Abstract
We investigated the effects of recombinant human erythropoietin (rh-EPO) in splanchnic artery occlusion (SAO) shock. Sham operated animals were used as controls. Survival rate, mean arterial blood pressure (MAP), serum Tumor Necrosis Factor (TNF-α), plasma nitrite/nitrate concentrations, red blood cell (RBC) count, blood haemoglobin (Hb), the responsiveness of aortic rings to phenylephrine (PE, 1 nM–10 μM) and the activity of inducible nitric oxide synthase (iNOS) were studied.
SAO shocked rats had a decreased survival rate (0% at 4 h of reperfusion, while sham shocked rats survived more than 4 h), enhanced serum TNF-α concentrations, increased plasma nitrite/nitrate levels (60±9.5 μM; sham shocked rats=2±0.4 μM), decreased MAP, unchanged RBC count and blood Hb and enhanced iNOS activity in the aorta. Moreover aortic rings from shocked rats showed a marked hyporeactivity to PE.
Rh-EPO (25, 50 and 100 U 100 g−1, 5 min following the onset of reperfusion) increased survival rate (70% at 4 h of reperfusion with the highest dose), reduced plasma nitrite/nitrate concentrations (10.3±3.3 μM), increased MAP, did not change RBC count and blood Hb, and inhibited iNOS activity in thoracic aortae. Furthermore rh-EPO, either in vivo or in vitro (10 U for 1 h in the organ bath), restored to control values the hyporeactivity to PE. Finally rh-EPO inhibited the activity of iNOS in peritoneal macrophages activated with endotoxin.
Our data suggest that rh-EPO protects against SAO shock by inhibiting iNOS activity.
Keywords: Splanchnic artery occlusion shock, vascular dysfunction, recombinant human erythropoietin, inducible nitric oxide synthase
Introduction
Erythropoietin, a sialoglycoprotein hormone produced primarily by the adult kidney, is a major regulator of red blood cell production in mammals, stimulating the proliferation of committed erythroid progenitor cells and their development into mature erythrocytes (Jelkmann, 1992). The potential benefits of replacement erythropoietin therapy in patients with anaemia associated with chronic renal failure have long been recognized (Erslev, 1991).
Recombinant human erythropoietin (rh-EPO) has been produced following isolation of the human gene and its expression in a Chinese hamster ovary cell line (Jacobs et al., 1985). The protein has an apparent molecular weight of about 30.4 kilodaltons, appears to be immunologically equivalent to the endogenous hormone, exhibits full biological activity and does not show species boundary (Egrie et al., 1986; Recny et al., 1987).
Besides its haematopoietic effects, it has been suggested that rh-EPO may also have cardiovascular effects (Neff et al., 1971; Casati et al., 1987; Raine, 1988; Mayer et al., 1989).
The increase in blood pressure directly after injection of rh-EPO, in spite of a slow increase of haematocrit, suggests that discrete pressure mechanisms may be involved (Edmunds & Walls, 1988; Acquit et al., 1987). Indeed it has been proposed that rh-EPO possesses a direct vasopressor effect on isolated vessels (Heidenreich, 1991). The rh-EPO pressor effects, however, are not always evident, and this suggests that they may depend upon some vascular mechanism(s) regulating blood pressure which may be turned on or off in subjects receiving the haematopoietic factor.
Based on these findings, previous data have pointed out that rh-EPO administration in rats subjected to hypovolaemic haemorrhagic shock increases blood pressure and enhances the resistance of animals to this experimental procedure (Buemi et al., 1993). In contrast no pressure effect was observed in sham shocked animals following the anti-shock dose of 100 U 100 g−1. However this preliminary study did not investigate the mechanism underlying the beneficial effect on vascular dysfunction.
Occlusion of the major splanchnic arteries followed by reperfusion in anaesthetized rats results in an irreversible circulatory failure and shock (splanchnic artery occlusion shock; SAO shock) (Squadrito et al., 1994b). It has been demonstrated that in SAO shock a marked and composite vascular dysfunction is present in which the L-arginine/nitric oxide (NO) pathway plays an important role (Squadrito et al., 1994a). Indeed aortic rings from shocked rats showed a marked hyporeactivity to phenylephrine and removal of endothelium did not restore the phenylephrine-induced contractile response to the values of the sham animals, thus suggesting that smooth muscle cells are involved in the hyporesponsiveness to phenylephrine. This complex dysfunction is probably the result of an increase in the endogenous NO produced by the inducible NO synthase.
Therefore the aim of our study was to investigate whether rh-EPO exerts protective effects on the pathological sequelae associated with SAO shock, by modulating vascular dysfunction. We found that rh-EPO may inhibit the activity of inducible nitric oxide synthase and therefore it may represent a new treatment for acute vascular failure during shock.
Methods
Animal preparation
Male Sprague-Dawley rats weighing 200–250 g were permitted access to food and water ad libitum. The rats were anaesthetized with urethane (1.3 g kg−1, i.p.). After midline laparotomy, the coeliac and superior mesenteric arteries were isolated near their aortic origins. During this procedure, the intestinal tract was maintained at 37°C by placing it between gauze pads soaked with warmed 0.9% NaCl solution. Rats were given heparin (1000 U kg−1, i.v.) and were observed for a 30 min stabilization period prior to either splanchnic ischaemia or sham ischaemia. Splanchnic artery ischaemia-reperfusion injury (SAO) was induced by clamping both the superior mesenteric artery and the coeliac trunk so as to produce a total occlusion of these arteries for 45 min. The clamps were then removed. Following reperfusion the rats were observed for 4 h. Sham-operated rats were subjected to the same surgical procedures as SAO rats except the arteries were not occluded.
Survival evaluation and arterial blood pressure monitoring
A first group of animals (n=90) was used to study survival and arterial blood pressure. These animals were implanted with cannulae (PE 50) into the left common carotid artery, as described elsewhere (Caputi et al., 1980). The arterial catheter was connected to a pressure transducer. The pressure pulse triggered a cardiotachometer, and arterial blood pressure was displayed on a polygraph. Arterial blood pressure is reported as mean arterial pressure (MAP) in mmHg.
Rh-EPO (25, 50 and 100 U 100 g−1) or vehicle (1 ml kg−1) were injected 5 min following the onset of reperfusion. This range of doses was chosen because it does not cause any significant change in blood pressure of anaesthetized sham shocked rats (Buemi et al., 1993). Survival was evaluated for 4 h after onset of reperfusion and expressed either as survival rate or survival time. Animals surviving at 4 h were sutured and allowed to recover from anaesthesia. In these animals survival was monitored for an additional 20 h.
Biological assay for TNF-α activity
Killing of L929 mouse tumour cells was used to measure TNF-α levels in serum on the basis of a standard micro-Elisa assay (Squadrito et al., 1994a). L929 cells in RPMI 1640 medium containing 5% foetal calf serum were seeded at 3×10−4 cells per well in 96-well microdilution plates and incubated overnight at 37°C in an atmosphere of 5% CO2 in air. Serial 1 : 2 dilutions of serum (drawn at different time points) were made in the above-described medium containing 1.0 μg of actinomycin D per ml and 100 μl volumes of each dilution were added to the wells. One TNF-α unit was defined as the amount giving 50% cell cytotoxicity. The TNF-α content in the sample was calculated by comparison with a calibration curve obtained with recombinant murine TNF-α.
Isolated aortic rings
Thoracic aorta were removed 80 min after reperfusion and placed in cold Krebs' solution of the following composition (nM): NaCl 118.4, KCL 4.7, MgSO4 1.2, CaCl2 2.5, KH2PO4 1.2, NaHCO3 25.0 and glucose 11.7; then aortae were cleaned of adherent connective and fat tissue and cut into rings of approximately 2 mm in length. In some rings, the vascular endothelium was removed mechanically by gently rubbing the luminal surface with a thin wooden stick. Rings were then placed under 1 g of tension in an organ bath containing 10 ml of Krebs' solution at 37°C and bubbled with 95% O2 and 5% CO2 (pH 7.4). All experiments were carried out in the presence of indomethacin (10 μM) in order to exclude the involvement of prostaglandins and their metabolites. Developed tension was measured with an isometric force transducer and recorded on a polygraph (Ugo Basile, Varese, Italy). After an equilibration period of 60 min during which time the rings were washed with fresh Krebs' solution at 15–20 min intervals and basal tension was readjusted to 1 g, the tissue was exposed to phenylephrine (PE, 100 nM). When the contraction was stable, the functional integrity of endothelium was assessed by a relaxant response to acetylcholine (ACh, 100 nM). The tissue was then washed occasionally for 30 min. Concentration-response curves were obtained by cumulative concentrations of PE (1 nM–10 μM) to intact or endothelium denuded aortic rings. The results (mean±s.e.mean) are expressed as g of tension mg−1 tissue.
Evaluation of red blood cell count and haemoglobin
Peripheral blood for smears to evaluate the absolute number of circulating red cells and for haemoglobin was obtained by tail bleeding at 0 min before the initiation of reperfusion and at 80 min after reperfusion. Red blood cell count was performed on stained smears, according to the standard morphological criteria for the rat. Blood haemoglobin was evaluated as previously described (Hulse, 1964).
Nitrite/nitrate measurement
Nitric oxide release was determined spectrophotometrically by measuring the accumulation of both nitrite and nitrate (the latter is reduced to nitrite) in plasma. Nitrate was stoichometrically reduced to nitrite by incubation of sample (100 μl plasma) for 2 h at 37°C, in the presence of 0.1 unit ml−1 nitrate reductase (NAD[P]H: nitrate oxidoreductase, EC 1.6.6.2; Aspergillus species; Sigma Chemical Co. St. Louis, MO, U.S.A.) 120 μM, NADPH and 5 μM FAD (flavinadenine dinucleotide, Sigma Chemical Co. St. Louis, MO, U.S.A.) in final volume of 103 μl. After nitrate had been reduced to nitrite, NADPH which interfered with the subsequent nitrite determination was oxidized with 10 units ml−1 L-lactic dehydrogenase (EC 1.1.1.27; type XI; from rabbit muscle; Sigma Chemical Co. St. Louis, MO, U.S.A.) and 10 mM sodium pyruvate for 30 min at 37°C in a final volume of 114 μl. Sodium nitrate was used as a standard. Nitrite/nitrate concentration in plasma was assayed by a standard Griess reaction (Ding et al., 1988). Briefly, 100 μl plasma was incubated with an equal volume of Griess reagent (1% sulpanilamide/0.1% napthylenediamine dihydrochloride/2.5% H3PO4) at room temperature for 10 min. The absorbance of the chromophore formed was determined at 540 nm using a microtiter plate reader. Sodium nitrite was used as a standard with control baseline plasma as a blanck.
To study the effects of rh-EPO on the inducible nitric oxide (iNOS) activity, peritoneal rat macrophages (harvested from normal rats) were cultured in Dulbecco's modified Eagle's medium (DMEM) with L-glutamine (4×10−3 M) and 10% foetal calf serum (FCS) as previously described (Szabo' et al., 1994). Each sample contained 1×10−6 cells per ml. To induce nitric oxide synthase (iNOS), fresh culture medium containing E. coli lipopolysaccharide (LPS, 50 μg ml−1) was added. Nitrite accumulation in the cell culture medium was measured after 24 h. To study the effects of rh-EPO on the production of nitrite, it was added to the medium 6 h after induction of iNOS with LPS. At this time there is no detectable increase in the concentration of nitrite, and agents such as glucocorticoids, that inhibit the induction but not the activity of iNOS, have no effect on subsequent nitrite production (Szabo' et al., 1994). Nitrite production, an indicator of NO synthesis, was measured in the supernatant of macrophages, as above described.
iNOS activity assay
Thoracic aortae were removed 80 min following the onset of reperfusion. Frozen tissues were homogenized on ice in Tris buffer (in mM: Tris HCL (pH 7.4) 50, EDTA 0.1, EGTA 0.1, 2-mercaptoethanol 12, phenylmethyl-sulfonyl fluoride 1). To determine calcium-dependent iNOS activity in the homogenates, production of L-[3H]-citrulline from L-[3H]-arginine (7,4 kBq per tube) was measured in the presence of 10 μM L-arginine/1 mM NADPH/300 units of calmodulin per ml/5 μM tetrahydrobiopterin/50 mM L-valine/1 mM EGTA for 30 min at room temperature. Reactions were stopped by adding 1 ml of ice cold HEPES, pH 5.5 (20 mM), EDTA (2 mM), EGTA (2 mM). After separation by using Dowex 50W (sodium form), L-[3H]-citrulline activity was measured by counting scintillation (Beckman analytical instruments S.p.A, Milan Italy). Experiments performed in the absence of NADPH determined the extent of L-[3H]-citrulline formation independent of iNOS activity.
Drugs
Acetylcholine chloride, phenylephrine hydrochloride, indomethacin, EGTA, EDTA, NADPH, nitrate reductase, sodium nitrate, L-Arginine, and Dowex 50W anion exchange resin were obtained from Sigma Chemical Co., St. Louis, MO, U.S.A. Rh-EPO was a kind gift from Janssen-Cilag (S.p.A, Latina, Italy). Specific monoclonal antibodies against erytropoietin (mAb EPO) were obtained from Genzyme (Cambridge, England). Mercaptoethylguanidine (MEG) was a kind gift from Dr Salvatore Guarini, (University of Modena, Italy).
Statistical analysis
Data are expressed as means±s.e.mean and were analysed by analysis of variance for multiple comparison of results. Duncan's multiple range test was used to compare group means. In all cases, a probability error of less than 0.05 was selected as criterion for statistical significance. For survival data, statistical analysis was done with Fisher's exact probability test.
Results
Survival
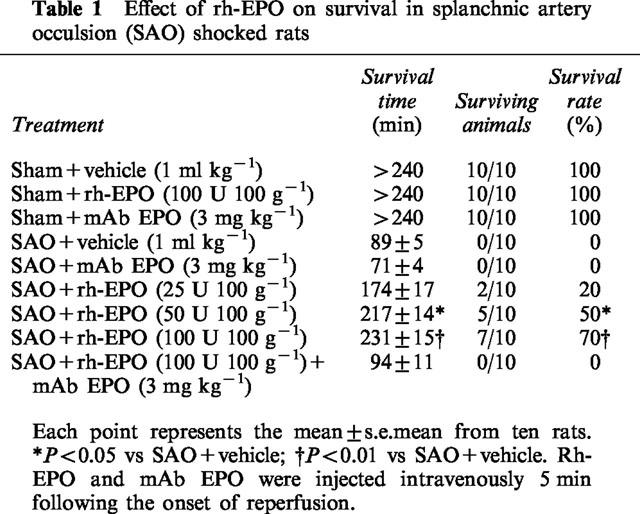
Table 1 summarizes survival rate, percentage survival and survival time for the groups of rats subjected to splanchnic ischaemia-reperfusion injury or sham ischaemia. All sham rats survived the entire 4 h observation period. In contrast in rats treated with the vehicle, occlusion and reperfusion of the splanchnic region produced a profound shock state characterized by a high lethality: no rat survived at 2 h of reperfusion (survival time=89±5 min). Administration of rh-EPO increased in a dose-dependent manner survival rate and time in SAO rats (Table 1). The most effective dose was 100 U 100 g−1 and therefore we used it in the further studies. Furthermore rats surviving at 4 h (these animals were sutured and allowed to recover from anaesthesia) were still alive 24 h after the surgical procedure. Shocked rats treated with a specific antibody raised against erythropoietin (mAb EPO; 3 mg kg−1) had a reduced resistance to the experimental procedure of splanchnic artery occlusion shock (Table 1). Furthermore mAb EPO (injected simultaneously with rh-EPO) abrogated the beneficial effects of the glycoproteic hormone in this form of experimental shock (Table 1).
Table 1.
Effect of rh-EPO on survival in splanchnic artery occulsion (SAO) shocked rats
Serum TNF-α
Serum TNF-α levels were undetectable in sham operated rats treated either with vehicle or the highest dose of rh-EPO (results not shown). Figure 1 depicts the time course of the pleiotropic cytokine during splanchnic artery occlusion shock. The serum levels of TNF-α promptly rose 20 min following the onset of occlusion and remained sustained for the entire duration of the experimental protocol (Figure 1). Administration of rh-EPO did not significantly modify the serum levels of the inflammatory cytokine (Figure 1).
Figure 1.
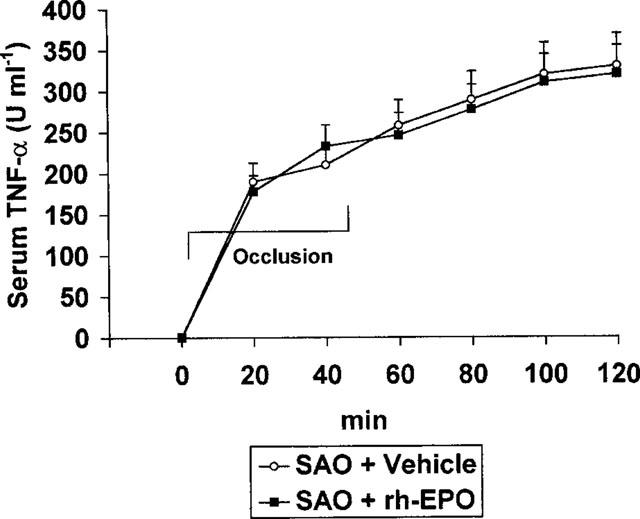
Effects of vehicle (1 ml kg−1 i.v., 5 min after the onset of reperfusion) or rh-EPO (100 U 100 g−1 i.v., 5 min following the onset of reperfusion) on serum TNF-α in rats subected to splanchnic ischaemia-reperfusion injury (SAO). Each point represents the mean±s.e.mean of seven experiments.
Plasma nitrite/nitrate
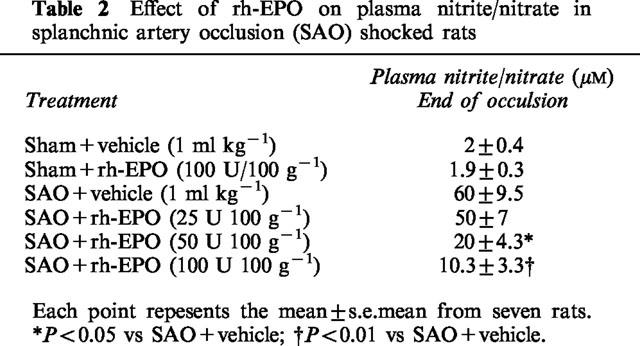
Plasma levels of nitrite/nitrate were very low in sham operated rats treated either with vehicle or the highest dose of rh-EPO (Table 2). Nitrite/nitrate levels were significantly increased in plasma collected from SAO rats at the end of the reperfusion period. The administration of rh-EPO blunted in a dose dependent manner plasma nitrite/nitrate concentrations (Table 2).
Table 2.
Effect of rh-EPO on plasma nitrite/nitrate in splanchnic artery occlusion (SAO) shocked rats
Red blood cell (RBC) count and blood haemoglobin
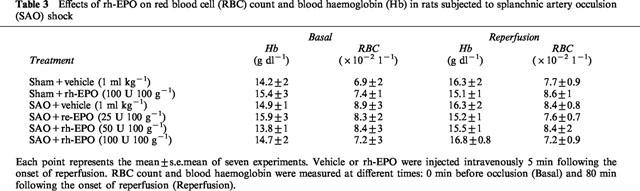
RBC count and haemoglobin were measured in rats at different times: 0 min before occlusion (basal; at the beginning of the experiment) and 80 min following the onset of reperfusion. RBC count and haemoglobin were not changed by the surgical procedures of SAO shock (Table 3). In addition rh-EPO did not modify RBC count and blood haemoglobin either in sham rats or in SAO shocked rats (Table 3).
Table 3.
Effects of rh-EPO on red blood cell (RBC) count and blood haemoglobin (Hb) in rats subjected to splanchnic artery occulsion (SAO) shock
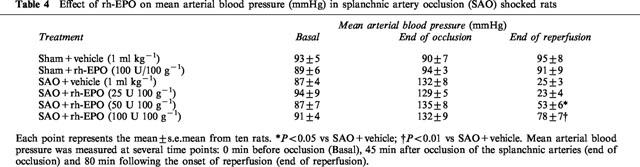
Mean arterial blood pressure
Occlusion of the splanchnic arteries produced a marked increase in mean arterial blood pressure. Subsequently mean arterial blood pressure decreased upon the release of the occlusion (Table 4). The administration of rh-EPO significantly blunted the reduction in mean arterial blood pressure (Table 4).
Table 4.
Effect of rh-EPO on mean arterial blood pressure (mmHg) in splanchnic artery occlusion (SAO) shocked rats
Vascular reactivity
In endothelium denuded aortic rings prepared from SAO rats, the contractile response to PE (1 nM–10 μM) was significantly reduced. The maximum force of contraction induced by 10 μM PE in aortic rings from sham rats was 1.9±0.2 g mg−1 tissue, whereas it was 1.05±0.1 g mg−1 tissue in rings from SAO shocked rats (Figure 2). Administration of rh-EPO (100 U 100 g−1) improved the impaired contractile response to PE in SAO shocked rats (Figure 2).
Figure 2.
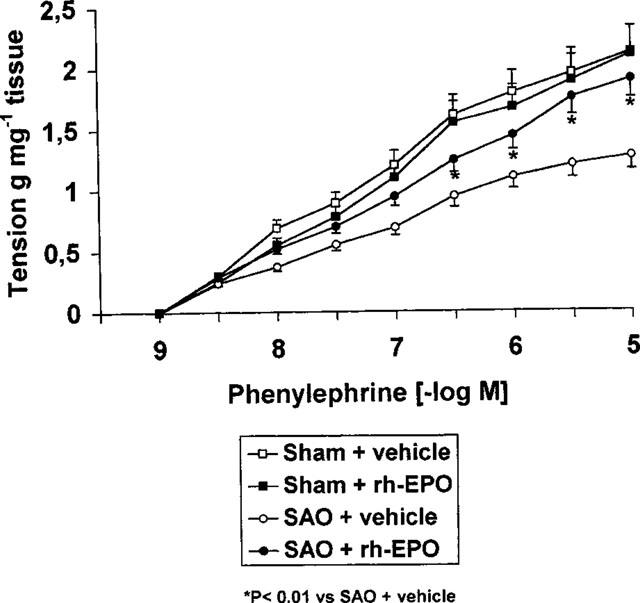
Contractile response to cumulative doses of phenylephrine (PE) in endothelium denuded aortic rings from sham-operated rats and rats subjected to splanchnic ischaemia-reperfusion injury (SAO) treated with vehicle (1 ml kg−1 i.v., 5 min after the onset of reperfusion) or rh-EPO (100 U 100 g−1 i.v. 5 min following the onset of reperfusion). Each point represents the mean±s.e.mean of seven experiments. *P<0.01 vs SAO+vehicle.
In endothelium denuded aortic rings from shocked rats rh-EPO (10 U) added for 1 h in the organ bath restored PE-sensitivity to control value (Figure 3), while it did not modify the contractile response to PE of aortic rings collected from sham rats (Figure 3).
Figure 3.
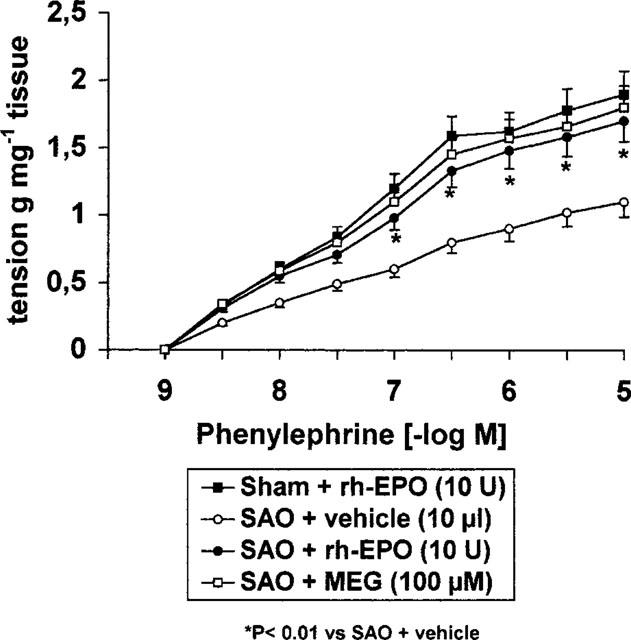
Contractile response to cumulative concentrations of phenylephrine in aortic rings without endothelium from untreated sham occluded or splanchnic artery occlusion (SAO) shocked rats. The rings were incubated for 1 h with rh-EPO (10 U), vehicle (10 μl) or MEG (100 μM). Each point represents the mean±s.e.mean of six experiments. *P<0.01 vs vehicle.
Incubation of endothelium-denuded aortic rings from shocked rats with mercaptoethylguanidine (MEG; 100 μM) also resulted in a marked increase in the contractile response to PE (Figure 3).
Nitric oxide (iNOS) activity
Splanchnic artery occlusion shock resulted in a significant increase in iNOS activity in thoracic aortae removed 80 min following the onset of reperfusion (Figure 4).
Figure 4.
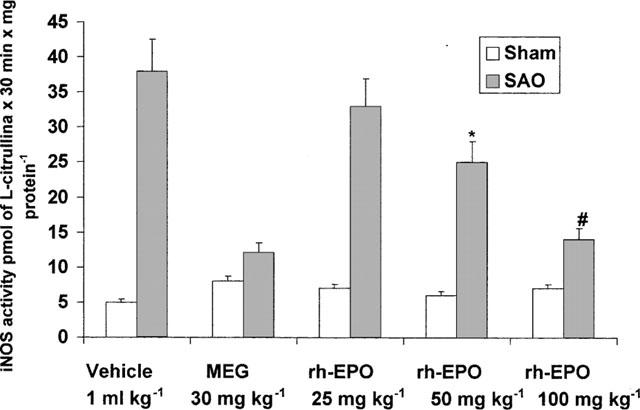
Effect of rh-EPO on iNOS activity in aortae collected 80 min following the onset of reperfusion in splanchnic artery occlusion (SAO) shocked rats. Each point represents the mean±s.e.mean from 3–5 rats. *P<0.05 vs SAO+vehicle: #P<0.01 vs SAO+vehicle. Rh-EPO (i.v.) or MEG (i.p.) were inected 5 min following the onset of reperfusion.
In contrast no significant change in iNOS activity was observed in sham operated rats treated either with vehicle or rh-EPO (Figure 4). Administration of either rh-EPO or MEG blunted the activity of iNOS in shocked animals (Figure 4).
To further investigate the potential effects of rh-EPO on iNOS activity, the ability of the haematopoietic factor to inhibit nitrite production in activated macrophages was tested. Endotoxin significantly increased nitrite production (Figure 5). Rh-EPO, when applied 6 h after lipopolysaccharide (LPS) significantly blunted nitrite production by stimulated macrophages. At this time there is no detectable increase in the concentration of nitrite, and agents such as glucocorticoids, that inhibit the induction but not the activity of iNOS, have no effect on subsequent nitrite production (Szabo' et al., 1994). To confirm the idea that rh-EPO does not influence the induction of iNOS, the haematopoietic hormone was added in the macrophage culture simultaneously together with endotoxin. Rh-EPO did not modify LPS-induced nitrite release under these experimental conditions (Figure 5).
Figure 5.
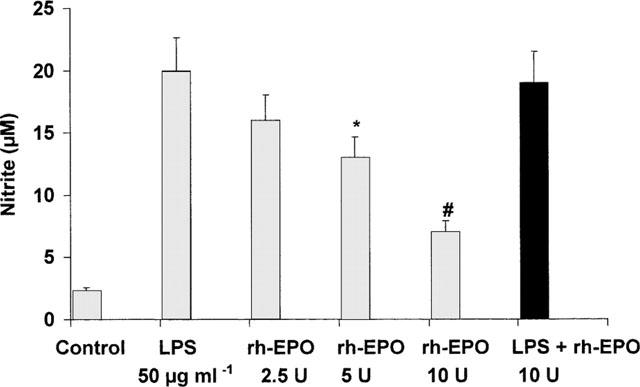
Effect of rh-EPO on nitrite accumulation in the supernatant of the cultured macrophages activated with endotoxin (LPS). Data are expressed as means±s.e.mean of eight wells from 3–4 independent experiments. Macrophages were stimulated for 24 h with LPS and rh-EPO was applied 6 h after LPS. The black column depicts the effect of rh-EPO incubated simultaneously together with LPS without any previous stimulation. *P<0.05 vs LPS+vehicle; #P<0.001 vs LPS+vehicle.
Discussion
Our data confirm that rh-EPO possesses beneficial effects in low-flow state, as previously showed in acute haemorrhage (Buemi et al., 1993) and furthermore add new information on the underlying mechanism of action.
We found that rh-EPO administration was able to increase the resistance of rats to the pathophysiological consequence of SAO shock: the effect was remarkable in terms of survival rate and improvement in vascular failure. Indeed this latter parameter was positively affected by rh-EPO treatment: in fact the hematopoietic factor, injected 5 min after the onset of reperfusion, produced a marked increase in blood pressure and furthermore aortic rings of rh-EPO treated rats exhibited a greater contractile response to phenylephrine. Furthermore mAb EPO treatment abrogated the beneficial effects of rh-EPO in SAO shock, thus confirming the specificity of the protective effect. Taken together these findings suggest that rh-EPO was able to revert vascular dysfunction that occurs during shock.
The mechanisms underlying the irreversible circulatory failure have been the subject of intense investigations.
It has been proposed that the L-arginine/nitric oxide (NO) pathway plays an important role in the pathogenesis of circulatory shock. In fact the production of large amount of NO by the inducible isoform of NOS (iNOS) contributes to the delayed vascular decompensation and to the hyporeactivity of the vasculature to vasoconstrictor agents observed in several experimental models of circulatory shock (Szabo' & Thiemermann, 1994).
As far as splanchnic artery occlusion shock is concerned, previous findings have indicated that an increase in NO derived by the iNOS plays an important role in the pathophysiology of this type of circulatory shock (Squadrito et al., 1996). In agreement with this hypothesis, selective inhibitors of iNOS display beneficial effects in SAO shock (Squadrito et al., 1996). The present study extends and confirms the previous data: in fact our shocked rats showed a marked increase in iNOS activity in the thoracic aortae collected at the end of reperfusion.
The increased formation of NO by the iNOS implies that this inducible form of NOS must be expressed in response to certain immunological stimuli. TNF-α induces the expression of iNOS in a number of cells including fibroblasts, glial cells, cardiac myocytes and more specifically vascular cells. iNOS requires at least 2 h to be expressed in vivo. In agreement with this pattern of activation, increased serum levels of TNF-α were already observed 10–20 min following the onset of occlusion.
It is well known that free haemoglobin can modulate the biological action of NO in vitro (Moncada et al., 1991) by binding this molecule to generate either methaemoglobin or nitrosylated haemoglobin, depending on the haemoglobin oxygenation (Moncada et al., 1991; Rimar & Gillis, 1993; Wennmain et al., 1992). In contrast, the possibility that blood haemoglobin concentration could regulate NO availability in vivo, and therefore modulate vascular tone, has not been specifically addressed. More specifically the effects of increased blood haemoglobin concentration on NO availability and its consequences on vascular tone have not been investigated so far in splanchnic artery occlusion shock, although it is known that erythrocytosis promoted by erythropoietin attenuates the hyperdynamic circulation associated with anaemia during chronic renal failure (Iwamoto & Morin, 1993). Our data show that splanchnic artery occlusion shock did not produce any change in RBC count and in blood haemoglobin levels. Moreover those parameters were not affected by erythropoietin treatment in sham operated rats and in SAO shocked rats.
Taken together these findings clearly suggest that the acute procedures of SAO shock are not accompanied by relevant change in erythrocytes count and blood haemoglobin concentration and that the doses of rh-EPO administered in our study and the timing of its injection (RBC count and haemoglobin were evaluated 75 min following rh-EPO injection) do not cause any significant haematopoietic effect.
These latter results are in keeping with the kinetics and time-course of rh-EPO effects on haematopoiesis (Stein et al., 1991). Therefore the hypothesis that acute administration of erythropoietin may regulate NO availability via an increase in blood haemoglobin concentration and in turn ameliorate vascular tone in circulatory shock, can be easily ruled out.
However plasma nitrite/nitrate levels were in a dose dependent manner reduced by rh-EPO treatment. These findings led us to conclude that, at least under our experimental conditions, other mechanism(s) are involved in rh-EPO-induced blunting of NO production.
Our results also showed the presence of a reduced vascular sensitivity to vasoconstrictor stimuli. This impaired vascular reactivity, as suggested for other models of experimental shock (Thiemermann et al., 1993), is a consequence of an overproduction of NO by the inducible NO synthase (iNOS) (Squadrito et al., 1996). Indeed aortae collected from shocked rats showed a significant increase in iNOS activity.
In the present paper aortic rings collected from rats subjected to ischaemia-reperfusion injury and treated with rh-EPO exhibited a greater contractile response to PE when compared to vehicle treated rats. The effect seems to be a direct phenomenon, since it was observed even when the recombinant haematopoietic hormone was added in the organ bath containing aortic rings taken from SAO shocked animals. Furthermore the in vivo administration of the haematopoietic factor suppressed iNOS activity in thoracic aortae. Similar results were obtained with mercaptoethylguanidine, a selective inhibitor of iNOS (Southan & Szabo', 1996).
Collectively, these results would suggest that rh-EPO inhibits iNOS activity. This could explain why erythropoietin caused a reduction in plasma nitrite/nitrate levels, ameliorated vascular tone and protected against splanchnic artery occlusion shock.
To further confirm the mechanism by which the hormone interferes with iNOS we performed experiments in macrophages activated with endotoxin. Our results suggest that rh-EPO reduces nitrite production, thus strongly indicating that this haematopoietic hormone may inhibit the activity of inducible nitric oxide synthase. At this time, in fact, there is no detectable increase in the concentration of nitrite, and agents such as glucocorticoids, that inhibit the induction but not the activity of iNOS, have no effect on subsequent nitrite production (Szabo' et al., 1994). In agreement with this hypothesis rh-EPO, incubated in the macrophage culture together with endotoxin, did not cause any change in nitrite production, thus confirming that rh-EPO does not affect iNOS induction. These latter data corroborate the results obtained when rh-EPO was added to endothelium denuded aortic rings produced from rats subjected to splanchnic artery occlusion shock.
In conclusion we have shown that rh-EPO inhibits either ‘in vitro' and ‘in vivo' iNOS activity: the rh-EPO-induced inhibition of NO production, at least in splanchnic ischaemia-reperfusion injury, enhances blood pressure, increases survival, and improves vascular dysfunction. These findings would suggest that iNOS inhibition may contribute, at least in part, to the acute vasoprotective effects of rh-EPO during low flow states such as circulatory shock.
Acknowledgments
This project was supported in part by a grant from CNR Italy (95.02181.CT04).
Abbreviations
- Hb
blood haemoglobin
- iNOS
inducible nitric oxide synthase
- MAP
mean arterial blood pressure
- RBC
red blood cell count
- rh-EPO
recombinant human erythropoietin
- SAO
splanchnic artery occlusion shock
- TNF-α
Tumor Necrosis Factor-α
References
- ACQUIT C.H.I., FERRAGU-HAGUET M., LEFEBVRE A., BERTHELOT J.M., PETERLONGO F., CASTAINGNE J.P. Recombinant erythropoietin and blood pressure. Lancet. 1987;2:1083. doi: 10.1016/s0140-6736(87)91504-2. [DOI] [PubMed] [Google Scholar]
- BUEMI M., ALLEGRA A., SQUADRITO F., BUEMI A.L., LAGANA' A., ALOISI C., FRISINA N. Effects of intravenous administration of recombinant human erythropoietin in rats subjected to hemorrhagic shock. Nephron. 1993;65:440–443. doi: 10.1159/000187526. [DOI] [PubMed] [Google Scholar]
- CAPUTI A.P., ROSSI F., CARNEY K., BREZENOFF H.E. Modulatory effect of brain acetylcholine on reflex-induced bradycardia and tachycardia in conscious rats. J. Pharmacol. Exp. Ther. 1980;215:309–316. [PubMed] [Google Scholar]
- CASATI S., PASSERINI P., CAMPISE M.R., GRAZIANI G., CESANA B. Benefits and risk of protracted treatment with human recombinant erythropoietin in patients having haemodialysis. Br. Med. J. 1987;23:1017–1020. doi: 10.1136/bmj.295.6605.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING A.H., NATHAN C.F., STUEHR D.J. Release of reactive nitrogen intermediates from mouse peritoneal macrophages. J. Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- EDMUNDS M.E., WALLS J. Blood pressure and erythropoietin. Lancet. 1988;1:352–353. [Google Scholar]
- EGRIE J.C., STRICKLAND T.W., LANE J., AOKI K., COHEN A.M. Characterization and biological effects of recombinant human erythropoietin. Immunobiology. 1986;172:213–224. doi: 10.1016/S0171-2985(86)80101-2. [DOI] [PubMed] [Google Scholar]
- ERSLEV A.J. Erythropoietin. New. Engl. J. Med. 1991;324:1339–1344. doi: 10.1056/NEJM199105093241907. [DOI] [PubMed] [Google Scholar]
- HEIDENREICH S., RAHN K.H., ZIDEK W. Direct vasopressor effect of recombinant human erythropoietin on renal resistance vessels. Kidney Int. 1991;39:259–265. doi: 10.1038/ki.1991.31. [DOI] [PubMed] [Google Scholar]
- HULSE E.V. Quantitative cell counts of the bone marrow and blood and their secular variations in the normal adult rats. Acta Haemat. 1964;31:50–63. doi: 10.1159/000209613. [DOI] [PubMed] [Google Scholar]
- IWAMOTO J., MORIN F.C. Nitric oxide inhibition varies with hemoglobin saturation. J. Appl. Physiol. 1993;75:2332–2336. doi: 10.1152/jappl.1993.75.5.2332. [DOI] [PubMed] [Google Scholar]
- JACOBS K., SHOEMAKER C., RUDERSDORF R. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Lancet. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- JELKMANN W. Erythropoietin: structure, control of production and function. Physiol. Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- MAYER G., CADA E.M., WATZINGER U., LUDVIK G., BARBAS U., GRAF H. Pathophysiology of hypertension in dialysis patients treated with erythropoietin (abstract) Kidney Int. 1989;35:319. [Google Scholar]
- MONCADA S., PLAMER R.M.J., HIGGS E.A. RIMAR S., GILLIS C.M. Nitric oxide: physiologySelective pulmonary vasodilation by inhaled nitric oxide is pathophysiology and pharmacology. Pharmacol. Rev. 1991;1993;43:109–142. [PubMed] [Google Scholar]
- NEFF M.S., KIM K.E., PERSOFF M., ONESTI G., SWARTZ C. Hemodynamics of uremic anemia. Circulation. 1971;43:876–883. doi: 10.1161/01.cir.43.6.876. [DOI] [PubMed] [Google Scholar]
- RAINE A.E.G. Hypertension, blood viscosity and cardiovascular morbidity in renal failure: Implications of erythropoietin therapy. Lancet. 1988;1:97–98. doi: 10.1016/s0140-6736(88)90293-0. [DOI] [PubMed] [Google Scholar]
- RECNY M.A., SCOBLE H.A., KIM Y. Structural characterization of natural human urinary and recombinant DNA-derived erythropoietin: identification of des-arginine 166 erythropoietin. J. Biol. Chem. 1987;262:17156–17163. [PubMed] [Google Scholar]
- RIMAR S., GILLIS C.N. Selective pulmonary vasodilation by inhaled nitric oxide is due to hemoglobin inactivation. Circulation. 1993;88:288–2887. doi: 10.1161/01.cir.88.6.2884. [DOI] [PubMed] [Google Scholar]
- SOUTHAN R.S., ABELS R.I., KRANTS S.B. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- SQUADRITO F., ALTAVILLA D., CANALE P., IOCULANO M., CAMPO G.M., AMMENDOLIA L., FERLITO M., ZINGARELLI B., SQUADRITO G., SAITTA A., CAPUTI A.P. Participation of tumor necrosis factor and nitric oxide in the mediation of vascular dysfunction in splanchnic artery occlusion shock. Br. J. Pharmacol. 1994a;113:1153–1158. doi: 10.1111/j.1476-5381.1994.tb17118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUADRITO F., ALTAVILLA D., CANALE P., IOCULANO M., CAMPO G.M., AMMENDOLIA L., SQUADRITO G., SAITTA A., CALAPAI G., CAPUTI A.P. Contribution of intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of splanchnic artery occlusion shock in the rat. Br. J. Pharmacol. 1994b;113:912–916. doi: 10.1111/j.1476-5381.1994.tb17079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUADRITO F., ALTAVILLA D., SQUADRITO G., CAMPO G.M., IOCULANO M., CANALE P., ROSSI F., SAITTA A., CAPUTI A.P. Effects of S-ethylisothiourea, a potent inhibitor of nitric oxide synthase, alone or in combination with a nitric oxide donor in splanchnic artery occlusion shock. Br. J. Pharmacol. 1996;119:23–28. doi: 10.1111/j.1476-5381.1996.tb15672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN R.S., ABELS R.I., KRANTS S.B. Pharmacological doses of recombinanthuman erythropoietin in the treatment of myelodysplastic syndromes. Blood. 1991;75:1658–1663. [PubMed] [Google Scholar]
- SZABO' C., SOUTHAN G.J., WOOD E., THIEMERMANN C., VANE J.R. Spermine inhibits the production of nitric oxide in immune-stimulated J774.2 macrophages: requirement of a serum factor. Br. J. Pharmacol. 1994;112:335–356. doi: 10.1111/j.1476-5381.1994.tb13078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO' C., THIEMERMANN C. Invited opinion: role of nitric oxide in hemorrhagic, traumatic and anaphylactic shock and thermal injury. Shock. 1994;2:145–155. [PubMed] [Google Scholar]
- THIEMERMANN C., SZABO' C., MITCHELL J.A., VANE J.R. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1993;90:267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENNMAIN A., BENTHIN G., PETERSSON A.S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br. J. Pharmacol. 1992;106:507–508. doi: 10.1111/j.1476-5381.1992.tb14365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


