Abstract
The effect of intrastriatally-administered morphine on striatal dopamine (DA) release was studied in freely moving rats. Morphine (1, 10 or 100 μM) was given into the striatum by reversed microdialysis, and concentrations of DA and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were simultaneously measured from the striatal dialysates.
Intrastriatally-administered morphine significantly and dose-dependently decreased the extracellular concentration of DA, the concentrations of the acidic DA metabolites were only slightly decreased. The effect of morphine was antagonized by naltrexone (2.25 mg kg−1, s.c.). Pretreatment with a preferential κ-opioid receptor antagonist, MR2266 [(−)-5,9 alpha-diethyl-2-(3-furylmethyl)-2′-hydroxy-6,7-benzomorphane; 1 mg kg−1, s.c.], had no effect on the decrease of extracellular DA evoked by intrastriatal morphine (100 μM).
Intrastriatal administration of the selective μ-opioid receptor agonist [D-Ala2,MePhe4,Gly-ol5] enkephalin (DAMGO; 1 μM), significantly decreased the extracellular concentration of DA in the striatum.
When the rats were given morphine repeatedly in increasing doses (10–25 mg kg−1, s.c.) twice daily for 7 days and withdrawn for 48 h, the decrease of extracellular DA induced by morphine (100 μM) was significantly less than that seen in saline-treated controls.
Our results show that besides the well-known stimulatory effect there is a local inhibitory component in the action of morphine on striatal DA release in the terminal regions of nigrostriatal DA neurones. Tolerance develops to this inhibitory effect during repeated morphine treatment. Furthermore, our results suggest that the effect of intrastriatally-administered morphine is mediated by the μ-opioid receptors.
Keywords: Morphine, opioids, dopamine release, striatum, microdialysis, naltrexone, tolerance, MR2266, DAMGO, sensitization
Introduction
It is well known that systemic administration of opioids increases the synthesis, metabolism and release of dopamine (DA) in the striatum thereby increasing dopaminergic transmission. However, contrary to the increase of the acidic DA metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) we have repeatedly found that the post-mortem concentration of 3-methoxytyramine (3-MT), a metabolite considered to reflect the release of DA (for a review see Wood & Altar, 1988) tends to decrease in the striatum of rats or mice given relatively large doses of morphine (Ahtee et al., 1989; 1990; Airio & Ahtee, 1997). Morphine (Celsen & Kuschinsky, 1974; Kuschinsky et al., 1975; Marien et al., 1983; Westfall et al., 1983) and the selective μ-receptor agonist [D-Ala2, MePhe4, Gly-ol5]enkephalin (DAMGO) (Widdowson & Holman, 1992) have been found to decrease the stimulated release of DA from striatal slices as well. Furthermore, there is preliminary evidence that intrastriatally-administered morphine decreases the release of striatal DA in freely moving rats (Rossetti et al., 1990). Thus, it appears that morphine has a dual effect on striatal DA release: an inhibitory effect in the terminal regions and a stimulatory effect in the somatodendritic regions of the nigrostriatal dopaminergic neurones. To investigate the inhibitory effect of morphine on DA release, we administered morphine directly into the striatum by reverse dialysis through a microdialysis probe and simultaneously measured the extracellular concentrations of DA and its metabolites DOPAC and HVA. Indeed, we found that intrastriatal morphine dose-dependently and in a naltrexone-reversible manner reduced the extracellular concentration of DA in the striatum indicating a reduced release of DA.
Morphine is a relatively selective agonist for the μ-opioid receptors, but it also has weak affinity for the rat κ-opioid receptors (Chen et al., 1993; Meng et al., 1993). κ-Opioid agonists have been consistently shown to decrease DA release in striatal tissue preparations (Mulder et al., 1984; 1991; Schoffelmeer et al., 1988). Thus, theoretically it is possible that the effect of morphine on striatal DA release is mediated through κ-opioid receptors, because naltrexone, which was used to show that the effect of morphine was mediated by opioid receptors, has high affinity to both μ- and κ-opioid receptors (Meng et al., 1993; Zastawny et al., 1994), cannot differentiate these receptor types. Therefore, we tested whether κ-opioid receptors are involved in this effect by using the relatively selective κ-opioid receptor antagonist, MR-2266 (Bhargava et al., 1989; Clark et al., 1989). To further verify the involvement of the μ-opioid receptors in this effect, we tested whether the selective μ-receptor agonist, DAMGO, is able to decrease the extracellular concentration of DA when given intrastriatally.
Initially, in rats large doses of morphine induce behavioural depression and catalepsy, which are followed by behavioural stimulation (Ahtee, 1974; Babbini & Davis, 1972; Vasko & Domino, 1978). During repeated administration, tolerance develops to the sedative and cataleptic effects of morphine and simultaneously, the stimulatory effects are potentiated and stereotypies occur with increasing frequency (Ahtee, 1974; Babbini & Davis, 1972). The stereotypies induced by morphine and other μ-opioids in rats treated repeatedly with opioids have been linked to the activation of the striatal dopaminergic system (Morelli et al., 1989). Indeed, we have shown that the effect of morphine on striatal DA transmission is augmented during withdrawal from chronic morphine treatment (Ahtee et al., 1989; Attila & Ahtee, 1984; Honkanen et al., 1994). Therefore, we investigated whether tolerance develops to the inhibitory component of morphine on striatal DA release, which in turn might contribute to the sensitization of DA release in the withdrawal from repeated opioid treatment.
Methods
Animals
Male Wistar rats weighing 250–350 g were used in the experiments. They were kept under a 12 h light/dark cycle (lights on at 06 00 h) at an ambient temperature of 24±2°C. Rat chow and tab water were available ad libitum. The animal experiments were approved by the local institutional animals care and use committee and the chief veterinarian of the county administrative board, and were conducted according to the ‘European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes'.
Microdialysis
An I-shaped microdialysis probe (Santiago & Westerink, 1990) was implanted into the rat striatum (A+1.0, L+3.0, D−6.0; Paxinos & Watson, 1986) under pentobarbitone (60–80 mg kg−1, i.p.) anaesthesia. The dialysis tube was prepared from a polyacrylonitrile/sodium metasulphonate copolymer (Filtral 20; o.d/i.d. 310 : 220 μm; Hospal, France). The exposed tip of the dialysis membrane was 4 mm. After the surgery, the rats were placed individually in test cages and allowed to recover for approximately 40 h. On the morning of the experimental day, the probe was connected via polyethylene tubing to a 1 ml microsyringe and Ringer solution (in mM: NaCl 147, CaCl2 1.2, KCl 2.7, MgCl2 1.0, ascorbic acid 0.04) was infused (2 μl min−1) through the microdialysis probe with a microinjection pump. The samples (40 μl) were collected every 20 min. The basal output of DA and its metabolites was defined as an average of the first four stable samples after a stabilization period of 1 h, and was defined as 100%. The last baseline sample was collected at time point 0. It is the first sample used in the statistical analyses.
Study design
Immediately after the collection of the final baseline sample (time point 0), the intrastriatal perfusion medium was switched to a modified Ringer solution containing morphine (1, 10 or 100 μM) or DAMGO (0.3 or 1 μM). Control rats were perfused continuously with Ringer solution. The lag time for the Ringer solution containing morphine to reach the microdialysis probe in the brain through the inlet tubing was about 40 min. In addition, the outlet tubing introduced a lag time of about 20 min. Thus, the effects of morphine and DAMGO were first detected at time point 60 min. Indeed, the electrochemical signal induced by morphine in the microdialysis samples was detected first in the third microdialysis sample (time point 60 min).
To study the effects of opioid antagonists, the rats were administered subcutaneously either naltrexone (2.25 mg kg−1) or MR2266 (1 mg kg−1) immediately after the collection of the last baseline sample (time point 0). Control animals received corresponding volumes (2 ml kg−1) of saline s.c. The effect of opioid antagonists are corrected for the lag time of 20 min (outlet tubing).
To study the effect of repeated morphine administration, the rats were given morphine s.c. twice daily (at 08.00 and 18.00 h) for 7 days according to the following schedule: day 1: 10 and 10 mg kg−1; day 2: 15 and 10 mg kg−1; day 3: 15 and 15 mg kg−1; day 4: 20 and 15 mg kg−1; day 5: 20 and 20 mg kg−1; day 6: 25 and 20 mg kg−1; day 7: 25 and 25 mg kg−1. The control animals received saline s.c. using the same schedule. On the morning of day 8, a further dose of morphine (15 mg kg−1 to avoid respiratory depression during the surgery) was given. The rats were operated upon about 4 h after the last dose of morphine. The rats were thereafter withdrawn from repeated morphine or saline treatment for approximately 48 h, and then microdialysis samples were taken from the striatum and morphine (100 μM) was administered intrastriatally as described above.
Analytical procedure
Extracellular concentrations of DA and its metabolites DOPAC and HVA were measured by h.p.l.c. with electrochemical detection. Slightly different analytical procedures were used in the experiments with morphine and DAMGO, because relatively large concentrations of morphine in the perfusion fluid induce interferences with the analysis of DA when ordinary reverse phase columns are used.
Experiments with morphine
Thirty μl and 5 μl of the dialysate sample were injected for the analysis of DA and DOPAC/HVA, respectively, with a CMA/200 autoinjector (CMA, Stockholm, Sweden). The system for determining DA consisted of an ESA Coulochem 5100A detector (ESA Inc., MA, U.S.A.) equipped with a model 5014A analytical cell and a Pharmacia LKB model 2150 h.p.l.c. pump (Pharmacia LKB, Sweden) with a SSI model 20-0225 pulse damper (Scientific Systems Inc., PA, U.S.A.). The separation and quantification of DA was performed as described by Lagerqvist (1991). The column (Nucleosil SA 5 μm, 20 cm, i.d. 4 mm) was kept at 45°C with a Bio-Rad column heater. The mobile phase was 15/85 (v v−1) mixture of solutions A (citric acid 300 mM, NaOH 700 mM) and B (citric acid 75 mM, NaOH 175 mM, 30 v v−1 methanol) and contained 0.004% (w v−1) EDTA. The flow rate of mobile phase was 0.6 ml min−1.
The system for determining DOPAC and HVA consisted of a Waters 464 amperometric detector, a Beckman model 110B pump (Beckman Instruments Inc., U.S.A.) with a SSI model LP-21 pulse damper and a Spherisorb 5 μm 25 cm column. The mobile phase was prepared by mixing 0.1 M citric acid and 0.2 M Na2HPO4 in order to set the pH to 4.3. The mobile phase also contained 10% (v v−1) methanol and EDTA (0.2 mM) and the flow rate was 1.2 ml min−1.
Experiments with DAMGO
Twenty μl of the dialysate sample were injected into the chromatographic system. The column (Spherisorb ODS2, 3 μm, 4.6×100 mm) was kept at 40°C with a column heater (Croco-Cil, France). The mobile phase consisted of 0.5 M NaH2PO4 buffer, pH 4.0 (adjusted with 1.0 mM citric acid), octane sulphonic acid (1–2 mM), 16% (v v−1) methanol and 0.004% (w v−1) EDTA. The flow rate of the mobile phase was 1.0 ml min−1. DA was reduced with the amperometric detector (potential −80 mV) and DOPAC and HVA were oxidized with the coulometric detector (+300 mV).
In vitro recovery of the probes
In vitro recovery rates of DA, DOPAC and HVA through the membrane of the probe were determined by placing probes (n=3) in Ringer solution (22°C) containing 8 nM of DA and 400 nM of DOPAC and HVA. The probes were perfused with Ringer solution at a flow rate of 2 μl min−1. After a stabilization period of 2 h, six consecutive 20 min samples were collected and assayed as described above (experiments with morphine), and their mean concentrations of DA, DOPAC and HVA were determined (concentrations inside the dialysis membrane). To estimate the concentrations of DA, DOPAC and HVA outside the dialysis membrane, the same Ringer solution to which the probes had been added was also assayed, and the recoveries were calculated as the ratio of concentrations inside and outside the dialysis membrane.
The recovery rate of morphine was not analysed in detail. However, the peaks in the chromatograms identified to be morphine were approximately 12% smaller in the dialysates from rats receiving 100 μM of morphine intrastriatally than in the Ringer solution containing 100 μM of morphine, suggesting that the in vivo recovery of morphine was about 12%. The recovery rate of DAMGO was not analysed.
Drugs
Intrastriatally-administered morphine hydrochloride (supplied by the University Pharmacy, Helsinki, Finland) or DAMGO (Bachem, Switzerland) were dissolved in the Ringer solution. Subcutaneously-administered morphine hydrochloride, naltrexone hydrochloride (Sigma, MO, U.S.A.) and MR2266 [(−)-5,9 alpha-diethyl-2-(3-furymethyl)-2′-hydroxy-6,7-benzomorphane, generously supplied by Boehringer Ingelheim KG, Germany] were dissolved in 0.9% NaCl solution (saline). Doses of morphine, naltrexone and MR2266 refer to the base form.
Statistics
Statistical analysis was carried out using one-way analysis of variance (ANOVA) for repeated measures (0–220 min). When appropriate, multiple comparisons were conducted using the contrast analysis with Bonferroni levels. P values <0.05 were considered to be statistically significant. All results are presented as mean±s.e.mean.
Results
The basal levels of DA, DOPAC and HVA in the striatal dialysates were 132.6±7.2 fmol, 14.1±0.8 pmol and 10.0±0.5 pmol per 40 μl sample (in 20 min), respectively. In vitro experiments showed that the recovery rates of the probes for DA, DOPAC and HVA were 47.9±2.5, 35.5±2.3 and 28.5±1.8%, respectively.
Morphine dose-response
Intrastriatally-administered morphine dose-dependently decreased the extracellular concentration of DA in the striatum [Figure 1, Dose effect F(3, 16)=7.02, P<0.0032; Dose×Time interaction F(3, 33)=5.687, P<0.0001]. Multiple comparisons showed that only the largest dose (100 μM) of morphine significantly decreased (P<0.001) the concentration of DA in the dialysates (maximally by about 50%) as compared with the control. The decrease induced by 10 μM of morphine (maximally by about 30%) was not statistically significant (P<0.1) and 1 μM of morphine had no effect on the concentration of DA in the dialysates. The concentrations of DOPAC and HVA in the dialysates were not significantly decreased by morphine [Figure 2, Dose effect F(3, 16)=2.57, P<0.1; F(3, 16)=1.313, P>0.3 for DOPAC and HVA, respectively].
Figure 1.
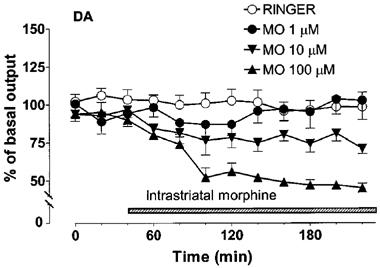
Effect of intrastriatal morphine (1, 10 or 100 μM) on the extracellular concentration of dopamine (DA) in the rat striatum. Morphine (MO) was adminstered intrastriatally via microdialysis probe instead of Ringer solution as shown by the shaded horizontal bar. All values are presented as percentages of the basal level±s.e.mean (n=5). Contrast analysis after analysis of variance for repeated measurements; 0–220 min: Morphine 100 μM vs Ringer P<0.001.
Figure 2.
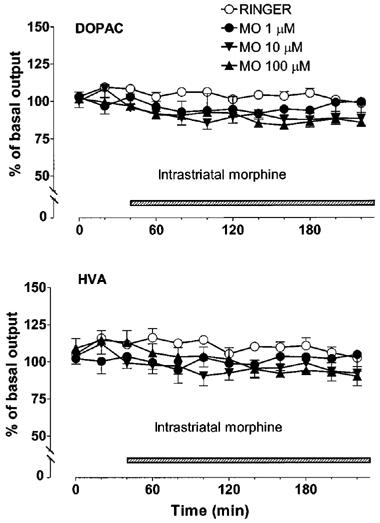
Effect of intrastriatal morphine (1, 10 or 100 μM) on the extracellular concentrations of DOPAC and HVA in the rat striatum. Morphine (MO) was administered intrastriatally via microdialysis probe instead of Ringer solution as shown by the shaded horizontal bar. All values are presented as percentages of the basal level±s.e.mean (n=5).
Effects of opioid antagonists
In rats pretreated with saline, intrastriatal morphine (100 μM) decreased the concentration of DA in the dialysate by about 40%. Pretreatment with naltrexone (2.25 mg kg−1, s.c., 40 min before morphine) completely prevented the effect of morphine on extracellular DA [saline+morphine vs naltrexone+morphine P<0.01, Figure 3]. The κ-opioid receptor antagonist, MR2266 (1 mg kg−1, s.c. 40 min before morphine), had no significant effect on the morphine-induced decrease of extracellular DA (Figure 4). Administration of naltrexone or MR2266 alone had no significant effect on striatal extracellular DA concentration.
Figure 3.
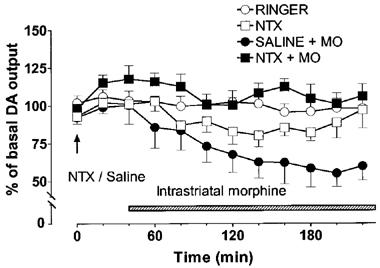
Effect of naltrexone (NTX, 2.25 mg kg−1) alone or in combination with intrastriatal morphine (100 μM) on the extracellular concentration of dopamine (DA) in the rat striatum. Morphine (MO) was administered intrastriatally via microdialysis probe instead of Ringer solution as shown by the shaded horizontal bar. Naltrexone or saline was given at the time indicated by the arrow. All values are presented as percentages of the basal level±s.e.mean (n=5–6). Contrast analysis after analysis of variance for repeated measurements; 0–220 min: Saline+morphine vs naltrexone+morphine P<0.01.
Figure 4.
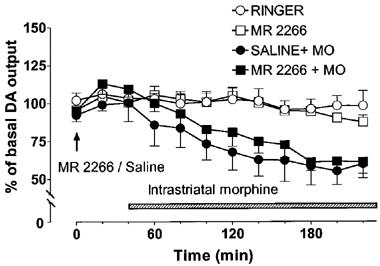
Effect of MR2266 (1 mg kg−1) alone or in combination with intrastriatal morphine (100 μM) on the extracellular concentration of dopamine (DA) in the rat striatum. Morphine (MO) was administered intrastriatally via microdialysis probe instead of Ringer solution as shown by the shaded horizontal bar. MR2266 or saline was given at the time indicated by the arrow. All values are presented as percentages of the basal level±s.e.mean (n=5–7).
Effects of DAMGO
Intrastriatally-administered DAMGO decreased the extracellular concentration of DA in the striatum [Figure 5, Dose effect F(2, 14)=4.25, P<0.0362; Dose×Time interaction F(2, 22)=3.581, P<0.0006]. The larger dose (1 μM) of DAMGO significantly decreased (P<0.05) the concentration of DA in the dialysates (maximally by about 40%) as compared with the control. The smaller dose (0.3 μM) of DAMGO had no effect on the concentration of DA in the dialysates. The larger dose of DAMGO slightly (by about 15%), but not significantly decreased the output of DOPAC, the output of HVA was not altered (data not shown).
Figure 5.
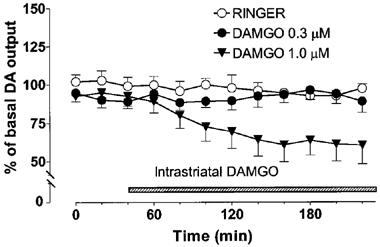
Effect of intrastriatal DAMGO (0.3 or 1 μM) on the extracellular concentration of dopamine (DA) in the rat striatum. DAMGO was administered intrastriatally via microdialysis probe instead of Ringer solution as shown by the shaded horizontal bar. All values are presented as percentages of the basal level±s.e.mean (n=5–6). Contrast analysis after analysis of variance for repeated measurements; 0–220 min: Ringer vs DAMGO 1 μM P<0.05.
Effects of repeated morphine administration
In rats treated repeatedly with saline (twice daily for 7 days), intrastriatal morphine (100 μM) decreased the output of DA by about 40% (Figure 6). In the rats withdrawn for 2 days from repeated morphine treatment (20–50 mg kg−1 daily for 7 days, see Methods), the reduction of DA levels evoked by intrastriatal morphine (100 μM) was significantly smaller (maximally by about 20%) than in the saline treated controls given morphine intrastriatally (repeated saline+morphine vs repeated morphine+morphine P<0.05).
Figure 6.
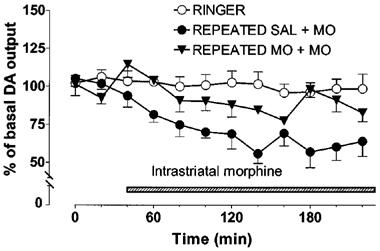
Effect of intrastriatal morphine (100 μM) on the extracellular concentration of dopamine (DA) in the striatum of rats withdrawn for 48 h from repeated saline (SAL) or morphine (10 to 25 mg kg−1, s.c. twice daily for 7 days) treatment. Morphine (MO) was administered intrastriatally via microdialysis probe instead of Ringer solution as shown by the shaded horizontal bar. All values are presented as percentages of the basal level±s.e.mean (n=5). Contrast analysis after analysis of variance for repeated measurements; 0–220 min: Repeated saline+morphine vs repeated morphine+morphine P<0.05.
Discussion
Although the main effect of opioids is to enhance nigrostriatal dopaminergic transmission (Ahtee, 1974; Ahtee & Attila, 1987; Di Chiara & Imperato, 1988; Kuschinsky & Hornykiewicz, 1974), there are indications (see Introduction) that opioids may also have inhibitory effects on this system. Some previous studies showed that acute systemic administration of opioids did not increase the release of nigrostriatal DA (Ahtee et al., 1989; 1990; Wood & Rao, 1991; Wood & Richard, 1982; Wood et al., 1980; Yonehara & Clouet, 1984). Also, in studies in which DA release was measured from striatal slices, it has been shown that μ-opioids somewhat retard the release of DA (Celsen & Kuschinsky, 1974; Kuschinsky et al., 1975; Loh et al., 1976; Schlosser et al., 1995; Widdowson & Holman, 1992). The results of the present study confirm that there is an inhibitory, naltrexone-sensitive component in the effect of opioids on striatal DA release. Furthermore, we found that tolerance develops to this inhibitory effect by repeated morphine administration.
Electrophysiological studies have shown that morphine increases the firing activity of nigrostriatal dopaminergic neurones (Gysling & Wang, 1983; Iwatsubo & Clouet, 1977; Matthews & German, 1984; Ostrowski & Caggiula, 1991). However, as discussed above, μ-opioids when administered locally seem to decrease striatal DA release in spite of the increased activity of dopaminergic neurones. When morphine is given systemically the predominant effect is enhanced release of DA, as the increased firing activity induced by a μ-opioid receptor mediated input at the dopaminergic cell somas can overcome this presynaptic ‘clamp'. However, when morphine is given directly into the striatum, the inhibitory effects predominate and the net effect on DA release is inhibition, as seen in our study. Indeed, Rossetti et al., (1990) reported that intrastriatally-administered opioid antagonist naloxone enhanced the effect of systemically administered morphine on striatal DA release.
In our study the output of the acidic DA metabolites, DOPAC and HVA, was not decreased by intrastriatal morphine or DAMGO. This could simply result from the fact that the decreased activity of dopaminergic neurones evoked by intrastriatal morphine increases the rate of DA synthesis (Roth et al., 1976; Walters & Roth, 1974). This increase in synthesis could lead to elevation of DA metabolites, DOPAC and HVA, in the dialysate. Indeed, local striatal administration of morphine increases the tissue concentration of DOPAC in the striatum (Moroni et al., 1979; Wood & Richards, 1982).
It is well documented that repeated administration of opioids results in development of both neurochemical and behavioural sensitization. This sensitization is more apparent in the mesolimbic dopaminergic system (for a review see Kalivas & Stewart, 1991), but the nigrostriatal system also sensitizes during repeated morphine treatment. Thus, during withdrawal from repeated morphine administration, acute challenge with morphine increases the release of DA considerably more than in naïve animals (Ahtee, 1974; Attila & Ahtee, 1984; Honkanen et al., 1994). Also behavioural effects of opioids linked to the activation of the nigrostriatal dopaminergic system undergo sensitization during repeated morphine administration (Ahtee, 1974; Babbini & Davis, 1972). However, the sensitization in these two systems seems to develop differently; at a molecular level, the alterations induced by repeated opioid administration are strikingly different in the somatodendritic regions of nigrostriatal and mesolimbic dopaminergic systems (Beitner-Johnson et al., 1992; Beitner-Johnson & Nestler, 1991). In addition, mesolimbic dopaminergic transmission can be sensitized by repeated administration of opioids in the somatodendritic region of this system (Vezina et al., 1987), whereas no such effect has been shown to occur in the nigrostriatal system. While we have now shown that tolerance develops to the inhibitory effect of morphine on nigrostriatal DA release, it is possible that the sensitization of this system results from the disappearance of the inhibitory component of morphine on DA release.
As relatively high concentrations of morphine were needed to decrease the release of DA in the striatum, it is possible that opioid receptor types other than μ are involved in this effect. δ-Opioid receptors are not likely to be involved since they have been shown to either increase striatal DA release in vivo (Dourmap & Costentin, 1994; Dourmap et al., 1992; Pentney & Gratton, 1991) and in vitro (Chesselet et al., 1982; Lubetzki et al., 1982; Widdowson & Holman, 1992), or to have no effect on striatal DA release (Mulder et al., 1984; Schoffelmeer et al., 1988). To our knowledge, there is only one study where δ-opioids were found to inhibit the release of striatal DA in vitro (Schlosser et al., 1995). Furthermore, the effect of intrastriatal morphine on striatal DA release was readily antagonized by naltrexone, the affinity of which for the cloned δ-opioid receptors is very poor (Yasuda et al., 1993). In contrast, the activation of κ-opioid receptors has consistently been shown to inhibit the release of striatal DA both in vivo (Di Chiara & Imperato, 1988) and in vitro (Mulder et al., 1984; 1991; Schoffelmeer et al., 1988). However, the fact that the inhibitory effect of morphine on DA release was not antagonized by the relatively selective κ-opioid antagonist, MR2266, does not support the involvement of κ-opioid receptors in this effect. Finally, we have now shown that similarly to morphine the selective μ-receptor agonist, DAMGO, when given intrastriatally decreases the release of DA in the striatum. This finding combined with the fact that the affinity of morphine for the cloned δ- and κ-opioid receptors is poor as compared with that for the μ-opioid receptors (Fukuda et al., 1993; Minami & Satoh, 1995; Raynor et al., 1994) seems to rule out the involvement of δ- and κ-opioid receptors in this effect of morphine.
The mechanism by which morphine locally inhibits the release of DA in the striatum remains obscure. The most straightforward explanation for this effect is that there are μ-opioid receptors on the nerve terminals of nigrostriatal dopaminergic neurones that mediate the inhibition of DA release. However, the inhibitory effect of μ-opioids on nigrostriatal DA release seems to be indirect involving other neurotransmitters locally in the striatum. Thus, recent studies have indicated that μ-opioid receptors are not located in the terminals of nigrostriatal dopaminergic neurones (Smith et al., 1993; Trovero et al., 1990; Waksman et al., 1987), although selective lesions of nigrostriatal dopaminergic neurones induce considerable loss (about 30%) of μ-opioid binding sites in the striatum (Bodnar et al., 1988; Eghbali et al., 1987; Smith et al., 1993). Striatal dopamine release is under the regulatory control of multiple excitatory and inhibitory neurotransmitters including glutamate, acetylcholine, dynorphins, and GABA (Cheramy et al., 1990; Ronken et al., 1993), and morphine might either activate the inhibitory mechanisms or inhibit excitatory mechanisms controlling DA release within the striatum. μ-Opioids have, indeed, been shown to presynaptically inhibit excitatory postsynaptic potentials, identified to be glutamatergic, in the striatum (Jiang & North, 1992). The involvement of indirect mechanisms in this effect is also supported by the fact that in none of the studies using striatal synaptosomes, which do not contain interneurones, was any effect of μ-opioids on DA release reported (Bosse & Kuschinsky, 1978; Clouet & Williams, 1974; Das et al., 1994; Kruk & Zarrindast, 1978; Ronken et al., 1993).
In conclusion, it appears that systemically-administered morphine simultaneously increases the release of striatal DA by activating dopaminergic neurones in the substantia nigra and presynaptically inhibits the release of DA in the striatum. When opioids are given systemically, their stimulatory effects on DA release predominate, but when μ-opioids are given directly to terminal areas of these neurones, it is the inhibitory effects that are predominant. Tolerance develops to the inhibitory effect of morphine on DA release, which might contribute to the sensitization of nigrostriatal dopaminergic transmission during repeated morphine administration.
Acknowledgments
This work was supported by grants from the Finnish Cultural Foundation, the University of Helsinki, the Emil Aaltonen Foundation and the Research Council for Health of the Academy of Finland.
Abbreviations
- DA
dopamine
- DAMGO
[D-Ala2,MePhe4,Gly-ol5]enkephalin
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MR2266
[(−)-5,9 alpha-diethyl-2-(3-furylmethyl)-2′-hydroxy-6,7-benzomorphane]
- 3-MT
3-methoxytyramine
- MO
morphine
- NTX
naltrexone
- SAL
saline
References
- AHTEE L. Catalepsy and stereotypies in rats treated with methadone; relation to striatal dopamine. Eur. J. Pharmacol. 1974;27:221–230. doi: 10.1016/0014-2999(74)90149-6. [DOI] [PubMed] [Google Scholar]
- AHTEE L., ATTILA L.M.J. Cerebral monoamine neurotransmitters in opioid withdrawal and dependence. Med. Biol. 1987;65:113–119. [PubMed] [Google Scholar]
- AHTEE L., ATTILA L.M.J., CARLSON K.R. Augmentation of morphine-induced changes in brain monoamine metabolism after chronic naltrexone treatment. J. Pharmacol. Exp. Ther. 1990;255:803–808. [PubMed] [Google Scholar]
- AHTEE L., ATTILA L.M.J., CARLSON K.R., HAIKALA H. Changes in brain monoamine metabolism during withdrawal from chronic oral self-administration of morphine and in response to a morphine challenge in the withdrawn state. J. Pharmacol. Exp. Ther. 1989;249:303–310. [PubMed] [Google Scholar]
- AIRIO J., AHTEE L. Role of cerebral dopamine and noradrenaline in the morphine-induced locomotor sensitisation in mice. Pharmacol. Biochem. Behav. 1997;58:379–386. doi: 10.1016/s0091-3057(97)00252-9. [DOI] [PubMed] [Google Scholar]
- ATTILA L.M.J., AHTEE L. Retardation of cerebral dopamine turnover after morphine withdrawal and its enhanced acceleration by acute morphine administration in rats. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;327:201–207. doi: 10.1007/BF00502450. [DOI] [PubMed] [Google Scholar]
- BABBINI M., DAVIS W.M. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Brit. J. Pharmacol. 1972;46:213–224. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEITNER-JOHNSON D., GUITART X., NESTLER E.J. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J. Neurosci. 1992;12:2165–2176. doi: 10.1523/JNEUROSCI.12-06-02165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEITNER-JOHNSON D., NESTLER E.J. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J. Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- BHARGAVA H.N., KREMER E.K., GIBBONS M.O., PHILIPS B.J., DRIVER J.W., CHOU M. Stereospecific effects of a kappa-opiate antagonist on the actions of morphine in morphine-tolerant rats. Eur. J. Pharmacol. 1989;173:159–164. doi: 10.1016/0014-2999(89)90513-x. [DOI] [PubMed] [Google Scholar]
- BODNAR R.J., CLARK J.A., COOPER M.L., PASTERNAK G.W. Loss of striatal mu1 opiate binding by substantia nigra lesions in the rat. Life Sci. 1988;43:1697–1700. doi: 10.1016/0024-3205(88)90480-8. [DOI] [PubMed] [Google Scholar]
- BOSSE A., KUSCHINSKY K. Potassium-induced release of 14C-dopamine from synaptosomes of corpus striatum of rats: effects of morphine. Arzneimittel-Forschung. 1978;28:2100–2102. [PubMed] [Google Scholar]
- CELSEN B., KUSCHINSKY K. Effects of morphine on kinetics of 14C-dopamine in rat striatal slices. Naunyn-Schmiedebergs Arch. Pharmakol. 1974;284:159–165. doi: 10.1007/BF00501120. [DOI] [PubMed] [Google Scholar]
- CHEN Y., MESTEK A., LIU J., YU L. Molecular cloning of a rat kappa opioid receptor reveals sequence similarities to the mu and delta opioid receptors. Biochem. J. 1993;295:625–628. doi: 10.1042/bj2950625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERAMY A., BARBEITO L., GODEHEU G., DESCE J.M., PITTALUGA A., GALLI T., ARTAUD F., GLOWINSKI J. Respective contributions of neuronal activity and presynaptic mechanisms in the control of the in vivo release of dopamine. J. Neural Transm. Supplementum. 1990;29:183–193. doi: 10.1007/978-3-7091-9050-0_18. [DOI] [PubMed] [Google Scholar]
- CHESSELET M.F., CHERAMY A., REISINE T.D., LUBETZKI C., GLOWINSKI J., FOURNIE-ZALUSKI M.C., ROQUES B. Effects of various opiates including specific delta and mu agonists on dopamine release from nigrostriatal dopaminergic neurons in vitro in the rat and in vivo in the cat. Life Sci. 1982;31:2291–2294. doi: 10.1016/0024-3205(82)90140-0. [DOI] [PubMed] [Google Scholar]
- CLARK J.A., LIU L., PRICE M., HERSH B., EDELSON M., PASTERNAK G.W. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J. Pharmacol. Exp. Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- CLOUET D.H., WILLIAMS N. The effect of narcotic analgesic drugs on the uptake and release of neurotransmitters in isolated synaptosomes. J. Pharmacol. Exp. Ther. 1974;188:419–428. [PubMed] [Google Scholar]
- DAS D., ROGERS J., MICHAEL-TITUS A.T. Comparative study of the effects of mu, delta and kappa opioid agonists on 3H-dopamine uptake in rat striatum and nucleus accumbens. Neuropharmacology. 1994;33:221–226. doi: 10.1016/0028-3908(94)90012-4. [DOI] [PubMed] [Google Scholar]
- DI CHIARA G., IMPERATO A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- DOURMAP N., COSTENTIN J. Involvement of glutamate receptors in the striatal enkephalin-induced dopamine release. Eur. J. Pharmacol. 1994;253:R9–R11. doi: 10.1016/0014-2999(94)90210-0. [DOI] [PubMed] [Google Scholar]
- DOURMAP N., MICHAEL-TITUS A., COSTENTIN J. Differential effect of intrastriatal kainic acid on the modulation of dopamine release by mu- and delta-opioid peptides: a microdialysis study. J. Neurochem. 1992;58:709–713. doi: 10.1111/j.1471-4159.1992.tb09775.x. [DOI] [PubMed] [Google Scholar]
- EGHBALI M., SANTORO C., PAREDES W., GARDNER E.L., ZUKIN R.S. Visualization of multiple opioid-receptor types in rat striatum after specific mesencephalic lesions. Proc. Natl. Acad. Sci. U.S.A. 1987;84:6582–6586. doi: 10.1073/pnas.84.18.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUDA K., KATO S., MORI K., NISHI M., TAKESHIMA H. Primary structures and expression from cDNAs of rat opioid receptor delta- and μ-subtypes. FEBS Lett. 1993;327:311–314. doi: 10.1016/0014-5793(93)81011-n. [DOI] [PubMed] [Google Scholar]
- GYSLING K., WANG R.Y. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- HONKANEN A., PIEPPONEN T.P., AHTEE L. Morphine-stimulated metabolism of striatal and limbic dopamine is dissimilarly sensitized in rats upon withdrawal from chronic morphine treatment. Neurosci. Lett. 1994;180:119–122. doi: 10.1016/0304-3940(94)90501-0. [DOI] [PubMed] [Google Scholar]
- IWATSUBO K., CLOUET D.H. Effects of morphine and haloperidol on the electrical activity of rat nigrostriatal neurons. J. Pharmacol. Exp. Ther. 1977;202:429–436. [PubMed] [Google Scholar]
- JIANG Z.G., NORTH R.A. Pre- and postsynaptic inhibition by opioids in rat striatum. J. Neurosci. 1992;12:356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALIVAS P.W., STEWART J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- KRUK Z.L., ZARRINDAST M.R. Effects of morphine on uptake and release of dopamine in mouse and rat striatal synaptosomes [proceedings] Brit. J. Pharmacol. 1978;63:386P. [PMC free article] [PubMed] [Google Scholar]
- KUSCHINSKY K., CELSEN B., HUPPERTZ A. Studies on dopamine kinetics in rat brain tissue under the influence of morphine. Int. J. Clin. Pharmacol. Biopharm. 1975;12:129–133. [PubMed] [Google Scholar]
- KUSCHINSKY K., HORNYKIEWICZ O. Effects of morphine on striatal dopamine metabolism: possible mechanism of its opposite effect on locomotor activity in rats and mice. Eur. J. Pharmacol. 1974;26:41–50. doi: 10.1016/0014-2999(74)90072-7. [DOI] [PubMed] [Google Scholar]
- LAGERQVIST S.Sample splitting provides a fast and selective method for determining brain dialysate dopamine and its metabolites Monitoring molecules in neuroscience 1991Groningen: University Centre for Pharmacy; 136–138.eds. Rollema, H., Westerink, B.H.C. & Drifthout, W.J. pp [Google Scholar]
- LOH H.H., BRASE D.A., SAMPATH-KHANNA S., MAR J.B., WAY E.L., LI C.H. beta-Endorphin in vitro inhibition of striatal dopamine release. Nature. 1976;264:567–568. doi: 10.1038/264567a0. [DOI] [PubMed] [Google Scholar]
- LUBETZKI C., CHESSELET M.F., GLOWINSKI J. Modulation of dopamine release in rat striatal slices by delta opiate agonist. J. Pharmacol. Exp. Ther. 1982;222:435–440. [PubMed] [Google Scholar]
- MARIEN M., BRIEN J., JHAMANDAS K. Regional release of [3H]dopamine from rat brain in vitro: effects of opioids on release induced by potassium, nicotine, and L-glutamic acid. Can. J. Physiol. Pharmacol. 1983;61:43–60. doi: 10.1139/y83-005. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R.T., GERMAN D.C. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- MENG F., XIE G.X., THOMPSON R.C., MANSOUR A., GOLDSTEIN A., WATSON S.J., AKIL H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMI M., SATOH M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci. Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- MORELLI M., FENU S., DI CHIARA G. Substantia nigra as a site of origin of dopamine-dependent motor syndromes induced by stimulation of mu and delta opioid receptors. Brain Res. 1989;487:120–130. doi: 10.1016/0006-8993(89)90947-5. [DOI] [PubMed] [Google Scholar]
- MORONI F., PERALTA E., CHENEY D.L., COSTA E. On the regulation of gamma-aminobutyric acid neurons in caudatus, pallidus and nigra: effects of opioids and dopamine agonists. J. Pharmacol. Exp. Ther. 1979;208:190–194. [PubMed] [Google Scholar]
- MULDER A.H., BURGER D.M., WARDEH G., HOGENBOOM F., FRANKHUYZEN A.L. Pharmacological profile of various kappa-agonists at kappa-, mu- and delta-opioid receptors mediating presynaptic inhibition of neurotransmitter release in the rat brain. Brit. J. Pharmacol. 1991;102:518–522. doi: 10.1111/j.1476-5381.1991.tb12203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER A.H., WARDEH G., HOGENBOOM F., FRANKHUYZEN A.L. Kappa- and delta-opioid receptor agonists differentially inhibit striatal dopamine and acetylcholine release. Nature. 1984;308:278–280. doi: 10.1038/308278a0. [DOI] [PubMed] [Google Scholar]
- OSTROWSKI N.L., CAGGIULA A.R. Correlation between locomotor stimulation and the electrophysiological effects of low doses of morphine on substantia nigra dopamine neurons. I. Acute drug administration. J. Pharmacol. Exp. Ther. 1991;257:72–81. [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotaxic coordinates 1986Academic Press, San Diego; 2nd edn [Google Scholar]
- PENTNEY R.J., GRATTON A. Effects of local delta and mu opioid receptor activation on basal and stimulated dopamine release in striatum and nucleus accumbens of rat: an in vivo electrochemical study. Neuroscience. 1991;45:95–102. doi: 10.1016/0306-4522(91)90106-x. [DOI] [PubMed] [Google Scholar]
- RAYNOR K., KONG H., CHEN Y., YASUDA K., YU L., BELL G.I., REISINE T. Pharmacological characterization of the cloned kappa-, delta- and mu-opioid receptors. Mol. Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- RONKEN E., MULDER A.H., SCHOFFELMEER A.N. Interacting presynaptic kappa-opioid and GABAA receptors modulate dopamine release from rat striatal synaptosomes. J. Neurochem. 1993;61:1634–1639. doi: 10.1111/j.1471-4159.1993.tb09797.x. [DOI] [PubMed] [Google Scholar]
- ROSSETTI Z.L., CARBONI S., MELIS F., NEFF N.H., GESSA G.L.Locally perfused morphine inhibits striatal DA release: reversal by systemic morphine Soc. Neurosci. Abstr. 1990161047(part 2) [Google Scholar]
- ROTH R.H., MURRIN L.C., WALTERS J.R. Central dopaminergic neurons: effects of alterations in impulse flow on the accumulation of dihydroxyphenylacetic acid. Eur. J. Pharmacol. 1976;36:163–171. doi: 10.1016/0014-2999(76)90268-5. [DOI] [PubMed] [Google Scholar]
- SANTIAGO M., WESTGERINK B.H. Characterization of the in vivo release of dopamine as recorded by different types of intracerebral microdialysis probes. Naunyn-Schmiedebergs Arch. Pharmacol. 1990;342:407–414. doi: 10.1007/BF00169457. [DOI] [PubMed] [Google Scholar]
- SCHLOSSER B., KUDERNATSCH M.B., SUTOR B., TEN BRUGGENCATE G. Delta, mu and kappa opioid receptor agonists inhibit dopamine overflow in rat neostriatal slices. Neurosci. Lett. 1995;191:126–130. doi: 10.1016/0304-3940(94)11552-3. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., RICE K.C., JACOBSEN A.E., VAN GELDEREN J.G., HOGENBOOM F., HEIJNA M.H., MULDER A.H. Mu-, delta- and kappa-opioid receptor-mediated inhibition of neurotransmitter release and adenylate cyclase activity in rat brain slices: studies with fentanyl isothiocyanate. Eur.J. Pharmacol. 1988;154:169–178. doi: 10.1016/0014-2999(88)90094-5. [DOI] [PubMed] [Google Scholar]
- SMITH J.A., LESLIE F.M., BROIDE R.S., LOUGHLIN S.E. Long-term changes in striatal opioid systems after 6-hydroxydopamine lesion of rat substantia nigra. Neuroscience. 1993;55:935–951. doi: 10.1016/0306-4522(93)90309-4. [DOI] [PubMed] [Google Scholar]
- TROVERO F., HERVE D., DESBAN M., GLOWINSKI J., TASSIN J.P. Striatal opiate mu-receptors are not located on dopamine nerve endings in the rat. Neuroscience. 1990;39:313–321. doi: 10.1016/0306-4522(90)90270-e. [DOI] [PubMed] [Google Scholar]
- VASKO M.R., DOMINO E.F. Tolerance development to the biphasic effects of morphine on locomotor activity and brain acetylcholine in the rat. J. Pharmacol. Exp. Ther. 1978;207:848–858. [PubMed] [Google Scholar]
- VEZINA P., KALIVAS P.W., STEWART J. Sensitization occurs to the locomotor effects of morphine and the specific mu opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res. 1987;417:51–58. doi: 10.1016/0006-8993(87)90178-8. [DOI] [PubMed] [Google Scholar]
- WAKSMAN G., HAMEL E., DELAY-GOYET P., ROQUES B.P. Neutral endopeptidase-24.11, mu and delta opioid receptors after selective brain lesions: an autoradiographic study. Brain Res. 1987;436:205–216. doi: 10.1016/0006-8993(87)91663-5. [DOI] [PubMed] [Google Scholar]
- WALTERS J.R., ROTH R.H. Dopaminergic neurons: drug-induced antagonism of the increase in tyrosine hydroxylase activity produced by cessation of impulse flow. J. Pharmacol. Exp. Ther. 1974;191:82–91. [PubMed] [Google Scholar]
- WESTFALL T.C., GRANT H., NAES L., MELDRUM M. The effect of opioid drugs on the release of dopamine and 5-hydroxytryptamine from rat striatum following activation of nicotinic-cholinergic receptors. Eur. J. Pharmacol. 1983;92:35–42. doi: 10.1016/0014-2999(83)90105-x. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON P.S., HOLMAN R.B. Ethanol-induced increase in endogenous dopamine release may involve endogenous opiates. J. Neurochem. 1992;59:157–163. doi: 10.1111/j.1471-4159.1992.tb08886.x. [DOI] [PubMed] [Google Scholar]
- WOOD P.L., ALTAR C.A. Dopamine release in vivo from nigrostriatal, mesolimbic, and mesocortical neurons: utility of 3-methoxytryramine measurements. Pharmacol. Rev. 1988;40:163–187. [PubMed] [Google Scholar]
- WOOD P.L., RAO T.S. Morphine stimulation of mesolimbic and mesocortical but not nigrostriatal dopamine release in the rat as reflected by changes in 3-methoxytyramine levels. Neuropharmacology. 1991;30:399–401. doi: 10.1016/0028-3908(91)90066-k. [DOI] [PubMed] [Google Scholar]
- WOOD P.L., RICHARD J.W. Morphine and nigrostriatal function in the rat and mouse: the role of nigral and striatal opiate receptors. Neuropharmacology. 1982;21:1305–1310. doi: 10.1016/0028-3908(82)90138-1. [DOI] [PubMed] [Google Scholar]
- WOOD P.L., STOTLAND M., RICHARD J.W., RACKHAM A. Actions of mu, kappa, sigma, delta and agonist/antagonist opiates on striatal dopaminergic function. J. Pharmacol. Exp. Ther. 1980;215:697–703. [PubMed] [Google Scholar]
- YASUDA K., RAYNOR K., KONG H., BREDER C.D., TAKEDA J., REISINE T., BELL G.I. Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEHARA N., CLOUET D.H. Effects of delta and mu opiopeptides on the turnover and release of dopamine in rat striatum. J. Pharmacol. Exp. Ther. 1984;231:38–42. [PubMed] [Google Scholar]
- ZASTAWNY R.L., GEORGE S.R., NGUYEN T., CHENG R., TSATSOS J., BRIONES-URBINA R., BF O.D. Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. J. Neurochem. 1994;62:2099–2105. doi: 10.1046/j.1471-4159.1994.62062099.x. [DOI] [PubMed] [Google Scholar]


