Abstract
Both the 5-HT1D and 5-HT1B receptors are implicated in migraine pathophysiology. Recently isochromans have been discovered to bind primate 5-HT1D receptors with much higher affinity than 5-HT1B receptors. In the guinea-pig, a primary animal model for anti-migraine drug testing, however, isochromans bound the 5-HT1D receptor with lower affinity than the gorilla receptor.
This species-specific pharmacology was investigated, using site-directed mutagenesis on cloned guinea-pig receptors heterologously expressed in human embryonic kidney 293 cells. Mutations of threonine 100 and arginine 102 at the extracellular side of transmembrane II of the guinea-pig 5-HT1D receptor to the corresponding primate residues, isoleucine and histidine, respectively, enhanced its affinity for isochromans to that of the gorilla receptor, with little effects on its affinities for serotonin, sumatriptan and metergoline. Free energy change from the R102H mutation was about twice as much as that from the T100I mutation.
For G protein-coupling, serotonin marginally enhanced GTPγ35S binding in membranes expressing the guinea-pig 5-HT1D receptor and its mutants, but robustly in membranes expressing the gorilla receptor. Sumatriptan enhanced GTPγ35S binding in the latter nearly as much as serotonin, and several isochromans by 30–60% of serotonin.
We discovered key differences in the function and binding properties of guinea-pig and gorilla 5-HT1D receptors, and identified contributions of I100 and H102 of primate 5-HT1D receptors to isochroman binding. Among common experimental animals, only the rabbit shares I100 and H102 with primates, and could be useful for studying isochroman actions in vivo.
Keywords: Gorilla 5-HT1D receptor, GTPγ35S binding, guinea-pig 5-HT1D receptor, 5-HT1B receptor, 5-HT1D-selective ligands, isochromans
Introduction
The emergence of sumatriptan as an effective anti-migraine drug has brought about much attention on two serotonin receptors, 5-HT1B and 5-HT1D, with which the drug interacts as an agonist of high affinity (Boess et al., 1994; Humphrey et al., 1989; Deliganis & Peroutka, 1991; Buzzi & Moskowitz, 1991; Rebeck et al., 1994; Moskowitz, 1993; Lee & Moskowitz, 1993; Weinshank et al., 1992). Both receptors are located in the human trigeminal ganglia (Bouchelet et al., 1996), a proposed site of neurogenic inflammation for headache, and the 5-HT1B receptor is also widely distributed in blood vessels. Two plausible mechanisms of action for sumatriptan have been advanced, its blocking of plasma protein extravasation in the trigeminal ganglia (Rebeck et al., 1994; Moskowitz, 1993; Lee & Moskowitz, 1993) and/or its cranial vasoconstriction (Friberg et al., 1991; Caekebeke et al., 1992; Schmetterer et al., 1996). Further dissection of the roles of the two receptors in migraine pathophysiology could be facilitated by development of ligands selective for each receptor.
Recently, isochromans have been found to be highly selective for primate 5-HT1D (human and non-human primate receptors) over 5-HT1B receptors by three orders of magnitude (Ennis et al., 1998). In the guinea-pig, a primary animal model for anti-migraine drug testing, however, isochromans bound the 5-HT1D receptor with low affinity (by nearly two orders of magnitude) than primate counterparts. Among serotonin receptors, species-specific pharmacologies are frequently observed, particularly between experimental animal models and primates (Adham et al., 1992; Kao et al., 1992). In this study, we search for residues in the guinea-pig receptor responsible for this isochroman pharmacology, using site-directed mutagenesis of divergent residues in transmembrane regions which primarily contribute to catecholamine binding. Mutants and wild type receptors, as heterologously expressed in human embryonic kidney (HEK) 293 cells, were examined here for their binding properties with several isochromans and standard serotonergic ligands (sumatriptan, metergoline and methiothepin), and also for their abilities to enhance GTPγ35S binding, representing target G protein activation.
Methods
Receptor cloning
Cloning of guinea-pig and gorilla 5-HT1D receptors was described elsewhere (Pregenzer et al., 1997). For cloning of the guinea-pig 5-HT1B receptor, a pair of primers (sense primer, 5′ GCCACCATGGGGAACCCTGAGGCTTCG3′; antisense primer, 5′ AAGTCAGGTTGTGCACTTAAAGCG3′) were designed from its known cDNA sequence (Zgombick et al., 1996). Polymerase chain reactions (PCR) were performed in a 50 μl reaction mixture containing 1 U Amplitaq DNA polymerase in the vender supplied buffer, 200 μM each dNTP, 10 pmol the primers, 5 ng guinea-pig genomic library (Clonetech). The cycle parameters were at 94°C for 30 s, 54°C for 30 s and 72°C for 90 s with a final extension for 10 min after 35 cycles. For the gorilla 5-HT1B receptor, primers (sense primer, 5′ GCCGCCATGGAGGAACCGGGTGCTCAG3′; antisense primer, 5′ AAGTCAACTTGTGCACTTAAAACG3′) were designed from the published sequence of the human 5-HT1B receptor (Jin et al., 1992). PCR with the two primers and genomic DNAs from the gorilla as a template yielded fragments of the expected size. Final PCR products from the gorilla and guinea-pig were purified through agarose gel electrophoresis, were directly sequenced using a PRISM Ready Reaction DyeDeoxy Terminal Cycle Sequencing Kit from Perkin-Elmer/Applied Biosystems Division, and were cloned into a PCRScript vector via blunt end ligation. Insert cDNAs, after amplification from selected clones, resequenced. The correct insert was transferred to a PCI-Neo™ vector, and used to transfect HEK293 cells using Ca2+ phosphate precipitation techniques. Transfected cells were selected for a month in the presence of G418 at the concentration of 500 μg ml−1. Cell membranes were prepared as described elsewhere (Pregenzer et al., 1993).
Mutation
Point mutations of T100I or/and R102H in the guinea-pig 5-HT1D receptor were produced using the procedure of gene splicing by overlap extension (Horton et al., 1989). Briefly, we obtained sense primers (base pairs (bp) 289–315, 5′ GCATACACCAC(T)CACCCGC(AT)ACCTGGAAC3′, the underlined bases in the parenthesis representing the altered ones for I and H, respectively) that are complementary to the cDNA region of interest except for the altered codons for target residues in the middle of the sequences, and their corresponding antisense primers. Also we used another pair of outside primers, containing a unique restriction site at the 3′ and 5′ ends for each mutation (the StyI site at the bp 269, and the BsgI site at the bp 381). Following polymerase chain reactions (PCR) as described elsewhere (Horton et al., 1989), we obtained 5-HT1D receptor cDNA fragments with specific mutations in the middle and unique restriction sites at the 5′ and 3′ ends. Final PCR fragments were digested with proper restriction enzymes to yield sticky ends, and then were subcloned into the PCR-script™ (Stratagene) vector containing the guinea-pig 5-HT1D receptor cDNA with the complementary ends. Similarly, the point mutation of A222R was generated with a sense primer (bp 648–672, 5′ CTACAGCCGCATCTACCGGGCCGCC3′, the underlined bases representing the codon for arginine). All mutants were confirmed initially with restriction digestion maps, and subsequently with dideoxy sequencing. Receptor cDNA inserts (or their mutants) in the PCR-script™ were transferred to a mammalian expression vector, PCI-neo™, and the vectors were used to transfect HEK293 cells using Ca2+-phosphate precipitation techniques.
Ligand Binding
Binding of [3H]-serotonin was measured in membranes, using filtration techniques as described elsewhere (Pregenzer et al., 1993). Briefly, the medium contained (in mM): NaCl 150, MgCl2 2, EDTA 1, HEPES/Tris (pH 7.4) 20, [3H]-serotonin at varying concentrations (0.1–30 nM), and 20–80 μg membrane protein, in a total volume of 500 μl. The mixture was incubated at 23°C for 60 min, and then filtered over a Whatman GF/B filter under vacuum. The filters were washed three times with 4 ml of ice cold 50 mM Tris/HCl buffer (pH 7.4). Non-specific binding was estimated in the presence of excess clozapine (100 μM). Displacement of [3H]-serotonin by test compounds (competition assays) was carried out in the same assay buffer with the radioactive ligand at 0.5–2 nM.
GTPγ35S binding was measured following the procedure reported earlier (Chabert et al., 1994) in medium containing (in mM): NaCl 100, EDTA 1, MgCl2 3 dithiothreitol 0.5, HEPES (pH 8.0) 25, digitonin 0.003% GTPγ35S (5–3×105 c.p.m./assay) 2 nM, and about 100 μg membrane protein in a volume of 120 μl. Test ligands were included at 1 μM unless indicated otherwise. Membranes, after preincubation with 10 μM GDP for 10 min on ice, were added to the reaction mixture, then incubated for 30 min at 30°C. Reaction mixtures were filtered over Whatman GF/B filters under vacuum, followed by washing three times with 4 ml of an ice-cold buffer containing (in mM): NaCl 100, Tris/HCl 20, pH 8.0, MgCl2 25. Agonist-induced GTPγ35S binding was calculated by subtracting that observed without test agonists. Binding data were analysed using a non-linear regression method (Sigma Plot), and presented as mean values±standard errors from three or more experiments.
The following isochromans were synthesized in Pharmacia and Upjohn, INC: PNU-109291, ( ( s )-3,4-dihydro-1-[2-[4-(4-methoxyphenyl ) - 1 -piperazinyl]ethyl] - N-methyl-1H-2-benzopyran-6-carboximide); PNU-142093, ((s)-3,4-dihydro-1-[2-[4-[4-(trifluoromethyl) - phenyl] - 1 - piperazinyl]ethyl] - N - methyl- 1H - 2-benzopyran-6-carboximide; PNU-142633, ((s)-3,4-dihydro-1-[2-[4-[4-(aminocarbonyl) phenyl]-1 piperazinyl] ethyl] -N-methyl-1H-2-benzopyran-6-carboximide.
Results
We examined [3H]-serotonin binding properties in cell membranes heterologously expressing the 5-HT1B and 5-HT1D receptors from the gorilla and guinea-pig, and guinea-pig 5-HT1D receptor mutants (T100I, R102H or in combination). Levels of specific [3H]-serotonin binding in these cell membranes were at least three times greater than non-specific binding as measured in the presence of excess clozapine (100 μM). No appreciable specific binding of [3H]-serotonin was observed in membranes of mock transfected HEK293 cells. Binding data of [3H]-serotonin at various concentrations showed good fits (linearity) for Scatchard plot with a binding isotherm for a single class of binding sites. As shown in Table 1, the [3H]-serotonin dissociation constant (KD) was 1.4±0.2, 1.3±0.2, 0.72±0.07 and 5.7±0.7 nM for the 5-HT1D and 5-HT1B receptors from the gorilla and those from the guinea-pig, respectively, with maximal binding sites of 1.2±0.2, 0.5±0.1, 0.25±0.02 and 2.4±0.3 pmol mg−1 protein, respectively. T100I and R102H mutations in the guinea-pig 5-HT1D receptor hardly affected the KD for serotonin, ranging from 0.4–0.5 nM. The maximal binding varied from 0.12–0.44 pmol mg−1 protein.
Table 1.
Comparison of ligand binding properties of 5-HT1D and 5-HT1B receptors from the gorilla and guinea-pig, and mutants of the guinea-pig 5-HT1D receptors
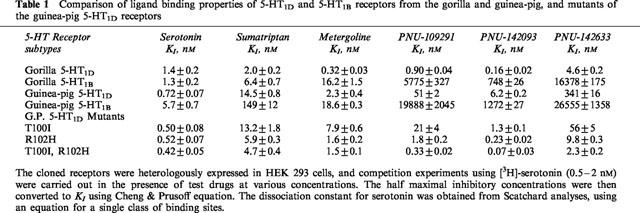
Competition binding experiments using [3H]-serotonin were carried out with sumatriptan, metergoline (a generic serotonergic ligand) and three isochromans (PNU-109201, -142093 and -142633). As shown previously for primate receptors (Weinshank et al., 1992), sumatriptan bound the gorilla 5-HT1D and 5-HT1B receptors with high affinity (inhibition constant (KI) of 2.0 and 6.4 nM, respectively), and little subtype selectivity, while metergoline was moderately selective for the 5-HT1D over 5-HT1B receptor (Ki of 0.32 and 16.2 nM, respectively). The isochromans were highly selective for 5-HT1D: PNU-109291, - 142093 and -142633 displayed 6400, 4600 and 3560 fold higher affinity, respectively, for the 5-HT1D over 5-HT1B receptor. In the guinea-pig receptors, their selectivity for the 5-HT1D over 5-HT1B receptor were less (ranging from 80 to 380 fold), primarily due to their lower affinities to the 5-HT1D receptor. Sumatriptan and metergoline, on the other hand, showed no appreciable affinity difference between the gorilla and guinea-pig 5-HT1D receptor, only 6 fold or less changes in KI values.
Upon mutations of T100I/R102H in the guinea-pig receptor, the isochroman affinities improved to those observed with the gorilla receptor. The mutations changed the Ki value for PNU-109291 from 51±2 to 0.33±0.02 nM, for PNU-142093 from 6.2±0.2 to 0.07±0.03 nM, and for PNU-142633 from 341±16 to 2.3±0.2 nM. Individual contributions of the two mutated residues were also accessed. With the T100I mutation, isochroman affinities improved only 3–6 fold as compared to the wild type values, but with the R102H mutation, their affinities improved 27–35 fold. Thus, the free energy changes for the two mutations appear to be additive. For instance, the free energy changes for PNU-142633 were 1.0, 2.0 and 3.0 kcal mol−1 with the T100I, R102H and T100I/R102H mutant, respectively. It should be noted again that the mutations produced no appreciable changes in affinity for serotonin, sumatriptan and metergoline.
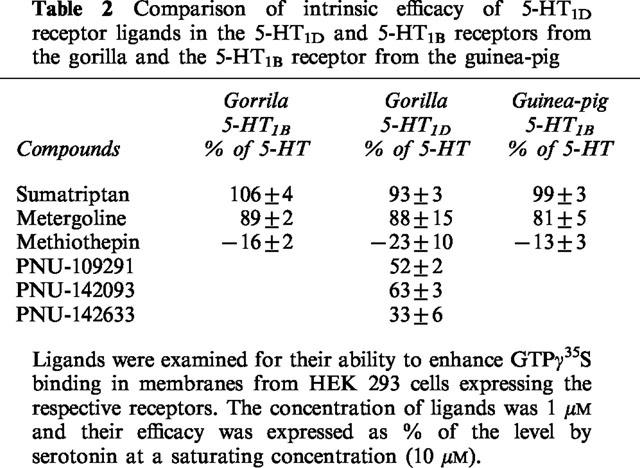
The functional hallmark for G-protein coupled receptors is agonist-induced GTPγ35S binding to Gα subunits. Serotonin dose-dependently enhanced GTPγ35S binding in the guinea-pig 5-HT1B and 5-HT1D receptor, with the half maximal concentration of 34±4 and 53±7 nM, respectively, and maximal binding of 226±4 and 36±12 fmoles mg−1 protein, respectively (Figure 1). In the gorilla receptors, the half maximal concentration for serotonin were 41±3 and 21±4 nM for the 5-HT1B and 5-HT1D receptor, respectively, and maximal binding of 171±4 and 147±17 fmoles mg−1 protein, respectively (Figure 1). Methiothepin at 10 μM reduced the basal level of GTPγ35S by 16, 23 and 13% in the gorilla 5-HT1D and 5-HT1B, and the guinea-pig 5-HT1B receptor, respectively, (Table 2), and also completely blocked serotonin action at 1 μM. Sumatriptan at a saturating concentration (10 μM) enhanced GTPγ35S binding as high as serotonin in both gorilla 5-HT1D and 5-HT1B receptors and the guinea-pig 5-HT1B receptor (Table 2). Metergoline also robustly enhanced GTPγ35S binding, nearly 80% of the level observed with serotonin in these receptors. In the gorilla 5-HT1D receptor, PNU-109291, PNU-142093 and PNU-142633 enhanced GTPγ35S binding to 52±2, 63±3 and 36±6% of the level observed with serotonin, respectively. We did not test the drugs in the guinea pig 5-HT1D receptor because of its marginal response in serotonin-dependent GTPγ35S binding. The guinea-pig receptor has Alanine 222 at the 3rd intracellular loop near the membrane while the corresponding residue in the gorilla receptor is arginine. Because this region has been reported to be critical for G protein couplings, we generated the A222R mutant of the guinea-pig 5-HT1D receptor. The mutant, however, also displayed no appreciable enhancement of serotonin-induced GTPγ35S binding (data not shown).
Figure 1.
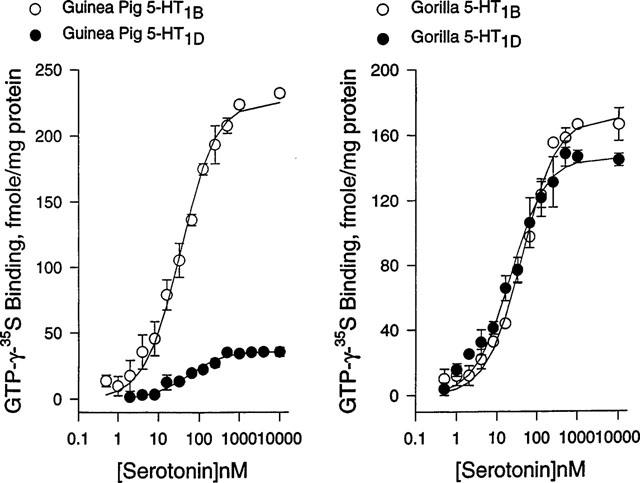
Dose-response profiles for serotonin to enhance GTPγ35S binding in the 5-HT1D and 5-HT1B receptors from the gorilla and the guinea-pig. GTPγ35S binding was measured in the presence of serotonin at various concentrations in cell membranes expressing indicated receptors. Serotonin-dependent GTPγ35S binding was plotted as a function of serotonin concentrations, and fitted to a binding isotherm for a single class of binding sites. The data represent mean values±standard errors from three experiments.
Table 2.
Comparison of intrinsic efficacy of 5-HT1D receptor ligands in the 5-HT1D and 5-HT1B receptors from the gorilla and the 5-HT1B receptor from the guinea-pig
Discussion
High affinity interaction of 5-HT1D and 5-HT1B receptors with sumatriptan, and efficacious anti-migraine drug, implies their involvements in migraine pathophysiology, but their individual contributions have not been clearly established yet (Boess et al., 1994; Humphrey et al., 1989; Deliganis & Peroutka, 1991; Buzzi & Moskowitz, 1991; Rebeck et al., 1994; Moskowitz, 1993; Lee & Moskowitz, 1993; Weinshank et al., 1992). Further understanding on this issue should be possible in the near future with a discovery of isochromans which are highly selective for 5-HT1D over 5-HT1B receptors (Ennis et al., 1998). In the course of our study with isochromans, we discovered their species-specific pharmacology. Isochromans bound the 5-HT1D receptor from the guinea-pig, an animal model for anti-migraine drug testing, with lower affinities than the receptor from the gorilla, a primate model with its receptor properties nearly identical to those of the human receptor (only four divergent residues, I47V, V164A, T302I and V336A) (Pregenzer et al., 1997). Serotonin receptors are known for pharmacological dissimilarities across animal species, as reflected by divergences in their primary sequences. For example, the human 5-HT1D receptor differs in the primary sequence from the rabbit, guinea-pig and dog by 31, 33 and 43 residues respectively (Hardwood et al., 1995; Libert et al., 1989; Zgombick et al., 1996). Often these divergences in primary sequence contribute not only to differential ligand binding properties, but also to differential functional properties, as noted here with serotonin-induced enhancement of GTPγ35S binding, which was robust for the gorilla receptor in HEK 293 cells, but low for the guinea-pig receptor. These species divergences could provide the opportunity to discover the roles of divergent residues in receptor ligand interactions, and also to evaluate various animal models for drug testing.
With respect to isochroman binding, we searched responsible residues at the transmembrane segments and adjacent extracellular regions, which are known to contribute primarily to catecholamine binding. Twelve of the 33 divergent amino acid residues of the guinea-pig receptor are located in the transmembrane segments and nearby extracellular loops, and only five of them are non-conservatively substituted, T100I, R102H, A157T, A161I and S218G (the first letter code for the guinea-pig residue and the last for the gorilla residue which is the same as human). Among those, the T100I and R102H were our primary mutational targets because these residues are fully exposed to the extracellular surface (TMII) where ligand-receptor interactions likely occur, while the rest of the divergent residues (A157T, A161I and S218G) are located near the intracellular surface (TMIV and V). As shown here, the mutations of the both TMII residues to the corresponding primate residues (T100I and R102H) improved the affinity of the guinea-pig 5-HT1D receptor for isochromans to that of the gorilla receptor. Individually, R102H mutation contributes to free energy change twice as much as T100I mutation. Further examination of a broad spectrum of isochroman analogues for their interactions with the mutants could aid us in identifying a chemical moiety interacting with these residues. This line of information, along with already known residues for receptor-ligand interactions (e.g., D113 as a counterion for the amine group of catecholamines), would be valuable for constructing a spatial binding map for isochromans. Inspection of primary sequences of 5-HT1D receptors from various experimental animals reveals that only the rabbit shares I100 and H102 with primates, while the dog, rat and mouse share T100 and R102 with the guinea-pig. Further comparison reveals that the rabbit 5-HT1D receptor display only eight transmembrane residues divergent from the guinea-pig receptor, and four of them match the human residues, two are conservatively substituted, and only the remaining two (T51A in TMII and G311V in TMVII) represent non-conservative substitutions as compared to the human sequence. This higher sequence homology of the rabbit 5-HT1D receptor to the primate receptor, including the two key residues, would render the receptor to exhibit isochroman pharmacology more closely related to human than the guinea-pig.
With respect to G-protein coupling, critical contributions have been reported to come from intracellular loops and C-terminal regions of G-protein-coupled receptors (GPCR), particularly, six to seven residues near membrane (Eason & Liggett, 1996). Only two residues in the guinea-pig 5-HT1D receptor in the regions are divergent from the primate receptors, A222R (non-conservative substitution) and S224N (semi-conservative substitution). In this study we generated the A222R mutant, and the mutant showed no appreciable enhancement of serotonin-induced GTPγ35S binding (data not shown). Outside of the putative critical regions, there are eight divergent residues from the third intracellular loop of the guinea-pig receptor (including a deletion of S267 in primate receptors) and three divergent residues from the C-terminal region. These multiple potential targets for mutation and also a number of studies reporting that the coupling processes have been altered via mutations on various parts of receptor led us to exclude further search for the residues responsible for G-protein interactions from the scope of this study.
With respect to the 5-HT1B receptor, the guinea-pig also displays 43 and 42 divergent residues from the human and gorilla receptors, respectively. Although our current study with several generic serotonergic ligands revealed no appreciable differences between the guinea-pig and the gorilla receptors, their differential pharmacologies would be revealed with discovery of ligands highly selective for 5-HT1B over 5-HT1D receptors.
In summary, we probed differences in functional and binding properties between the guinea-pig and gorilla 5-HT1D receptors using isochromans (5-HT1D-selective ligands), and discovered the contributions of I100 and H102 near the extracellular side of TMII of primate 5-HT1D receptors to isochroman binding. Among common experimental animals, the rabbit has I100 and H102 like primates, but the dog, rat and mouse share T100 and R102 with the guinea-pig.
Abbreviations
- bp
base pairs
- HEK
human embryonic kidney
- HEPES
4-(2-hydroxyethyl)-1-piperazineehthanesulphonic acid
- KD
dissociation constant
- Ki
inhibition constant
- PCR
polymerase chain reaction
- TM
transmembrane segment
References
- ADHAM N., ROMANIENCO P., HART P., WEISHANK R.L., BRANCHEK T. The rat 5-hydroxytryptamine 1B receptor is the species homologue of the human 5-hydroxytryptamine 1D beta receptor. Mol. Pharmacol. 1992;41:1–7. [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacol. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., MOSKOWITZ M.A. Evidence for 5-HT1B/1D receptors mediating the anti-migraine effect of sumatriptan and dihydroergotamine. Cephalalgia. 1991;11:165–168. doi: 10.1046/j.1468-2982.1991.1104165.x. [DOI] [PubMed] [Google Scholar]
- BOUCHELET I., COHEN Z., CASE B., SEGUELA P., HAMEL E. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol. Pharmacol. 1996;50:219–223. [PubMed] [Google Scholar]
- CAEKEBEKE J.F.V., FERRARI M.D., ZWETSLOOT C.P., JANSEN J., SAXENA P.R. Anti-migraine drug sumatriptan increases blood flow velocity in large cerebral arteries during migraine attack. Neurology. 1992;42:1522–1526. doi: 10.1212/wnl.42.8.1522. [DOI] [PubMed] [Google Scholar]
- CHABERT C., CAVEGN C., BERNARD A., MILLS A. Characterization of the functional activity of dopamine ligands at human recombinant dopamine D4 receptors. J. Neurochem. 1994;63:62–65. doi: 10.1046/j.1471-4159.1994.63010062.x. [DOI] [PubMed] [Google Scholar]
- DELIGANIS A.V., PEROUTKA S.J. 5-Hydroxytryptamine1D receptor agonism predicts anti-migraine efficacy. Headache. 1991;31:228–231. doi: 10.1111/j.1526-4610.1991.hed3104228.x. [DOI] [PubMed] [Google Scholar]
- EASON M.G., LIGGETT S.B. Chimeric mutagenesis of putative G-protein coupling domains of the α2A-adrenergic receptor. J. Biol. Chem. 1996;271:12826–12832. doi: 10.1074/jbc.271.22.12826. [DOI] [PubMed] [Google Scholar]
- ENNIS M.D., GHAZAL N.B., HOFFMAN R.L., SMITH M.W., SCHLACHTER S.K., LAWSON C.F., IM W.B., PREGENZER J.F., SVENSSON K.A., LEWIS R.A., HALL E.D., SUTTER D.M., HARRIS L.T., MCCALL R.B. Isochroman-6-carboxamides as highly selective 5-HT1D agonists: potential new treatment for migraine without cardiovascular side effects. J. Med. Chem. 1998;41:2180–2183. doi: 10.1021/jm980137o. [DOI] [PubMed] [Google Scholar]
- FRIBERG L., OLESEN J., IVERSEN H.K., SPERLING B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- HARWOOD G., LOCKYER M., GILES H., FAIRWEATHER N. Cloning and characterization of the rabbit 5-HT1Dα and 5-HT1Dβ receptors. FEBS Lett. 1995;377:73–76. doi: 10.1016/0014-5793(95)01308-3. [DOI] [PubMed] [Google Scholar]
- HORTON R.M., HUNT H.D., HO S.N., PULLEN J.K., PEASE L.R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- HUMPHREY P.P.A., PERRIN M.J., FENJUK W., OXFORD A.W. The pharmacology of the novel 5-HT1-like receptor agonist GR453175. Cephalagia. 1989;9 suppl. 9:23–33. doi: 10.1111/J.1468-2982.1989.TB00069.X. [DOI] [PubMed] [Google Scholar]
- JIN H., OKSENBERG D., ASHKENAZI A., PEROUTKA S.J., DUNCAN A.M.V., ROZMAHEL R., YANG Y., MENGOD G., PALACIOS J.M., O'DOWD B.F. Characterization of the human 5-hydroxytryptamine-1B receptor. J. Biol. Chem. 1992;267:5735–5738. [PubMed] [Google Scholar]
- KAO H.-T., ADHAM N., OLSEN M.A., WEINSHANK R.L., BRANCHEK T.A., HART P.R. Site-directed mutagenesis of a single residue changes the binding properties of the serotonin 5-HT2 receptor from a human to a rat pharmacology. FEBS Lett. 1992;307:324–328. doi: 10.1016/0014-5793(92)80705-l. [DOI] [PubMed] [Google Scholar]
- LEE W.S., MOSKOWITZ M.A. Conformationally restricted sumatriptan analogues, CP-122,288 and CP-122,638 exhibit enhanced potency against neurogenic inflammation in dura mater. Brain Res. 1993;626:303–305. doi: 10.1016/0006-8993(93)90591-a. [DOI] [PubMed] [Google Scholar]
- LIBERT F., PARMENTIER M., LEFORT A., DUMONT J.E., VASSART G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43 suppl 3:S16–S20. [PubMed] [Google Scholar]
- PREGENZER J.F. , ALBERTS G.L., BOCK J.H., SLIGHTOM J.L., IM W.B. Characterization of ligand binding properties of the 65-HT1D receptors cloned from chimpanzee, gorilla and rhesus monkey in comparison with those from the human and guinea pig receptors. Neurosci. Lett. 1997;235:117–120. doi: 10.1016/s0304-3940(97)00728-3. [DOI] [PubMed] [Google Scholar]
- PREGENZER J.F., IM W.B., CARTER D.B., THOMSEN D.R. Comparison of interaction of [3H]muscimol, t-butylbicyclophosphoro[35S]thionate, and [3H]flunitrazepam with cloned GABAA receptors of the α1β2 and α1β2γ2 subtypes. Mol. Pharmacol. 1993;43:801–806. [PubMed] [Google Scholar]
- REBECK G.W., MAYNARD K.I., HYMAN B.T., MOSKOWITZ M.A. Selective 5-HT1Dα serotonin receptor gene expression in trigeminal ganglia: Implications for anti-migraine drug development. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3666–3669. doi: 10.1073/pnas.91.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMETTERER L., WOLZT M., KREJCY K., GRASELLI U., FINDL O., EICHLER H.-G., SINGER E.A. Cerebral and ocular hemodynamic effects of sumatriptan in the nitroglycerin headache model. Clin. Pharmacol. Ther. 1996;60:199–205. doi: 10.1016/S0009-9236(96)90136-8. [DOI] [PubMed] [Google Scholar]
- WEINSHANK R.L., ZGOMBICK J.M., MACCHI M.J., BRANCHEK T.A., HART P.R. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1Dα and 5-HT1Dβ. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZGOMBICK J.M., BARD J.A., KUCHAREWICZ S.A., URQUHART D.A., WEINSHANK R.L., BRANCHEK T.A. Cloning and characterization of guinea-pig 5-HT1B and 5-HT1D receptor subtypes. Soc. Neurosci. Abs. 1996;22:528.7. [Google Scholar]


