Abstract
Bradykinin is suggested to play a role in the pathophysiology of several acute and chronic diseases, including allergic disorders such as asthma. In the present study, we have investigated the importance of bradykinin in mediating allergic inflammation in rats.
To this end we have tested the effects of the B2 receptor antagonists Hoe 140, FR173657 or FR172357 on the pleural inflammatory response triggered by intrapleural (i.pl.) injection of allergen (ovalbumin, 12 μg cavity−1) in 14 day-actively sensitized Wistar rats. Analysis of the pleural fluid effluent revealed a sequence of mast cell-dependent inflammatory events, including early protein exudation and neutrophilia and late pleural eosinophil influx.
Local treatment with Hoe 140 (0.1 and 1 μg cavity−1), FR173657 (1 and 10 μg cavity−1) or FR172357 (1 and 10 μg cavity−1) inhibited dose-dependently allergen-induced mast cell activation with impairment of pleural plasma leakage, neutrophil accumulation and late eosinophil influx.
Moreover, the B2 receptor antagonists also dose-dependently inhibited the allergic like inflammatory pleurisy triggered by bradykinin (50 μg cavity−1), which is characterized by acute mast cell degranulation, protein leakage and pleural eosinophil infiltration.
Taken together, our findings provide substantial evidence to suggest that bradykinin acting on its B2 receptors play a critical role in mediating allergic mast cell-dependent inflammation in rats, and suggest that B2 receptor antagonists may be useful therapeutically to control allergic dysfunction.
Keywords: Bradykinin, B2 receptor antagonists, allergic pleurisy, eosinophils, neutrophils, oedema
Introduction
Bradykinin is a bioactive nonapeptide formed in pathophysiological states by the enzymatic action of circulating or tissue kallikreins on plasma globulin precursors. Elevated levels of bradykinin in circulating and bronchoalveolar lavage fluid have been demonstrated in patients with allergic disorders, including asthma, allergic rhinitis and anaphylactic shock (for review see Bhoola et al., 1992). Moreover, bradykinin produces several biological alterations which are observed in allergic inflammatory disorders. Thus, bradykinin induces arteriolar dilatation, venular permeability, oedema and tissue leukocyte infiltration. In addition, bradykinin has potent direct bronchoconstrictory action in allergic subjects and stimulates the release of several other mediators associated with allergic inflammation (Farmer, 1991). For example Polosa et al. (1990) have shown that bradykinin induced severe bronchospasm as well as cough and wheezing in asthmatic but not in normal subjects. Likewise, Brunnée et al. (1991) have reported that allergic patients show more microvascular leakage to the topical application of bradykinin to the nasal mucosa than non-allergic individuals.
The pharmacological effects of bradykinin are mediated via activation of, at least, two distinct typical G-protein coupled cell surface receptors designated B1 and B2 (Regoli & Barabé, 1980; Vavrek & Stewart, 1985; Roberts, 1989; Regoli et al., 1990). Most of the acute responses induced by bradykinin seem to be mediated by the constitutively expressed B2 receptors (Stewart et al., 1997). In the last two decades, a number of selective B2 receptor antagonists have been developed and these have been critical in the evaluation of the role of bradykinin in several disease models. For example, the peptidic B2 receptor antagonists, NPC 567 and NPC 16731, have been shown to inhibit allergen-induced airway inflammation and hyperresponsiveness in sensitized sheep and guinea-pigs (Soler et al., 1990; Abraham et al., 1991; Farmer et al., 1992). In addition, treatment with a ‘second generation' B2 antagonist, Hoe 140, was found to be effective in relieving nasal blockade in patients with seasonal allergic rhinitis (Austin et al., 1994) and in certain forms of asthma (Akbary et al., 1996).
Therefore, there is substantial evidence to support a role for bradykinin in the pathophysiology of allergic diseases. However, the putative mechanism by which bradykinin effect allergic inflammation in vivo is not fully elucidated. We have previously shown that bradykinin desensitizes the rat pleural cavity to a further injection of bradykinin or to an injection of allergen suggesting that allergic pleurisy in rats may be dependent on the activation of bradykinin receptor(s) (Martins et al., 1992). Here, we have investigated the effects of the administration of distinct B2 receptor antagonists on allergen-induced pleurisy in rats and assessed whether inhibition of the activation of mast cells was involved in the actions of these antagonists. Three B2 receptor antagonists were used, the peptidic antagonist Hoe 140 and two non-peptidic antagonists FR 173657 and FR 172357.
Methods
Animals
Wistar rats of either sex and weighing 150–200 g from the Oswaldo Cruz Foundation Breeding Unit (Rio de Janeiro, Brazil) were used.
Allergic pleurisy in actively sensitized rats
Rats were actively sensitized by means of a dorsal subcutaneous injection of a mixture containing 50 μg of ovalbumin and 5 mg of AI(OH)3, in a final volume of 200 μl. Fourteen days later, the sensitized and non-sensitized animals received an intrapleural injection of ovalbumin (12 μg cavity−1) which was dissolved in sterile 0.9% NaCl (saline) immediately before use. All intrapleural injections were performed during ether anaesthesia in a final volume of 100 μl using a 27.5-gauge needle adjusted to be 3 mm in length. At different times after pleural stimulation, the animals were killed with terminal ether anaesthesia, and the thoracic cavity was rinsed with 3 ml of saline containing heparin (10 IU ml−1). Pleural washing was collected and its volume measured with a graduated syringe.
Pleurisy triggered by bradykinin, compound 48/80 or PAF
Non-sensitized rats were stimulated intrapleurally with bradykinin (50 μg cavity−1), compound 48/80 (25 μg cavity−1) or platelet-activating factor (PAF) (1 μg cavity−1). Control animals were injected with the same volume of vehicles. PAF was diluted with sterile saline containing 0.01% bovine serum albumin (BSA), whereas all other agents were dissolved in saline. As described above, the pleural fluid was collected for analysis of plasma leakage and eosinophil accumulation in time-points ranging from 30 min to 24 h post-challenge.
Treatment
The B2 receptor antagonists Hoe 140 (0.1 and 1 μg cavity−1), FR173657 (1 and 10 μg cavity−1) and FR172357 (1 and 10 μg cavity−1) were given intrapleurally 5 min before stimulation. In control groups, the antagonists were replaced by their vehicles. Hoe 140 was diluted in sterile saline and both FR173657 and FR172357 were dissolved in dimethyl sulphoxide (DMSO) and further diluted with saline. All solutions were prepared immediately before use.
Measurement of total protein extravasated
Analysis of the plasma protein leakage was performed 4 h after injection of allergen. The fluid collected from the pleural cavity was centrifuged (2500 r.p.m.) for 10 min and the protein content of the supernatant quantified in a spectrophotometer (540 nm) by Biuret technique (Gornall et al., 1949).
Cell analysis
Total leukocyte from pleural cavity and mast cell counts were performed in Neubauer chamber by means of an optical microscope after dilution with Türk solution (2% acetic acid) and toluidine blue dye (Mota, 1966) respectively. Differential analysis of cells from pleural cavity was made in cytospin preparations stained with May-Grünwald-Giemsa dye under an oil immersion objective.
Histamine measurement
Histamine stored in cells recovered by washing the pleural cavity with heparinized saline was spectrofluorimetrically determined according to Shore et al. (1959). The unpurified cellular suspension was centrifuged at 300 r.p.m. for 10 min, and 0.4 n perchloric acid (0.5 ml) was added to the pellet leading to cell lysis and protein precipitation. After centrifugation at 3000 r.p.m. for 10 min, the supernatants were collected and stored at −20°C until histamine quantification.
Drugs
Hoe 140 was a kind gift from Dr Wirth (Hoechst, Frankfurt, Germany). FR173657 and FR172357 were a donation from Dr Aramori (Fujisawa Pharmaceutical Co., Japan. Ovalbumin was provided by Biochemika Fluka (Switzerland); PAF (1-O-hexadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine) was purchased from Bachem (Bubendorf, Switzerland); bradykinin, compound 48/80 and histamine from Sigma Chemical Co. (St. Louis, U.S.A.); and sodium heparine from CEME (Brasília, Brazil).
Statistical analysis
Data were reported as mean±s.e.mean and statistically analysed by means of analysis of variance (ANOVA) followed by the Newman-Keuls-Student's test. Differences were considered to be statistically significant when P<0.05.
Results
Effects of B2 receptor antagonists on allergen-induced pleurisy
In agreement with our previous observations (Lima et al., 1991), the intrapleural injection of allergen (ovalbumin, 12 μg cavity−1) into actively sensitized rats led to an intense pleural inflammatory response, which was characterized by a massive mast cell degranulation, plasma leakage and neutrophil accumulation noted at 4 h (Table 1), and a later eosinophil accumulation observed 24 h post-challenge (Figure 1).
Table 1.
Effect of B2 receptor antagonists on histamine release, mast cell degranulation, protein exudation and neutrophil influx induced by allergen in sensitized rats
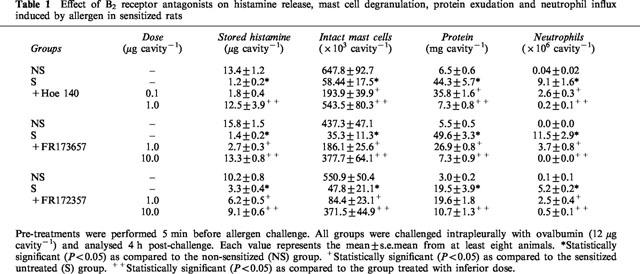
Figure 1.
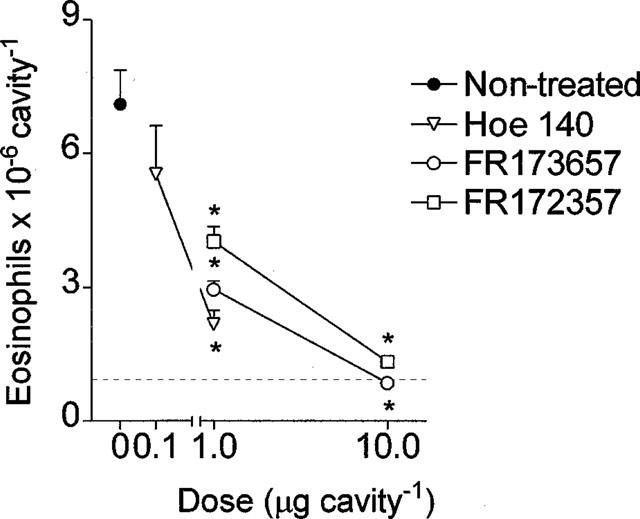
Inhibition by Hoe 140 (0.1 and 1 μg cavity−1), FR173657 or FR172357 (1 and 10 μg cavity−1) of pleural eosinophil accumulation observed 24 h after allergen (ovalbumin, 12 μg cavity−1) in actively sensitized rats. The antagonists were injected locally 5 min before challenge, as described in Methods. Non-sensitized ovalbumin challenged control group is represented by the dashed line. Results were expressed as the mean±s.e.mean from at least six animals. *P<0.001 compared to the allergen-challenged sensitized group.
As shown in Table 1, allergen-induced mast cell degranulation (attested by both a significant reduction in the number of intact mast cells recovered from the pleural cavity and a decrease in the stored histamine content of these cells) was clearly prevented by local pretreatment with bradykinin B2 receptor antagonists. Almost complete blockade of these phenomena was obtained following administration of Hoe 140 at 1 μg cavity−1 or FR173657 and FR172357 injected at 10 μg cavity−1. The effectiveness of these treatments on plasma leakage and neutrophil recruitment at 4 h, and eosinophil recruitment observed 24 h post-challenge is illustrated in Table 1 and Figure 1 respectively. Hoe 140 (0.1 and 1 μg cavity−1), FR173657 and FR172357 (1 and 10 μg cavity−1) clearly inhibited the phenomena in a dose-dependent manner. Hoe 140 was always the most potent inhibitor in all parameters evaluated.
Effects of B2 receptor antagonists on bradykinin-induced pleurisy
The intrapleural injection of bradykinin (50 μg cavity−1) is followed by a quite similar sequence of events to that evoked by allergen. Thus, bradykinin caused an early degranulation of mast cells, as revealed by a fall in the number of intact mast cells (from 413.9±61.7 to 51.3±17.2×103 mast cells cavity−1, n=8, P<0.001) and a significant reduction in the amount of mast cell-stored histamine (from 8.4±0.6 to 4.5±0.8 μg cavity−1; n=7; P<0.01). Moreover, the intrapleural administration of bradykinin into naive rats led to a marked plasma exudation noted 30 min post-stimulation (from 3.95±0.32 (n=6) to 26.62±2.16 mg of protein cavity−1, n=7, P<0.001), and a significant pleural eosinophil accumulation 24 h post-stimulation (from 1.0±0.1 to 2.1±0.2×106 eosinophils cavity−1, n=8, P<0.01).
Pretreatment with Hoe 140, FR173657 and FR172357 inhibited in a dose-dependent manner the effects of bradykinin on intact mast cells numbers (Figure 2) and the amount of mast cell-stored histamine (data not shown). Pretreatment with Hoe 140 (1 μg cavity−1), FR173657, or FR172357 (10 μg cavity−1) had no effect on the basal population of mast cells or protein content of the pleural space in naive rats (data not shown). Local administration of Hoe 140 (0.1 and 1 μg cavity−1), FR173657 or FR172357 (1 and 10 μg cavity−1) also suppressed in a dose-dependent manner the plasma leakage induced by bradykinin (Figure 3A). In contrast, pleural plasma leakage caused by intrapleural injection of compound 48/80 (25 μg cavity−1, a standard non-immunological mast cell degranulating agent) remained unaltered following pretreatment with these drugs (Figure 3B). Finally, local pretreatment with either Hoe 140 (1 μg cavity−1), FR173657 or FR172357 (10 μg cavity−1) significantly inhibited the pleural eosinophilia evoked by bradykinin (Figure 4A) but not that evoked by PAF (Figure 4B).
Figure 2.

Effect of Hoe 140 (0.1 and 1 μg cavity−1), FR173657 or FR172357 (1 and 10 μg cavity−1) on reduction in intact mast cell numbers evoked by intrapleural injection of bradykinin (50 μg cavity−1) in naive rats. The analysis was performed 30 min post-challenge and the antagonists were injected locally just 5 min before stimulation. Saline challenged control group is represented by the dashed line. Results were expressed as the mean±s.e.mean from at least six animals. *P<0.001 compared to the bradykinin-injected group.
Figure 3.

(A) effect of Hoe 140, FR173657 or FR172357 on plasma leakage evoked by intrapleural injection of bradykinin (50 μg cavity−1). Saline challenged control group is represented by the dashed line. (B) effect of Hoe 140 (1 μg cavity−1), FR173657 or FR172357 (10 μg cavity−1) on plasma leakage evoked by intrapleural injection of compound 48/80 (25 μg cavity−1). The analyses were performed 30 min post-challenge and the antagonists locally injected just 5 min before stimulation. Results were expressed as the mean±s.e.mean from at least six animals. *P<0.001 compared to the Bradykinin-injected group. †P<0.001 compared to saline-injected group.
Figure 4.

Effect of Hoe 140 (1 μg cavity−1), FR173657 or FR172357 (10 μg cavity−1) on eosinophil infiltration induced by intrapleural injection of (A) bradykinin (50 μg cavity−1) or (B) PAF (1 μg cavity−1). The analyses were performed 24 h post-challenge and the antagonists locally injected just 5 min before stimulation. Results were expressed as the mean±s.e.mean from at least six animals. *P<0.001 compared to the saline injected group. +P<0.001 compared to the bradykinin injected group.
Discussion
The present study suggests an important role for bradykinin in the allergic inflammatory response. Treatment with three distinct B2 receptor antagonists (Hoe 140, FR173657 and FR172357) suppressed in a dose-dependent manner the pleural anaphylactic reaction in sensitized rats. We found that these antagonists clearly inhibited allergen- and bradykinin-induced mast cell degranulation. The findings suggest that there is a complex interaction between the bradykinin system and the IgE-mast cell system in the regulation of vascular and cellular changes following allergen challenge. Moreover they suggest that inhibition of mast cell function may underlie the ability of bradykinin B2 antagonists to inhibit allergic pleurisy.
Bradykinin has been suggested to play a pivotal role in allergic inflammatory disorders (Farmer, 1991). Elevated levels of bradykinin have been found in tissues and lavage effluents in allergic patients (Christiansen et al., 1987; Abe et al., 1967). The implication of bradykinin in allergy is also clearly supported by the ability of this peptide to cause bronchoconstriction, nasal blockade and a broad-spectrum of inflammatory actions that resemble findings in allergic individuals (review Bhoola et al., 1992). In the current study, we have evaluated the hypothesis that bradykinin mediates vascular and cellular changes triggered by allergen in actively sensitized rats. This was accomplished by evaluating the inhibitory effects of selective bradykinin B2 receptor antagonists, including Hoe 140, FR173657 and FR172357 (Wirth et al., 1991; Lembeck et al., 1991; Inamura et al., 1997; Majima et al., 1997; Griesbacher & Legat, 1997), on both bradykinin- and allergen-evoked rat pleurisy.
Present and previous findings have shown that Wistar rats sensitized to ovalbumin react to an intrapleural injection of ovalbumin with massive mast cell degranulation, plasma leakage and leukocyte recruitment (Lima et al., 1991; 1996). We found that plasma exudation, neutrophil and eosinophil accumulation evoked by pleural allergen challenge were clearly sensitive to the local pretreatment with either Hoe 140, FR173657 or FR172357. Interestingly, when actively sensitized rats were submitted to bradykinin-induced homologous tachyphylaxis, pleural plasma leakage and leukocyte infiltration evoked by allergen challenge were significantly impaired (Martins et al., 1992). Bradykinin receptors undergo ligand-induced desensitization in response to chronic bradykinin exposure (Roberts & Gullick, 1990). All these data are in line with the interpretation that receptor-mediated actions of bradykinin may play a pivotal role in the development of allergic pleurisy.
There have been conflicting reports as to the efficacy of bradykinin B2 receptor antagonists on allergen-induced plasma extravasation. For instance, Featherstone et al. (1996) and Sakamoto et al. (1996) reported that Hoe 140 failed to alter allergen-induced airway microvascular leakage in sensitized guinea-pigs, whereas Bertrand et al. (1993) found a partial but significant blockade of Evans blue dye extravasation caused by allergen challenge in guinea-pig trachea. Hoe 140 also prevented allergen-evoked plasma leakage in the nasal mucosa of hamsters (Gao et al., 1998) and in the bladder of sensitized rats (Ahluwalia et al., 1998), whereas other selective B2 antagonists such as NPC 17731 and FR173657 were shown to impair airway and cutaneous plasma leakage in primates and guinea-pig respectively (Hogan et al., 1997; Mori & Imamura, 1998). Overall, it seems that the anti-allergic efficacy of B2 receptor antagonists may vary in function of the system (or tissue) investigated, but in general these drugs tend to be active in the case of a strong neurogenic component (Lindström & Andersson, 1997; Gao et al., 1998; Ahluwalia et al., 1998).
We demonstrated here that bradykinin can reproduce the vascular and cellular changes observed in allergic pleurisy. In addition, bradykinin-induced plasma leakage and eosinophil influx were inhibited in a dose-dependent manner by Hoe 140, FR173657 and FR172357. The effects were shown to be specific since the antagonists failed to alter plasma leakage and eosinophil infiltration triggered by compound 48/80 and PAF, respectively. Furthermore, the antagonists also showed the same rank order of potency (Hoe 140>FR173657> FR172357) at inhibiting both allergen- and bradykinin-evoked pleurisy, adding credibility to the interpretation of specificity.
Previous studies have shown that a localized mast cell granule depletion significantly inhibited allergen-induced plasma leakage and leukocyte accumulation in the pleural cavity, emphasizing the pivotal role of mast cells in this process (Lima et al., 1996; Diaz et al., 1996). Bradykinin pleurisy was also marked by mast cell degranulation, and all the three antagonists used here were able to prevent bradykinin- and allergen-induced pleural mast cell degranulation. In fact, after treatment with the bradykinin B2 antagonists, the number of intact mast cells was increased and their stored histamine content returned to baseline levels. In line with these data, it has been reported that the exudatory actions of bradykinin in human nasal mucosa are in part accounted for by histamine release, presumably from mast cells (Austin et al., 1996). There are at least two possibilities to explain the ability of bradykinin to cause degranulation of mast cells. First, B2 receptor antagonists could be acting directly upon mast cells to prevent bradykinin-induced degranulation. However, bradykinin-induced histamine-releasing effect on rat and mouse mast cells in vitro have been shown to be unrelated to either B1 or B2 receptor activation (Ishizaka et al., 1985; Devillier et al., 1989). The second and more likely possibility is that these antagonists are preventing the action of bradykinin on an intermediate cell type (e.g. sensory neurones or macrophages) which in turn leads to a decreased mast cell activation. It is noteworthy that sensory nerve endings and macrophages (Saria et al., 1988; Tiffany & Bruch, 1989; Lindström & Andersson, 1997; Sato et al., 1996) are sensitive to bradykinin B2 activation. Studies are now underway in an attempt to assess whether bradykinin-induced release of neuropeptides from sensory nerve endings may participate in the vascular and cellular changes evoked by antigen in the rat.
In conclusion, our findings support the interpretation that there is a pivotal role for bradykinin in the pathophysiology of the allergic inflammatory response. Moreover, it is suggested that activation of mast cell degranulation via B2 receptors may underlie the ability of bradykinin to participate in allergy. According to these data, B2 receptor antagonists may provide important therapeutic benefit for treatment of allergic disorders.
Acknowledgments
We are indebted to Mr Edson Alvarenga for his technical assistance. This work was supported by grants from FAPERJ, FAPEMIG (to M.M.Teixeira) and CNPq (Brazil).
Abbreviations
- BSA
bovine serum albumin
- DMSO
dimethyl sulphoxide
- PAF
platelet-activating factor
References
- ABE K., WATANABE N., KUMAGAI N., MOURI T., SEKI T., YOSHINAGA K. Circulating plasma kinin in patients with bronchial asthma. Experientia. 1967;23:626–627. doi: 10.1007/BF02144161. [DOI] [PubMed] [Google Scholar]
- ABRAHAM W.M., BURCH R.M., FARMER S.G., AHMED A., CORTES A. A bradykinin antagonist modifies allergen-induced mediator release and late bronchial responses in sheep. Am. Rev. Respir. Dis. 1991;143:787–796. doi: 10.1164/ajrccm/143.4_Pt_1.787. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA A., GIULIANI S., SCOTLAND R., MAGGI C.A. Ovalbumin-induced neurogenic inflammation in the bladder of sensitized rats. Br. J. Pharmacol. 1998;124:190–196. doi: 10.1038/sj.bjp.0701793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKBARY A.M., WIRTH K.J., SCHÖLKENS B.A. Efficacy and tolerability of Icatibant (Hoe 140) in patients with moderately severe chronic bronchial asthma. Immunopharmacol. 1996;33:238–242. doi: 10.1016/0162-3109(96)00065-3. [DOI] [PubMed] [Google Scholar]
- AUSTIN C.E., DEAR J.W., NEIGHBOUR H., LUNG V., FOREMAN J.C. The contribution of histamine to action of bradykinin in the human nasal airway. Immunopharmacol. 1996;34:181–189. doi: 10.1016/0162-3109(96)00136-1. [DOI] [PubMed] [Google Scholar]
- AUSTIN C.E., FOREMAN J.C., SACDDING G.K. Reduction by Hoe 140, the B2 kinin receptor antagonist, of antigen-induced nasal blockage. Br. J. Pharmacol. 1994;111:969–971. doi: 10.1111/j.1476-5381.1994.tb14835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTRAND C., NADEL J.A., YAMAWAKI I., GEPPETTI P. Role of kinins in the vascular extravasation evoked by antigen and mediated by tachykinins in guinea pig trachea. J. Immunol. 1993;151:4902–4907. [PubMed] [Google Scholar]
- BHOOLA K., FIGUEROA C.D., WORTHY K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- BRUNNÉE T., NIGAM S., KUNKEL G., BAUMGARTEM C.R. Nasal challenge studies with bradykinin: influence upon mediator generation. Clin. Exp. Allergy. 1991;21:425–431. doi: 10.1111/j.1365-2222.1991.tb01682.x. [DOI] [PubMed] [Google Scholar]
- CHRISTIANSEN S.C., PROUD D., COCHRANE C.G. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J. Clin. Invest. 1987;79:188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVILLIER P., DRAPEAU G., RENOUX M., REGOLI D. Role of the n-terminal arginine in the histamine-releasing activity of substance P, bradykinin and related peptides. Eur. J. Pharmacol. 1989;168:53–60. doi: 10.1016/0014-2999(89)90632-8. [DOI] [PubMed] [Google Scholar]
- DIAZ B.L., SERRA M.F., ALVES A.C., PIRES A.L.A., CORREA F.M.A., CORDEIRO R.S.B., MARTINS M.A., SILVA P.M.R. Alloxan diabetes reduces pleural mast cell numbers and the subsequent eosinophil influx induced by allergen in sensitized rats. Int. Arch. Allergy. Immunol. 1996;111:36–43. doi: 10.1159/000237343. [DOI] [PubMed] [Google Scholar]
- FARMER S.C. Role of kinins in airway diseases. Immunopharmacology. 1991;22:1–20. doi: 10.1016/0162-3109(91)90051-y. [DOI] [PubMed] [Google Scholar]
- FARMER S.G., WILKINS D.E., MEEKER S.A., SEEDS E.A.M., PAGE C.P. Effects of bradykinin receptor antagonists on antigen-induced respiratory distress, airway hyperresponsiveness and eosinophil in guinea-pigs. Br. J. Pharmacol. 1992;107:653–659. doi: 10.1111/j.1476-5381.1992.tb14502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEATHERSTONE R.L., PARRY J.E., CHURCH M.K. The effects of a kinin antagonist on changes in lung function and plasma extravasation into the airways following challenge of sensitized guinea-pigs. Clin. Exp. Allergy. 1996;26:235–240. doi: 10.1111/j.1365-2222.1996.tb00085.x. [DOI] [PubMed] [Google Scholar]
- GAO X.P., AKHTER S.R., RUBINSTEIN I. Ovalbumin increases macromolecular efflux from the in situ nasal mucosa of allergic hamsters. J. Appl. Physiol. 1998;84:169–176. doi: 10.1152/jappl.1998.84.1.169. [DOI] [PubMed] [Google Scholar]
- GRIESBACHER T., LEGAT F.J. Effects of FR173657, a non-peptide B2 antagonist, on kinin-induced hypotension, visceral and peripheral oedema formation and bronchoconstriction. Br. J. Pharmacol. 1997;120:933–939. doi: 10.1038/sj.bjp.0700966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORNALL A.G., BARDAWILL C.J., DAVID M.M. Determination of serum protein by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- HOGAN M.B., HARRIS K.E., PROTTER A. & A., PATTERSON R. A bradykinin antagonist inhibits both bradykinin- and the allergen-induced airway response in primates. Proc. Assoc. Am. Physicians. 1977;109:269–274. [PubMed] [Google Scholar]
- INAMURA N., ASANO M., KAYAKIRI H., HATORI C., OKU T., NAKAHARA K. Characterization of FR173657, a novel nonpeptide B2 antagonist: in vitro and in vivo studies. Can. J. Physiol. Pharmacol. 1997;75:622–628. [PubMed] [Google Scholar]
- ISHIZAKA T., IWATA M., ISHIZAKA K. Release of histamine and arachidonate from mouse and mast cells induced by glycosilation-enhancing factor and bradykinin. J. Immunol. 1985;134:1880–1887. [PubMed] [Google Scholar]
- LEMBECK F., GRIENSBACHER T., ECKHARDT M., HENKE S., BREIPOHL G., KNOLLE J. New long-acting, potent bradykinin antagonists. Br. J. Pharmacol. 1991;102:297–304. doi: 10.1111/j.1476-5381.1991.tb12169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIMA M.C.R., CHAGAS M.S.S., SILVA P.M.R., CALHEIROS A.S., PROUVOST-DANON A., FARIA-NETO H.C.C., CORDEIRO R.S.B., MARTINS M.A. Histamine-potentiating activity in rats anaphylactic pleural fluid: role of serotonin. Braz. J. Med. Biol. Res. 1996;29:1049–1056. [PubMed] [Google Scholar]
- LIMA M.C.R., MARTINS M.A., PEREZ S.A.C., SILVA P.M.R., CORDEIRO R.S.B., VARGAFTIG B.B. Effect of azelastine on platelet-activating factor and antigen-induced pleurisy in rats. Eur. J. Pharmacol. 1991;197:201–207. doi: 10.1016/0014-2999(91)90522-r. [DOI] [PubMed] [Google Scholar]
- LINDSTRÖM E.G., ANDERSSON R.G.G. Neurokinin A-LI release after antigen challenge in guinea-pig bronchial tubes: influence of histamine and bradykinin. Br. J. Pharmacol. 1997;122:417–422. doi: 10.1038/sj.bjp.0701382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJIMA M., KAWASHIMA N., HIROSHI I., KATORI M. Effects of an orally active non-peptide bradykin B2 receptor antagonist, FR173657, on plasma exudation in rat carrageenin-induced pleurisy. Br. J. Pharmacol. 1997;121:723–730. doi: 10.1038/sj.bjp.0701194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINS M.A., PASQUALE C.P., BOZZA P.T., SILVA P.M.R., CASTRO H.C., CORDEIRO R.S.B. Homologous tachyphylaxis to bradykinin and its interference with allergic pleurisy in actively sensitized rats. Eur. J. Pharmacol. 1992;220:55–61. doi: 10.1016/0014-2999(92)90011-r. [DOI] [PubMed] [Google Scholar]
- MORI T., IMAMURA T. Inhibition of guinea pig skin allergic reactions by nonpeptide bradykinin B2 receptor antagonist FR 173657. Int. Arch. Allergy Immunol. 1988;116:278–283. doi: 10.1159/000023956. [DOI] [PubMed] [Google Scholar]
- MOTA I.Release of histamine from mast cells Histamine and Antihistaminics 1966Berlin: Springer-Verlag; 569–636.Handbook of Experimental Pharmacology. ed. Rocha e Silva, M. pp [Google Scholar]
- POLOSA R., LAI C.K.W., ROBINSON C., HOLGATE S.T. The influence of cyclooxygenase inhibition on the loss of bronchoconstrictor response to repeated bradykinin challenge in asthma. Eur. Respir. J. 1990;3:914–921. [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- REGOLI D., RHALEB N.-E., DRAPEAU G., DION S. Kinin receptor subtypes. J. Cardiovasc. Pharmacol. 1990;15:S30–S38. [PubMed] [Google Scholar]
- ROBERTS R.A. Bradykinin receptors: characterization, distribution and mechanisms of signal transduction. Prog. Growth Factors Res. 1989;1:237–252. doi: 10.1016/0955-2235(89)90013-6. [DOI] [PubMed] [Google Scholar]
- ROBERTS R.A., GULLICK W.J. Bradykinin receptors undergo ligand induced desensitization. Biochemistry. 1990;29:1975–1979. doi: 10.1021/bi00460a002. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO T., BARNES P.J., CHUNG K.F. Lack of a role for bradykinin in allergen-induced airway microvascular leakage and bronchoconstriction in the guinea pig. Inflamma. Res. 1996;45:123–126. doi: 10.1007/BF02265164. [DOI] [PubMed] [Google Scholar]
- SARIA A., MARTLING C.-R., YAN Z., THEODORSSON-NORHEIM E., GAMSE R., LUNDBERG J.M. Release of multiple tachykinins from capsaicin-sensitive sensory nerves in the lung by bradykinin, histamine, dimethylphenyl piperazinium, and vagal nerve stimulation. Am. Rev. Respir. Dis. 1988;137:1330–1335. doi: 10.1164/ajrccm/137.6.1330. [DOI] [PubMed] [Google Scholar]
- SATO E., KOYAMA S., NOMURA H., KUBO K., SEKIGUCHI M. Bradykinin stimulates alevolar macrophages to release neutrophil, monocyte and eosinophil chemotactic activity. J. Immunol. 1996;157:3122–3129. [PubMed] [Google Scholar]
- SHORE P.A., BURKHALTER A., COHN V.H. A method for a fluorimetric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959;127:182–186. [PubMed] [Google Scholar]
- SOLER M., SIELCZAK M., ABRAHAM W.M. A bradykinin antagonist blocks antigen-induced airway hyperresponsiveness and inflammation in sheep. Pulm. Pharmacol. 1990;3:9–15. doi: 10.1016/0952-0600(90)90003-2. [DOI] [PubMed] [Google Scholar]
- STEWART J.M., GERA L., CHAN D.C., WHALLEY E.T., HANSON W.L., ZUZACK J.S. Potent, long-acting bradykinin antagonists for a wide range of applications. Can. J. Physiol. Pharmacol. 1997;75:719–724. [PubMed] [Google Scholar]
- TIFFANY C.W., BURCH R.M. Bradykinin stimulates tumour necrosis factor and interleukin-1 release from macrophages. FEBS Lett. 1989;247:189–192. doi: 10.1016/0014-5793(89)81331-6. [DOI] [PubMed] [Google Scholar]
- VAVREK R., STEWART J.M. Competitive antagonists of bradykinin. Peptides. 1985;6:161–164. doi: 10.1016/0196-9781(85)90033-6. [DOI] [PubMed] [Google Scholar]
- WIRTH K., HOCK F.J., ALIBIS U., LIZ W., ALDERMAN H.G., ANAGNOSTOPOULOS H., HENKE S., BREIPOHL G., KÖNING W., KNOLLE J., SHÖLKENS B.A. Hoe 140, a new potent and long acting bradykinin-antagonist: In vivo studies. Br. J. Pharmacol. 1991;102:774–778. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


