Abstract
We have investigated the possibility that ET-1 can induce an increase in myofilament calcium sensitivity in pulmonary artery smooth muscle. Arterial rings were permeabilized using α-toxin (120 μg ml−1), in the presence of A23187 (10 μM) to ‘knock out' Ca2+ stores, and pre-constricted with pCa 6.8 (buffered with 10 mM EGTA). In the presence of this fixed Ca2+ concentration, 1 μM ET-1 induced a sustained, reversible constriction of 0.15 mN.
Pulmonary arterial rings were freeze-clamped at the peak of the induced constriction (time matched). Subsequent densitometric analysis revealed that ET-1 (1 μM) increased the level of phosphorylated myosin light chains by 34% compared to an 11% increase in the presence of pCa 6.8 alone.
In contrast to ET-1, the selective ETB receptor agonist Sarafotoxin S6C (100 nM) failed to induce a significant constriction.
The constriction induced by 1 μM ET-1 was reversibly inhibited when the preparation was pre-incubated (15 min) with the ETA receptor antagonist BQ 123 (100 μM). The constriction measured 0.13 mN in the absence and 0.07 mN in the presence of 100 μM BQ 123.
In contrast, the constriction induced by 1 μM ET-1 measured 0.19 mN in the absence and 0.175 mN following a 15 min pre-incubation with the ETB antagonist BQ 788 (100 μM).
The constriction induced by 1 μM ET-1 measured 0.14 mN in the presence and 0.13 mN following pre-incubation with the tyrosine kinase inhibitor Tyrphostin A23 (100 μM).
We conclude that ET-1 induced an increase in myofilament calcium sensitivity in rat pulmonary arteries via the activation of ETA receptors and by a mechanism(s) independent of tyrosine kinase.
Keywords: Smooth muscle, pulmonary artery, ET-1, ETA, ETB, calcium sensitization
Introduction
It is well known that endothelin-1 (ET-1), a member of a family of vasoactive peptides, is one of the most potent constrictors of vascular smooth muscle, and that its effects are mediated by at least two identified receptor subtypes, namely ETA and ETB (Yanagisawa et al., 1988; Arai et al., 1990; Leach et al., 1994; Sakurai et al., 1990; Sakamoto et al., 1991; Yoshida et al., 1994; Maguire et al., 1996). Recent investigations have suggested that ET-1 may play an important role in the regulation of pulmonary vascular tone and may promote the development of pulmonary hypertension (Chen et al., 1997; Bonvallet et al., 1994; Prie et al., 1997; Langleben et al., 1993; Yoshibayashi et al., 1991). During pulmonary hypoxia, plasma ET-1 levels are elevated due to increased expression of ET-1 mRNA, and release of ET-1 from pulmonary artery endothelial cells (Giaid et al., 1993; Elton et al., 1992; Li et al., 1994a; 1995), and this is accompanied by an increase in endothelin receptor gene expression in human and rat pulmonary vasculature (Li et al., 1994a,1994b). Furthermore, investigations have shown that orally active ETA receptor antagonists attenuate hypoxic pulmonary vasoconstriction (HPV) in rats, and suppress the development of hypoxic pulmonary hypertension, right ventricular hypertrophy and pulmonary vascular remodelling (Chen et al., 1997; Bonvallet et al., 1994). In contrast, the ETA receptor antagonists had little or no effect on pulmonary arterial pressure in normoxic animals.
When one considers the possible role of ET-1 in mediating HPV, it is of some interest to note that endothelin receptor activation can induce constriction of arterial smooth muscle not only by raising the intracellular Ca2+ concentration (Highsmith et al., 1992; Pang et al., 1989; Kasuya et al., 1989; Goto et al., 1989; Enoki et al., 1995), but by increasing the sensitivity of the contractile apparatus to Ca2+ (Nishimura et al., 1992; Ohanian et al., 1997). The significance of this observation becomes apparent when taken together with the fact that calcium sensitization has also been implicated in the development of HPV (Robertson et al., 1995). Hence, pulmonary arteries constrict biphasically when exposed to hypoxia (⩽50 Torr). An initial transient constriction (phase 1) is followed by a slowly developing and sustained increase in tone (Albarwani et al., 1994; Bennie et al., 1991; Jin et al., 1992; Kovitz et al., 1993; Robertson et al., 1995), (phase 2). Phase 1 occurs independently of the endothelium and is thought to result from depolarization of the smooth muscle cell membrane (Evans et al., 1996; Osipenko et al., 1997; Yuan et al., 1993; Post et al., 1992) and Ca2+ influx through voltage-gated Ca2+ channels (Harder et al., 1985; McMurty et al., 1976; Madden et al., 1992; Cornfield et al., 1993; Salvatera & Goldman, 1993; Vadula et al., 1993). In contrast, Phase 2 requires the release of a vasoconstrictor from the intact endothelium (Leach et al., 1994) and may develop in the presence of a constant cytoplasmic free Ca2+ concentration (Robertson et al., 1995). It seems possible, therefore, that this endothelially derived vasoconstrictor may be ET-1, and that ET-1 may promote HPV in part by inducing myofilament Ca2+ sensitization, which may in turn promote the development of hypoxic pulmonary hypertension.
Until now, however, there have been no investigations of the possibility that ET-1 may induce pulmonary arterial constriction by increasing the sensitivity of the contractile apparatus to calcium. We have, therefore, addressed this question in the present investigation. Our findings suggest that ETA receptors are the primary mediators of the myofilament calcium sensitization induced by ET-1 in rat pulmonary arteries. The observed calcium sensitization appears to result from an increase in myosin light chain phosphorylation and is mediated by a mechanism(s) independent of the tyrosine kinase pathway which has been proposed to mediate the ET-1 induced increase in myofilament Ca2+ sensitivity in systemic arteries (Ohanian et al., 1997).
Methods
Permeabilized arterial rings
Male Sprague Dawley rats (313 g) were killed by cervical dislocation. The heart and lungs were removed together and placed in chilled HEPES buffered normal Krebs' solution. In all cases, second order branches off the main intrapulmonary arteries (i.d. 100–400 μm, length 1–2 mm) were dissected out and cleaned of connective tissue using a dissecting microscope. The endothelium of the arteries was then removed by rubbing the inner surface with thin surgical thread (o.d. about 0.1 mm). Vessels were always obtained from the same anatomical position within the lung. Pulmonary artery rings were then tied with thread fibres to a force transducer (AE801, SensoNor a.s., U.K.). The transducer was mounted on a ‘bubble plate' (Kitazawa et al., 1991) and tension recorded with a flat-bed pen recorder. Pulmonary artery rings were stretched to a length which gave the greatest constriction in 153 mM K+ solution. After determining the contractile response to K+ in HEPES buffered Krebs' solution, the strips were incubated in normal relaxing solution (G1) containing 1 mM EGTA. Details of the solutions used have been described previously (Horiuti et al., 1986). The calcium concentration of pCa solutions was regulated by the ratio of K2EGTA and CaEGTA. Strips were permeabilized with 50 μM β-escin or 120 μg ml−1 α-toxin (purified from Staph. Aureus), respectively, and in the presence of 10 μM A23187 (to deplete intracellular calcium stores), using pCa 6.0 solution at 24°C (Iizuka et al., 1994). In all solutions used with β-escin permeabilized strips, 0.1 μM calmodulin was added to compensate for leakage of calmodulin from permeabilized cells.
A maximal calcium response to pCa 4.5 was obtained. After relaxing in G1 solution, the strips were submaximally constricted in a calcium buffer; pCa 6.5–6.8. Once a plateau was reached, ET-1 or Sarafotoxin S6C (SX6C) were added to the bathing solution to study ET receptor mediated calcium sensitization.
Myosin light chain 20 phosphorylation
In order to determine changes in myosin light chain (MLC) phosphorylation in permeabilized pulmonary arterial smooth muscle, rings were rapidly frozen in liquid Freon 22 either at the peak of the ET-1-induced constriction or at the plateau level of a sustained, submaximal Ca2+-induced constriction. Rings were then transferred to frozen acetone containing 10% trichloroacetic acid. The acetone/trichloroacetic acid was exchanged for 100% acetone and rings left to air dry at room temperature. Each sample was pooled from 4 rings in order to provide sufficient protein. Dried rings were homogenized in glycerol sample buffer (containing 1% SDS, 10% glycerol and 20 mM DTT) and subjected to 2-dimensional gel electrophoresis (Nixon et al., 1995) consisting of isoelectric focusing (ampholyte range pH 4.5–5.5, Pharmacia Biotech, St. Albans, U.K.) in the first dimension followed by SDS-polyacrylamide gel electrophoresis in the second dimension on 12% polyacrylamide gels. Resolved proteins were transferred onto nitrocellulose (Biorad Labs Ltd., Hemel Hempstead, U.K.) and the membrane stained with colloidal gold solution to reveal protein spots of unphosphorylated, monophosphorylated and diphosphorylated myosin light chains. MLC phosphorylation was quantitatively assessed using a Biorad GS690 densitometer.
Data analysis
The force of constriction is given in mN of tension developed, or normalized to the maximal constriction obtained to pCa 4.5 solution. Data are expressed as the mean±s.e.mean unless stated. Data were compared using a Student's t-test, unless otherwise indicated, and statistical significance was assumed if P<0.05.
Drugs and solutions
All compounds used in this study were obtained from RBI (SIGMA-ALDRICH, U.K.), with the exception of Tyrphostin A23 which was obtained from Semat Technical (U.K.). Endothelin-1, Sarafotoxin S6C, BQ 123 and BQ 788 were dissolved in de-oxygenated H2O. Tyrphostin A23 was dissolved in DMSO. Stock solutions in DMSO were diluted at least 1 : 1000, after which the vehicle alone had no effect on the response of the preparation to Ca2+ or to ET-1.
Results
The effect of endothelin-1 and sarafotoxin S6C in permeabilized pulmonary arterial rings
Either β-escin (50 μM) or α-toxin (120 μg ml−1) permeabilized small pulmonary arterial rings were used to provide a preparation which allowed us to ‘clamp' the intracellular calcium concentration ([Ca2+]) at a defined level, while retaining receptor coupled intracellular second messenger pathways (Kitazawa et al., 1989; Iizuka et al., 1994). Under these conditions a submaximal constriction was first induced by raising the [Ca2+] in the bathing solution from 0 to between pCa 6.8 and pCa 6.5, before the application of ET receptor agonists. Under these ‘calcium clamped' conditions the threshold concentration for the ET-1 induced constriction was in the range 30–100 nM (not shown), while 1 μM ET-1 was found to induce a constriction of rat pulmonary arterial rings which measured approximately 50% of the maximum tension developed in the presence of pCa 4.5 solution. Clearly, with the [Ca2+] clamped and the intracellular stores depleted, the observed constriction could only be due to an increased sensitivity of the contractile apparatus to Ca2+. This concentration of ET-1 was used to provide the standard submaximal response for comparison in the present investigation. It should be noted that although this concentration is at least an order of magnitude greater than that required to induce a maximal constriction in intact (non-permeabilized) pulmonary arteries (Kato et al., 1999; see also McCulloch et al., 1998), it is to be expected that higher agonist concentrations may be required as thorough permeabilization of smooth muscle preparations, including the larger conduit pulmonary arteries of the rabbit results in a reduction in the efficiency of receptor coupling (Himpens et al., 1989). This probably results from the loss/dilution of intracellular messengers and enzymes with time. For this reason, β-escin permeabilized preparations were found to run down dramatically over 30 min even in the presence of added calmodulin (0.1 μM) and GTP (50 μM). We therefore, carried out all subsequent studies using α-toxin permeabilized pulmonary arterial rings.
Figure 1 shows the response of an α-toxin permeabilized pulmonary arterial ring first to an increase in Ca2+ from 0 to pCa 6.8 and then to subsequent addition of the selective ETB receptor agonist SX6C (100 nM) and ET-1 (1 μM), respectively, in the continued presence of pCa 6.8. Addition of pCa 6.8 induced a constriction of 0.07±0.01 mN (24±5% of maximum constriction to pCa 4.5; n=3). Subsequent application of the selective ETB receptor agonist SX6C (100 nM) failed to induce a significant increase in the force of constriction relative to that induced by pCa 6.8 alone. The tension developed by pCa 6.8 alone was 0.07±0.01 mN (n=3) and increased to 0.1±0.02 (n=3) in the presence of SX6C (an increase of 0.03±0.01 mN; 10±6% of the maximum constriction to pCa 4.5). In marked contrast to the effects of SX6C, a pronounced increase in the sensitivity of the myofilaments to Ca2+ was induced by the addition of ET-1 (1 μM), which generated a further 0.15±0.03 mN of tension (49±3% of the maximum constriction to pCa 4.5; n=3). Note: In intact rat pulmonary arteries (endothelium denuded), 1 nM SX6C was sufficient to induce a constriction equivalent to that induced by 1 μM ET-1 (Kato et al., 1999; see also McCulloch et al., 1998McCulloch et al., 1998).
Figure 1.
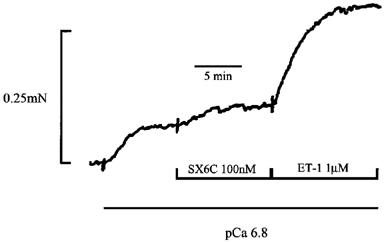
Effect of the selective ETB receptor agonist SX6C and ET-1 on permeabilized pulmonary artery rings. This figure shows an initial submaximal constriction induced by raising the Ca2+ concentration in the bathing solution from 0 to pCa 6.8. In addition the effects of subsequent application of 100 nM SX6C, a selective ETB receptor agonist, and 1 μM ET-1, respectively, in the continued presence of pCa 6.8 are shown. In this and all subsequent figures pulmonary artery rings were permeabilized using α-toxin (120 μg ml−1), and in the presence of 10 μM A23187 (to deplete intracellular calcium stores), using pCa 6.0 solution at 24°C.
Effect of ETA and ETB receptor antagonists on the response of permeabilized pulmonary arteries to ET-1
In α-toxin permeabilized pulmonary artery rings, application of 1 μM ET-1 increased the constriction induced by pCa 6.8 solution from 0.11±0.02 mN to 0.27±0.02 mN (an increase of 0.15±0.02 mN; n=9). Under these conditions the ET-1 (1 μM) induced constriction measured 52±6% of the maximum constriction to pCa 4.5 solution (n=9). Figure 2A shows a control response to 1 μM ET-1 in an artery pre-constricted with pCa 6.8 and GTP (50 μM), respectively. Figure 2B shows the constriction induced by 1 μM ET-1 following a 15 min pre-incubation with the selective ETA receptor antagonist BQ 123 (100 μM; Bonvallet et al., 1994). The constriction to 1 μM ET-1 was reduced from 0.13±0.03 mN (53.12±0.05% of the maximum response to pCa 4.5 solution; n=4) in the absence to 0.067±0.003 mN (27.92±0.03% of the maximum response to pCa 4.5 solution; n=4) in the presence of 100 μM BQ 123 (P<0.05). The constriction to 1 μM ET-1 recovered to 0.12±0.025 mN (43.30±0.01% of the maximum response to pCa 4.5 solution; n=4) following a 15 min wash (Figure 2C). In contrast to its effects on the constriction induced by ET-1, 100 μM BQ 123 did not alter the magnitude of the constriction induced by pCa 6.8 solution, which measured 0.32±0.11 mN in the absence and 0.32±0.11 mN in the presence of the ETA receptor antagonist (n=4). Note: The ET-1 induced constriction of rat pulmonary artery rings was unaffected by the presence or absence of applied GTP. We, therefore, excluded GTP from the bathing solution in all other experiments.
Figure 2.
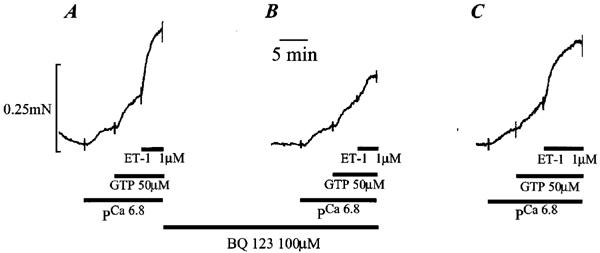
Effect of the ETA receptor antagonist BQ 123 on the ET-1 induced constriction of permeabilized pulmonary artery rings. (A) shows a typical submaximal constriction to pCa 6.8 and to 50 μM GTP, and the subsequent constriction induced by 1 μM ET-1 in the continued presence of pCa 6.8 and 50 μM GTP. (B) shows the effect of a 15 min pre-incubation with 100 μM BQ 123 on the constriction to pCa 6.8, 50 μM GTP and 1 μM ET-1 respectively. (C) shows the reversal of the effect of 100 μM BQ 123 following a 15 min wash.
Figure 3 shows the response induced by 1 μM ET-1 in an artery pre-constricted with pCa 6.8 (Figure 3A) before and (Figure 3B) after a 15 min incubation with the selective ETB receptor antagonist BQ 788 (100 μM). In marked contrast to the effects of the selective ETA receptor antagonist BQ 123 (Figure 2), BQ 788 (100 μM) had no significant effect on the constriction induced by 1 μM ET-1, the tension developed being 0.19±0.05 mN in the absence and 0.175±0.031 mN in the presence of the ETB receptor antagonist (n=3), respectively. A small reduction in the ET-1-induced constriction was observed in the presence of BQ 788 in two out of three preparations. However, a further decline was observed on washing (not shown), which suggests that this was probably due to rundown rather than to the presence of the antagonist per se.
Figure 3.
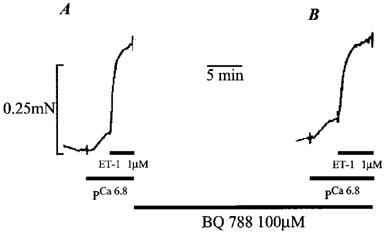
Effect of the ETB receptor antagonist BQ 788 on the ET-1 induced constriction of permeabilized pulmonary artery rings. (A) shows a submaximal constriction to pCa 6.8, and the subsequent constriction induced by 1 μM ET-1 in the continued presence of pCa 6.8. (B) shows the effect of a 15 min pre-incubation with 100 μM BQ 788 on the constriction to pCa 6.8 and 1 μM ET-1, respectively.
Effect of ET-1 on myosin light chain phosphorylation
Figure 4A shows the increase in myosin light chain phosphorylation following the incubation of α-toxin permeabilized pulmonary artery rings with pCa 6.8 solution for 7 min (time-matched to ET-1 treated samples). Three different myosin spots could be distinguished by this method; nonphosphorylated (NP), monophosphorylated (P1) and diphosphorylated (P2) myosin light chains. The diphosphorylated spot appears denser than the monophosphorylated spot due to the presence of unphosphorylated non-muscle myosin light chains which run at the same position as diphosphorylated muscle myosin light chains (Mougios & Barany, 1986). Previous studies of arterial smooth muscle have shown non-muscle myosin light chains to account for 6% of the total myosin (Kitazawa et al., 1991) and this has therefore been subtracted from the phosphorylated myosin calculations. The mean densitometric analysis of myosin light chain phosphorylation in pCa 6.8 was 11% (n=2 samples, n=4 preparations per sample). This is not the peak phosphorylation produced by pCa 6.8 as permeabilized tissues were exposed to Ca2+ buffer for 7 min (time-matched experiment) and phosphorylation has been shown to decrease quickly with prolonged exposure (Driska et al., 1981). Figure 4B shows the myosin light chain phosphorylation after exposure to pCa 6.8 and 1 μM ET-1. Clearly, ET-1 induced an increase in the monophosphorylated myosin light chains relative to control (pCa 6.8 alone), as would be expected for agonist-induced calcium sensitization (Kitazawa et al., 1991). Strips frozen at the peak of the ET-1 induced constriction produced a total phosphorylation (adjusted for non-muscle myosin) of 34% compared to the 11% observed in the controls (n=2 samples, n=4 preparations per sample). The Ca2+ sensitization induced by ET-1 in permeabilized pulmonary arterial rings results, therefore, from a 23% increase in myosin light chain phosphorylation.
Figure 4.
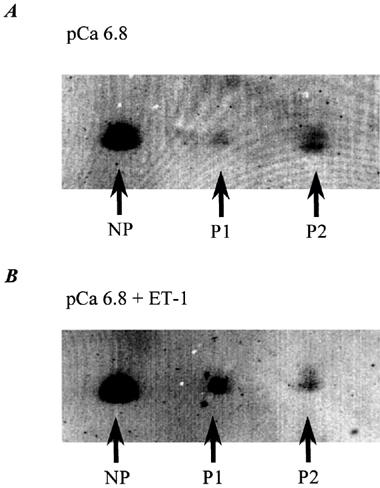
Effect of ET-1 on myofilament phosphorylation in permeabilized pulmonary artery rings. (A) shows the level of unphosphorylated (NP), monophosphorylated (P1) and diphosphorylated (P2) myosin light chains observed in the permeabilized pulmonary artery rings constricted by pCa 6.8 solution alone. (B) shows the level of unphosphorylated (NP), monophosphorylated (P1) and diphosphorylated (P2) myosin light chains observed in the permeabilized pulmonary artery rings constricted by pCa 6.8 solution and 1 μM ET-1. Both (A) and (B) represent the results from four permeabilized artery rings, which were rapidly frozen in liquid Freon 22 at the peak of the induced constriction (time matched at 7 min). The rings were dried and homogenized in glycerol sample buffer and subjected to two-dimensional gel electrophoresis. Resolved proteins were transferred on to nitrocellulose and the membrane stained with colloidal gold solution to reveal protein spots. The phosphorylation level of myofilament phosphorylation was quantitatively assessed using a Biorad GS690 densitometer.
Effect of the tyrosine kinase inhibitor Tyrphostin A23 on the response of permeabilized pulmonary arteries to ET-1
Figure 5A shows a control response to 1 μM ET-1 in an α-toxin permeabilized pulmonary artery ring which had been pre-constricted by raising the Ca2+ concentration to pCa 6.8. Figure 5B shows the constriction induced by 1 μM ET-1 following a 15 min pre-incubation with the tyrosine kinase inhibitor Tyrphostin A23 (100 μM). The constriction to 1 μM ET-1 was unaffected by the presence of 100 μM Tyrphostin A23, the increase in tension measuring 0.143±0.04 mN (43±10% of the maximum response to pCa 4.5 solution; n=4) in the absence and 0.124±0.06 mN (37±12% of the maximum response to pCa 4.5 solution; n=4) in the presence of 100 μM Tyrphostin A23. Although not significant, 100 μM Tyrphostin A23 was found to inhibit the pre-constriction to pCa 6.8 solution in three out of four preparations. The increase in tension induced by pCa 6.8 solution was 0.1±0.03 mN (30±8% of the maximum response to pCa 4.5 solution; n=4) in the absence and 0.069±0.03 mN (23±9% of the maximum response to pCa 4.5 solution; n=4) in the presence of 100 μM Tyrphostin A23. In addition, exposure of pulmonary arterial rings to 100 μM Tyrphostin A23 resulted in an increase in the resting tone in Ca2+ free solution to 20±4% of the maximum response to pCa 4.5 solution (n=4), which may in some way be related to the observed inhibition of the Ca2+-induced constriction.
Figure 5.
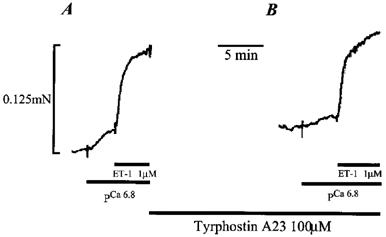
Effect of the tyrosine kinase inhibitor Tyrphostin A23 on the ET-1 induced constriction of permeabilized pulmonary artery rings. (A) shows a submaximal constriction to pCa 6.8, and a subsequent constriction induced by 1 μM ET-1 in the continued presence of pCa 6.8. (B) shows the effect of a 15 min pre-incubation with 100 μM Tyrphostin A23 on the constriction to pCa 6.8 and 1 μM ET-1, respectively.
Discussion
ET-1 increases myofilament calcium sensitivity in pulmonary artery rings by increasing myosin light chain 20 phosphorylation
We have investigated the possibility that ET-1 may induce a constriction of rat pulmonary arteries, at least in part, by increasing the sensitivity of the contractile apparatus to Ca2+. ET-1 was found to induce a sustained and reversible constriction in both β-escin and α-toxin permeabilized pulmonary artery rings, respectively, when the intracellular Ca2+ concentration was ‘clamped' at a defined level. Importantly, these preparations retain receptor coupled intracellular second messenger pathways, even though the intracellular Ca2+ concentration is not allowed to vary in response to applied agonists (Kitazawa et al., 1989; Iizuka et al., 1994). The constriction induced by ET-1 under these conditions must, therefore, result from an increase in the sensitivity of the contractile apparatus to Ca2+. Moreover, we have shown that the observed calcium sensitization likely results from a concomitant increase in phosphorylated MLC, as would be expected given the findings of previous investigations (e.g. Kitazawa et al., 1989). The concentration of ET-1 used to induce a significant increase in myofilament calcium sensitivity was 1–3 orders of magnitude greater than those concentrations required to produce near maximal constriction of intact (i.e. non-permeabilized) rat pulmonary resistance arteries by ourselves and others (Kato et al., 1999; McCulloch et al., 1998McCulloch et al., 1998). However, this is not unexpected as thorough permeabilization of smooth muscle preparations, including the larger conduit pulmonary arteries of the rabbit, results in a reduction in the efficiency of receptor coupling via a variety of receptor subtypes which probably results from the loss/dilution of intracellular messengers and enzymes with time (Himpens et al., 1989). Such a reduction in agonist potency is not, however, observed in poorly/partially permeabilized preparations, but such preparations do not offer a reliable ‘calcium clamp'.
The fact that ET-1 is capable of inducing an increase in myofilament calcium sensitivity may be of some significance when taken together with the fact that a vasoconstrictor-induced increase in myofilament Ca2+ sensitivity has been implicated in the development of hypoxic pulmonary vasoconstriction (HPV). As mentioned previously, the second phase of acute HPV requires the release of a vasoconstrictor from the intact endothelium (Leach et al., 1994) and appears to develop in the presence of a constant cytoplasmic free Ca2+ concentration (Robertson et al., 1995). Moreover, recent investigations have suggested that ET-1 may play an important role in the regulation of pulmonary vascular tone and may promote the development of hypoxic pulmonary hypertension (Chen et al., 1997; Bonvallet et al., 1994; Prie et al., 1997; Langleben et al., 1993; Yoshibayashi et al., 1991). Our findings suggest, therefore, that ET-1, or a related peptide, may be responsible for the increase in myofilament Ca2+ sensitivity associated with hypoxic pulmonary hypertension. Indirect support for this conclusion comes from the finding that pulmonary hypoxia raises plasma ET-1 levels by increasing the expression of ET-1 mRNA, and increases the release of ET-1 from pulmonary artery endothelial cells (Giaid et al., 1993; Elton et al., 1992; Li et al., 1994a; 1995). However, it should be noted that the suggestion that ET-1 acts as a mediator of HPV remains controversial and a number of authors have shown that ET receptor antagonists are unable to inhibit HPV and it has been proposed that an unidentified vasoconstrictor distinct from ET-1 may act as the primary mediator of HPV (Wadsworth, 1994; Douglas et al., 1993; Gaine et al., 1998; Ward & Robertson, 1995). Thus, it may be more likely that ET-1 contributes to the development of chronic hypoxic pulmonary hypertension rather than to the acute response to hypoxia (Chen et al., 1997; Bonvallet et al., 1994; Prie et al., 1997; Barton et al., 1998).
ETA but not ETB receptors mediate the increase in myofilament calcium sensitivity induced by ET-1
In contrast to the effects of ET-1, the selective ETB receptor agonist SX6C was found to induce little or no constriction when applied at high concentrations (100 nM) to permeabilized pulmonary artery rings, when the intracellular Ca2+ concentration was ‘clamped' at a defined level. This finding suggests that ETB receptor activation may not be directly coupled to those second messenger pathways which promote an increase in myofilament Ca2+ sensitivity. Further support for this conclusion was obtained from the finding that high concentrations (100 μM) of the selective ETB receptor antagonist BQ 788 failed to inhibit the increase in myofilament calcium sensitivity induced by ET-1. However, quite different findings were obtained with the selective ETA receptor antagonist BQ 123. Pre-incubation of the α-toxin permeabilized pulmonary artery rings with BQ 123, again at 100 μM, caused a marked inhibition (approximately 50%) of the ET-1 induced increase in myofilament Ca2+ sensitivity. These findings strongly suggest that the ET-1 induced increase in myofilament calcium sensitivity in resistance-sized rat pulmonary arteries is mediated by ETA receptors, but not by ETB receptors and that each receptor subtype may regulate discrete cellular events. These data also suggest that while ETA receptors may induce a constriction by raising the intracellular calcium concentration and also via inducing an increase in myofilament calcium sensitivity, ETB receptor activation may be exclusively coupled to pathways which mediate calcium influx and intracellular calcium release. This finding is not without significance with respect to HPV, as recent investigations have shown that new orally active ETA receptor antagonists attenuate HPV in rats, and suppress the development of hypoxic pulmonary hypertension (Chen et al., 1997; Bonvallet et al., 1994; Prie et al., 1997; Barton et al., 1998). Thus, we suggest that hypoxic pulmonary hypertension may be due, in part, to the release of ET-1, or a related peptide, from the endothelium in response to hypoxia and that the subsequent activation of ETA receptors on the smooth muscle cells increases myofilament Ca2+ sensitivity, resulting in a sustained vasoconstriction and an increase in pulmonary artery perfusion pressure. Interestingly, previous studies have suggested that ETA receptors are the primary mediators of the ET-1-induced constriction observed in human pulmonary arteries (Fukuroda et al., 1994; Buchan, 1994), although a more recent study has confirmed that vasoconstrictor ETB receptors are also present in human pulmonary resistance arteries (McCulloch et al., 1998).
E-1 induced myofilament Ca2+ sensitization may also be involved in post-natal adaption of the pulmonary vasculature, as plasma endothelin levels and ETA receptor density are both high at birth and exposure to hypoxia from birth prevents their normal reduction and may even increase ETA receptor numbers (Noguchi et al., 1997 ).
The tyrosine kinase pathway does not mediate the calcium sensitization induced by ET-1 in resistance sized rat pulmonary arteries
A recent investigation by Ohanian et al. (1997) suggested that ET-1 may induce an increase in myofilament Ca2+ sensitivity in mesenteric artery smooth muscle by activating a tyrosine kinase and by subsequent protein tyrosine phosphorylation. We, therefore, investigated the possibility that this pathway mediated the ET-1 induced calcium sensitization observed in rat pulmonary artery smooth muscle. We found that the selective tyrosine kinase inhibitor Tyrphostin A23 failed to inhibit the constriction of α-toxin permeabilized pulmonary artery rings induced by ET-1. In marked contrast, the same concentration of Tyrphostin A23 inhibited the ET-1 induced constriction of rat mesenteric arteries by approximately 50% (Ohanian et al., 1997). In agreement with the findings of Ohanian et al. (1997) we did, however, find that Tyrphostin A23 inhibited the Ca2+-induced constriction of α-toxin permeabilized pulmonary arteries. These findings suggest that ET-1 may increase myofilament Ca2+ sensitivity by a tyrosine kinase independent pathway in rat pulmonary artery smooth muscle, whilst increasing myofilament Ca2+ sensitivity through a tyrosine kinase dependent pathway in rat systemic (mesenteric) arteries.
We conclude that the ET-1-induced increase in Ca2+ sensitivity in pulmonary artery smooth muscle is primarily mediated by ETA receptors. Furthermore, our findings suggest that the observed increase in Ca2+ sensitivity results from an increase in phosphorylated MLC, which is mediated by a mechanism(s) independent of the tyrosine kinase pathway which has been proposed to mediate ET-1 induced Ca2+ sensitization in rat mesenteric arteries. These observations may be of some importance, as the identification of a distinct signal transduction mechanism associated with HPV and the development of pulmonary hypertension could lead to the development of new more effective therapies for this disorder.
Acknowledgments
This work was supported by the Wellcome Trust. Dr A. Mark Evans is a Wellcome Trust Career Development Research Fellow. We would also like to thank Professor A. David Smith for his support and encouragement, and Margaret Jackson for her helpful comments.
Abbreviations
- ET
endothelin
- ET-1
endothelin-1
- ETA
endothelin A receptor
- ETB
endothelin B receptor
- HPV
hypoxic pulmonary vasoconstriction
- Ca2+
calcium
- SX6C
sarafotoxin S6C
- MLC
myosin light chains
- [Ca2+]
calcium concentration
- GTP
guanosine 5′ Triphosphate
- NP
non-phosphorylated
- P1
monophosphorylated
- P2
diphosphorylated
References
- ALBARWANI S., TANDON K., XIA N., NYE P.C.G. Thapsigargin inhibits the first phase of hypoxic contraction of small isolated pulmonary vessels of the rat. J. Physiol. 1994;475:122P. [Google Scholar]
- ARAI H., HORI S., ARAMORI I., OHKUBA H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- BARTON M., D'USCIO L.V., SHAW S., MEYER P., MOREAU P., LUSCHER T.F. ETA receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension. 1998;31:499–504. doi: 10.1161/01.hyp.31.1.499. [DOI] [PubMed] [Google Scholar]
- BENNIE R.E., PACKER C.S., POWELL D.R., JIN N., RHOADES R.A. Biphasic contractile response of the pulmonary artery to hypoxia. Am. J. Physiol. 1991;261:L156–L163. doi: 10.1152/ajplung.1991.261.2.L156. [DOI] [PubMed] [Google Scholar]
- BONVALLET S.T., ZAMORA M.R., HASUNUMA K., SATO K., HANASATO N., ANDERSON D., SATO K., STELZNER T. BQ123, an ETA-receptor antagonist, attenuates hypoxic pulmonary hypertension in rats. Am. J. Physiol. 1994;266:H1327–H1331. doi: 10.1152/ajpheart.1994.266.4.H1327. [DOI] [PubMed] [Google Scholar]
- BUCHAN K.W., MAGNUSSON H., RABE K.F., SUMMER M.J., WATTS I.S. Characterization of the endothelin receptor mediating contraction of human pulmonary artery using BQ123 and Ro 46-2005. Eu. J. Pharmacol. 1994;260:221–226. doi: 10.1016/0014-2999(94)90340-9. [DOI] [PubMed] [Google Scholar]
- CHEN S.J., CHEN Y.-F., OPGENORTH T.J., WESSALE J.L., MENG Q.C., DURAND J., DICARLO V.S., OPARIL S. The Orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia induced pulmonary hypertension and pulmonary vascular remodeling in Sprague-Dawley rats. J. Cardiovascular Pharmacol. 1997;29:713–725. doi: 10.1097/00005344-199706000-00003. [DOI] [PubMed] [Google Scholar]
- CORNFIELD D., STEVENS T., MCMURTRY I., ABMAN S., RODMAN D. Acute hypoxia increases cytosolic calcium in fetal pulmonary artery smooth muscle cells. Am. J. Physiol. 1993;265:L1–L4. doi: 10.1152/ajplung.1993.265.1.L53. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., VICKERY-CLARK L.M., OHLSTEIN E.H. Endothelin-1 does not mediate hypoxic pulmonary vasoconstriction in canine isolated blood vessels: effect of BQ123. Br. J. Pharmacol. 1993;108:418–421. doi: 10.1111/j.1476-5381.1993.tb12819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRISKA S.P., AKSOY M.O., MURPHY R.A. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am. J. Physiol. 1981;240:C222–C233. doi: 10.1152/ajpcell.1981.240.5.C222. [DOI] [PubMed] [Google Scholar]
- ELTON T.S., OPARIL S., TAYLOR G.R., HICKS P.H., YANG R.-H., JIN H., CHEN Y.F. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. Am. J. Physiol. 1992;263:R1260–R1264. doi: 10.1152/ajpregu.1992.263.6.R1260. [DOI] [PubMed] [Google Scholar]
- ENOKI T., MIWA S., SAKAMOTO A., MINOWA T., KOMURA T., KOBAYASHI S., NINOMIYA H., MASAKI T. Long lasting activation of cation current by low concentration of endothelin-1 in mouse fibroblasts and smooth muscle cells of rabbit aorta. Br. J. Pharmacol. 1995;115:479–485. doi: 10.1111/j.1476-5381.1995.tb16358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS A.M., OSIPENKO O.N., GURNEY A.M. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. J. Physiol. 1996;496 2:407–420. doi: 10.1113/jphysiol.1996.sp021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKURODA T., KOBAYASHI M., OZAKI S., YANO M., MIYAUCHI T., ONIZUKA M., SUGISHITA Y., GOTO K., NISHIKIBE M. Endothelin receptor subtypes in human versus rabbit pulmonary arteries. J. Appl. Physiol. 1994;76:1976–1982. doi: 10.1152/jappl.1994.76.5.1976. [DOI] [PubMed] [Google Scholar]
- GAINE S.P., HALES M.A., FLAVAHAN N.A. Hypoxic pulmonary endothelial cells release a diffusable contractile factor distinct from endothelin. Am. J. Physiol. 1998;274:L657–L664. doi: 10.1152/ajplung.1998.274.4.L657. [DOI] [PubMed] [Google Scholar]
- GIAID A., YANAGISAWA M., LANGLEBEN D., MICHEL R.P., LEVY R., SHENNIB H., KIMURA S., MASAKI T., DUGUID W., STEWART D. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. Engl. J. Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- GOTO K., KASUYA Y., MATSUKI N., TAKUWA Y., KURIHARA H., ISHIKAWA T., KIUMURA S., YANAGISAWA M., MAKAKI T. Endothelin activates the dihydropyridine-sensitive voltage-dependent Ca2+ channel in vascular smooth muscle. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDER D., MADDEN J., DAWSON C. Hypoxic induction of C++-dependent action potentials in small pulmonary arteries of the cat. J. Appl. Physiol. 1985;59:1389–1393. doi: 10.1152/jappl.1985.59.5.1389. [DOI] [PubMed] [Google Scholar]
- HIGHSMITH R.F., BLACKBURN K., SCHMIDT D.J. Endothelin and calcium dynamics in vascular smooth muscle. Ann. Rev. Physiol. 1992;54:257–278. doi: 10.1146/annurev.ph.54.030192.001353. [DOI] [PubMed] [Google Scholar]
- HIMPENS B., MATTHIJS G., SOMLYO A.P. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J. Physiol. 1989;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIUTI K. Some properties of the contractile system and sarcoplasmic reticulum of skinned slow fibres from xenopus muscle. J. Physiol. 1986;373:1–23. doi: 10.1113/jphysiol.1986.sp016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIZUKA K., IKEBE M., SOMLYO A.V., SOMLYO A.P. Introduction of high molecular weight (IgG) proteins into receptor coupled, permeabilized smooth muscle. Cell Calcium. 1994;16:431–445. doi: 10.1016/0143-4160(94)90073-6. [DOI] [PubMed] [Google Scholar]
- JIN N., PACKER C.S., RHOADES R.A. Pulmonary arterial hypoxic contraction: signal transduction. Am. J. Physiol. 1992;263:L73–L78. doi: 10.1152/ajplung.1992.263.1.L73. [DOI] [PubMed] [Google Scholar]
- KASUYA Y., TAKUWA Y., YANAGISAWA M., KIMURA S., GOTO K., MASAKI T. Endothelin I induces vasoconstriction through two functionally distinct pathways in porcine coronary artery: contribution of phosphoinositide turnover. Biochem. Biophys. Res. Commun. 1989;161:1049–1055. doi: 10.1016/0006-291x(89)91349-1. [DOI] [PubMed] [Google Scholar]
- KATO K., EVANS A.M., KOZLOWSKI R.Z.K. Reversal of ET-1 mediated small pulmonary arterial constriction by niflumic acid and 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) does not involve chloride channels. J. Pharm. Exp. Ther. 1999;288:1242–1250. [PubMed] [Google Scholar]
- KITAZAWA T., GAYLINN B.D., DENNY G.H., SOMLYO A.P. G-protein mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1991;266:1708–1715. [PubMed] [Google Scholar]
- KITAZAWA T., KOBAYASHI S., HORIUCHI K., SOMLYO A.V., SOMLYO A.P. Receptor-coupled, permeabilized smooth muscle. J. Biol. Chem. 1989;264:5339–5342. [PubMed] [Google Scholar]
- KOVITZ K.L., ALESKOWITCH J.T., SYLVESTER J.T., FLAVAHAN N.A. Endothelium-derived contracting and relaxing factors contribute to hypoxic responses of pulmonary arteries. Am. J. Physiol. 1991;260:L516–L521. doi: 10.1152/ajpheart.1993.265.4.H1139. [DOI] [PubMed] [Google Scholar]
- LANGLEBEN D., DEMARCHIE M., LAPORTA D., SPANIER A.H., SCHLESINGER R.D., STEWART D.J. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993;148:1646–1650. doi: 10.1164/ajrccm/148.6_Pt_1.1646. [DOI] [PubMed] [Google Scholar]
- LEACH R.M., ROBERTSON T.P., TWORT C.H.C., WARD J.P.T. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am. J. Physiol. 1994;266:L223–L231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- LI H.B., CHEN S.J., CHEN Y.F., OPARIL S. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J. Appl. Physiol. 1994b;77:1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- LI H.B., CHEN S.J. , ELTON T.S., OPARIL S. Hypoxia stimulates endothelin-1 gene expression in human pulmonary microvessel endothelial cells by a mechanism that involves a heme containing protein. J. Invest. Med. 1995;43 suppl. 1:45(A). [Google Scholar]
- LI H.B., ELTON T.S., CHEN S.J., OPARIL S. Increased endothelin receptor gene expression in hypoxic rat lung. Am. J. Physiol. 1994a;266:L553–L560. doi: 10.1152/ajplung.1994.266.5.L553. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH K.M., DOCHERTY C.C., MACLEAN M.R. Endothelin receptors mediating contraction of rat and human pulmonary resistance arteries: effects of chronic hypoxia in the rat. Br. J. Pharmacol. 1998;123:1621–1630. doi: 10.1038/sj.bjp.0701785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH K.M., DOCHERTY C.C., MOORECROFT I., MACLEAN Endothelin B receptors mediate contraction of rat and human pulmonary and resistance arteries. Br. J. Pharmacol. 1998;119:1125–1130. doi: 10.1111/j.1476-5381.1996.tb16013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADDEN J., VADULA M., KURUP V. Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am. J. Physiol. 1992;263:L384–L393. doi: 10.1152/ajplung.1992.263.3.L384. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., ROUS B.A., DAVENPORT A.P. Failure of BQ123, a more potent antagonist of sarafotoxin 6b than of endothelin-1, to distinguish between these agonists in binding experiments. Br. J. Pharmacol. 1996;118:335–342. doi: 10.1111/j.1476-5381.1996.tb15407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCMURTRY I., DAVIDSON B., REEVES J., GROVER R. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ. Res. 1976;38:99–104. doi: 10.1161/01.res.38.2.99. [DOI] [PubMed] [Google Scholar]
- MOUGIOS V., BARANY M. Isoforms of the phosphorylatable myosin light chain in arterial smooth muscle. Biochem. Biophys. Acta. 1986;872:305–308. doi: 10.1016/0167-4838(86)90284-0. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., MORELAND S., AHH H.Y., KAWASE T., MORELAND R.S., VANBREEMAN Endothelin increases Ca2+ sensitivity in alpha-toxin-permeabilized rabbit mesenteric artery. Circ. Res. 1992;71:951–959. doi: 10.1161/01.res.71.4.951. [DOI] [PubMed] [Google Scholar]
- NIXON G.F., IIZUKA K., HAYSTEAD T.A.J., SOMLYO A.P., SOMLYO A.V. Phosphorylation of caldesmon by mitogen activated protein kinase with no effect on Ca2+ sensitivity in smooth muscle. J. Physiol. 1995;487:283–290. doi: 10.1113/jphysiol.1995.sp020879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOGUCHI Y., HISLOP A.A., HAWORTH S.G. Influence of hypoxia on endothelin-1 binding sites in neonatal porcine pulmonary vasculature. Am. J. Physiol. 1997;272:H669–H678. doi: 10.1152/ajpheart.1997.272.2.H669. [DOI] [PubMed] [Google Scholar]
- OHANIAN J., OHANIAN V., SHAW L., BRUCE C., HEAGERTY AM. Involvement of tyrosine phosphorylation in endothelin-1-induced calcium-sensitization in rat small mesenteric arteries. Br. J. Pharmacol. 1997;120:653–661. doi: 10.1038/sj.bjp.0700950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSIPENKO O.N., EVANS A.M., GURNEY A.M. Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current. Br. J. Pharmacol. 1997;120:1461–1470. doi: 10.1038/sj.bjp.0701075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANG D.C., JOHNS A., PATTERSON K., BOTELHO L.H., RUBANYI G.M. Endothelin-1 stimulates phosphatidylinositol hydrolysis and calcium uptake in isolated canine coronary arteries. J. Cardiovasc. Pharmacol. 1989;13 suppl. 5:S75–S79. doi: 10.1097/00005344-198900135-00018. [DOI] [PubMed] [Google Scholar]
- POST J., HUME J., ARCHER S., WEIR E. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am. J. Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- PRIE S., LEUNG T.K., CERNACEK P., RYAN J.W., DUPUIS J. The orally active ETA receptor antagonist (+)-(S)-2-(4,6-dimethoxy-pyrimidin-2-yloxyl)-3 -methoxy-3,3-diphenyl-propionic acid (Lu 135252) prevents the development of pulmonary hypertension and endothelial metabolic dysfunction in monocrotaline treated rats. J. Pharmacol. Exp. Ther. 1997;282:1312–1318. [PubMed] [Google Scholar]
- ROBERTSON T.P., AARONSON P.I., WARD J.P.T. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am. J. Physiol. 1995;268:H301–H307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO A., YANAGISAWA M., SAKURAI T., TAKUWA Y., YANAGISAWA H., MASAKI T. Cloning and functional expression of human cDNA for the ETB endothelin receptor. Biochem. Biophys. Res. Commun. 1991;178:656–663. doi: 10.1016/0006-291x(91)90158-4. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., TAKUWA Y., MIYAZAKI H., KIMURA S., GOTO K., MASAKI T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- SALVATERRA C., GOLDMAN W. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am. J. Physiol. 1993;264:L323–L328. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- VADULA M., KLEINMAN J., MADDEN J. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am. J. Physiol. 1993;265:L591–L597. doi: 10.1152/ajplung.1993.265.6.L591. [DOI] [PubMed] [Google Scholar]
- WADSWORTH R.M. Vasoconstrictor and vasodilator effects of hypoxia. Trends. Pharmacol. Sci. 1994;15:47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- WARD J.P.T., ROBERTSON T.P. The role of the endothelium in hypoxic pulmonary vasoconstriction. Experimental Physiol. 1995;80:793–801. doi: 10.1113/expphysiol.1995.sp003887. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- YOSHIBAYASHI M., NISHIOKA K., NAKAO K., SAITO Y., MATSUMURA M., UEDA T., TEMMA S., SHIRAKAMI G., IMURA H., MIKAWA H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects; Evidence for increased productions of endothelin in pulmonary circulation. Circulation. 1991;84:2280–2285. doi: 10.1161/01.cir.84.6.2280. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M., SUZUKI A., IOH T. Mechanisms of vasoconstriction induced by endothelin-1 in smooth muscle of rabbit mesenteric artery. J. Physiol. 1994;477:253–265. doi: 10.1113/jphysiol.1994.sp020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN X-J., GOLDMAN W., TOD M., RUBIN L., BLAUSTEIN M. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am. J. Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]


