
Effect of CB 34 in Vogel's anticonflict test
Intraperitoneal administration of CB 34 elicited a dose-dependent anticonflict effect in rats. This effect was significant at doses of 25 and 50 mg kg−1, at which the number of licking periods during punishment was increased 3 and 4 fold, respectively (Figure 7). This effect was similar to that exerted by 0.5 mg kg−1 (i.p.) of diazepam. CB 34 had no effect on the number of licking periods under the nonpunishment condition.
Figure 7.
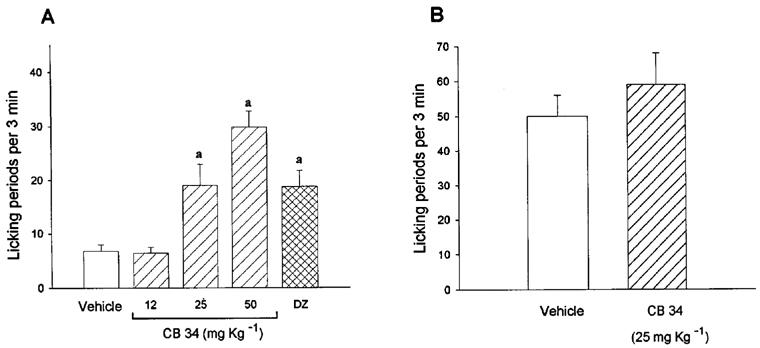
Effect of CB 34 in Vogel's anticonflict test. Rats were injected intraperitoneally with vehicle or the indicated dose of CB 34, or 0.5 mg of diazepam, 25 min before administration of the Vogel anticonflict test under punishment (A) or nonpunishment (B) conditions. Data are means±s.e.mean of 7–12 rats per group and represent the number of licking periods per 3 min. aP<0.01 vs respective vehicle-treated group.
Discussion
We have shown that the three 2-phenyl-imidazo[1,2-a]pyridine derivatives investigated in the present study are potent and selective ligands for PBRs and stimulate steroidogenesis in both the brain and periphery. These three compounds, CB 34, CB 50 and CB 54, competed with [3H]-PK 11195 for binding to membrane preparations of both the cerebral cortex and ovary. However, CB 34 and CB 50 showed no affinity, and CB 54 showed only a low affinity, for central benzodiazepine binding sites labelled by [3H]-flunitrazepam. These binding data, together with those of a previous study (Trapani et al., 1997), demonstrate that small changes on the pyridine moiety can influence receptor selectivity. Thus, the dichloro substituents at both the 6 and 8 positions of the pyridine moiety of CB 34 and CB 50 appear to confer high affinity and high selectivity for peripheral versus central benzodiazepine receptors. Indeed, the nature and position of the substituent on the pyridine nucleus seem to be key factor promoting PBR selectivity of these compounds. In fact, the bromo and methyl groups at 6 and 8 positions of CB 54 appear to confer some affinity for central benzodiazepine receptors. The respective potencies of CB 34, CB 50 and CB 54 in displacing [3H]-PK 11195 were similar for membranes prepared from cerebral cortex or ovary. In contrast, these compounds had no effect on the binding of ligands selective for GABAB, N-methyl-D-aspartate-sensitive glutamate, dopamine, ACh or opiate receptors.
Consistent with the binding data, CB 34 and CB 50, as well PK 11195, at concentrations of 10 μM, had minimal (⩽20%) effects on GABA-evoked Cl− currents in voltage-clamped Xenopus oocytes expressing human α1β2γ2S GABAA receptors. The lower efficacy of CB 54 in stimulating GABA-evoked Cl− currents with respect to alprazolam, suggests a partial agonist property of this compound. Moreover, the 33% increase in the GABA-evoked current induced by 10 μM CB 54 is consistent with the capability of this compound to interact with [3H]-flunitrazepam binding sites. In fact, the GABA-evoked Cl− currents measured with this oocyte expression system are very sensitive to the action of benzodiazepine receptor ligands, as revealed by the marked enhancement elicited by alprazolam, a full benzodiazepine receptor agonist. The inhibition of the Cl− currents by CB 34 and CB 50 at a concentration of 100 μM does not appear to be mediated by an interaction of these compounds with the central benzodiazepine recognition site, as demonstrated by the insensitivity of this action to the central benzodiazepine receptor antagonist flumazenil. This inhibitory effect is likely due to a direct interaction of CB 34 and CB 50 with the ‘Ro 5 4864 site' of the Cl− channel (Gee, 1987), which is thought to mediate the proconvulsant-convulsant action of Ro 5 4864 (Weissman et al., 1983; File et al., 1984).
CB 34, CB 50 and CB 54 each exhibited potent and marked effects on the peripheral and central synthesis of neuroactive steroids. CB 34 induced dose-dependent increases in the cortical concentrations of pregnenolone, progesterone, allopregnanolone and THDOC, with maximal effects of about 100% apparent around 30 min after i.p. administration. Surprisingly, allopregnanolone was elevated only at single time point (30 min), while its precursor (progesterone) was still high at 60 min. Since both allopregnanolone and THDOC are progesterone metabolites, it seems that after 30 min all progesterone is converted to THDOC. In fact, the levels of this steroid are, at 60 min, as high (+87%) as those measured at 30 min (+74%). A similar phenomenon was observed when the time course of the increase in steroid content was measured after CO2 inhalation (Barbaccia et al., 1996). In that case, the increase of allopregnanonolone lasted for 60 min, while the levels of DOC were increased at 30 but not at 60 min after stress. The increases in the brain concentrations of neuroactive steroids were associated with increases in the corresponding plasma concentrations. PK 11195 also induced significant increases in both the cortical and plasma concentrations of neuroactive steroids, consistent with previous studies showing a similar efficacy of PK 11195 in stimulating steroidogenesis in brain mitochondria and in Y1 cells (McCauley et al., 1995; Zisterer & Williams, 1997). In contrast, Korneyev et al. (1993) showed that PK 11195 is a specific blocker of PBR-mediated steroidogenesis but itself lacks steroidogenic activity. The later result may be due to the fact that in this study animals were killed 80 min after injection of PK 11195. In the present study, despite the apparently similar affinities of CB 34 and PK 11195 for the PBR revealed by the binding data, the potency and efficacy of PK 11195 in stimulating steroid synthesis were less than those for CB 34. Thus, a higher dose of PK 11195 (40 mg kg−1) was necessary to obtain a stimulation of steroid formation similar to that produced by CB 34 at 25 mg kg−1. Our observation that the administration of PK 11195 prior to CB 34 resulted in a reciprocal inhibition of their stimulatory effects on steroid biosynthesis is not readily explained. It is possible that these compounds bind to different conformational states of the PBR, or that the PK 11195 binding site partially overlaps or is allosterically coupled to the CB 34 binding site. Several studies support the existence of two distinct sites on the PBR, one for benzodiazepine derivatives (Ro 5 4864) and one for isoquinoline carboxamides (PK 11195) (Papadopoulos, 1993; Raghavendra & Butterworth, 1997).
The ability of CB 34 to stimulate the synthesis of neurosteroids in the brain of ADX-ORX rats demonstrates that this compound penetrates the blood–brain barrier and selectively interacts with the PBR in vivo, as well as in vitro. Consistent with previous observations (Robel & Baulieu, 1985; Baulieu, 1991), the basal concentration of pregnenolone in the brain was unchanged after castration and adrenalectomy, whereas the basal brain concentrations of progesterone and allopregnanolone were reduced by about 50% as a result of such surgery. Our observation that CB 34 increased the concentrations of neuroactive steroids in the brains of sham-operated rats to a greater extent than in the brains of ADX-ORX rats suggest that the increase in brain neurosteroid content in the former animals results, in part, from peripheral synthesis, but that this compound can also increase the amount of neurosteroids in the brain independently of peripheral sources. Our data (Tables 3 and 4; Figure 6) suggest that CB 34 stimulates steroidogenesis in the periphery to a greater extent than it does in the brain. Although higher affinity for the receptor site does not necessarily mean higher intrinsic activity, the 3 fold higher affinity of CB 34 for cortical versus ovarian [3H]-PK 11195 binding sites may have relevance for the increase of brain neurosteroid levels in ADX/ORX rats induced by this drug. On the other hand, this difference may also reflect the distribution of CB 34 into different compartments.
Allopregnanolone and THDOC are the most potent and efficacious of known endogenous positive allosteric modulators of GABAA receptors (Puia et al., 1990; Paul & Purdy, 1992), and they may play an important role in the control of emotional state. Thus, in vivo administration of allopregnanolone has a marked anticonflict effect and antagonizes the action of stress on the release of various neurotransmitters in freely moving rats (Crawley et al., 1986; Bitran et al., 1991; Wieland et al., 1991; Dazzi et al., 1996; Motzo et al., 1996). The marked increase in the brain content of these two neuroactive steroids induced by CB compounds might therefore be expected to reduce the effects of stress on neuronal excitability and associated behaviour. We have now shown that the administration of CB 34 (25 or 50 mg kg−1, i.p.) elicited an anticonflict action in rats, as demonstrated by an increase in the number of licking periods under the punishment condition of Vogel's test. This observation thus supports the notion that neurosteroids may play an important role in regulating emotional state (Purdy et al., 1991; Paul & Purdy, 1992; Biggio et al., 1996; Barbaccia et al., 1994; 1996; 1997) and suggest that PBR agonists might prove beneficial for the treatment of stress and anxiety-related diseases. It is possible that aberrant central or peripheral synthesis of neuroactive steroids may contribute to defects in neurotransmission that result in the variety of emotional and affective disorders sometimes associated with physiological conditions (such as the response to stress, pregnancy and the menstrual cycle) in which central and peripheral steroidogenesis is either enhanced or reduced (Barbaccia et al., 1994; 1996; 1997; Rapkin et al., 1997; Concas et al., 1998). Again, the availability of high-affinity PBR ligands, able to stimulate the production of neuroactive steroids with great selectivity and efficacy, may prove of benefit in the treatment of such disorders.
In conclusion, we have shown that a new class of PBR ligands, 6,8-disubstituted acetamidopyridines, stimulates both peripheral and central steroidogenesis in rats. Given the high affinity and selectivity of these compounds for PBRs, they may represent starting points for the synthesis of additional selective ligands with different affinities and intrinsic activities (antagonists and partial agonists) that may help to increase our understanding of the pharmacological and physiological roles of PBRs and neuroactive steroids.
Abbreviations
- ADX-ORX
adrenalectomized-orchiectomized
- PBR
peripheral benzodiazepine receptor
- PBS
phosphate-buffered saline
- THDOC
allotetrahydrodeoxycorticosterone
References
- BARBACCIA M.L., ROSCETTI G., TRABUCCHI M., CUCCHEDDU T., CONCAS A., BIGGIO G. Neurosteroids in the brain of handling-habituated and naive rats: effects of CO2 inhalation. Eur. J. Pharmacol. 1994;261:317–320. doi: 10.1016/0014-2999(94)90123-6. [DOI] [PubMed] [Google Scholar]
- BARBACCIA M.L., ROSCETTI G., TRABUCCHI M., MOSTALLINO M.C., CONCAS A., PURDY R.H., BIGGIO G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- BARBACCIA M.L., ROSCETTI G., TRABUCCHI M., PURDY R.H., MOSTALLINO M.C., CONCAS A., BIGGIO G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br. J. Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAULIEU E.E. Neurosteroids: a new function in the brain. Biol. Cell. 1991;17:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- BELELLI D., LAN N.C., GEE K.W. Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci. Biobehav. Rev. 1990;14:315–322. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- BENAVIDES J., BEGASSAT F., PHAN T., TUR C., UZAN A., RENAULT C., DUBROEUCQ M.C., GUEREMY C., LE FUR G. Histitine modification with diethylpyrocarbonate induces a decrease in the binding of an antagonist, PK 11195, but not of an agonist, Ro 5-4864, of the peripheral benzodiazepine receptors. Life Sci. 1984;35:1249–1256. doi: 10.1016/0024-3205(84)90095-x. [DOI] [PubMed] [Google Scholar]
- BIGGIO G., CONCAS A., MOSTALLINO M.C., PURDY R.H., TRABUCCHI M., BARBACCIA M.L.Inhibition of GABAergic transmission enhances neurosteroid concentrations in the rat brain The Brain: Source and Target for Sex Steroid Hormones 1996London: Parthenon; 43–62.Genazzani, A.R., Petraglia, F. & Purdy, R.H. (eds) [Google Scholar]
- BITRAN D., HILVERS R.J., KELLOGG C.K. Anxiolytic effect of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- CONCAS A., MOSTALLINO M.C., PORCU P., FOLLESA P., BARBACCIA M.L., TRABUCCHI M., PURDY R.H., GRISENTI P., BIGGIO G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONCAS A., SERRA M., ATSOGGIU T., TOFFANO G., BIGGIO G. Foot-shock stress and anxiogenic β-carbolines increase t-[35S]butylbicyclophosphorothionate binding in the rat cerebral cortex, an effect opposite to anxiolytics and γ-aminobutyric acid mimetics. J. Neurochem. 1988;51:1868–1876. doi: 10.1111/j.1471-4159.1988.tb01170.x. [DOI] [PubMed] [Google Scholar]
- CORDA M.G., BLAKER W.D., MENDELSON W.B., GUIDOTTI A., COSTA E. β-Carbolines enhance shock-induced suppression of drinking in rats. Proc. Natl. Acad. Sci. U.S.A. 1983;80:2072–2076. doi: 10.1073/pnas.80.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWLEY J.N., GLOWA J.R., MAJEWSKA M.D., PAUL S.M. Anxiolytic activity of endogenous adrenal steroids. Brain Res. 1986;339:382–386. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- DAZZI L., SANNA A., CAGETTI E., CONCAS A., BIGGIO G. Inhibition by the neurosteroid allopregnanolone of basal and stress-induced acetylcholine release in the brain of freely moving rats. Brain Res. 1996;710:275–280. doi: 10.1016/0006-8993(95)01478-0. [DOI] [PubMed] [Google Scholar]
- FILE S.E., GREEN A.R., NUTT D.J., VINCENT D.N. On the convulsant action of Ro 5 4864 and the existence of a micromolar benzodiazepine binding site in rat brain. Psychopharmacology. 1984;82:199–202. doi: 10.1007/BF00427773. [DOI] [PubMed] [Google Scholar]
- GEE K.W. Phenylquinolines PK 8165 and PK 9084 allosterically modulate [35S]t-butylbicyclophosphorothionate binding to a chloride ionophore in the brain via a novel Ro 5 4864 binding site. J. Pharmacol. Exp. Ther. 1987;240:747–753. [PubMed] [Google Scholar]
- GUARNIERI P., PAPADOPOULOS V., PAN B., COSTA E. Regulation of pregnenolone synthesis in C6-2B glioma cells by 4′-chlordiazepam. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5118–5122. doi: 10.1073/pnas.89.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P.B., WAFFORD K.A., BAIN C., KEMP P.A., PALMER K.J., WILSON A.W., WILCOX A.S., SIKELA J.M., RAGAN C.I., WHITING P.J. Role of β-subunits in determining the pharmacology of human γ-aminobutyric acid type A receptors. Mol. Pharmacol. 1993;44:1211–1218. [PubMed] [Google Scholar]
- HARRISON N.L., MAJEWSKA M.D., HARRINGTON J.W., BARKER J.L. Structure-activity relationships for steroid interaction with the γ-aminobutyric acidA receptor complex. J. Pharmacol. Exp. Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- HILL-VENNING C., BELELLI D., PETERS J.A., LAMBERT J.J. Electrophysiological studies of neurosteroid modulation of the γ-aminobutyric acid type A receptor. Neuroscience. 1994;22:446–467. [Google Scholar]
- HOLZBAUER M., BIRMINGHAM M.K., DE NICOLA A.F., OLIVER J.T. In vivo secretion of 3α-hydroxy-5α-pregnane-20-one, a potent anaesthetic steroid, by the adrenal gland of the rat. J Steroid Biochem. 1985;22:97–102. doi: 10.1016/0022-4731(85)90147-5. [DOI] [PubMed] [Google Scholar]
- KOKATE T.G., SVENSSON B.E., ROGAWSKI M.A. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J. Pharmacol. Exp. Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- KORNEYEV A., PAN B.S., POLO A., ROMEO E., GUIDOTTI A., COSTA E. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J. Neurochem. 1993;61:1515–1524. doi: 10.1111/j.1471-4159.1993.tb13647.x. [DOI] [PubMed] [Google Scholar]
- LAMBERT J.J., BELELLI D., HILL-VENNING G., PETERS J.A. Neurosteroids and GABAA receptor function. Trends Pharmacol. Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- LE FUR G., PERRIER M.L., VAUCHER N., IMBANT F., FLAMIER A., UZAN A., RENAULT C., DUBROEUCQ M.C., GUEREMY C. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 1983;32:1839–1847. doi: 10.1016/0024-3205(83)90062-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–276. [PubMed] [Google Scholar]
- MAJEWSKA M.D. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- MAJEWSKA M.D., HARRISON N.L., SCHWHARTZ R.D., BARKER J.L., PAUL S.M. Steroid hormones metabolites are barbiturate-like modulators of GABA receptors. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- MAO C.C., GUIDOTTI A., COSTA E. Interaction between γ-aminobutyric acid and guanosine cyclic 3′,5′-monophosphate in rat cerebellum. Mol. Pharmacol. 1974;10:736–745. [Google Scholar]
- MCCAULEY L.D., PARK C.H., LAN N.C., TOMICH J.M., SHIVELY J.E., GEE K.W. Benzodiazepines and peptides stimulate pregnenolone synthesis in brain mitchondria. Eur. J. Pharmacol. 1995;276:145–153. doi: 10.1016/0014-2999(95)00036-k. [DOI] [PubMed] [Google Scholar]
- MENDELSON W.B., MARTIN J.V., PERLIS M., WAGNER R., MAJEWSKA M.D., PAUL S.M. Sleep induction by adrenal steroids in the rat. Psychopharmacology. 1987;93:226–229. doi: 10.1007/BF00179939. [DOI] [PubMed] [Google Scholar]
- MOTZO C., PROCEDDU M.L., MAIRA G., FLORE G., CONCAS A., DAZZI L., BIGGIO G. Inhibition of basal and stress-induced dopamine release in the cerebral cortex and nucleus accumbens of freely moving rats by the neurosteroid allopregnanolone. J. Psychopharmacol. 1996;10:266–272. doi: 10.1177/026988119601000402. [DOI] [PubMed] [Google Scholar]
- PAPADOPOULOS V. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocrine Rev. 1993;14:222–240. doi: 10.1210/edrv-14-2-222. [DOI] [PubMed] [Google Scholar]
- PAPADOPOULOS V., GUARNIERI P., KRUEGER K.E., GUIDOTTI A., COSTA E. Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: regulation by a mitochondrial diazepam binding inhibitor receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5113–5117. doi: 10.1073/pnas.89.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL S.M., PURDY R.H. Neuroactive steroids. FASEB J. 1992;6:2311–2320. [PubMed] [Google Scholar]
- PUIA G., SANTI M.R., VICINI S., PRITCHETT D.B., PURDY R.H., PAUL S.M., SEEBURG P.H., COSTA E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- PURDY R.H., MORROW A.L., BLINN J.R., PAUL S.M. Synthesis, metabolism and pharmacological activity of 3-α-hydroxy-steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J. Med. Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- PURDY R.H., MORROW A.L., MOORE P.H., PAUL S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5453–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGHAVENDRA V.L., BUTTERWORTH R.F. Characterization of binding sites for the ω3 receptor ligands [3H]PK11195 and [3H]RO5-4864 in human brain. Eur. J. Pharmacol. 1997;340:89–99. doi: 10.1016/s0014-2999(97)01395-2. [DOI] [PubMed] [Google Scholar]
- RAPKIN A.J., MORGAN M., GOLDMAN L., BRANN D.W., SIMONE D., MAHESH V.B. Progesterone metabolite allopregnanolone in women withe premestrual syndrome. Obst. Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- ROBEL P., BAULIEU E.E. Neurosteroids: 3β-hydroxy-Δ5-derivatives in the rodent brain. Neurochem. Int. 1985;7:953–958. doi: 10.1016/0197-0186(85)90143-3. [DOI] [PubMed] [Google Scholar]
- ROMEO E., AUTA J., KOZIKOWSKI A.P., PAPADOPOULOS V., PUIA G., COSTA E., GUIDOTTI A. 2-Aryl-3-indoacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR) J. Pharmacol. Exp. Ther. 1992;262:971–978. [PubMed] [Google Scholar]
- SANNA E., MASCIA M.P., KLEIN R.L., WHITING P.J., BIGGIO G., HARRIS R.A. Actions of the general anesthetic propofol on recombinant human GABAA receptors: influence of receptor subunits. J. Pharmacol. Exp. Ther. 1995;274:353–360. [PubMed] [Google Scholar]
- TRAPANI G., FRANCO M., RICCIARDI L., LATROFA A., GENCHI G. , SANNA E., TUVERI F., CAGETTI E., BIGGIO G., LISO G. Synthesis and binding affinity of 2-phenylimidazo[1,2-a]pyridine derivatives for both central and peripheral benzodiazepine receptors. A new series of high-affinity and selective ligands for peripheral type. J. Med. Chem. 1997;40:3109–3118. doi: 10.1021/jm970112+. [DOI] [PubMed] [Google Scholar]
- WEISMANN B.A., COTT J., PAUL S.M., SKOLNICK P. Ro 5 4864: a potent benzodiazepine convulsant. Eur. J. Pharmacol. 1983;90:149–150. doi: 10.1016/0014-2999(83)90229-7. [DOI] [PubMed] [Google Scholar]
- WIELAND S., LAN N.C., MIRASEDEGHI S., GEE K.W. Anxiolytic activity of the progesterone metabolite 5α-pregnan-3α-o1-20-one. Brain Res. 1991;565:263–268. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]
- ZISTERER D.M., WILLIAMS D.C. Calmidazolium and other imidazole compounds affect steroidogenesis in Y1 cells: lack of involvement of the peripheral-type benzodiazepine receptor. J. Steroid Biochem. Mol. Biol. 1997;60:189–195. doi: 10.1016/s0960-0760(96)00189-6. [DOI] [PubMed] [Google Scholar]


