Abstract
The respiratory response to microinjection of capsaicin into the commissural nucleus of the solitary tract (cNTS) of urethane-anaesthetized rats was investigated in the absence and presence of the competitive vanilloid (capsaicin) antagonist, capsazepine, and selective tachykinin NK1, NK2 and NK3 antagonists (RP 67580, SR 48968 and SR 142801, respectively).
Microinjection of capsaicin reduced respiratory frequency but not tidal volume (VT), leading to an overall reduction in minute ventilation (V{dot above]E). The effect was dose-dependent between 0.5 and 2 nmol capsaicin. Doses greater than 2 nmol produced apnoea. Tachyphylaxis was observed following repeated injection of capsaicin (1 nmol, 30 min apart).
Capsazepine (1 nmol) had no effect on frequency or VT when injected alone but completely blocked the respiratory response to capsaicin (1 nmol).
RP 67580 (1 but not 5 nmol) alone depressed frequency and VT slightly. Moreover, RP 67580 appeared to potentiate the bradypnoeic effect of capsaicin. In contrast, SR 48968 and SR 142801 (1 and 5 nmol) alone had no significant effect on respiration. However, both agents significantly attenuated the reduction in frequency produced by capsaicin.
In conclusion, microinjection of capsaicin into the cNTS decreases overall ventilation, primarily by reducing frequency. The action of capsaicin appears from the data to be mediated by vanilloid receptors since it is blocked by the competitive vanilloid antagonist capsazepine and is subject to tachyphylaxis. However, since NK2 (SR 48968) and NK3 (SR 142801) receptor antagonists block the actions of capsaicin, we propose that capsaicin acts also by releasing tachykinins from central afferent terminals in the cNTS.
Keywords: Capsaicin, capsazepine, tachykinin antagonists, nucleus of the solitary tract, respiration
Introduction
Vanilloids, including capsaicin and resiniferatoxin (RTX), are thought to act via specific vanilloid receptors located on the central and peripheral terminals of unmyelinated C-fibres or lightly myelinated Aδ-fibres. The distribution of vanilloid receptors (assessed using [3H]-RTX binding) in the CNS is confined to the dorsal horn of the spinal cord and discrete regions of the brain stem, namely in and around the nucleus of the solitary tract (NTS; Szallasi et al., 1995). There are numerous studies which describe the actions of capsaicin on primary afferent C-fibres and nociceptive processing at the level of the spinal cord (for review, see Bevan & Szolcsányi, 1990; Scolcsányi, 1993). However, to date there is little information on the role of vanilloid receptors at supraspinal (medullary) sites.
The NTS receives input from many sources, including peripheral chemo- and baroreceptor, and vagal pulmonary afferents, i.e., slowly adapting stretch receptors (SARs), rapidly adapting (irritant) receptors and J-receptors (Kalia & Mesulam, 1980; Jordan & Spyer, 1986). Many of these afferent fibres contain tachykinins, inlcuding substance P (SP) and neurokinin A (NKA), and excitatory amino acids (EAAs) in their central terminals (Kalia et al., 1984; Helke & Hill, 1988; Chen et al., 1994; Mizusawa et al., 1994). SP-preferring neurokinin1 (NK1) and NKB-preferring NK3 receptors have been identified in the NTS (Stoessl & Hill, 1990; Nakaya et al., 1994; Ding et al., 1996; Mazzone et al., 1997). Moreover, several studies have documented an excitatory role for SP in baro- and chemoreceptor reflex integration (Chen et al., 1990; Couture et al., 1995; Mazzone et al., 1998), although the role played by tachykinins in other respiratory reflexes (e.g., Hering-Breuer (H-B) reflex) is not clear. In contrast, despite functional evidence (Tschöpe et al., 1992; Lepre et al., 1994; Picard et al., 1994; Couture et al., 1995), localization studies have failed to detect NKA-preferring NK2 receptors in the brain stem (Dam et al., 1987; Tsuchida et al., 1990; Zerari et al., 1998).
Capsaicin releases tachykinins from C- and Aδ-afferents, resulting in a dramatic Ca2+-dependent increase in SP release from tissue slices of rat NTS in vitro (Helke et al., 1981). Moreover, systemic capsaicin pretreatment of neonatal rats, which destroys the majority of C- and Aδ-fibres, causes a marked decrease (50%) in the SP content of the NTS compared with vehicle-pretreated controls (Takano et al., 1988). The respiratory response to hypoxia (increased ventilation) is reduced by 40% in adult rats pretreated with capsaicin as neonates (De Sanctis et al., 1991), suggesting that this may in part be related to the loss of SP. However, there has been no detailed studies addressing the acute effects of vanilloids on the central control of respiration.
The aim of the present study was to investigate whether activation of vanilloid receptors in the region of the NTS modifies respiration, and subsequently to determine the role played by tachykinins (if any) in the central respiratory actions of capsaicin.
Methods
Surgery
All experimental procedures were approved by the University of Tasmania Ethics Committee (Animal Experimentation; project 97044). A total of 88 male Hooded Wistar rats 240–290 g) were anaesthetized using urethane (0.5 g kg−1 i.p. and 0.5 kg−1 s.c.) and allowed to breathe room air throughout all procedures. The level of anaesthesia was assessed by monitoring limb withdrawal and head-shake reactions. Body core temperature was kept constant at 37°C by placing the rat in the prone position on a thermostatically controlled water bed. The animal's head was stabilized in a Kopf stereotaxic apparatus and the dorsal aspect of the brain stem and cerebellum was exposed by a midline incision and partial occipital craniotomy. The dura mater was temporarily left intact. Animals were allowed to stabilize for 20 min before continuing.
Respiration, which was spontaneous and rhythmic, was recorded using subcutaneous electrodes (inserted along the sixth intercostal space) and a calibrated impedance converter (UFI, Morro Bay, California, U.S.A.) as previously described by our laboratory (Maskrey & Hinrichsen, 1994; Mazzone et al., 1997; 1998). The response of the impedance converter was linear over a volume range of 0.1–5 ml and varied less than 5% over the frequency range of 50–150 breaths min−1.
Injection of agents
The dura mater was cut and retracted. In preliminary experiments to determine the dose-effect relationship of capsaicin on respiration, a 1 μl microsyringe (o.d., 470 μm; Hamilton, Reno, Nevada, U.S.A.) was mounted in a micromanipulator and using obex as a reference point, the needle was inserted into the cNTS: AP −15 to −15.3 mm; L 0 to 0.3 mm relative to bregma and 0.4 mm into the dorsal surface of the brain stem (Paxinos & Watson, 1986). Capsaicin (0.5–3.0 nmol in 500 nl) or vehicle (25% ethanol in normal saline) was then injected over 30 s into the cNTS. Each animal received only a single injection of capsaicin or vehicle. Respiratory movements were recorded for 60 min following the injection.
In a separate series of experiments, one group of animals (n=4) received three injections of capsaicin (1 nmol, 30 min apart) and respiratory movements were recorded for 30 min following each injection.
In all subsequent experiments, agents were injected using a two-barrel micropipette (o.d. 100 μm). One barrel was used to inject capsaicin and the other for vehicle injection or injection of antagonists prior to capsaicin. Thus, each animal received a maximum of two injections (i.e., an injection of vehicle or antagonist followed by an injection of capsaicin). The competitive vanilloid antagonist, capsazepine (1 nmol) was injected 10 min prior to capsaicin (1 nmol). The role of NK1, NK2 and NK3 receptors in the respiratory effects of capsaicin was investigated by injecting 1 and 5 nmol of the highly selective NK1, NK2 and NK3 antagonists, RP 67580, SR 48968 and SR 142801, respectively, 10 min prior to injecting 1 nmol of capsaicin. At the end of each experiment, rats were killed with an overdose of pentobarbitone and the brain stems were removed and rapidly frozen. Cryostat-cut serial sections were thaw-mounted onto gelatine-coated slides and stained with thionine to ascertain the exact injection site.
Data analysis
Prior to removal of the brain stem, a polyethylene tube attached to a 5 ml syringe was inserted into the trachea and the impedance converter was calibrated by graded inflation of the lung. Respiratory movements were measured over a 20 s time interval and then converted to frequency, tidal volume (VT) and minute ventilation (V̇E). Volumes were subsequently normalized to body weight. Data obtained from animals which showed evidence of the needle tract in the cNTS were compared using ANOVA followed by Fisher's Least Significant Difference (LSD) test. For antagonist studies, all statistical comparisons were made against time-matched, vehicle-injected controls. Where capsaicin was injected repetitively, comparisons were to the first injection. P<0.05 was considered statistically significant.
Drugs and materials
Capsaicin (>99% purity) and capsazepine were purchased from the Sigma Chemical Company. Nonpeptide antagonists were generous gifts: (3aR, 7aR)-2-(1-imino-2-(2-methoxy-phenyl)-ethyl)-7,7-diphenyl-4-perhydroisoindolone (RP 67580) from Dr C. Garret, Rhône-Poulenc Rorer, Vitry sur Seine, France); (S)-N-methyl-N[4-(4-acetylamino-4-phenyl piperidino)-2-(3,4-dichlorophenyl)butyl]benzamide (SR 48968) and (R)-(N)-[1-[3-[1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]propyl ] - 4 - phenylpiperidin - 4 - yl ] - N - methylacetamide (SR 142801) from Dr X. Emonds-Alt, Sanofi Recherche, Montpellier, France. All agents were dissolved in 25–35% ethanol in normal saline (except for RP 67580 which was dissolved in 0.01 M HCl in normal saline) and stored in frozen aliquots. All other reagents were of analytical grade.
Results
Injection of capsaicin
Microinjection of capsaicin (0.5–2.0 nmol) into the cNTS decreased frequency in a dose-dependent manner (Figures 1 and 2a). The maximum decrease in frequency was observed between 5 and 10 min after the injection (minimum frequency values were 105±4 breaths min−1 for vehicle and 90±6, 75±7, 51±6 breaths min−1 for 0.5, 1.0 and 2.0 nmol capsaicin, respectively). Respiratory frequency slowly returned to pre-injection values by 60 min. Doses of capsaicin greater than 2 nmol produced apnoea (only two animals were tested due to death being the end-point of the experiment; Figure 1). Thus, the dose range producing a response from zero to maximum effect (i.e., apnoea) was less than one order of magnitude.
Figure 1.
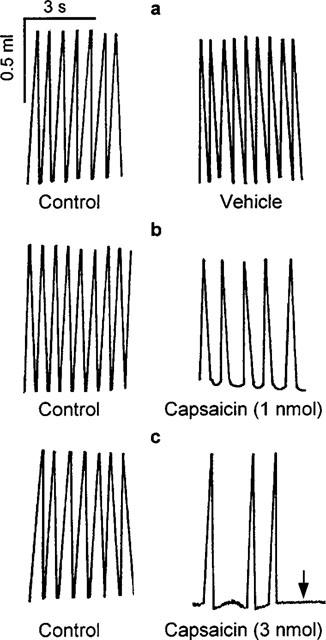
Representative chart tracings which show respiratory movements before (control) and 10 min after microinjection of 500 nl vehicle (a) and 1 nmol capsaicin (b) into the commissural nucleus of the solitary tract of urethane-anaesthetized, spontaneously breathing rats. The bottom panels (c) show the effect of 3 nmol capsaicin, which produced irreversible apnoea (arrow) 2 min after injection.
Figure 2.
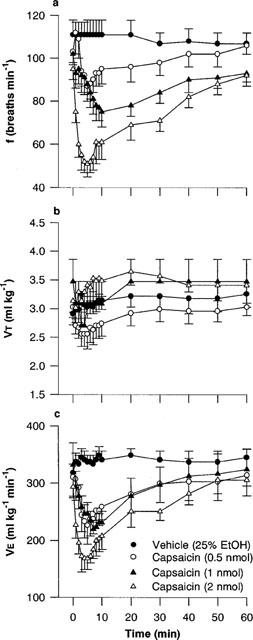
(a) Respiratory frequency (f), (b) tidal volume (VT) and (c) minute ventilation (V̇E) in rats following microinjection of capsaicin (0.5–2.0 nmol) or vehicle (25% ethanol in normal saline) into the commissural nucleus of the solitary tract of urethane-anaesthetized, spontaneously breathing rats. Values are the mean of four to eight animals and vertical bars show s.e.mean.
VT was not generally affected by capsaicin (Figure 2b). However, the lowest dose of capsaicin (0.5 nmol) produced a slight depression of VT (3.03±0.19 ml kg−1 versus 2.55±0.26 ml kg−1 5 min after injection of vehicle and capsaicin, respectively), whereas 1 and 2 nmol capsaicin both produced a small increase in VT. As a result, V̇E followed essentially the same time-course as frequency after injection of capsaicin (minimum V̇ E values were 333±15 ml kg−1 min−1 for vehicle and 226±33, 220±12, 169±20 ml kg−1 min−1 for 0.5, 1.0 and 2.0 nmol capsaicin, respectively; Figure 2c). The effect of vehicle (500 nl, 25% ethanol in normal saline) on respiration was negligible (Figures 1 and 2). One nmol capsaicin was used as the reference dose in all subsequent experiments since it produced consistent respiratory slowing without apnoeic periods.
Figure 3 shows the respiratory response to multiple injections of capsaicin (1 nmol×three injections, 30 min apart). All three injections produced the characteristic reduction in frequency. However, the bradypnoeic response to the second and third injection was attenuated when compared with the bradypnoea following the first injection. The attenuation of frequency, together with a slightly elevated VT response, resulted in a significantly (P<0.05) reduced V̇E response to the second and third capsaicin injections (e.g., 193±26, 261±26 and 265±30 ml kg−1 min−1 for the first, second and third injections, respectively).
Figure 3.
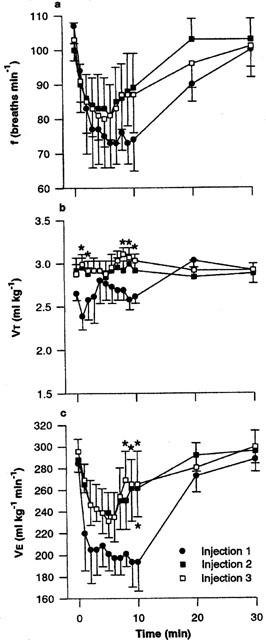
(a) Respiratory frequency (f), (b) tidal volume (VT) and (c) minute ventilation (V̇E) in rats following repeated injection of capsaicin (×3, 1 nmol, 30 min apart) into the commissural nucleus of the solitary tract of urethane-anaesthetized, spontaneously breathing rats. Values are the mean of four animals and vertical bars show s.e.mean. *P<0.05 versus injection one (ANOVA followed by Fisher's LSD test).
Injection of capsaicin and capsazepine
Microinjection of capsazepine (1 nmol) or capsazepine vehicle (35% ethanol in normal saline) alone had no effect on basal respiration (Figure 4). However, capsazepine completely blocked the bradypnoeic response (and hence, the decrease in V̇E) to capsaicin (1 nmol) whereas injection of the vehicle had no effect on the action of capsaicin (Figure 4). Although the degree of bradypnoea produced by capsaicin (1 nmol) following vehicle injection was comparable to that obtained in dose-response experiments where capsaicin was injected alone (72±5 and 75±7 breaths min−1, respectively) the response to capsaicin following vehicle injection was faster (maximum decreases in frequency were observed after 2 and 10 min, respectively) (compare Figures 2a and 4a).
Figure 4.
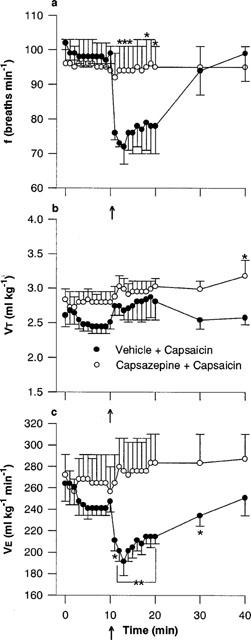
Effect of capsazepine on the respiratory response to microinjection of capsaicin into the commissural nucleus of the solitary tract: (a) respiratory frequency (f), (b) tidal volume (VT) and (c) minute ventilation (V̇E). Capsazepine (1 nmol) or vehicle (35% ethanol in normal saline) was injected into the cNTS of urethane-anaesthetized, spontaneously breathing rats and allowed to act for 10 min prior to injection of capsaicin (1 nmol, arrow). Values are the mean of four animals and vertical bars show s.e.mean. *P<0.05; **P<0.01 versus vehicle-capsaicin (ANOVA followed by Fisher's LSD test).
Injection of capsaicin and tachykinin antagonists
The possible involvement of tachykinins in the respiratory actions of capsaicin following injection into the cNTS was investigated by injecting (separately) RP 67580, SR 48968 and SR 142801 to block NK1, NK2 and NK3 receptors respectively, prior to 1 nmol capsaicin (Figures 5,6,7).
Figure 5.
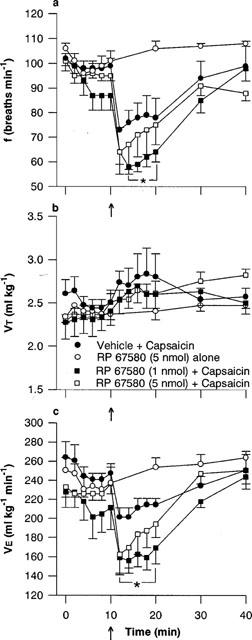
Effect of the NK1 tachykinin receptor antagonist, RP 67580, on the respiratory response to microinjection of capsaicin into the commissural nucleus of the solitary tract (cNTS): (a) respiratory frequency (f), (b) tidal volume (VT) and (c) minute ventilation (V̇E). RP 67580 was injected into the cNTS of urethane-anaesthetized, spontaneously breathing rats and allowed to act for 10 min prior to microinjection of capsaicin (arrow, 1 nmol) or vehicle (25% ethanol in normal saline). Values are the mean of three to five animals and vertical bars show s.e.mean. *P<0.05, response in presence of 1 nmol RP 67580 significantly different from vehicle-capsaicin (ANOVA followed by Fisher's LSD test).
Figure 6.
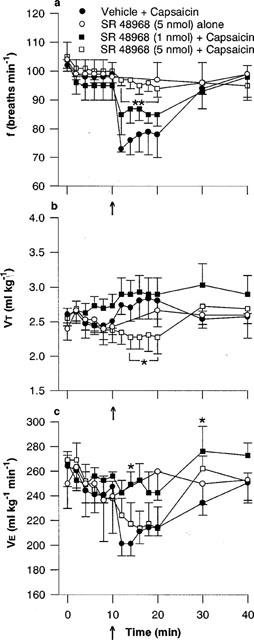
Effect of the NK2 tachykinin receptor antagonist SR 48968, on the respiratory response to microinjection of capsaicin into the commissural nucleus of the solitary tract (cNTS): (a) respiratory frequency (f), (b) tidal volume (VT) and (c) minute ventilation (V̇E). SR 48968 was injected into the cNTS of urethane-anaesthetized, spontaneously breathing rats and allowed to act for 10 min prior to microinjection of capsaicin (arrow, 1 nmol) or vehicle (25% ethanol in normal saline). Values are the mean of three to five animals and vertical bars show s.e.mean. * and **, P<0.05 and P<0.01 respectively, response in presence of SR 48968 significantly different from vehicle-capsaicin (ANOVA followed by Fisher's LSD test).
Figure 7.
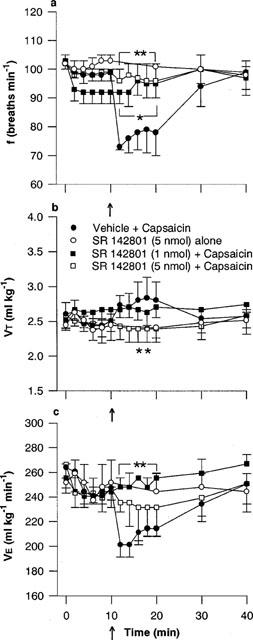
Effect of the NK3 tachykinin receptor antagonist, SR 142801, on the respiratory response to microinjection of capsaicin into the commissural nucleus of the solitary tract (cNTS): (a) respiratory frequency (f), (b) tidal volume (VT) and (c) minute ventilation (V̇E). SR 142801 was injected into the cNTS of urethane-anaesthetized, spontaneously breathing rats and allowed to act for 10 min prior to microinjection of capsaicin (arrow, 1 nmol) or vehicle (25% ethanol in normal saline). Values are the mean of three to five animals and vertical bars show s.e.mean. * and **, P<0.05 and P<0.01 respectively, response in presence of SR 142801 antagonist significantly different from vehicle-capsaicin (ANOVA followed by Fisher's LSD test).
One nmol of the selective NK1 receptor antagonist, RP 67580, depressed frequency slightly (98±3 versus 87±5 breaths min−1, 5 min following injection of vehicle and RP 67580, respectively), which remained stable for a further 5 min prior to the injection of capsaicin (Figure 5a). The depression of frequency following injection of RP 67580 appeared to be additive to the capsaicin response (Figure 5a). That is, rats pretreated with 1 nmol RP 67580 displayed a significantly (P<0.05) greater maximum bradypnoeic response to capsaicin compared to vehicle-injected controls (59±3 and 72±5 breaths min−1, respectively). Although some potentiation of the frequency, and hence V̇ E, responses to capsaicin were observed after 5 nmol RP 67580, the effect was not statistically significant.
In contrast, the selective NK2 receptor antagonist, SR 48968, did not alter basal respiration at either low (1 nmol) or high (5 nmol) doses. However, the maximum response (i.e., decrease in frequency) to capsaicin was significantly (P<0.05) attenuated by 1 nmol SR 48968 (85±3 breaths min−1) and completely blocked by 5 nmol SR 48968 (96±3 breaths min−1; vehicle-injected animals, 72±5 breaths min−1; Figure 6a). In the presence of SR 48968 (5 nmol), capsaicin injection produced a small reduction in VT, which was significantly less than the control VT response (Figure 6b). As a result of the reduction in VT, 5 nmol SR 142801 did not significantly alter the overall V̇E response to capsaicin (Figure 6c).
Similarly, neither 1 nor 5 nmol of the selective NK3 antagonist, SR 142801, had any effect on respiration when injected alone. However, both concentrations completely blocked the characteristic reduction in frequency induced by capsaicin (minimum frequency; 72±5, 91±6 and 96±6 breaths min−1, for vehicle-, 1 nmol and 5 nmol SR 142801-pretreated rats, respectively; Figure 7a). Again, the VT response following capsaicin injection in rats pretreated with high dose SR 142801 (5 nmol) was significantly (P<0.05) less than control (vehicle-pretreated) animals (Figure 7b).
Discussion
The present functional study shows that microinjection of the vanilloid capsaicin into the cNTS decreases respiratory frequency and has minimal effects on tidal volume. The respiratory actions of capsaicin in the rat brain stem satisfy three established criteria for vanilloid receptor classification: viz, steep dose-effect relationship, desensitization (tachyphylaxis) with repeated administration, and antagonism by the competitive antagonist capsazepine. Indeed, to our knowledge, this is the first study to report the characterization of brain stem vanilloid receptors in vivo. Moreover, the bradypnoeic action of capsaicin was blocked by selective tachykinin NK2 and NK3 receptor antagonists (SR 48968 and SR 142801, respectively) which suggests that the effects of capsaicin are mediated, at least partly, by the release of tachykinins from the central terminals of sensory neurones and subsequent stimulation of NK2 and/or NK3 receptors.
Functional evidence for vanilloid receptors in the brain stem
Although an endogenous ligand for vanilloid receptors has yet to be identified, a vanilloid receptor has recently been cloned and there is some suggestion that central and peripheral isoforms or subtypes exist (Griffiths et al., 1996; 1998; Caterina et al., 1997; Liu et al., 1998; reviewed by Szallasi, 1994). Two hallmarks of vanilloid action are rapid desensitization following repeated administration and attenutation of responses by the competitive antagonist capsazepine and non-competitive antagonist ruthenium red (Maggi et al., 1993; Szallasi, 1994). In the present study, the magnitude of the bradypnoeic response to capsaicin declined with repeated administration (desensitization) and was abolished in the presence of equimolar concentrations of capsazepine.
An additional criterion for an action on vanilloid receptors is an extremely steep dose-response relationship which reflects the positive cooperative nature of vanilloid receptor-ligand interaction. The concentration range of individual vanilloids producing zero to maximum response in vivo or inhibition of [3H]-RTX binding in vitro is generally one order of magnitude (Acs et al., 1994; Eldershaw et al., 1994; Szallasi et al., 1995; Griffiths et al., 1996; 1998). In the present study, the dose range of capsaicin which produced zero to maximum response (i.e., apnoea) was less than one order of magnitude, suggesting a positive cooperative interaction between capsaicin and its receptors in the brain stem.
Involvement of tachykinin receptors in the central respiratory actions of capsaicin
Given the selectivity of capsaicin for unmyelinated and lightly myelinated afferents, many of which contain SP and/or NKA together with other neurotransmitters, one would expect one or more of the tachykinin antagonists to attenuate the effects of capsaicin on respiration. Moreover, previous studies have shown that capsaicin increases SP release from slices of NTS in vitro (Helke et al., 1981). Thus, one of our objectives was to determine whether stimulation of tachykinin NK1 receptors was involved in the bradypnoeic action of capsaicin.
There are at least two distinct types of SP-containing terminals in the cNTS (Kalia et al., 1984; Kubota et al., 1985; Chen et al., 1994). One source of SP is undoubtedly the central terminals of chemoreceptor, baroreceptor and/or pulmonary afferents, whereas the remainder may represent interneurones or the terminals of projections from other brain stem regions, including the parabrachial nucleus, ventrolateral medulla regions of the raphe (Helke & Hill, 1988; Chen et al., 1994). In addition, the adult rat NTS possesses a very high density of [125I]-SP binding sites (Quirion & Dam, 1986; Mazzone et al., 1997) and microinjection of SP into the NTS of anaesthetized rats stimulates ventilation (Chen et al., 1990; Mazzone et al., 1998). In a previous study, we have shown that injection of SP (750 pmol) into the cNTS (midline, ∼0.5–0.8 mm caudal to obex) substantially elevates VT and slightly reduces frequency (Mazzone et al., 1998). However, we did not identify the receptor(s) responsible for these effects. Given that injection of capsaicin into the cNTS produces a profound bradypnoea but no significant change in VT, suggests that SP may not be involved in the respiratory actions of capsaicin. Moreover, the respiratory actions of capsaicin were not blocked by either low (1 nmol) or high (5 nmol) doses of the selective NK1 receptor antagonist, RP 67580. Since previous studies have shown that at doses ranging from 100 pmol–2.5 nmol RP 67580 selectively antagonize the actions of SP at central NK1 receptors (Culman et al., 1995), the present data show that NK1 receptors are unlikely to be involved in the respiratory actions of capsaicin.
Although the present data do not support a role for NK1 receptors, it would appear that tachykinins released from capsaicin-sensitive afferent terminals in the region of the NTS may reduce frequency by interactiing with brain stem NK3 and possibly NK2 receptors. In the present study, injection of nonpeptide NK3 receptor antagonist, SR 142801, abolished the respiratory actions of capsaicin. Indeed, our data support immunohistochemical and autoradiographic studies which have localized both NKB and NK3 receptors in regions of the NTS and suggest that NK3 receptors play an important role in the reflex control of respiration. Nevertheless, SR 142801 possesses greater affinity for human and guinea-pig (pA2∼9) than for rat NK3 receptors (pA2∼7) and at high doses 6.5–65 nmol i.c.v.) may act as an agonist at rat central NK3 receptors involved in cardiovascular regulation (Emonds-Alt et al., 1995; Nguyen-Le et al., 1996; Cellier et al., 1997). In the present study, injection of SR 142801 (1–5 nmol) alone had no effect on respiration, and thus appears to be devoid of agonist activity at brain stem NK3 receptors involved in respiratory control.
We were somewhat surprised that SR 48968, a NK2 receptor antagonist, attenuated the central respiratory effects of capsaicin. There is considerable controversy surrounding the presence and function of NK2 receptors in the adult rat CNS. Although Zerari et al. (1998) recently demonstrated NK2 receptors on rat spinal cord astrocytes, attempts to detect supraspinal NK2 receptors using autoradiography or NK2 receptor-encoding mRNA by in-situ hybridization have not been successful (Dam et al., 1987; Tsuchida et al., 1990). In contrast, functional studies suggest that NK2 receptors are present at both the spinal and supraspinal level (Picard et al., 1994; Couture et al., 1995). Some actions of NKA ascribed to NK2 receptors may simply reflect the promiscuity of tachykinins, since all three endogenous tachykinins interact to some degree with the three established tachykinin receptor types. However, the use of nonpeptide NK2 antagonists strongly supports the presence of NK2 receptors in the CNS (Tschöpe et al., 1992; Lepre et al., 1994; for review, see Mussap et al., 1993; Maggi, 1995). Our studies employing SR 48968 provide functional evidence that NK2 receptors are involved in central (brain stem) control of respiration.
Although the nonpeptide antagonists used in the present study have high affinity for specific tachykinin receptor types, there have been no studies to date addressing whether SR 48968, SR 142801 and other nonpeptide antagonists interact directly with vanilloid receptors. Thus, one cannot exclude the possibility that these compounds prevent the central effects of capsaicin by blocking vanilloid receptors in the brain stem. Given the intimate relationship between capsaicin (vanilloid)-sensitive neurones, tachykinins and tachykinin receptors, such an interaction could only be tested in a tachykinin-free environment. The [3H]-RTX binding assay developed by Szallasi & Blumberg (1990) would be ideal to determine whether an interaction occurs between tachykinin antagonists and capsaicin at the vanilloid binding site. Alternatively, the effects of tachykinin antagonists on capsaicin-stimulated Ca2+ influx in Xenopus oocytes or human HEK cells expressing cloned vanilloid receptors would be a useful functional system (Caterina et al., 1997). However, until [3H]-RTX and the cloned vanilloid receptor are available commercially, this hypothesis remains to be tested.
The identity of capsaicin-sensitive afferents in the region of the NTS
The extremely low solubility of capsaicin in aqueous solvents necessitated using large injection volumes (500 nl) to avoid excessively high concentrations of ethanol. Presumably, capsaicin would be able to diffuse some distance from the site of injection in the cNTS and interact with neurones throughout the NTS and the nearby area postrema and dorsal motor nucleus of the vagus (DVN). Since vagal afferents terminate in each of these nuclei, the exact identity and location of neurones in the region of the NTS which are stimulated by capsaicin cannot be determined. Nevertheless, the respiratory effects of capsaicin are probably due to stimulation of only sensory neurones since [3H]-RTX binding is limited to sensory nuclei (viz, NTS, DVN and area postrema) in the brain stem and is abolished by neonatal capsaicin pretreatment, which is known to selectively destroy sensory fibres (Szallasi & Blumberg, 1994; Szallasi et al., 1995). Moreover, we have obtained some preliminary evidence that the bradypnoeic response to microinjection of capsaicin into the cNTS is markedly reduced in rats systemically pretreated at birth with capsaicin (50 mg kg−1 s.c.; data not shown).
Functional studies in the rat and mouse have shown that peripheral chemoreceptor, pulmonary, cardiac and baroreceptor afferents all terminate in the NTS and neighbouring nuclei (Kalia & Mesulam, 1980; Jordan & Spyer, 1986). The profound bradypnoea (and apnoea at higher doses) which occurs following capsaicin injection is similar to that observed when EAAs are injected into H-B region of the cNTS of urethane-anaesthetized rats (Bonham et al., 1993) and may suggest that stimulation of SAR afferent terminals is involved in the respiratory actions of capsaicin. Indeed, our injection site correlates with the location of the central terminals of SAR afferents. Moreover, unlike arterial chemoreceptor afferents, these fibres appear not to use SP and NK1 receptors as the primary sensory neurotransmitter system. Bonham et al. (1993) reported that injection of SP (0.03–4.0 pmol) alone into the H-B region of the NTS (0.1–0.6 mm caudal to obex and 0.5–0.9 mm lateral to midline) had no effect on basal respiration, but potentiated the bradypnoeic effect of EAAS injected into the same region.
Although the slowing of respiratory rate following capsaicin injection observed in the present study may correspond to an activation of SAR terminals, one cannot exclude an involvement of other reflex pathways. Indeed, stimulation of baroreceptor, laryngeal chemoreceptor and J-receptor (pulmonary C-fibre) may all produce apnoea. Interestingly, a recent study by Butcher et al. (1998) reported that respiratory responses to stimulation of pulmonary vagal C-fibre afferents were unchanged in urethane-anaesthetized NK1 receptor knockout mice, supporting a lack of involvement of NK1 receptors in pulmonary chemoreceptor reflexes. If capsaicin is acting on pulmonary afferent terminals in the brain stem, and given the apparent lack of involvement of SP in the integration of such reflexes, it is not surprising that RP 67580 had no effect on the action of capsaicin observed in the present study. Nevertheless, since both SR 48968 and SR 142801 attenuated the response to capsaicin, pulmonary afferents may employ tachykinins as sensory neurotransmitters.
In conclusion, these data provide functional evidence for the presence of vanilloid receptors in the brain stem of adult rats. Moreover, the findings suggest that the respiratory actions of capsaicin are probably due to the release of tachykinins from the central terminals of sensory neurones, since responses to local injection of capsaicin were blocked by NK2 and NK3 but not NK1 receptor antagonists. Although the exact identity of the neurones which are stimulated by capsaicin have not been identified, SAR, pulmonary and/or larynegeal chemo-sensitive afferents are undoubtedly involved.
Acknowledgments
This study was supported by a grant from the Australian Research Council. We thank Dr Xavier Emonds-Alt, Sanofi Recherche (Montpellier, France) for the gift of SR 48968 and SR 142801, and Dr Claude Garret, Rhône-Poulenc Rorer (Vitry sur Seine, France) for the gift of RP 67580.
Abbreviations
- H-B
Hering-Breuer
- NK
neurokinin
- NTS
nucleus of the solitary tract
- RTX
resiniferatoxin
- SAR
slowly adapting stretch receptors
- SP
substance P
- V̇E
minute ventilation
- VT
tidal volume
References
- ACS G., PALKOVITS M., BLUMBERG P.M. [3H]-Resiniferatoxin binding by the human vanilloid (capsaicin) receptor. Mol. Brain Res. 1994;23:185–190. doi: 10.1016/0169-328x(94)90225-9. [DOI] [PubMed] [Google Scholar]
- BEVAN S., SZOLCSANYI J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. TIPS. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- BONHAM A.C., COLES S.K., MCCRIMMON D.R. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J. Physiol. 1993;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER J.W., DE FELIPE C., SMITH A.J.H., HUNT S.P., PATON J.F.R. Comparison of cardiorespiratory reflexes in NK1 receptor knockout, heterozygous and wild-type mice in vivo. J. Autonom. Nerv. Sys. 1998;69:89–95. doi: 10.1016/s0165-1838(98)00018-6. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CELLIER E., BARBOT L., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of intracerebroventricularly administered tachykinin NK3 receptor antagonists in the conscious rat. Br. J. Pharmacol. 1997;122:643–654. doi: 10.1038/sj.bjp.0701435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN I.-L., CUSICK C.G., WEBER J.T., YATES R.D. Synaptic morphology of substance P terminals on catecholamine neurons in the commissural subnucleus of the nucleus tractus soliarii in the rat. Microscopy Res. Tech. 1994;29:177–183. doi: 10.1002/jemt.1070290216. [DOI] [PubMed] [Google Scholar]
- CHEN Z., HEDNER J., HEDNER T. Local effects of substance P on respiratory regulation in the rat medulla oblongata. J. Appl. Physiol. 1990;68:693–699. doi: 10.1152/jappl.1990.68.2.693. [DOI] [PubMed] [Google Scholar]
- COUTURE R., PICARD P., POULAT P., PRAT A. Characterization of the tachykinin receptors involved in spinal and supraspinal cardiovascular control. Can. J. Physiol. Pharmacol. 1995;73:892–902. doi: 10.1139/y95-123. [DOI] [PubMed] [Google Scholar]
- CULMAN J., WIEGAND B., SPITZNAGEL H., KLEE S., UNGER T. Effects of the tachykinin NK1 receptor antagonist, RP 67580, on central cardiovascular and behavioural effects of substance P, neurokinin A and neurokinin B. Br. J. Pharmacol. 1995;114:1310–1316. doi: 10.1111/j.1476-5381.1995.tb13348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAM T.-V., ESCHER E., QUIRION R. Evidence for the existence of three classes of neurokinin receptors in brain. Differential ontogeny of neurokinin-1, neurokinin-2 and and neurokinin-3 binding sites in rat cerebral cortex. Brain Res. 1987;453:372–376. doi: 10.1016/0006-8993(88)90181-3. [DOI] [PubMed] [Google Scholar]
- DE SANCTIS G.T., GREEN F.H.Y., REMMERS J.E. Ventilatory responses to hypoxia and hypercapnia in awake rats pretreated with capsaicin. J. Appl. Physiol. 1991;70:1168–1174. doi: 10.1152/jappl.1991.70.3.1168. [DOI] [PubMed] [Google Scholar]
- DING Y.-Q., SHIGEMOTO R., TAKADA M., OHISHI H., NAKANISHI S., MIZUNO N. Localization of the neuromedin K receptor (NK3) in the CNS of the rat. J. Comp. Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- ELDERSHAW T.P.D., COLQUHOUN E.Q., BENNET K.L., DORA K.A., CLARK M.G. Resiniferatoxin and piperine: capsaicin-like stimulators of oxygen uptake in the perfused rat hindlimb. Life Sci. 1994;55:389–397. doi: 10.1016/0024-3205(94)00650-4. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., BICHON D., DUCOUX J.P., HEAULME M., MILOUX B., PONCELET M., PROIETTO V., VAN BROECK D., VILAIN P., NELIAT G., SOUBRIE P., LE FUR G., BRELIERE J.C. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:PL27–32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS C.D., ELDERSHAW T.P.D., GERAGHTY D.P., HALL J.L., COLQUHOUN E.Q. Capsaicin-induced biphasic oxygen uptake in rat musle: antagonism by capsazepine and ruthenium red provides further evidence for peripheral vanilloid receptor subtypes (VN1/VN2) Life Sci. 1996;59:105–117. doi: 10.1016/0024-3205(96)00267-6. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS C.D., GERAGHTY D.P., ELDERSHAW T.P.D., COLQUHOUN E.Q. Acute and chronic effects of capsaicin in perfused rat muscle: the role of tachykinins and calcitonin gene-related peptide. J. Pharmacol. Exp. Ther. 1998;287:697–704. [PubMed] [Google Scholar]
- HELKE C.J., JACOBOWITZ D., THOA N.B. Capsaicin and potassium evoked substance P release from the nucleus tractus solitarius and spinal trigeminal nucleus in vitro. Life Sci. 1981;29:1779–1785. doi: 10.1016/0024-3205(81)90188-0. [DOI] [PubMed] [Google Scholar]
- HELKE C.J., HILL K.M. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience. 1988;26:539–551. doi: 10.1016/0306-4522(88)90166-2. [DOI] [PubMed] [Google Scholar]
- JORDAN D., SPYER K.M.Brainstem integration of cardiovascular and pulmonary afferent activity Progress in Brain Research 1986volume 67New York: Elsevier Science Publishers; 295–314.ed. Cervero, F. & Morrison, J.F.B. [DOI] [PubMed] [Google Scholar]
- KALIA M., FUXE K., HÖKFELT T., JOHANSSON O., LANG R., GATEN D., CUELLO C., TERENIUS L. Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J. Comp. Neurol. 1984;222:409–444. doi: 10.1002/cne.902220308. [DOI] [PubMed] [Google Scholar]
- KALIA M., MESULAM M.M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac and gastrointestinal branches. J. Comp. Neurol. 1980;191:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- KUBOTA Y., TAKAGI H., MORISHIMA Y., POWELL J.F., SMITH A.D. Synaptic interaction between catecholaminergic neurons and substance P-immunoreactive axons in the caudal part of the nucleus of the solitary tract of the rat: demonstration by the electron microscopic mirror technique. Brain Res. 1985;333:188–192. doi: 10.1016/0006-8993(85)90145-3. [DOI] [PubMed] [Google Scholar]
- LEPRE M., OLPE H.-R., EVANS R., BRUGGER F. Physiological characterization of the spinal NK2 receptor. Eur. J. Pharmacol. 1994;258:23–31. doi: 10.1016/0014-2999(94)90053-1. [DOI] [PubMed] [Google Scholar]
- LIU L., SZALLASI A., SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenlyacetate 13-acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neuroscience. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., BEVAN S., WALPOLE C.S.J., RANG H.P., GIULIANAI S. A comparison of capsazepine and ruthenium red as capsaicin antagonists in the rat isolated urinary bladder and vas deferens. Br. J. Pharmacol. 1993;108:801–805. doi: 10.1111/j.1476-5381.1993.tb12881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASKREY M., HINRICHSEN C.F. Respiratory responses to combined hypoxia and hypothermia in rats after posterior hypothalamic lesions. Eur. J. Physiol. 1994;426:371–377. doi: 10.1007/BF00388299. [DOI] [PubMed] [Google Scholar]
- MAZZONE S.B., HINRICHSEN C.F., GERAGHTY D.P. Substance P receptors in brain stem respiratory centres of the rat: regulation of NK1 receptors by hypoxia. J. Pharmacol. Exp. Ther. 1997;282:1547–1556. [PubMed] [Google Scholar]
- MAZZONE S.B., HINRICHSEN C.F., GERAGHTY D.P. Hypoxia attenuates the respiratory response to microinjection of substance P into the nucleus of the solitary tract of the rat. Neurosci. Lett. 1998;256:9–12. doi: 10.1016/s0304-3940(98)00743-5. [DOI] [PubMed] [Google Scholar]
- MIZUSAWA A., OGAWA H., KIKUCHI Y., HIDA W., KUROSAWA H., OKABE S., TAKISHIMA T., SHIRATO K. In vivo release of glutamate in the nucleus tractus solitarii of the rat during hypoxia. J. Physiol. 1994;478:55–65. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSAP C.J., GERAGHTY D.P., BURCHER E. Tachykinin receptors: a radioligand binding perspective. J. Neurochem. 1993;60:1987–2009. doi: 10.1111/j.1471-4159.1993.tb03484.x. [DOI] [PubMed] [Google Scholar]
- NAKAYA Y., KANEKO T., SHIGEMOTO R., NAKANISHI S., MIZUNO N. Immunohistochemical localization of substance P receptor in the CNS of the adult rat. J. Comp. Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- NGUYEN-LE X.K., NGUYEN Q.T., GOBEIL F., PHENG L.H., EMONDS-ALT X., BRELIERE J.C., REGOLI D. Pharmacological characterization of SR 142801: a new non-peptide antagonist of the neurokinin NK-3 receptor. Pharmacology. 1996;52:283–291. doi: 10.1159/000139393. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotaxic coordinates 1986Sydney: Academic Press Inc; (2nd edition) [Google Scholar]
- PICARD P., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of centrally administered tachykinins in the rat: characterization of receptors with selective antagonists. Br. J. Pharmacol. 1994;112:240–249. doi: 10.1111/j.1476-5381.1994.tb13058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIRION R., DAM T. Ontogeny of substance P receptor binding sites in rat brain. J. Neurosci. 1986;6:2187–2199. doi: 10.1523/JNEUROSCI.06-08-02187.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOESSL A.J., HILL D.R. Autoradiographic visualization of NK-3 tachykinin binding sites in the rat brain, utilizing [3H]senktide. Brain Res. 1990;534:1–7. doi: 10.1016/0006-8993(90)90105-k. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. The vanilloid (capsaicin) receptor: receptor types and species differences. Gen. Pharmacol. 1994;25:223–243. doi: 10.1016/0306-3623(94)90049-3. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 1990;524:106–111. doi: 10.1016/0006-8993(90)90498-z. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Comparison of [3H]resiniferatoxin binding by the vanilloid (capsaicin) receptor in dorsal root ganglia, spinal cord, dorsal vagal complex, sciatic and vagal nerve and urinary bladder of the rat. Life Sci. 1994;55:1017–1026. doi: 10.1016/0024-3205(94)00636-9. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., NILSSON S., FARKAS-SZALLASI T., BLUMBERG P.M., HOKFELT T., LUNDBERG J.M. Vanilloid receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- SZOLCSÁNYI J.Actions of capsaicin on sensory receptors Capsaicin in the Study of Pain 1993London: Academic Press; 1–27.ed. Wood, J.N., pp [Google Scholar]
- TAKANO Y., NAGASHIMA A., KAMIYA H., KUROSAWA M., SATO A. Well-maintained reflex responses of sympathetic nerve activity to stimulation of baroreceptor, chemoreceptor and cutaneous mechanoreceptors in neonatal capsaicin-treated rats. Brain Res. 1988;445:188–192. [PubMed] [Google Scholar]
- TSCHÖPE C., PICARD P., CULMAN J., PRAT A., ITOI K., REGOLI D., UNGER T., COUTURE R. Use of selective antagonists to dissociate the central cardiovascular and behavioural effects of tachykinins on NK1 and NK2 receptors in the rat. Br. J. Pharmacol. 1992;107:750–755. doi: 10.1111/j.1476-5381.1992.tb14518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIDA K., SHIGEMOTO R., YOKOTA Y., NAKANISHI S. Tissue distribution and quantitation of the mRNAs for three rat tachykinin receptors. Eur. J. Biochem. 1990;193:751–757. doi: 10.1111/j.1432-1033.1990.tb19396.x. [DOI] [PubMed] [Google Scholar]
- ZERARI F., KARPITSKIY V., KRAUSE J., DESCARRIES L., COUTURE R. Astroglial distribution of neurokinin-2 receptor immunoreactivity in the rat spinal cord. Neuroscience. 1998;84:1233–1246. doi: 10.1016/s0306-4522(97)00548-4. [DOI] [PubMed] [Google Scholar]


