Abstract
The neurotrophic factor promoting activity of the neuroprotective compound SR57746A was evaluated in primary cultures of neonatal rat cortical astrocytes by studying the synthesis of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF).
A concentration- and time-dependent increase of nerve growth factor mRNA was induced by SR57746A (10 nM–1 μM). In these astrocytes, BDNF mRNA contents were increased to a significant but smaller extent, and β-actin mRNA showed no variation.
SR57746A (1 μM) induced increases of both de novo protein translation after 6 h of incubation and NGF release into the extracellular medium after 6–24 h.
These effects were preceded by a transient augmentation of junB, c-fos and c-jun mRNA contents. These increases of AP-1 family mRNA were associated with increased nuclear AP-1 binding activity.
The results show that SR57746A can increase the synthesis and release of NGF in rat cortical astrocytes. Such effects may contribute to the drug's previously described neuroprotective effects.
Keywords: NGF, BDNF, AP-1, astrocytes
Introduction
The non-peptide compound, SR57746A, has been found to possess a number of neurotrophic properties in vitro and in vivo (Fournier et al., 1993; Pradines et al., 1995). In a variety of experimental models, this compound elicits activities similar to those of neurotrophic factors, and particularly of nerve growth factor (NGF) (see Fournier et al., 1993). In vitro, SR57746A increases neurite elongation and survival of rat foetal septal cultures and potentiates the effect of NGF on neurite outgrowth in PC12 cells. In vivo, SR57746A reduces the histological deficits produced by transient global ischaemia and reverses the reduction of hippocampal choline acetyl transferase activity induced by septal injection of vincristine. Similarly, SR57746A has been found to antagonize the effects of an ibotenic acid-induced lesion of the nucleus basalis magnocellularis (Fournier et al., 1997).
It has now been well established that intercellular interactions mediated by functionally active cellular constituents, especially between astrocytes and neurons, are crucial for the normal development and maintenance of the brain (Barde, 1989; Thoenen, 1991). Astroglial neurotrophic factors may affect the migration, axonal growth and specific morphological pattern of neurons, while, reciprocally, factors produced by nerve cells may influence the development of astrocytes (Denis-Donini et al., 1984; Rudge et al., 1985; Hayashi et al, 1988). A number of neurotrophic factors have been identified and classified in different families according to structural homologies. The neurotrophin family includes NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5) (for review see Thoenen, 1991). A variety of experimental approaches have shown that each of these factors is able to support the survival or alleviate cell death of distinct classes of neurons (Davies, 1994; Snider, 1994; Isackson, 1995). In the adult brain, neurotrophins seem to be mainly expressed by neuronal cells (Isackson et al., 1991), which, in addition, could exert an inhibitory effect on astrocyte neurotrophin expression (Rudge et al., 1995). After neuronal damage, astroglial cells become reactive, and several lines of evidence indicate that astrocytes may be responsible for the increased expression of neurotrophins observed in these conditions (Spranger et al., 1990; Rossner et al., 1997). However, little is known about the regulation of neurotrophin expression. It has been demonstrated that interrelated cis- and trans-acting transcription factors may positively and negatively regulate NGF, BDNF or NT-3 gene transcription (D'Mello & Heinrich, 1991; Shintani et al., 1992; Leingartner & Lindholm, 1994). Such factors include immediate early genes (IEG), which are able to form AP-1 transcription complexes, and have been shown to be involved in the activation of NGF expression in vitro and in vivo (Hengerer et al., 1990; Jehan et al., 1993).
Preliminary results from our laboratory indicated that SR57746A increases NGF mRNA and protein contents in astrocytoma cells (Gauthier et al., 1993), suggesting that this compound could have neurotrophic factor promoting activities in glial cells. To further assess this possibility, we have examined the effects of SR57746A on neurotrophin expression and IEG activation in primary astrocyte cultures which display some of the characteristics of reactive astrocytes in the brain (McMillian et al., 1994). Because SR57746A has protective activities in cholinergic neuronal models, we studied the synthesis of some neurotrophins expressed in astroglial cells from cortex, a main projection region for forebrain cholinergic neurons.
Methods
Drugs
SR57746A (1-[2-(naphth-2-yl)-ethyl]-4-(3-trifluoromethylphenyl)-1,2,5,6 tetrahydropyridine, hydrochloride) was synthesized in the laboratories of Sanofi Recherche, Montpellier, France.
Dulbecco's modified Eagle's medium (DMEM) and F12 medium, foetal calf serum (FCS), glutamate and gentamycin solution were obtained from GIBCO, and sodium selenite, putrescine and transferrin from Sigma. Anti-NGF (M-20) polyclonal antibody was purchased from Santa Cruz Biotech. Inc., [35S]-Promix and [γ-32P]-ATP from Amersham, ±-8-hydroxy-dipropylaminotetralin HBr (8-OH-DPAT) from Research Biochemicals Inc., Nonidet P 40, dimethylsulphoxide (DMSO), iodoacetamide, phenylmethylsulphonyl fluoride (PMSF), aprotinin and 12-O-tetradecanoylphorbol 13-acetate (TPA), were purchased from Sigma, interleukin-1 β (IL-1) and protein A-agarose from Boehringer Mannheim. Oligonucleotides complementary to rat c-fos and β-actin was purchased from Oncogene Science and 63mer double-stranded oligonucleotide containing Oct-1 binding site from Pharmacia Biotech.
Astrocyte culture
Primary glial cultures were established from 4-day-old neonatal Sprague-Dawley rat cortices by a protocol approved by the Animal Care and Use Committee of Sanofi Recherche, and enriched for astrocytes by methods previously described (Hatten, 1985). The cells were kept in DMEM/F12 (50/50; v v−1) supplemented with 10% FCS, 1% glutamine and 0.1% gentamycin and grown in 5% CO2/90% air at 37°C. The medium was changed every fourth day and the cells were confluent after 3 weeks. More than 97% of cells were astrocytes, as indicated by positive glial fibrillary acidic protein immunohistochemistry in these conditions.
Before addition of SR57746A or vehicle (0.1% DMSO) for various times, the cultures were kept for 24 h in serum-free medium complemented with sodium selenite (30 nM), putrescine (100 μM) and transferrin (5 μg ml−1).
Isolation of RNA and S1 nuclease analysis
Briefly, cytoplasmic RNA was extracted from monolayer cells lysed in extraction buffer (in mM: Tris-HCl (pH 8) 50, NaCl 100, MgCl2 5, 0.5% Nonidet P-40). After proteinase K (0.15 mg ml−1) treatment and phenol extraction, RNA was ethanol precipitated, and final pellets were resuspended in water.
Quantitative S1 analyses were performed using 32P-labelled oligonucleotide probes complementary to the neurotrophins or IEG studied and β-actin. The sizes and complementary positions of oligonucleotide sequences (from GenBank) were: 41mer-NGF690-730; 51mer-BDNF153-203; 57mer-c-jun1609-1665; 33mer-junB1468-1500; 40mer-c-fos and -β-actin (Oncogene Science).
Solution hybridization was carried out on 25–50 μg of cytoplasmic RNA at 52–60°C overnight with 5×104 c.p.m. of each oligonucleotide (∼109 c.p.m. μg−1) in (NaCl 1 M, HEPES (pH 7.5) 0.16 M, ethylenediamine-tetra-acetate (EDTA) 0.33 mM). The excess of probe was digested with 270 μl of S1 nuclease digestion solution (NaCl 0.28 M, NaAcetate (pH 5.2) 0.05 M, ZnSO4 4.5 mM and 1000 U ml−1 of S1 nuclease) for an additional 60 min at 37°C. The reaction was stopped with EDTA (5 mM), and hybrids were ethanol precipitated using 10 μg of tRNA as carrier. The pellets were resuspended in loading buffer (80% formamide, EDTA 10 mM, 1 mg ml−1 of xylene cyanol and bromophenol blue) and analysed on denaturing polyacrylamide/8 M urea gel. Autoradiography of dry gels was performed at −80°C with an intensifying screen for 1 day, 4 days and 8 days for β-actin, IEG and neurotrophin probes, respectively. The amounts of mRNA were expressed as optical densities (OD) of protected bands normalized by the amount of hybridized cytoplasmic RNA.
NGF immunoprecipitation
After incubation with SR57746A (1 μM) for 6 h, the medium was removed and cells were kept in methionine-free medium containing 5% dialysed FCS for 30 min at 37°C. The medium was replaced with methionine-free medium containing 5% dialysed FCS and 100 μCi ml−1 [35S]-Promix and incubated for various times. Cell lysis and immunoprecipitation were performed in lysis buffer (in mM: Tris-HCl (pH 7.5) 50, NaCl 150, Iodoacetamide 1, 1% Nonidet P 40, 0.5% Sodium Deoxycholate, 0.1 mg ml−1 PMSF, 0.2 U ml−1 Aprotinin). Polyclonal anti-NGF antibody (0.2 μg per ml of lysis buffer) was added to cell extracts, and immune complexes were precipitated by protein A-agarose. Immunoprecipitates were analysed by tris-glycine-polyacrylamide gel electrophoresis (PAGE) after normalization by the protein content as determined by Biorad assay.
NGF ELISA
The NGF protein was assessed by ELISA (Emax™ Immunoassay System, Promega) according to the procedures of the manufacturer. After various times of incubation with SR57746A (1 μM), culture media were concentrated by centrifugation on Microsep 3K (Pall Filtron), and diluted to appropriate concentration with block buffer of the immunoassay kit. Duplicate aliquots were used for enzyme immunoassay.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared with a modification of the protocol described by Dignam et al. (1983). Briefly, cells were lysed in low-salt buffer (in mM: HEPES (pH 7.9) 10, KCl 25, EDTA 1, EGTA 1, DTT 0.5) containing 0.5% NP-40, PMSF 0.5 mM, 1 μg ml−1 aprotinin and 1 μg ml−1 leupeptin. The suspension was diluted with enough high-salt buffer (in mM: HEPES (pH 7.9) 10, EDTA 1, EGTA 1, DTT 1, 1 M KCl, 20% glycerol) to obtain an elution salt concentration of 420 mM. Nuclear proteins were extracted at 4°C with gentle agitation for 30 min. After centrifugation, protein concentration was determined by the Biorad assay.
For the binding reactions, nuclear extracts (7 μg) were mixed with 1.5×104 c.p.m. (i.e., 0.05 ng) of 32P-end-labelled double-stranded oligonucleotide (AP-1NGF, 5′-ATCGGTGAGTCAGGCTTGCG-3′; Colangelo et al., 1996), corresponding to the AP-1 sequence of the rat NGF gene, in 20 μl of buffer containing Tris-HCl (pH 7.5) 10 mM, NaCl 50 mM, 10% glycerol, DTT 0.5 mM and 1 μg poly[d(I-C)]. After 30 min at 10°C, the reaction products were separated on 8% non-denaturing polyacrylamide gel and analysed by autoradiography. Competition experiments were performed by incubating the extracts with unlabelled AP-1NGF or Oct-1 oligonucleotide (30 fold of molar excess), added 10 min prior to the binding reaction.
Statistical analysis
Data are presented as the mean±s.e.mean (n=3–4) of normalized values: mRNA as OD per μg of RNA, or NGF as pg ml−1 of medium, and expressed as a per cent of the respective control value. Differences between groups were analysed using ANOVA followed by Duncan's t-test for the concentration-response study. For the time-course experiments, the differences between the SR57746A-treated and vehicle-treated groups at each time point were analysed by Student's t-test.
Results
Effect of SR57746A on neurotrophin mRNA levels
In primary cultures of rat cortical astrocytes incubated in the presence of SR57746A (1 μM), the NGF mRNA levels were significantly increased to about 200% of control after 3 h of incubation (Figure 1B). The maximal NGF mRNA levels, 220% of control, were attained after 6 h of incubation and then declined at 9 h and returned to control levels by 18 h (Figure 1A and B). In the same experiment, BDNF mRNA levels showed a lower and more progressive increase to 140% of control values after 6 h of incubation with SR57746A and 176% of control after 9 h of incubation, before returning to basal levels at 18 h. The β-actin mRNA levels were not affected at any time of incubation (94±12 to 110±10% of control values, and Figure 1A). In a concentration-response experiment, 6 h of incubation with SR57746A induced a gradual, concentration-dependent increase of NGF mRNA, which attained statistical significance at the concentration of 10 nM (Figure 1C). No significant variation of BDNF or β-actin mRNA was seen in the same assay (data not shown).
Figure 1.
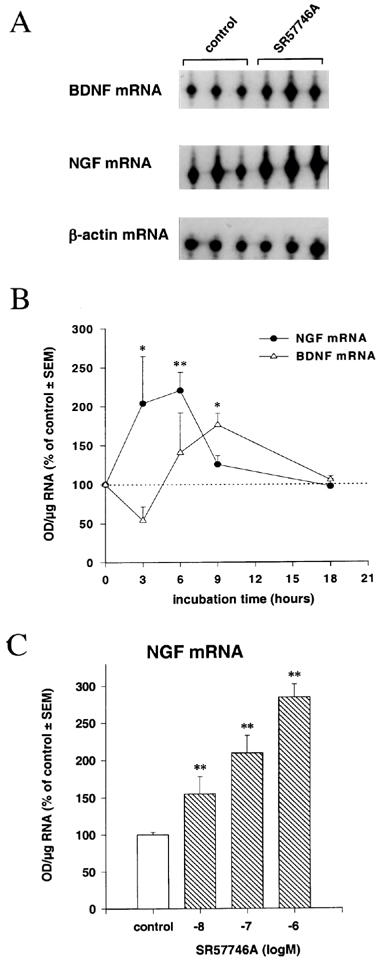
Effects of SR57746A on NGF, BDNF and β-actin mRNA levels in cortical astrocytes. (A) representative autoradiograph, from 6 h incubation experiment, with relative positions of protected oligonucleotides indicated. (B) Time course effect of SR57746A (1 μM); *P<0.05, **P<0.01, Student's t-test (n=3). (C) concentration effect of SR57746A, after 6 h of incubation, on NGF mRNA contents in astrocytes. **P<0.01, Duncan's t-test (n=3).
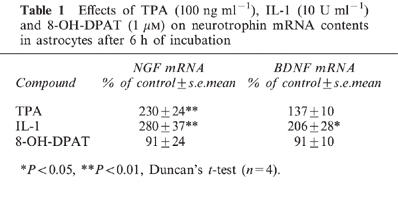
In control experiments, 6 h of incubation with TPA (100 ng ml−1) induced high increases of NGF mRNA to 230% of control levels in cortical astrocytes, but not of BDNF mRNA (Table 1). In contrast, 6 h of IL-1 (10 U ml−1) incubation induced significant increases of NGF mRNA (280% of control) and BDNF mRNA (206% of control; Table 1). While SR57746A has no or only very low affinity for the majority of neurotransmitter receptors, including β-noradrenergic receptors, whose stimulation can increase NGF production in cortical astrocytes (Schwartz & Mishler, 1990), the compound is a potent agonist for the 5HT1A receptor (Bachy et al., 1993). The effect of the 5HT1A receptor agonist 8-OH-DPAT was therefore evaluated on neurotrophin mRNA levels in astrocyte cultures. As shown in Table 1, after 6 h of incubation with 1 μM 8-OH-DPAT, no variation in neurotrophin messenger levels was seen in these cells.
Table 1.
Effects of TPA (100 ng ml−1), IL-1 (10 U ml−1) and 8-OH-DPAT (1 μM) on neurotrophin mRNA contents in astrocytes after 6 h of incubation
Effect of SR57746A on NGF translation
To determine whether the increases of neurotrophin mRNA contents induced by SR57746A were associated with increases in translation, we evaluated NGF translation rates after 6 h incubation with or without 1 μM SR57746A. The level of de novo NGF translation in cortical astrocytes was assessed by immunoprecipitation of neo-synthesized proteins with polyclonal antibody directed against the N-terminal part of mature β-NGF. The band pattern seen for NGF immunoprecipitation on the tris-glycine-PAGE autoradiogram clearly indicated that different proteins were precipitated (Figure 2). In the presence of SR57746A, a progressive increase of intensity of a band corresponding to the precursor proNGF (35 kDa) was seen from 1 to 3 h of labelling. The 42.5 kDa glycosylated intermediate NGF precursor (Seidah et al., 1996) was increased after 1 and 2 h of label incorporation, but was not further detectable after 3 h. The processed 13.5 kDa form of β-NGF appeared after 3 h of labelling, and the intensity of this band was also increased by incubation with SR57746A. In addition, immunoprecipitation produced high molecular weight protein background (150–200 kDa; see Seidah et al., 1996). Because these bands disappeared, as did the neurotrophin related proteins described above, upon addition of 5 μg of cold antigens in control experiments (data not shown), they can be attributed to residual high molecular complexes.
Figure 2.
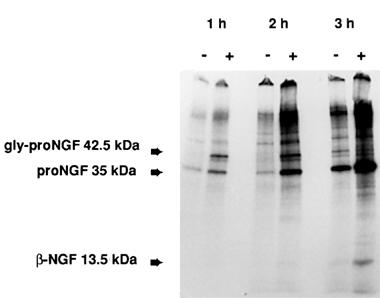
Effects of SR57746A on NGF protein synthesis. Autoradiography of tris-glycine-PAGE of neo-synthesized proteins from cortical astrocytes, immunoprecipitated by anti-NGF as described in the text. Astrocytes were incubated for 6 h with (+) SR57746A (1 μM), or with (−) 0.1% DMSO. Positions for glycosylated proNGF (42.5 kDa), proNGF (35 kDa) and β-NGF (13.5 kDa) are indicated.
Effect of SR57746A on NGF secretion
Treatment of cortical astrocytes with SR57746A (1 μM) significantly increased the NGF content of the culture medium. As seen in Figure 3, the levels of extracellular NGF were about 175% of control values after 6 h of incubation, increased to a maximum of 350% of control after 15 h of incubation, and then declined to 260% of control after 24 h. The mean value of the control (19.2±0.62 pg NGF ml−1 of medium) remained invariant during the course of the study (data not shown).
Figure 3.
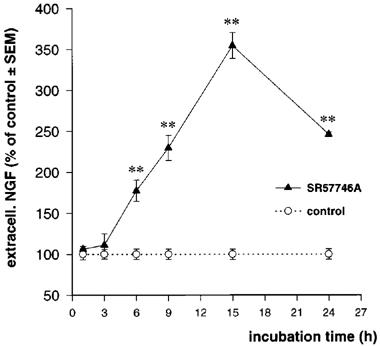
Time-course effect of SR57746A on secreted NGF. Astrocytes were treated with SR57746A (1 μM) for the indicated times. Extracellular NGF was determined by ELISA as described in Methods. Values are the means±s.e.mean of three independent determinations expressed as per cent of control values. *P<0.05, **P<0.01, Student's t-test (n=4).
Activation of AP-1 transcription factors in SR57746A-treated astrocytes
Exposure of astrocyte cultures to the minimal concentration of SR57746A (10 nM) capable of inducing neurotrophin mRNA increases produced transient and significant increases of c-fos and c-jun mRNA levels, together with a higher increase in junB mRNA contents (Figure 4). These increases were maximal after 15 min of incubation and returned to basal levels by 30 min for c-fos and c-jun, and 60 min for junB. Maximal c-fos and c-jun induction by SR57746A was about 2 fold, whereas junB mRNA was increased by 2.5 fold in these cells. The β-actin mRNA levels were not modified in any experiment (95.3±8.2 to 108.5±6.1% of control). To further assess the possible relationship between IEG activation and the increase of neurotrophin mRNA induced by SR57746A, we performed an EMSA with synthetic oligonucleotides corresponding to the rat NGF AP-1 site. Nuclear extracts prepared from astrocytes, exposed for 60 min to 10 nM SR57746A, were analysed for AP-1 binding activity. Figure 5 (lanes 2 and 3) shows that SR57746A enhanced the AP-1 band-shift induced by nuclear extracts. The specificity of the shifts was demonstrated by cold-oligonucleotide competition experiments (Figure 5: lanes 4 and 5).
Figure 4.
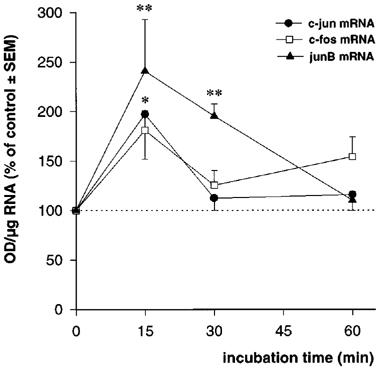
Time-course effect of SR57746A (10 nM) on IEG mRNA levels in cortical astrocytes. *P<0.05, **P<0.01, Student's t-test (n=4).
Figure 5.
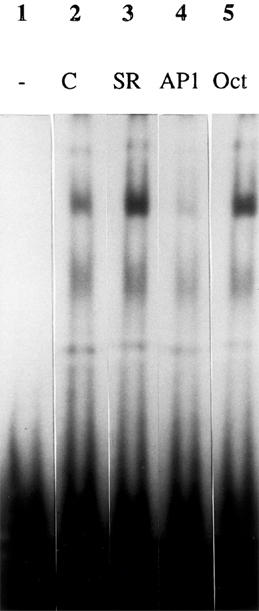
EMSA for 32P-labelled AP-1NGF oligonucleotide. Gel retardation assays were performed with AP-1NGF oligonucleotide (0.05 ng) and 7 μg of nuclear proteins from cortical (lanes 2–5) astrocytes. Lane 1, AP-1NGF without nuclear extract; lane 2, AP-1NGF with nuclear extracts from vehicle-treated cells (C); lane 3, AP-1NGF with nuclear extracts from cells incubated with SR57746A (10 nM, SR); lane 4, AP-1NGF with nuclear extracts from vehicle-treated cells and 30 fold molar excess of unlabelled AP-1NGF (AP1); lane 5, AP-1NGF with nuclear extracts from vehicle-treated cells and 30 fold molar excess of unlabelled Oct-1 oligonucleotide (Oct).
Discussion
The main result of this study is that the neuroprotective compound SR57746A possesses significant NGF-promoting activity in primary cultures of astrocytes from rat neonatal cortex.
In the first part of this study, we found that SR57746A induced a concentration- and time-dependent increase in NGF mRNA levels in these cultures. Increase of NGF mRNA to 220% to 270% of control was reached after 6 h of incubation. In the same cells, BDNF mRNA levels were enhanced to a maximum of 176% of the control values after 9 h. The fact that β-actin mRNA levels remained invariant over an 18 h incubation period indicates that the observed neurotrophin increases were not the result of an activation of cell growth. SR57746A may increase the levels of NGF and BDNF mRNA either independently, or, alternatively, the increase in BDNF mRNA observed in cortical cells could occur indirectly, as a consequence of the initial increase in NGF mRNA, as an NGF-induced increase of BDNF has previously been described (Apfel et al., 1996).
In the second part of the study, we found that the increase in NGF mRNA was accompanied by an increase in neo-synthesis of the corresponding protein. Following translation of neurotrophin mRNAs, their precursors are subject to post-translational modifications which include signal peptide cleavage, N-glycosylation and limited proteolysis (Seidah et al., 1996). We observed that the increase in NGF translation induced by SR57746A in cortical astrocytes was initially restricted to the glycosylated (42.5 kDa) and unglycosylated (35 kDa) forms of proNGF, which have previously been found to be present in cells which express NGF constitutively (Seidah et al., 1996). These increases in precursors were followed by the appearance of free monomeric β-NGF (13.5 kDa) after 3 h of labelling.
The functionality of the activation of NGF synthesis induced by SR57746A was further demonstrated by the drug-induced increase of NGF secretion. The amount of extracellular NGF was time-dependently increased by SR57746A with a maximum seen after 15 h of incubation, i.e., 6–9 h after the maximal increase of NGF mRNA, a delay very similar to the lag between NGF mRNA and protein secretion increases induced by TPA or IL-1 in cultured astrocytes (Pshenichkin et al., 1994).
As a first step to identifying the molecular processes involved in the neurotrophin-promoting activities of SR57746A in cortical astrocytes, we evaluated the effect of the compound on AP-1 transcription factors. At the minimal active concentration, increases in c-fos, c-jun and junB mRNA were found to precede those of NGF or BDNF mRNA. Furthermore, these IEG messenger increases were associated with functional activation of the AP-1 complex as shown by increase of AP-1NGF binding activities in nuclear extracts. As described by D'Mello & Heinrich (1991), various transcription factors could be involved in the NGF gene expression, and further experiments are currently under way to assess the effects of SR57746A on other IEG. Nevertheless, the present results indicate that this compound can activate the intracellular transcription machinery of astrocytes, and strongly suggest that the increases of neurotrophin mRNA are the result of increased gene transcription.
SR57746A has no affinity for the majority or neurotransmitter receptors except for the 5-HT1A receptor for which this compound is a potent agonist (Bachy et al., 1993). As shown in the present study, the compound's effects were not shared by 8-OH-DPAT, suggesting that the 5-HT1A receptor is not involved in, or is not sufficient by itself, to induce the neurotrophin-promoting effect of SR57746A. This is also in agreement with other studies suggesting that this receptor is not implicated in the neuroprotective effects of SR57746A (Fournier et al., 1992, 1997).
In conclusion, SR57746A promotes the synthesis of NGF and BDNF in neonatal rat cortical astrocytes. The extent to which such effects may contribute to the drug's neuroprotective activity in vivo remains to be established, and studies are ongoing to assess this point.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- DMSO
dimethylsulphoxide
- EMSA
electrophoretic mobility shift assay
- IEG
immediate early gene
- IL-1
interleukin-1β
- NGF
nerve growth factor
- 8-OH-DPAT
±-8-hydroxy-dipropylaminotetralin HBr
- TPA
12-O-tetradecanoylphorbol 13-acetate
References
- APFEL S.C., WRIGHT D.E., WIIDEMAN A.M., DORMIA C., SNIDER W.D., KESSLER J.A. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol. Cell. Neurosci. 1996;7:134–142. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- BACHY A., STEINBERG R., SANTUCCI V., FOURNIER M., LANDI M., HAMON M., MANARA L., KEANE P.E., SOUBRIÉ P., LE FUR G. Biochemical and electrophysiological properties of SR57746A, a new, potent 5-HT1A receptor agonist. Fund. Clin. Pharmacol. 1993;7:487–497. doi: 10.1111/j.1472-8206.1993.tb00253.x. [DOI] [PubMed] [Google Scholar]
- BARDE Y.-A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- COLANGELO A.M., PANI L., MOCCHETTI I. Correlation between increased AP-1NGF binding activity and induction of nerve growth factor transcription by multiple signal transduction pathways in C6-2B glioma cells. Mol. Brain Res. 1996;35:1–10. doi: 10.1016/0169-328x(95)00171-n. [DOI] [PubMed] [Google Scholar]
- DAVIES A.M. The role of neurotrophins in the developing nervous system. J. Neurobiol. 1994;25:1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- DENIS-DONINI S., GLOWINSKI J., PROCHIANTZ A. Glial heterogeneity may define the three-dimensional shape of mouse mesencephalic dopaminergic neurons. Nature. 1984;307:641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- DIGNAM J.D., LEBOVITZ R.M., ROEDER R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucl. Acid. Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'MELLO S.R., HEINRICH G. Structural and functional identification of regulatory regions and cis elements surrounding the nerve growth factor gene promoter. Mol. Brain Res. 1991;11:255–264. doi: 10.1016/0169-328x(91)90034-u. [DOI] [PubMed] [Google Scholar]
- FOURNIER J., GAUTHIER T., KEANE P.E., COUDE F.X., GUZZI U., SOUBRIE P., LE FUR G. Neurotrophic effects of SR57746A, a non peptide compound: in vitro studies. Br. J. Pharmacol. 1992;106 suppl:120P. [Google Scholar]
- FOURNIER J., KEANE P.E., FERRARA P., SOUBRIÉ P. SR57746A: an orally active non-peptide compound with neurotrophic and neuroprotective effects. C.N.S. Drug Reviews. 1997;3:148–167. [Google Scholar]
- FOURNIER J., STEINBERG R., GAUTHIER T., KEANE P.E., GUZZI U., COUDÉ F.X., BOUGAULT I., MAFFRAND J.P., SOUBRIÉ P., LE FUR G. Protective effects of SR57746A in central and peripheral models of neurodegenerative disorders in rodents and primates. Neuroscience. 1993;55:629–641. doi: 10.1016/0306-4522(93)90429-j. [DOI] [PubMed] [Google Scholar]
- GAUTHIER T., BORNIA J., REITH Y. , KEANE P.E., LE FUR G., SOUBRIÉ P. SR57746A: induction of NGF gene expression and synthesis in astrocytoma and fibroblast cell lines, and beneficial effects in peripheral degenerative model in rats. Fund. Clin. Pharmacol. 1993;7:359. [Google Scholar]
- HATTEN M.E. Neuronal regulation of astroglial morphology and proliferation in vitro. J. Cell. Biol. 1985;100:384–396. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI M., HAYASHI R., TANII H., HASHIMOTO K., PATEL A.J. The influence of neuronal cells on the development of glutamine synthetase in astrocytes in vitro. Brain Res. 1988;469:37–42. doi: 10.1016/0165-3806(88)90167-8. [DOI] [PubMed] [Google Scholar]
- HENGERER B., LINDHOLM D., HEUMANN R., RUTHER U., WAGNER E.F., THOENEN H. Lesion-induced increase in nerve growth factor mRNA is mediated by c-fos. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISACKSON P.J. Trophic factor response to neuronal stimuli or injury. Curr. Opin. Neurobiol. 1995;5:350–357. doi: 10.1016/0959-4388(95)80048-4. [DOI] [PubMed] [Google Scholar]
- ISACKSON P.J., HUNTSMAN M.M., MURRAY K.D., GALL C.M. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal pattern of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- JEHAN F., NEVEU I., NAVEILHAN P., BRACHET P., WION D. Complex interactions among second messenger pathways, steroid hormones, and protooncogenes of the Fos and Jun families converge in the regulation of the nerve growth factor gene. J. Neurochem. 1993;60:1843–1853. doi: 10.1111/j.1471-4159.1993.tb13411.x. [DOI] [PubMed] [Google Scholar]
- LEINGARTNER A, LINDHOLM D. Two promoters direct transcription of the mouse NT-3 gene. Eur. J. Neurosci. 1994;6:1149–1159. doi: 10.1111/j.1460-9568.1994.tb00613.x. [DOI] [PubMed] [Google Scholar]
- MCMILLIAN M.K., THAI L., HONG J.-S., O'CALLAGHAN J.P., PENNYPACKER K.R. Brain injury in a dish: a model for reactive gliosis. Trends Neurosci. 1994;17:138–142. doi: 10.1016/0166-2236(94)90086-8. [DOI] [PubMed] [Google Scholar]
- PRADINES A., MAGAZIN M., SCHILTZ P., LE FUR G., CAPUT D., FERRARA P. Evidence for nerve growth factor-potentiating activities of the non-peptidic compound SR57746A in PC 12 cells. J. Neurochem. 1995;64:1954–1964. doi: 10.1046/j.1471-4159.1995.64051954.x. [DOI] [PubMed] [Google Scholar]
- PSHENICHKIN S.P., SZEKELY A.M., WISE B.C. Transcriptional and posttranscriptional mechanisms involved in the interleukin-1, steroid, and protein kinase C regulation of nerve growth factor in cortical astrocytes. J. Neurochem. 1994;63:419–428. doi: 10.1046/j.1471-4159.1994.63020419.x. [DOI] [PubMed] [Google Scholar]
- ROSSNER S., SCHLIEBS R., HÄRTIG W., PEREZ-POLO J.R., BIGL V. Selective induction of c-jun and NGF in reactive astrocytes after cholinergic degeneration in rat basal forebrain. Neuroreport. 1997;8:2199–2202. doi: 10.1097/00001756-199707070-00022. [DOI] [PubMed] [Google Scholar]
- RUDGE J.S., MANTHORPE M., VARON S. The output of neurotrophic and neurite-promoting agents from rat brain astroglial cells: a microculture method of screening potential regulatory molecules. Brain Res. 1985;351:161–172. doi: 10.1016/0165-3806(85)90188-9. [DOI] [PubMed] [Google Scholar]
- RUDGE J.S., PASNIKOWSKI E.M., HOLST P., LINDSAY R.M. Changes in neurotrophic factor expression and receptor activation following exposure of hippocampal neuron/astrocyte cocultures to kainic acid. J. Neurosci. 1995;15:6856–6867. doi: 10.1523/JNEUROSCI.15-10-06856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J.P., MISHLER K. Beta-adrenergic receptor regulation, through cyclic AMP, of nerve growth factor expression in rat cortical and cerebellar astrocytes. Cell. Mol. Neurobiol. 1990;10:447–457. doi: 10.1007/BF00711186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDAH N.G., BENJANNET S., PAREEK S., SAVARIA D., HAMELIN J., GOULET B., LALIBERTE J., LAZURE C., CHRETIEN M., MURPHY R.A. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem. J. 1996;314:951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINTANI A., ONO Y., KAISHO Y., IGARASHI K. Characterization of the 5′-flanking region of the human brain-derived neurotrophic factor gene. Biochem. Biophys. Res. Commun. 1992;182:325–332. doi: 10.1016/s0006-291x(05)80148-2. [DOI] [PubMed] [Google Scholar]
- SNIDER W.D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- SPRANGER M., LINDHOLM D., BANDTLOW C., HEUMANN R., GNAHN H., NÄHER-NOÉ M., THOENEN H. Regulation of nerve growth factor (NGF) synthesis in the rat central nervous system: comparison between the effects of interleukin-1 and various growth factors in astrocyte cultures and in vivo. Eur. J. Neurosci. 1990;2:69–76. doi: 10.1111/j.1460-9568.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- THOENEN H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]


