Abstract
Gastrin stimulates rat stomach ECL cells to secrete histamine and pacreastatin, a chromogranin A (CGA)-derived peptide. The present report describes the effect of nine cholecystokinin2 (CCK2) receptor antagonists and one CCK1 receptor antagonist on the gastrin-evoked secretion of pancreastatin from isolated ECL cells.
The CCK2 receptor antagonists comprised three benzodiazepine derivatives L-740,093, YM022 and YF476, one ureidoacetamide compound RP73870, one benzimidazole compound JB 93182, one ureidoindoline compound AG041R and three tryptophan dipeptoids PD 134308 (CI988), PD135158 and PD 136450. The CCK1 receptor antagonist was devazepide.
A preparation of well-functioning ECL cells (∼80% purity) was prepared from rat oxyntic mucosa using counter-flow elutriation. The cells were cultured for 48 h in the presence of 0.1 nM gastrin; they were then washed and incubated with antagonist alone or with various concentrations of antagonist plus 10 nM gastrin (a maximally effective concentration) for 30 min. Gastrin dose-response curves were constructed in the absence or presence of increasing concentrations of antagonist. The amount of pancreastatin secreted was determined by radioimmunoassay.
The gastrin-evoked secretion of pancreastatin was inhibited in a dose-dependent manner. YM022, AG041R and YF476 had IC50 values of 0.5, 2.2 and 2.7 nM respectively. L-740,093, JB93182 and RP73870 had IC50 values of 7.8, 9.3 and 9.8 nM, while PD135158, PD136450 and PD134308 had IC50 values of 76, 135 and 145 nM. The CCK1 receptor antagonist devazepide was a poor CCK2 receptor antagonist with an IC50 of about 800 nM.
YM022, YF476 and AG041R were chosen for further analysis. YM022 and YF476 shifted the gastrin dose-response curve to the right in a manner suggesting competitive antagonism, while the effects of AG041R could not be explained by simple competitive antagonism. pKB values were 11.3 for YM022, 10.8 for YF476 and the apparent pKB for AG041R was 10.4.
Keywords: CCK2 receptors, CCK2 receptor antagonists, CCK-B/gastrin receptors, CCK-B/gastrin receptor antagonists, ECL cells, gastrin, pancreastatin
Introduction
The ECL cells constitute the quantitatively predominant endocrine cell population in the acid-producing part of the stomach. They operate under the control of circulating gastrin (Håkanson et al., 1992; 1993; 1994), which stimulates them to secrete histamine and pancreastatin, a chromogranin A(CGA)- derived peptide (Prinz et al., 1993; Chen et al., 1994; 1996; Lindström et al., 1997). In fact, the ECL cells represent the major source of circulating pancreastatin-like peptides in the rat (Håkanson et al., 1995; Kimura et al., 1997; Norlén et al., 1997). CGA and CGA-derived peptides including pancreastatin are thought to contribute to the formation and stabilization of secretory granules (Winkler et al., 1986; Winkler & Fischer-Colbrie, 1992). We have suggested that CGA is synthesized, packaged, stored and processed along with an anticipated peptide hormone and that the resulting products are secreted together with histamine (Lindström et al., 1997).
The receptors for the gastrin/cholecystokinin (CCK) family of peptide hormones are classified into CCK1 (formerly CCK-A) and CCK2 (formerly CCK-B/gastrin), based on cloning experiments (Kopin et al., 1992; Wank et al., 1992a,1992b; Miyake et al., 1994), binding studies (Innis & Snyder, 1980; Beinfeld 1983; Chang et al., 1989) and physiological/pharmacological characterization (for a recent review see Wank 1998). The gastrin-recognizing receptor on the ECL cells is of the CCK2 type (Kawabata et al., 1991; Sandvik & Waldum, 1991; Prinz et al., 1993; 1994; Ding et al., 1995; 1997a,1997b; Lindström et al., 1997), displaying high affinity for both sulphated and nonsulphated CCK-8 and for gastrin.
The compounds YF476 (Semple et al., 1997; Takinami et al., 1997), JB93182 (Hills et al., 1996; Kalindjian et al., 1996), AG041R (Baba et al., 1995; Kinoshita et al., 1996; 1998), YM022 (Nishida et al., 1994a,1994b; Miyake et al., 1994), RP73870 (Bertrand et al., 1994; Pendley et al., 1995) and L740,093 (Patel et al., 1994) are known to be potent and selective CCK2 receptor antagonists. YM022 and RP73870 were able to inhibit both gastrin-evoked acid secretion and activation of ECL cells in vivo (Ding & Håkanson, 1996a,1996b; Ding et al., 1997a,1997b). In another in vivo study, YF476, AG041R and JB93182 were found to inhibit gastrin-evoked activation of ECL-cell histidine decarboxylase and secretion of pancreastatin (Ding et al., 1997c).
We have established a method for isolating ECL cells to a purity of about 80% (Lindström et al., 1997) based on the protocol of Prinz et al. (1993). After 2 days of primary culture the cells are capable of secreting pancreastatin and histamine in response to gastrin and to pituitary adenylate cyclase activating peptide (PACAP) (Lindström et al., 1997). The effect of gastrin but not of PACAP was inhibited by YM022 (Lindström et al., 1997). Since ECL cells are rich in CCK2 receptors (Roche et al., 1991a,1991b; Chiba et al., 1991; Asahara et al., 1994), they offer an attractive test system for the assessment of the effectiveness of CCK2 receptor antagonists. The aim of the present study was to compare such drugs with respect to their ability to inhibit gastrin-evoked pancreastatin secretion from isolated rat stomach ECL cells.
Methods
Chemicals
YF476 (R)-1-[2,3-dihydro-2-oxo-1-pivaloylmethyl-5-(2-pyridyl)1H-1,4-benzo-diazepin-3-yl]-3-(3-methylaminophenyl)urea was provided by Dr Alan Harris (Ferring A/S, Vanlose, Denmark). JB93182 5[(3S)-2,5-diaza-5-(3,5-dicarboxyphenyl)-1,4 - dioxo-3- (methylphenyl) pentanyl] - 6 - [3- (1-adamantyl)-2-aza-1-oxopropyl]indole.bis N-methyl-d-glucamine salt was provided by Sir James W. Black (James Black Foundation, London, U.K.) AG041R [3R-1-(2,2-diethoxyethyl)-3-((4-methylphenyl)aminocarbonylmethyl)-3-((4-methylphenyl)ureido)-indoline-2-one was provided by Dr E. Hoshino (Chugai Laboratory, Shizuoka, Japan). YM022 {(R)-1-[2,3-dihydro-1-(2′-methylphenacyl) -2-oxo-5-phenyl-1H- 1, 4-benzodiazepin-3-yl]-3-(3-methylphenyl)urea} was provided by Dr K. Miyata (Yamanouchi Pharmaceutical, Ibaraki, Japan). RP73870 {{[N-(methoxy - 3 - phenyl ) - N- (N - methyl - N - phenyl - carbamoylmethyl) - carbamoyl-methyl] - 3-ureido} -3-phenyl} -2 - ethylsulphonate-(RS) was provided by Dr C. Guyon (Rhône-Poulenc Rorer, Vitry-sur-Seine, France). L-740,093 was supplied by Dr V.G. Matassa (Merck Sharpe & Dohme (MSD), Terlings Park, Harlow, U.K.). PD134308 (Cam 958, CI988), PD135158 (Cam 1028) and PD136450 (Cam 1189) were supplied by Dr John Hughes (Parke-Davis Research Unit, Cambridge, U.K.). Devazepide, a selective CCK1 receptor antagonist (Chang & Lotti, 1986) was from Dr R. Freidinger (MSD, West Point, PA, U.S.A.). Rat gastrin-17 was obtained from Research Plus (Bayonne, NJ, U.S.A.) and PACAP-27 from Peninsula Europe (St. Helens, Merseyside, U.K.). Stock solutions (10 mM) of JB93182, PD134308, PD135158, PD136450 and RP73870 were prepared by dissolving each compound in 0.9% saline. Stock solutions (10 mM) of YF476, AG041R, YM022, L740,093 and devazepide were prepared by dissolving the compounds in dimethylsulphoxide (DMSO). The final concentration of DMSO in the medium never exceeded 0.01% which did not affect ECL cell secretion.
Isolation, fractionation and primary culture of ECL cells
The ECL cells were purified as described in detail by Lindstöm et al. (1997). Four male, freely fed Sprague-Dawley rats, each weighing 250–300 g were used for each preparation. ECL cells were identified by immunofluorescence using an anti-rat-histidine decarboxylase antiserum (working dilution 1 : 750) raised in a guinea-pig (Dartsch & Persson, 1998; Dartsch et al., 1998). The purity of the isolated ECL cells was 75–85%. The cells were cultured in 96-well plates pre-coated with Matrigel® (20,000 cells per well) in a humid atmosphere with 5% CO2/95% air at 37°C for 48 h until the start of the experiments. The culture medium consisted of DMEM-Ham's F12 (1 : 1) supplemented with 2% foetal calf serum, glutamine 2 mM, penicillin 100 IU ml−1, streptomycin 100 μg ml−1, amphotgericin B, 250 ng ml−1, insulin 6.25 μg ml−1, transferrin 6.25 μg ml−1, selenious acid 6.25 μg ml−1, bovine serum albumin 1.25 mg ml−1, hydrocortisone 10 nM, HEPES 15 mM, pyridoxal-5-phosphate 10 μM and gastrin-17 0.1 nM.
Secretion experiments
The mobilization of pancreastatin from the isolated ECL cells was monitored by the measurement of pancreastatin in the medium. In preparation for the secretion experiments, the medium was aspirated and replaced with fresh serum-free medium without gastrin. After equilibration for 2–3 h, the medium was again aspirated and replaced with secretion medium (in mM: NaCl 150, KCl 5, CaCl2 2, HEPES (pH 7.0) 10) plus test substances. The cells were exposed to various concentrations of antagonist together with 10 nM gastrin (EC100; i.e. a concentration that results in maximum secretory effect) for 30 min. An incubation time of 30 min was chosen because longer times of exposure to gastrin can be expected to cause intracellular redistribution of stored secretory products as well as accelerated de novo synthesis of the secretory products. In a second set of experiments, gastrin dose-response curves (1 pM–1 μM) were constructed in the absence or presence of increasing concentrations of antagonist. After incubation, the plates were centrifuged at 220×g for 1 min. The supernatants were collected and stored at −20°C until measurement of pancreastatin.
Determination of pancreastatin-like peptides
The pancreastatin-like immunoreactivity was measured by radioimmunoassay using authentic rat pancreastatin as standard (Chen et al., 1994). The amount of pancreastatin released was expressed as per cent of control. Controls (basal or stimulated secretion) were set to 100%.
Data analysis and statistics
Dose-response curves illustrating antagonist-induced inhibition of secretion evoked by a maximal dose/concentration of gastrin (10 nM) (Lindström et al., 1997) were sigmoidally fitted and pIC50 (the negative log of the antagonist concentration that produces 50% inhibition of the gastrin effect) and midpoint slope parameters were calculated using Graph Pad Prism software. Each individual dose-response curve generated a pIC50. For each drug, the mean value and s.e.mean were calculated. For display purposes, the individual computed parameters for each treatment group were expressed as means and a single logistic curve was generated. pKB estimates were calculated using a modified Cheng-Prusoff equation (Leff & Dougall, 1993). Thirty-one gastrin dose-response curves obtained under identical experimental conditions were used to generate EC50 and the midpoint slope. Whenever the midpoint slope for an antagonist dose-response curve differed significantly from the midpoint slope of the control gastrin dose-response curve, the pKB obtained was referred to as apparent pKB (pKB′). As a result of these studies, YM022, AG041R and YF476 were selected for further analysis because of their potency. In this series of experiments, gastrin dose-response curves with or without antagonist were fitted to the Hill equation to provide midpoint slopes, upper asymptote parameters and EC50 concentrations (the gastrin dose producing a half-maximal response) (Black & Shankley, 1985). Gastrin concentration ratios were defined as the ratios between EC50 concentrations of gastrin with or without antagonist. If the antagonist produced parallel, rightward shifts of the gastrin dose-response curves with no change in upper asymptote, Schild plots were constructed by plotting log antagonist concentration against log (EC50 concentration ratio-1). The slope of the line gives the Schild plot slope while the point of intersection with the x-axis gives the antagonist concentration which gives a concentration ratio of 2 (A2). The Schild plot slope should be close to unity if the antagonist is to be regarded as a competitive antagonist (Schild, 1949). The goodness of fit to a straight line was assessed by linear regression analysis and expressed as the square of the correlation coefficient (r2). If the Schild plot slope parameter was not significantly different from unity, it was constrained to a value of unity (as recommended by Jenkinson (1991)) and the data refitted to provide the antagonist dissociation constant (KB). In the experiments using AG041R, the gastrin dose-response curves were significantly flatter the greater the concentration of the antagonist, so the criteria for competitive antagonism were not strictly met. Therfore, the pKB obtained is referred to as apparent pKB (pKB′). For display purposes, the individual computed parameters for each treatment group were expressed as means and a single Schild plot generated. The statistical analysis consisted of a one-way analysis of variance (ANOVA) followed by the Bonferroni test or Dunnett's test for multiple comparisons. P<0.05 was considered significant.
Results
Intrinsic activity of the antagonists
In the absence of gastrin, none of the antagonists tested (up to a concentration of 100 nM) stimulated or inhibited pancreastatin secretion from isolated ECL cells (data not shown). The PD compounds were tested at higher concentrations (up to 0.1 mM) but showed no effects.
Antagonism of gastrin-evoked secretion
All ten compounds exhibited a dose-dependent inhibition of pancreastatin secretion evoked by 10 nM gastrin (Figure 1a–c). The pIC50 value for each antagonist was calculated (Table 1). The antagonists could be divided into three groups according to their potencies; (1) very potent: YM022, YF476 and AG041R: (pIC50: 8.6–9.3), (2) potent: L740,093, JB93182 and RP73870 (pIC50: 8.0–8.1) and (3) weak: PD134308, PD135158 and PD 136450 (pIC50: 6.8–7.1). The classification of the antagonists into these groups was based on the statistical comparison of their pIC50 to the pIC50 of YM022.YM022 was not significantly more potent than AG041R or YF476, while being more potent than the rest of the compounds. However, classification into three groups was not statistically ‘clear-cut', for instance AG041R and YF476 in the first group were not significantly more potent than any of the antagonists in the second group. However, all antagonists in the first two groups were significantly more potent than the PD compounds that made up the third group. The CCK1 antagonist devazepide, having an estimated pIC50 of about 6, was only slightly less potent than the PD compounds. Higher concentrations of devazepide could not be tested due to inhibitory effects of the vehicle (DMSO). The poor antagonistic effect of devazepide suggests that gastrin-stimulated pancreastatin secretion does not involve CCK1 receptors. None of the antagonists inhibited PACAP-27-evoked secretion (not shown). This indicates that the antagonists inhibit ECL-cell secretion via a specific action on CCK2 receptors and not via a general suppression of the activity of the cells.
Figure 1.
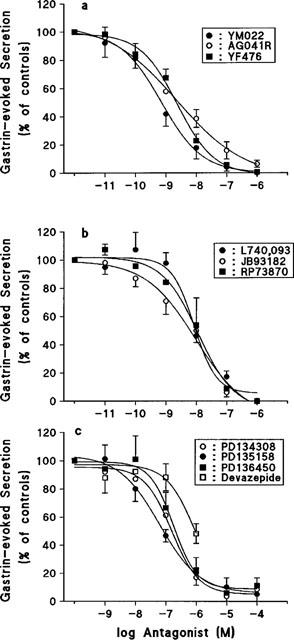
Dose-response curves illustrating the inhibition of gastrin-stimulated (10 nM) pancreastatin secretion from isolated ECL cells by (a): YM022, YF476 and AG041R (b): JB93182, RP73870 and L740,093 (c): PD134308, PD135158, PD136450 and the CCK1 receptor antagonist devazepide.
Table 1.
pIC50 values for the ten compounds tested for CCK2 receptor antagonism
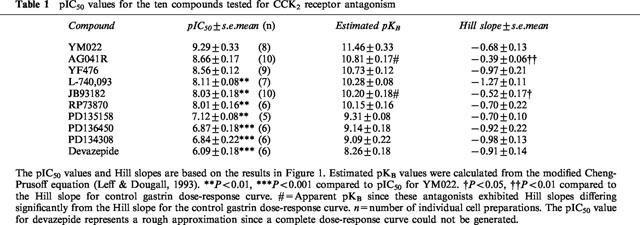
pKB values were calculated using a modified Cheng-Prusoff equation (Leff & Dougall, 1993). The midpoint slopes for AG041R and JB93182 differed significantly from that of the gastrin dose-response curve (0.39±0.06 and 0.52±0.17 versus 1.04±0.12). Thus, the pKB estimates for these two drugs were referred to as apparent pKB′ (Table 1). None of the other antagonists had Hill slopes that differed significantly from that of the gastrin dose-response curve.
Competitive analysis of YM022, YF476 and AG041R
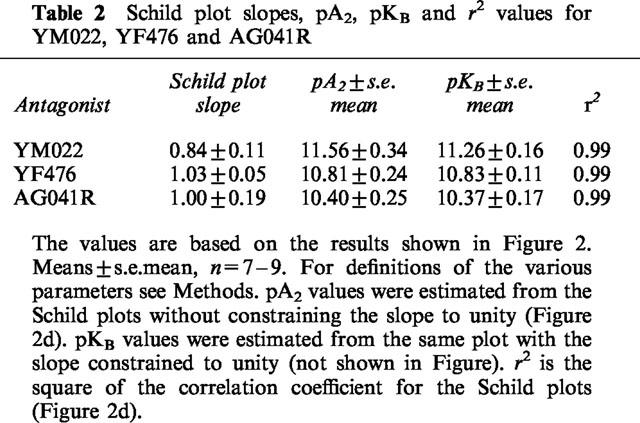
The most potent antagonists YM022, YF476 and AG041R were chosen for further analysis. Gastrin dose-response curves were generated in the absence or presence of increasing concentrations of antagonist (Figure 2a–c). All dose-response curves were subsequently fitted to the Hill equation. None of the antagonists had any significant effects on the curve upper asymptotes. AG041R produced flatter dose-response curves (midpoint slope values; control, 1.04±0.12; 0.1 nM AG041R, 0.84±0.12: 1 nM, 0.57±0.19; 10 nM, 0.46±0.06). YM022 also tended to ‘flatten' the dose-response curves, but the midpoint slopes were not significantly different from controls. Analysis of the Schild plots for all three drugs revealed slopes not differing significantly from unity and the intercepts on the x-axis could be used to provide pA2 (see Table 2). A second model fit, performed with the slope constrained to unity, yielded the pKB values shown in Figure 2d and Table 2. Schild plot slopes, pA2 values, pKB values and correlation coefficients are summarized in Table 2. The pKB values for YM022, AG041R and YF476 were very similar to those calculated in the first set of experiments using the modified Cheng-Prusoff equation.
Figure 2.
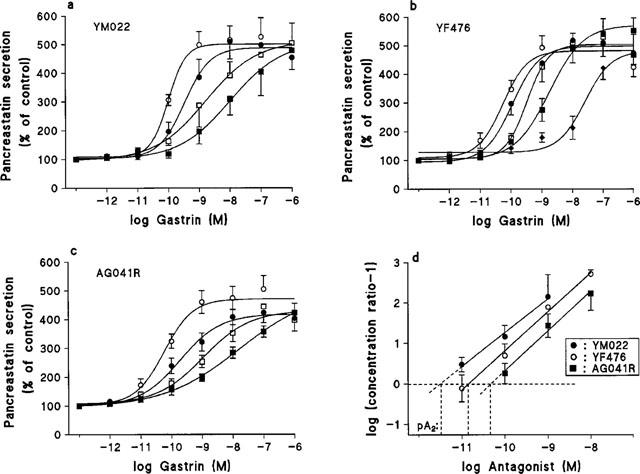
Rightward shift of the gastrin dose-response curve induced by increasing concentrations of (a): YM022 (○: control, •: 0.01 nM, □: 0.1 nM, ▪: 1 nM), (b): YF476 (○: control, •: 0.01 nM, □: 0.1 nM, ▪: 1 nM, ⧫: 10 nM) and (c) AG041R (○: control, •: 0.1 nM, □: 1 nM, ▪: 10 nM). (d) Schild plots illustrating the degree of competitive antagonism for YM022, YF476 and AG041R. The pA2 values for each drug is indicated (see Table 2).
Table 2.
Schild plot slopes, pA2, pKB and r2 values for YM022, YF476 and AG041R
Discussion
Recently, CCK2 receptor antagonists have attracted a great deal of attention, because of their ability to inhibit gastric acid secretion (Nishida et al., 1994a,1994b; Pendley et al., 1995; Ding & Håkanson, 1996a; Yuki et al., 1997; Ding et al., 1998). In fact, they are potentially useful drugs for peptic ulcer therapy (Jensen 1996; Makovec & D'Amato, 1997; Håkanson et al., 1999). We have argued that the ECL cells of the oxyntic mucosa are a major target for CCK2 receptor antagonists (Ding et al., 1997a,1997b,1997c). Gastrin stimulates acid secretion by mobilizing ECL-cell histamine which in turn activates the parietal cells (Andersson et al., 1996). Previous reports have shown that gastrin (and PACAP) induces a parallel secretion of histamine and pancreastatin from ECL cells (Chen et al., 1994; 1996; Lindström et al., 1997); and that the effect of gastrin is mediated by CCK2 receptors (Roche et al., 1991a, 1991b; Sandvik & Waldum, 1991; Prinz et al., 1993; Lindström et al., 1997). Thus, inhibition of gastrin-evoked secretion of pancreastatin from isolated rat stomach ECL cells would offer a convenient system for screening newly developed CCK2 receptor antagonists.
None of the antagonists tested displayed any agonistic action in the present experimental setting and none of them inhibited PACAP-evoked secretion. The fact that the potent CCK1 receptor antagonist devazepide was a poor antagonist of gastrin-stimulated pancreastatin release supports the view that CCK2 receptors and not CCK1 receptors are involved in ECL-cell stimulation. The PD compounds were originally characterized as competitive CCK2 receptor antagonists (Hughes et al., 1990). However, the results of subsequent studies found them to be partial agonists rather than pure antagonists at ECL-cell CCK2 receptors (Schmassmann et al., 1994; Ding et al., 1995), in that administration of the drugs stimulated rather than inhibited ECL-cell histidine decarboxylase activity in vivo (Ding et al., 1995). The results of the present study suggest that the CCK2 receptor on isolated cultured ECL cells differs from the receptor on ECL cells in vivo or that the agonistic effect of the PD compounds seen in vivo does not reflect a direct action on the ECL cells. The affinity estimates for YM022, YF476 and AG041R are consistent with literature values (see below), suggesting that CCK2 receptors on the ECL cells are the same whether in vitro or in vivo. However, the efficiency of coupling to the second messenger system and/or the receptor density could be reduced in the in vitro situation such that while the compounds bind to the receptors, their pharmacological efficacy is too low to translate into a response. Since none of the antagonists were able to inhibit basal pancreastatin secretion from the ECL cells, they do not seem to express any inverse agonist activity either. This suggests that the CCK2 receptor residing on isolated ECL cells is not constitutively active.
YM022 was the most potent antagonist tested. It produced parallel rightward-shifts of the gastrin dose-response curves without affecting upper asymptotes and midpoint slope parameters. There was a tendency for ‘flattening' of the inhibitory curve (Figure 1) and the gastrin dose-response curves (Figure 2a), however these effects were not significant using the statistical criteria chosen. The value of the Schild plot slope was not significantly different from unity, suggesting competitive, surmountable antagonism. This is in agreement with two previous reports (Nishida et al., 1994a; Ding & Håkanson, 1996b), but in disagreement with a recent report (Dunlop et al., 1997), in which it was claimed that YM022 acts as an irreversible antagonist in Chinese ovary cells transfected with the human CCK2 receptor gene. At present, we have no explanation for this discrepancy. However, CCK2 receptors from different species have been shown to have varying affinities to antagonists, possibly due to sequence variations in the 6th transmembrane domain of the receptor (Beinborn et al., 1993; Kopin et al., 1997). The possibility that isolated ECL cells have a high receptor reserve could also explain this discrepancy.
YF476 and AG041R were also found to be potent antagonists. While YF476 had the characteristics of a competitive surmountable antagonist, AG041R displayed a flat inhibitory curve and caused flattening of the gastrin dose-response curves. Nonetheless, the estimated pKB′ of AG041R in the first set of experiments (10.8) did not differ much from the pKB′ calculated in the second set of experiments (10.4). Despite the lack of effect on the upper asymptotes, the results suggest that AG041R does not act as a simple competitive antagonist. Conceivably, AG041R requires longer incubation times than 30 min to attain steady state/equilibrium. (The reasons for choosing 30 min as incubation time are given in Methods).
Information in the literature on affinity and effectiveness of CCK2 receptor antagonists is limited. On the whole, our results are in agreement with previously published data (Nishida et al., 1994a; Pendley et al., 1995; Hills et al., 1996; Kalindijan et al., 1996; Takinami et al., 1997).
A recent method to screen for gastrin (CCK2) receptor antagonists has been described (Letari et al., 1996). The model consisted of isolated rabbit parietal cells and the response measured was the increase in intracellular Ca2+. In the adult rat, the acid-stimulating effect of gastrin seems to be mediated by histamine mobilized from the ECL cells (Andersson et al., 1996). However, Hills et al. (1996) reported that pentagastrin produced a dose-dependent increase of acid secretion in the isolated immature rat stomach in the presence of the H2-receptor antagonist famotidine. This suggests that gastrin is able to directly stimulate acid secretion from parietal cells in the immature rat. Therefore, unless developmental changes occur in the way gastrin stimulates acid secretion, it seems that both ECL cells and parietal cells are respectively able to indirectly and directly contribute to gastrin-evoked acid secretion. At present there is no evidence to suggest that the CCK2 receptors of the ECL cells differ from those expressed by the parietal cells. We suggest therefore that monitoring gastrin-evoked secretion from isolated ECL cells rather than from parietal cells is an alternative, and perhaps more relevant method for the screening of CCK2 receptor antagonists.
References
- ANDERSSON K., CABERO J.L., MATTSSON H., HÅKANSON R. Gastric acid secretion after depletion of enterochromaffin-like cell histamine. Scand. J. Gastroenterol. 1996;31:24–30. doi: 10.3109/00365529609031622. [DOI] [PubMed] [Google Scholar]
- ASAHARA M., KINOSHITA H., NAKATA H., MATSUSHIMA Y., NARIBAYASHI Y., NAKAMURA A., MATSUI K., CHIHARA K., YAMAMOTO J., ICHIKAWA A., CHIBA T. Gastrin receptor genes are expressed in gastric parietal and enterochromaffin-like cells of mastomys natalensis. Dig. Dis. Sci. 1994;39:2149–2156. doi: 10.1007/BF02090363. [DOI] [PubMed] [Google Scholar]
- BABA A., HOSHINO E., ONOMA M., ESAKI T., TAKEDA K.Pharmacological profile of AG041R. A novel selective gastrin/CCK-B antagonist Gastroenterology 1995108A950(abstract) [Google Scholar]
- BEINBORN M., LEE Y.-M., MCBRIDE E.W., QUINN S.M., KOPIN A.S. A single amino acid of the cholecystokinin-B/gastrin receptor determines specificity for non-peptide antagonists. Nature. 1993;362:348–350. doi: 10.1038/362348a0. [DOI] [PubMed] [Google Scholar]
- BEINFELD M.C. Cholecystokinin in the central nervous system: A minireview. Neuropeptides. 1983;3:411–427. doi: 10.1016/0143-4179(83)90032-x. [DOI] [PubMed] [Google Scholar]
- BERTRAND P., BÖHME G.A., DURIEUX C., GUYON C., CAPET M., JEANTAUD B., BOUDEAU P., DUCOS B., PENDLEY C.E., MARTIN G.E. Pharmacological properties of ureido-acetamides, new potent and selective non-peptide CCK/gastrin receptor antagonists. Eur. J. Pharmacol. 1994;262:233–245. doi: 10.1016/0014-2999(94)90737-4. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., SHANKLEY N.P. The isolated stomach preparation of the mouse: a physiological unit for pharmacological analysis. Br. J. Pharmacol. 1985;86:571–579. doi: 10.1111/j.1476-5381.1985.tb08933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R.S.L., CHENG T.B., BOCK M.G., FREIDINGER R.M., CHEN R., ROSEGAY A., LOTTI V.J. Characterization of the binding of [3H] L-365,260: a new potent and selective brain cholecystokinin (CCK-B) and gastrin receptor antagonist radioligand. Mol. Pharm. 1989;35:803–810. [PubMed] [Google Scholar]
- CHANG R.S.L., LOTTI V.J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN D., MÅRVIK R., RONNING K., ANDERSSON K., WALDUM H.L., HÅKANSON R. Gastrin-evoked secretion of histamine, pancreastatin and acid from the isolated, vascularly perfused rat stomach. Effects of isobutylmethylxanthin and α-fluoromethylhistidine. Regul. Pept. 1996;65:133–138. doi: 10.1016/0167-0115(96)00082-1. [DOI] [PubMed] [Google Scholar]
- CHEN D., MONSTEIN H.-J., NYLANDER A.-G., ZHAO C.-M., SUNDLER F., HÅKANSON R. Acute responses of rat stomach enterochromaffin-like cells to gastrin: Secretory activation and adaptation. Gastroenterology. 1994;107:18–27. doi: 10.1016/0016-5085(94)90056-6. [DOI] [PubMed] [Google Scholar]
- CHIBA T., KINOSHITA Y., MORISHITA T., NAKATA H., NAKAMURA A., HOSODA S. Receptors for gastrin on gastric carcinoid tumor membranes of Mastomys Natalensis. Biochem. Biophys. Res. Commun. 1991;177:739–744. doi: 10.1016/0006-291x(91)91850-c. [DOI] [PubMed] [Google Scholar]
- DARTSCH C., CHEN D., PERSSON L. Multiple forms of rat histidine decarboxylase may reflect posttranslational activation of the enzyme. Regul. Pept. 1998;77:33–41. doi: 10.1016/s0167-0115(98)00045-7. [DOI] [PubMed] [Google Scholar]
- DARTSCH C., PERSSON L. Recombinant expression of rat histidine decarboxylase: generation of antibodies useful for western blot analysis. Int. J. Biochem. Cell Biol. 1998;30:773–782. doi: 10.1016/s1357-2725(98)00047-8. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., CHEN D., HÅKANSON R. Cholecystokinin-B receptor ligands of the dipeptoid series act as agonists on rat stomach histidine decarboxylase. Gastroenterology. 1995;109:1181–1187. doi: 10.1016/0016-5085(95)90577-4. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., HÅKANSON R. Effect of gastrin receptor blockade on gastric acid secretion in conscious rats. Pharmacol. Toxicol. 1996a;79:324–330. doi: 10.1111/j.1600-0773.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., HÅKANSON R. Evaluation of the specificity and potency of a series of cholecystokinin-B/gastrin receptor antagonists in vivo. Pharmacol. Toxicol. 1996b;79:124–130. doi: 10.1111/j.1600-0773.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., KITANO M., HÅKANSON R. Sustained cholecystokinin-B/gastrin receptor blockade does not impair basal and histamine-stimulated acid secretion in chronic gastric fistula rats. Pharmacol. Toxicol. 1998;87:177–182. doi: 10.1111/j.1600-0773.1998.tb01421.x. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., LINDSTRÖM E., HÅKANSON R. Time-course of deactivation of rat stomach ECL cells following cholecystokinin-B/gastrin receptor blockade. Br. J. Pharmacol. 1997a;122:1–6. doi: 10.1038/sj.bjp.0701316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING X.-Q., LINDSTRÖM E., HÅKANSON R. Cholecystokinin-B/gastrin receptor blockade suppresses the activity of rat stomach ECL cells. Pharmacol. Toxicol. 1997b;81:19–25. doi: 10.1111/j.1600-0773.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- DING X.-Q., LINDSTRÖM E., HÅKANSON R. Evaluation of three novel cholecytokinin-B/gastrin receptor antagonists: A study of their effects on rat stomach ECL cell activity. Pharmacol. Toxicol. 1997c;81:232–237. doi: 10.1111/j.1600-0773.1997.tb00052.x. [DOI] [PubMed] [Google Scholar]
- DUNLOP J., BRAMMER N., EVANS N., ENNIS C. YM022 [(R)-1-[2,3-dihdro-1-(2′-methylphenacyl)-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl]-3-(3-methylphenyl)urea]: an irreversible cholecystokinin type-B receptor antagonist. Biochem. Pharm. 1997;54:81–85. doi: 10.1016/s0006-2952(97)00139-1. [DOI] [PubMed] [Google Scholar]
- HÅKANSON R, , CHEN D., SUNDLER F.The ECL cells Physiology of the Gastrointestinal Tract 1994New York: Raven Press; 1171–1184.ed. Johnson, L.R. Volume 2, 3rd ed. pp [Google Scholar]
- HÅKANSON R, , DING X.-Q., NORLÉN P., CHEN D. Circulating pancreastatin is a marker for the enterochromaffin-like cells of the rat stomach. Gastroenterology. 1995;108:1445–1452. doi: 10.1016/0016-5085(95)90693-2. [DOI] [PubMed] [Google Scholar]
- HÅKANSON R, , DING X.-Q., NORLÉN P., LINDSTRÖM E. CCK2 receptor antagonists: pharmacological tools to study the gastrin-ECL cell-parietal cell axis. Regul. Pept. 1999;80:1–11. doi: 10.1016/s0167-0115(99)00008-7. [DOI] [PubMed] [Google Scholar]
- HÅKANSON R, , TIELEMANS Y., CHEN D., ANDERSSON K., RYBERG B., MATTSSON H., SUNDLER F. The biology and pathobiology of the ECL cells. Yale J. Biol. Med. 1992;65:761–774. [PMC free article] [PubMed] [Google Scholar]
- HÅKANSON R, , TIELEMANS Y., CHEN D., ANDERSSON K., MATTSSON H., SUNDLER F. Time-dependent changes in enterochromaffinlike cell kinetics in stomach of hypergastrinemic rats. Gastroenterology. 1993;105:15–21. doi: 10.1016/0016-5085(93)90005-w. [DOI] [PubMed] [Google Scholar]
- HILLS D.M., GERSKOWITCH V.P., ROBERTS S.P., WELSH N.J., SHANKLEY N.P., BLACK J.W. Pharmacological analysis of the CCKB/gastrin receptors mediating pentagastrin-stimulated gastric acid secretion in the isolated stomach of the immature rat. Br. J. Pharmacol. 1996;119:1401–1410. doi: 10.1111/j.1476-5381.1996.tb16052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES J., BODEN P., COSTALL B., DOMENEY A., KELLY E., HORWELL D.C., HUNTER J.C., PINNOCK R.D., WOODRUFF G.N. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INNIS R.B., SNYDER S.H. Distinct cholecystokinin receptors in brain and pancreas. Proc. Natl. Acad.Sci. U.S.A. 1980;77:6917–6928. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D.H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol. Sci. 1991;12:53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- JENSEN R.T. CCKB/gastrin receptor antagonists: recent advances and potential uses in gastric secretory disorders. Yale J. Biol Med. 1996;69:245–259. [PMC free article] [PubMed] [Google Scholar]
- KALINDIJAN S.B., BUCK I.M., DAVIS M.M.R., DUNSTONE D.J., HUDSON M.L., LOW C.M.R., MCDONALD I.M., PETHER M.J., STEEL K.I.M., TOZER M.J., VINTER J.G. Non-peptide cholecystokinin-B/gastrin receptor antagonists based on bicyclic, heteroaromatic skeletons. J. Med. Chem. 1996;39:1806–1815. doi: 10.1021/jm9508907. [DOI] [PubMed] [Google Scholar]
- KAWABATA S., KANAYAMA S., SHINOMURA Y., MIYAZAKI Y., IMAMURA I., MORIWAKI K., WADA H., TARUI S. Effect of CCK receptor antagonists, MK-329 and L-365,260 on CCK-induced acid secretion and HDC activity in the rat. Regul. Pept. 1991;35:1–10. doi: 10.1016/0167-0115(91)90248-f. [DOI] [PubMed] [Google Scholar]
- KIMURA K., CHEN D., LINDSTRÖM E., ZHAO C.-M., HÅKANSON R. Evidence that rat stomach ECL cells represent the main source of circulating pancreastatin. Regul. Pept. 1997;68:177–180. doi: 10.1016/s0167-0115(96)02117-9. [DOI] [PubMed] [Google Scholar]
- KINOSHITA Y., NAKATA H., ASAHARA M., KAWANAMI C., MATSUSHIMA Y., KISHI K., HOSHINO E., BABA A., CHIBA T.Effects of a new gastrin/cholecystokinin-B receptor antagonist AG-041R on ECL carinoid tumor cells of mastomys natalensis Regul. Pept. 199664suppl90(abstract) [Google Scholar]
- KINOSHITA Y., NAKATA H., KISHI K., KAWANAMAI C., SAWADA M., CHIBA T. Comparison of the signal transduction pathways activated by gastrin in enterochromaffin-like and parietal cells. Gastroenterology. 1998;115:93–100. doi: 10.1016/s0016-5085(98)70369-5. [DOI] [PubMed] [Google Scholar]
- KOPIN A.S., LEE Y.-M., MCBRIDE E.W., MILLER L.J., LU M., LIN H.Y., KOLAKOWSKI L.F., BEINBORN M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPIN A.S. , MCBRIDE E.W., GORDON M.C., QUINN S.M., BEINBORN M. Inter- and intrapsecies polymorphisms in the cholecystokinin-B/gastrin receptor alter drug efficacy. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11043–11048. doi: 10.1073/pnas.94.20.11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFF P., DOUGALL I.G. Further concerns over Cheng-Prusoff analysis. Trends Pharmacol. Sci. 1993;14:110–112. doi: 10.1016/0165-6147(93)90080-4. [DOI] [PubMed] [Google Scholar]
- LETARI O., MENNUNI L., REVEL L., COLOMBO S., MAKOVEC F. Cytosolic Ca2+ evaluation in rabbit parietal cells: a novel method to screen gastrin receptor antagonists. Eur. J. Pharmacol. 1996;306:325–333. doi: 10.1016/0014-2999(96)00222-1. [DOI] [PubMed] [Google Scholar]
- LINDSTRÖM E., BJÖRKQVIST M., BOKETOFT Å., CHEN D., ZHAO C.-M., KIMURA K., HÅKANSON R. Neurohormonal regulation of histamine and pancreAstatin secretion from isolated rat stomach ECL cells. Regul. Pept. 1997;71:73–86. doi: 10.1016/s0167-0115(97)01018-5. [DOI] [PubMed] [Google Scholar]
- MAKOVEC F., D'AMATO M. CCKB/gastrin receptor antagonists as potential drugs for peptic ulcer therapy. Drug Discovery Today. 1997;7:283–293. [Google Scholar]
- MIYAKE A., MOCHIZUKI S., KAWASHIMA H. Characterization of cloned human cholecystokinin-B receptor as a gastrin receptor. Biochem. Pharmacol. 1994;47:1339–1343. doi: 10.1016/0006-2952(94)90332-8. [DOI] [PubMed] [Google Scholar]
- NISHIDA A., MIYATA R., TSUTSUMI H., YUKI S., AKUZAWA A., KOBAYASHI T., KAMATO H., ITO M., YAMANO Y., KATAUYAMA M., SATOH M., OHTA M., HONDA K. Pharmacological profile of (R)-1-[2,3-dihydro-1-(2′methylphenacyl)-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-yl]-3-(methylphenyl)urea (YM022), a new potent and selective gastrin/cholecystokinin (CCK)-B receptor antagonist, in vitro and in vivo. J. Pharmacol. Exp. Ther. 1994a;269:725–731. [PubMed] [Google Scholar]
- NISHIDA A., TAKINAMI Y., YUKI H., KOBAYASHI A., AKUZAWA S., KAMATO T., ITO H., YAMANO M., NAGAKURA Y., MIYATA K. YM022 {(R)-1-[2,3-dihydro-1-)2′methylphenacyl) - 2 - oxo -5 - phenyl - 1 - H1, 4 - benzodiazepine -3 -yl] -3 -(3′methylphenyl)urea}, a potent and selective gastrin/cholecystokinin-B receptor antagonist, prevents gastric and duodenal lesions in rats. J. Pharmacol. Exp. Ther. 1994b;270:1256–1261. [PubMed] [Google Scholar]
- NORLÉN P., CURRY W.J., CHEN D., ZHAO C.-M., JOHNSTON C.F., HÅKANSON R. Expression of the chromogranin A-derived peptides pancreastatin and WE14 in rat stomach ECL cells. Regul. Pept. 1997;70:121–133. doi: 10.1016/s0167-0115(97)00021-9. [DOI] [PubMed] [Google Scholar]
- PATEL S., SMITH A.J., CHAPMAN K.L., FLETCHER A.E., KEMP J.A., MARSHALL G.R., HARGREAVES R.J., RYECROFT W., IVERSEN L.L., IVERSEN S.D., BAKER R., SHOWELL G.A., BOURRAIN S., NEDUVELIL J.G., MATASSA V.G., FREEDMAN S.B. Biological properties of the benzodiazepine amidine derivative L-740,093, a cholecystokinin-B/gastrin receptor antagonist with high affinity in vitro and high potency in vivo. Mol. Pharmacol. 1994;46:943–948. [PubMed] [Google Scholar]
- PENDLEY C.E., FITZPATRICK L.R., CAPOLINO A.J., DAVIS M.A., ESTERLINE N.J., JAKUBOWSKA A., BERTRAND P., GUYON C., DUBROEUCQ M.-C., MARTIN G.E. RP73870, a gastrin/cholecystokinin-B antagonist with potent anti-ulcer activity in the rat. J. Pharmacol. Exp. Ther. 1995;273:1015–1022. [PubMed] [Google Scholar]
- PRINZ C., KAJIMURA M., SCOTT D.R., MERCIER F., HELANDER H.F., SACHS G. Histamine secretion from rat enterochromafinlike cells. Gastroenterology. 1993;105:449–461. doi: 10.1016/0016-5085(93)90719-s. [DOI] [PubMed] [Google Scholar]
- PRINZ C., SCOTT D.R., HURWITZ D., HELANDER H.F., SACHS G. Gastrin effects on isolated rat enterochromaffin-like cells in primary culture. Am. J.Physiol. 1994;267:G663–G675. doi: 10.1152/ajpgi.1994.267.4.G663. [DOI] [PubMed] [Google Scholar]
- ROCHE S., GUSDINAR T., BALI J.P., MAGOUS R. ‘Gastrin'and ‘CCK' receptors on histamine- and somatostatin-containing cells from rabbit fundic mucosa-I. Characterization by means of agonists. Biochem. Pharmacol. 1991a;42:765–770. doi: 10.1016/0006-2952(91)90034-3. [DOI] [PubMed] [Google Scholar]
- ROCHE S., GUSDINAR T., BALI J.P., MAGOUS R. ‘Gastrin' and ‘CCK' receptors on histamine- and somatostain-containing cells from rabbit fundic mucosa-II. Characterization by means of selective antagonists (L-364,718 and L-365,260) Biochem. Pharmacol. 1991b;42:771–776. doi: 10.1016/0006-2952(91)90035-4. [DOI] [PubMed] [Google Scholar]
- SANDVIK A.K., WALDUM H.L. CCK-B (gastrin) receptor regulates histamine release and acid secretion. Am. J. Physiol. 1991;260:G925–G928. doi: 10.1152/ajpgi.1991.260.6.G925. [DOI] [PubMed] [Google Scholar]
- SCHILD H.O. pAx and competitive drug antagonism. Br. J. Pharmacol. 1949;4:277–280. doi: 10.1111/j.1476-5381.1949.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMASSMANN A., GARNER A., FLOGERZI B., HASAN M.Y., SANNER M., VARGA I., HALTER F. Cholecystokinin type B receptor antagonist PD-136,450 is a partial agonist in the stomach and a full agonist in the pancreas of the rat. Gut. 1994;35:270–274. doi: 10.1136/gut.35.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMPLE G., RYDER H., ROOKER D.P., BATT A.R., KENDRICK D.A., SZELKE M., OHTA M., SATOH M., NISHIDA A., AKUZAWA S., MIYATA K. (3R)-N-(1-(tert-Butylcarbonylmethyl) -2,3-dihdro-2-oxo-5- (2-pyridyl) -1H-1,4-benzodiazepin-3-yl)-N′-(3-(methylamino)phenyl)urea (YF476): A potent and orally active gastrin/CCK-B antagonist. J. Med. Chem. 1997;40:331–341. doi: 10.1021/jm960669+. [DOI] [PubMed] [Google Scholar]
- TAKINAMI Y., YUKI H., NISHIDA A., AKUZAWA S., UCHIDA A., TAKEMOTO Y., OHTA M., SATOH M., SEMPLE G., MIYATA K. YF476 is a new potent and selective gastrin/cholecystokinin-B receptor antagonist in vitro and in vivo. Aliment. Pharmacol. Ther. 1997;11:113–120. doi: 10.1046/j.1365-2036.1997.110281000.x. [DOI] [PubMed] [Google Scholar]
- WANK S.A. G protein-coupled receptors in gastrointestinal physiology I. CCK receptors: an exemplary family. Am. J. Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- WANK S.A., HARKINS R., JENSEN R.T., SHAPIRA H., WEERTH A.D., SLATTERY T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc. Natl. Acad. Sci. U.S.A. 1992a;89:3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANK S.A., PISEGNA J.R., WEERTH A.D. Brain and gastrointestinal cholecystokinin receptor family: Structure and functional expression. Proc. Natl. Acad. Sci. U.S.A. 1992b;89:8691–8695. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKLER H., APPS D.-K., FISCHER-COLBRIE R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986;18:261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- WINKLER H., FISCHER-COLBRIE R. The chromogranins A and B: the first 25 years and futrue perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUKI H., NISHIDA A., MIYAKE A., ITO H., AKUZAWA S., TAKINAMI Y., TAKEMOTO Y., MIYATA K. YM022, a potent and selective gastrin/CCK-B receptor antagonists, inhibits peptone meal-induced gastric acid secretion in Heidenhain pouch dogs. Dig. Dis. Sci. 1997;42:707–714. doi: 10.1023/a:1018887308280. [DOI] [PubMed] [Google Scholar]


