Abstract
We previously reported that substance P and insulin-like growth factor-1 (IGF-1) synergistically stimulate corneal epithelial wound healing in vitro and in vivo. We wished to identify which portion of the amino acid sequence of substance P might be responsible for this synergism.
Corneal epithelial migration was not affected by the addition of any one of the following factors: substance P; Phe-Gly-Leu-Met-NH2 (C-terminal of substance P); Val-Gly-Leu-Met-NH2 (C-terminal of neurokinin A, neurokinin B, and kassinin); Tyr-Gly-Leu-Met-NH2 (C-terminal of physalaemin); Ile-Gly-Leu-Met-NH2 (C-terminal of eledoisin); or Gly-Leu-Met-NH2 (common C-terminal of tachykinins).
In the presence of IGF-1, only substance P and Phe-Gly-Leu-Met-NH2 were synergistic in stimulating corneal epithelial migration in a dose-dependent fashion.
The combination of Phe-Gly-Leu-Met-NH2 and IGF-1 did not affect the incorporation of [3H]-thymidine into corneal epithelial cells.
Treatment with Phe-Gly-Leu-Met-NH2 and IGF-1, but not with Phe-Gly-Leu-Met-NH2 or IGF-1 alone, increased attachment of corneal epithelial cells to a fibronectin matrix.
The levels of α5 and β1 integrin were not affected by Phe-Gly-Leu-Met-NH2 or IGF-1 alone, but they were significantly increased by the combination of Phe-Gly-Leu-Met-NH2 and IGF-1.
Topical application of the same combination facilitated corneal epithelial wound closure in vivo.
These results demonstrated that Phe-Gly-Leu-Met-NH2, a sequence of 4 amino-acids of the C-terminal of substance P, is the minimum sequence necessary to produce the synergistic effects of substance P and IGF-1 on corneal epithelial wound healing.
Keywords: Substance P, insulin-like growth factor-1, wound healing, epithelial migration, cell attachment, integrin
Introduction
The epithelium is an important barrier that protects and maintains the structure and functions of the inside of the body against a variable external environment. Because the epithelium is often injured, however, it requires a speedy healing process. Various biological active substances, such as growth factors and extracellular matrix proteins, have been reported to modulate and regulate epithelial wound healing (Nishida, 1993; Woodley, 1996). However, the role of neural factors in epithelial wound healing is not yet fully understood. Clinically delayed wound healing has been noted in patients who have lost sensation through diseases such as diabetes mellitus.
The cornea is more heavily innervated with sensory nerve fibres than any other tissue in the body, and corneal innervatioan also plays an important role in the maintenance of corneal structure and functions. When corneal nerves from a branch of the trigeminal nerve are damaged by injury or disease, a variety of abnormalities result. In experimental studies, corneal denervation has been reported to result in epithelial changes: increased permeability, decreased proliferation, changed phenotype, and delayed wound healing (Mishima, 1957; Baker et al., 1993; Araki et al., 1994). In the clinical setting, after various types of corneal injuries or diseases, such as trauma, intraocular surgery, herpetic keratitis, or diabetic keratopathy, neutrotrophic keratopathy develops, and persistent corneal defects or trophic ulcer often occur (Dawson & Togni, 1976; Mackie, 1978; Groos, 1997). Therefore, intact corneal innervation is required to maintain the integrity of the normal corneal epithelium, and factors released from sensory nerves appear to play an important role in the physiology of the corneal epithelium.
Using an organ culture of the cornea, we recently found that substance P and insulin-like growth factor-1 (IGF-1) synergistically stimulate corneal epithelial migration (Nishida et al., 1996). Epithelial migration was not affected by the addition of either substance P or IGF-1 alone, but it was stimulated significantly by the combination of substance P and IGF-1. This action of substance P occurred specifically among various kinds of neurotransmitters and tachykinins. Furthermore, substance P and IGF-1 synergistically facilitated corneal epithelial wound closure in vivo (Nakamura et al., 1997b). In clinical situations, topical application of substance P and IGF-1 was effective in the treatment of severe neurotrophic and anhydrotic keratopathy (Brown et al., 1997).
Substance P is a constituent of sensory nerve fibres and has been postulated to mediate various physiological functions, such as vasodilatation and inflammation (Pernow, 1983; Payan, 1989). Substance P is, however, easily degraded and inactivated by neuropeptidases, such as carboxypeptidases and endopeptidases (Guyon et al., 1979; Matsas et al., 1983; 1984; LeBien & McCormack, 1989). Therefore, finding a smaller and more stable peptide is essential to developing further clinical use of substance P.
In the present study, we wished to identify which portion of the amino acid sequence of substance P might be responsible for this synergism of substance P and IGF-1 in corneal epithelial wound healing. First, based on a previous report showing that a sequence of 5 amino-acids of the C-terminal of substance P is sufficient to produce the synergistic effect with IGF-1 (Nishida et al., 1996), we used an organ culture system of rabbit cornea to examine whether tachykinin-related C-terminal peptides of various lengths are synergistic with IGF-1. Furthermore, we investigated the combined effects of a minimum essential peptide of substance P and IGF-1 on corneal epithelial wound closure of rabbit in vivo.
Methods
Materials
Albino rabbits weighing 2–3 kg were obtained from Kitayama Labs, Kyoto, Japan TC-199 culture medium, trypsin (0.25%), and EDTA (0.02%) were from the Research Foundation for Microbial Diseases of Osaka University, Suita, Osaka, Japan. Foetal bovine serum (FBS) was from Flow Laboratories, North Ryde, Australia. Dispase was from Godo Shusei, Tokyo, Japan. Bovine serum albumin fraction V (BSA) was from Nacalei Tesque, Kyoto, Japan. The [methyl-3H]-thymidine (37 MBq ml−1) was from Amersham Japan, Tokyo, Japan. Plastic multi-well culture dishes were from Costar, Cambridge, MA, U.S.A. Substance P was from Sigma Chemical Co., St. Louis, MO, U.S.A. Human plasma fibronectin and human recombinant IGF-1 were from Collaborative Research, Bedford, MA, U.S.A. Phe-Gly-Leu-Met-NH2 (FGLM), Val-Gly-Leu-Met-NH2 (VGLM), Tyr-Gly-Leu-Met-NH2 (YGLM), Ile-Gly-Leu-Met-NH2 (IGLM), and Gly-Leu-Met-NH2 (GLM) were synthesized from Peptide Institute, Osaka, Japan. Substance P (1-7), Arg-Pro-Lys-Pro-Gln-Gln-Phe, was from Neosystem Laboratories, Strasbourg, France. Care and treatment of animals adhered to the Guiding Principles in the Care and Use of Animals (DHEW Publication, NIH 86-23).
Epithelial migration
The length of the path of the epithelial migration over the cut stromal surface of a block of cultured rabbit cornea was measured as reported previously (Nishida et al., 1983; Nakamura et al., 1997a). In brief, full-thickness corneal blocks (approximately 2×4 mm) were cut with a razor blade from excised corneas and placed in each well of a multiwell tissue culture dish (24 wells) with one of the culture media indicated below and incubated at 37°C under humidified 5% CO2 in air. The culture media used were unsupplemented TC-199 (control) or TC-199 containing various agents to be examined. At the end of cultivation, specimens were fixed with a mixture of glacial acetic acid and absolute ethanol (5 : 95 in volume) at 4°C overnight. They were then dehydrated through graded ethanol, immersed in xylene, and embedded in paraffin. Thin sections (4 μm) were cut, deparaffinized, and stained with haemotoxylin-eosin. Specimens were observed under a light microscope; photographs were taken, and the length of the path of the corneal epithelium was measured on the printed photographs. Data were expressed as the mean (±s.e.mean) for six determinations.
Preparation of corneal epithelial cells
Corneal epithelial cells were prepared and cultured as described previously (Nishida et al., 1988). In brief, rabbit corneas were excised. The endothelial layers were removed mechanically, and the remaining corneas were incubated with dispase (2 mg ml−1 in TC-199) for 1 h. The epithelial layer was harvested as a sheet and washed with TC-199 to remove the debrise. The epithelial layer was incubated with trypsin (0.125%) and EDTA (0.01%) to make single-cell suspensions. The cells were then washed with TC-199 containing 15% FBS.
Characterization of corneal epithelial cells
To confirm the homogeneity and specificity of epithelial cells, we immunostained the cells with anti-cytokeratin antibody (AE1+AE3, epithelial cell marker) and anti-vementin antibody (V9, fibroblastic cell marker). All of the isolated rabbit corneal epithelial cells were immunoreactive with anti-cytokeratin but not with anti-vementin antibody. As a control, we isolated rabbit corneal fibroblasts, and the cells were immunoreactive with anti-vementin but not with antibody anti-cytokeratin antibody (Figure 1). Therefore, the cells used in our experiments were homogeneous epithelial cells. No contamination of stromal cells, corneal fibroblasts, was observed.
Figure 1.
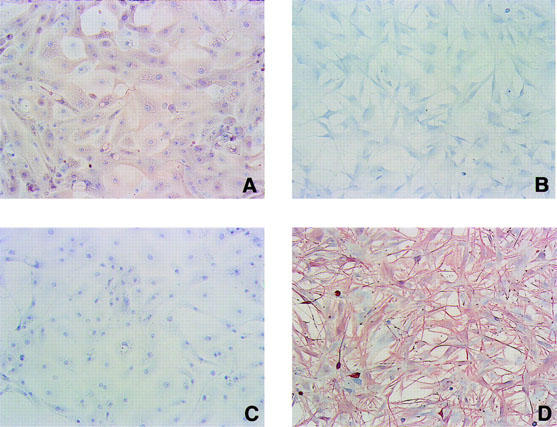
Characterization of rabbit corneal epithelial cells and fibroblasts. Cultured rabbit corneal epithelial cells (A and C) and fibroblasts (B and D) were immunostained with anti-cytokeratin antibody (AE1+AE3, epithelial cell marker) (A and B) or anti-vementin antibody (V9, fibroblastic cell marker) (C and D).
[3H]-thymidine uptake
Corneal epithelial cells were prepared as described above and were cultured on a 96-multiwell culture plate (5×104 cells well−1) for 4 days in TC-199 containing 15% FBS. The culture medium was then changed to TC-199 containing 1% FBS (low-serum medium) with or without substance P (2×10−5 M) or Phe-Gly-Leu-Met-NH2 (2×10−5 M) and/or IGF-1 (10 ng ml−1). No agent was added to the control cultures. As a positive control, 10 ng/ml of EGF was added to the culture medium. Twenty-four hours after the change of the medium, [3H]-thymidine (3.7 KBq well−1, 37 KBq ml−1) was added. The cells were incubated for a further 24 h and harvested, and the radioactivity was measured with a liquid scintillation counter. Data were expressed as means (±s.e.mean) c.p.m. well−1 from four determinations.
Cell attachment assay
The dissociated corneal epithelial cells were cultured in TC-199 culture medium containing 15% FBS for 4 days. The medium was changed to TC-199 containing 1% FBS with or without substance P (2×10−5 M) or Phe-Gly-Leu-Met-NH2 (2×10−5 M) and/or IGF-1 (10 ng ml−1), and incubation was continued for another 24 h. Cells were harvested by a 0.125% trypsin and 0.01% EDTA solution. The cells were washed with unsupplemented TC-199, and the cell suspension was adjusted to 1×104 cells ml−1. This cell suspension (0.1 ml) was plated on each well of a 96-well tissue culture plate, precoated with human plasma fibronectin (10 μg ml−1) plus BSA (1 mg ml−1), or with BSA alone (1 mg ml−1). After 45 min of incubation at 37°C, the cells were fixed with 3.7% formaldehyde and stained with 1% crystal violet. The numbers of attached cells were counted under an inverted phase contrast microscope. Data were expressed as the mean (±s.e.mean) of the number of attached cells per well in an assay performed in triplicate.
Reverse transcription-polymerase chain reaction (RT–PCR)
Total RNA was extracted from cultured rabbit corneal epithelial cells with ISOGEN from Nippongene, Toyama, Japan, and quantitated spectrophotometrically by absorption at 260 and 280 nm. The specific mRNA levels were estimated by means of a reverse transcription-polymerase chain reaction protocol using a Takara RNA PCR kit (AMV) ver. 2.1 (Takara Shuzo, Otsu, Shiga, Japan). One half μg of total RNA was reverse transcripted into cDNA by incubation with 0.25 U of avian myeloblastosis virus reverse transcriptase XL in a reaction buffer containing 1/10 volume of 10×RNA PCR buffer, MgCl2 5 mM, dNTP mixture 1 mM, randam 9 mers 2.5 pmol ml−1 and 1 U of RNase inhibitor in a final volume of 20 μl. The reaction mixture was incubated at 30°C for 10 min, and at 42°C for 30 min, and the reaction was stopped by heating at 99°C for 5 min and then cooling down to 4°C.
A polymerase chain reaction was carried out in a GeneAmp™ PCR System 9600 (Perkin-Elmer, Norwalk, CT, U.S.A.) by adding 80 μl of reaction mixture containing 8 μl of 10×RNA PCR buffer, 0.025 U of Taq DNA polymerase, and 100 pmol of sense and antisense primers to 20 μl of cDNA solution. The following sequence of primers was used in the PCR reactions: integrin α5 sense: 5′- GGCAGCTATGGCGTCCCACTGTGG-3′, integrin α5 antisense: 5′- GGCATCAGAGGTGGCTGGAGGCTT-3′, integrin β1 sense: 5′- GTGGTTGCTGGAATTGTTCTTATT-3′, integrin β1 antisense: 5′- TTTTCCCTCATACTTCGGATTGAC-3′, glyceraldehyde 3-phosphate dehydrogenase (G3PDH, internal control) sense: 5′- CGCGGGCCATTCATTGACCTCCACTAC-3′, G3PDH antisense: 5′- CGCGGGCTCCTGGAAGATGGTGAT-3′. These primers led to expected cDNA sequences of 171 base pairs (bp) for integrin α5, 189 bp for integrin β1, and 147 bp for G3PDH (Fini et al., 1995; Ohashi et al., 1995). The condition for the PCR reaction was: 25 cycles, 1 min at 94°C, 1 min at 50°C, and 2 min at 72°C. The products of amplification were electrophoresed in 1.5% of agarose gel (Sigma Chemical Co., St. Louis, MO, U.S.A.), containing 1 mg per ml of ethidium bromide (Nippongene, Toyama, Japan) and visualized on the u.v. transilluminator. The band area of integrin α5 or β1 and G3PDH was measured, and the band area of integrin α5 or β1 was normalized with the band area of G3PDH, and then the specific integrin α5 or β1 mRNA level was estimated.
In vivo corneal epithelial wound closure
Twenty-four albino rabbits were used. The animals were anaesthetized with an intramuscular injection of ketamine and xylazine and with oxybuprocaine eye drops. A corneal epithelial wound was produced in each eye according to the method of Cintron and others (Cintron et al., 1979). In brief, filter paper (6 mm in diameter) soaked with n-heptyl alcohol was placed on each cornea for 1 min, and then the damaged epithelial cells were washed out with saline. This method makes a uniform wound, and the denuded area appears quite clean and smooth, with a sharply demarcated wound edge. The basement membrane underlying the epithelium is not damaged (Cintron et al., 1979). This wound-healing model was used to evaluate the growth factors and peptides (Schultz et al., 1988; Takagi et al., 1994; Sotozono et al., 1995).
The animals were divided into six groups (eight eyes per group). The control group was treated with eye drops containing PBS alone; the experimental groups received PBS containing substance P or Phe-Gly-Leu-Met-NH2 at a concentration of 1 mM, or IGF-1 at a concentration of 1 μg ml−1, or a combination of substance P or Phe-Gly-Leu-Met-NH2 and IGF-1. In general, concentrations 10–100 fold higher than the effective doses in in vitro experiments are needed to evaluate the effects of growth factors and peptides in vivo (Schultz et al., 1988; Takagi et al., 1994; Sotozono et al., 1995). Based on our in vitro experiments, the concentrations of substance P, Phe-Gly-Leu-Met-NH2 and IGF-1 used in this study were 50 fold and 100 fold higher, respectively, than their in vitro effective doses. All eyes received one drop of the solution immediately after the n-heptyl alcohol treatment and again at 2, 4, 6, 8, 10, 24, 26, 28, 30, 32, and 34 h after debridement. The epithelial defects were stained with one drop of 2% fluorescein and photographed immediately following wounding and at 6, 12, 18, 24, 30, 36, and 48 h after wounding. The area of each epithelial defect was measured on the photographs with a computer-assisted digitizer and expressed as the mean, in square millimeters, for the eyes in each group. The healing rate of each eye was calculated with linear regression analysis of the data collected at 12, 18, 24, and 30 h and expressed in square millimeters per hour. The experiment was carried out in a double-masked fashion to avoid any bias. The rate of healing for each group was expressed as the mean (±s.e.mean). Pupil size was measured by Haab's pupillometer before instillation of eye drops at 10, 24, 34, and 48 h after debridement.
Statistical analysis
Statistical analysis was carried out by an unpaired Student's t-test for comparison of two groups, and by the Dunnett's multiple comparison test for comparison of three or more groups; pupil size was compared using a Scheffe-type multiple comparison test.
Results
Based on a previous report showing that the sequence of 5 amino acids of the C-terminal of substance P is sufficient to produce the synergistic effect with IGF-1 (Nishida et al., 1996), we examined whether tachykinin-related C-terminal peptides of various lengths are synergistic with IGF-1 (Figure 2). The addition of substance P (SP, 11 amino-acid peptides, SP1-11), Phe-Gly-Leu-Met-NH2 (FGLM, 4 amino-acid sequence at C-terminal of substance P, SP8-11), Val-Gly-Leu-Met-NH2 (VGLM, 4 amino-acid sequence at C-terminal of neurokinin A, neurokinin B and kassinin), Tyr-Gly-Leu-Met-NH2 (YGLM, 4 amino-acid sequence at C-terminal of physalaemin), Ile-Gly-Leu-Met-NH2 (IGLM, C-terminal of eledoisin), or Gly-Leu-Met-NH2 (GLM, 3 amino-acid sequence at common C-terminal of tachykinins) at a concentration of 2×10−5 M did not affect corneal epithelial migration. However, in the presence of IGF-1 at a concentration of 10 ng ml−1, substance P and Phe-Gly-Leu-Met-NH2 are synergistic in stimulating corneal epithelial migration. No synergistic effects with other peptides (Val-Gly-Leu-Met-NH2, Tyr-Gly-Leu-Met-NH2, Ile-Gly-Leu-Met-NH2, nor Gly-Leu-Met-NH2) were observed. In addition, the 7 amino-acid peptides at the N-terminal of substance P, SP1-7, which remained peptides of the Phe-Gly-Leu-Met-NH2, also did not affect corneal epithelial migration, whether or not IGF-1 was present (Figure 3). These results demonstrated that the sequence of the 4 amino-acids of the C-terminal of substance P is the minimum number of peptides required to produce the synergistic effect with IGF-1. Furthermore, among tachykinins (neurokinin A, neurokinin B, kassinin, physalaemin, eledoisin), only the C-terminal of substance P demonstrated a synergistic effect with IGF-1 on corneal epithelial migration when evaluated in our in vitro assay.
Figure 2.
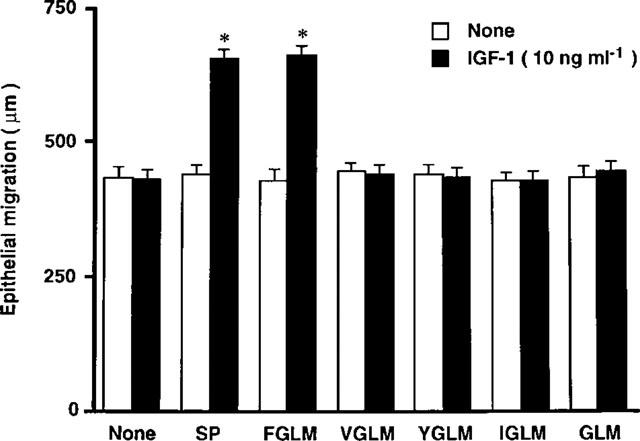
Synergistic effects with various types of C-terminal of tachykinin peptides with IGF-1 on corneal epithelial migration. Corneal blocks were cultured for 24 h in TC-199 containing various kinds of peptides (2×10−5 M) in the absence or presence of IGF-1 (10 ng ml−1). Error bars represent the s.e.mean from six determinations. *P<0.01 versus the corneal blocks cultured without IGF-1.
Figure 3.
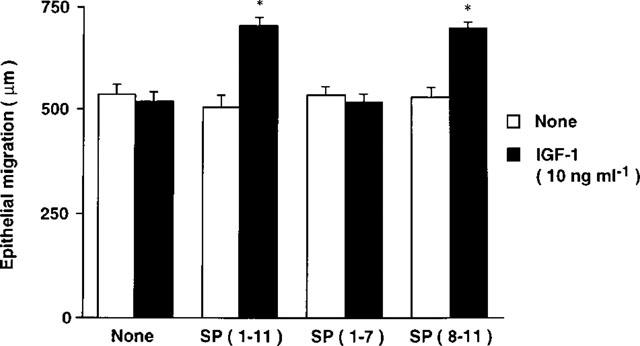
Synergistic effects with sequence of 7 amino-acids of N-terminal of substance P (SP), SP(1–7), and sequence of 4 amino-acids of C-terminal of SP, SP(8–11), with IGF-1 on corneal epithelial migration. Corneal blocks were cultured for 24 h in TC-199 containing peptides (2×10−5 M) in the absence or presence of IGF-1 (10 ng ml−1). Error bars represent the s.e.mean from six determinations. *P<0.01 versus the corneal blocks cultured without IGF-1.
We then examined whether the synergistic effect of Phe-Gly-Leu-Met-NH2 with IGF-1 was concentration dependent (Figure 4). When various concentrations of Phe-Gly-Leu-Met-NH2 (0–2×10−5 M) were added in the absence of IGF-1, no change in the length of epithelial migration was observed. In the presence of IGF-1 at a concentration of 10 ng ml−1, however, the length of the path of epithelial migration increased in proportion to the concentration of Phe-Gly-Leu-Met-NH2. At a concentration of 1 or 2×10−5 M of Phe-Gly-Leu-Met-NH2, the length of epithelial migration was significantly higher in the corneal blocks cultured with IGF-1 than in those cultured without IGF-1 (P<0.01) (Figure 4A). Corneal blocks were then cultured in varying concentrations of IGF-1 (0–10 ng ml−1) in the absence or presence of Phe-Gly-Leu-Met-NH2 (2×10−5 M) for 24 h (Figure 4B). The addition of IGF-1 did not affect the length of epithelial migration in the absence of Phe-Gly-Leu-Met-NH2. However, in the presence of Phe-Gly-Leu-Met-NH2, the length of epithelial migration increased in proportion to the concentration of IGF-1. At concentrations of 1 or 10 ng ml−1 of IGF-1 with Phe-Gly-Leu-Met-NH2, the length of epithelial migration was significantly higher than the length of epithelial migration in the corneal blocks cultured without Phe-Gly-Leu-Met-NH2 alone (P<0.01). These results demonstrate that the synergistic effects of Phe-Gly-Leu-Met-NH2 with IGF-1 are mutually concentration dependent.
Figure 4.
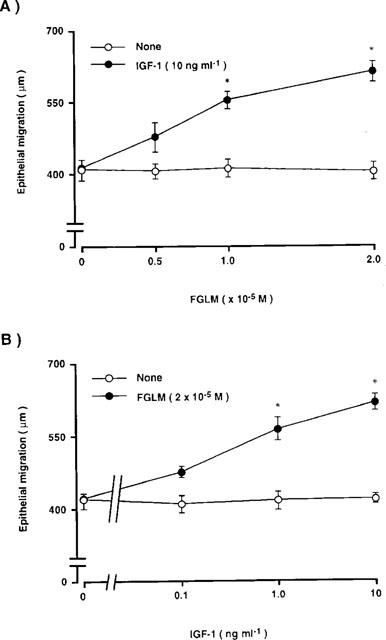
Synergistic effects of Phe-Gly-Leu-Met-NH2 (FGLM) with IGF-1 on corneal epithelial migration. Corneal blocks were cultured for 24 h in TC-199 containing: (A) FGLM (0.5, 1, 2×10−5 M) in the absence or presence of IGF-1 (10 ng ml−1); (B) IGF-1 (0.1, 1, 10 ng ml−1) in the absence or presence of FGLM (2×10−5 M). Error bars represent the s.e.mean from six determinations. *P<0.01 versus the corneal blocks cultured without FGLM or IGF-1.
Corneal epithelial migration depends on the increase in the proliferation of the cells and/or an increase in the attachment to extracellular matrix proteins, such as fibronectin. Therefore, we examined whether Phe-Gly-Leu-Met-NH2 and IGF-1 acted in a synergistic way on [3H]-thymidine uptake and/or the attachment of the epithelial cells to the fibronectin matrix.
The addition of only IGF-1 (10 ng ml−1) to cultured corneal epithelial cells did not affect [3H]-thymidine uptake. When substance P or Phe-Gly-Leu-Met-NH2 (2×10−5 M) was added to corneal epithelial cells, no change in [3H]-thymidine uptake was observed, whether IGF-1 was absent or present (Table 1). When EGF (10 ng ml−1) was added simultaneously, [3H]-thymidine uptake was 13,433±549 c.p.m./well, which demonstrated that the cells were not under contact inhibition. Thus, no synergistic effects of Phe-Gly-Leu-Met-NH2 and IGF-1 on corneal epithelial proliferation were observed.
Table 1.
Synergistic effects of substance p (SP) or Phe-Gly-Leu-Met-NH2 (FGLM) with IGF-1 on corneal epithelial cell proliferation
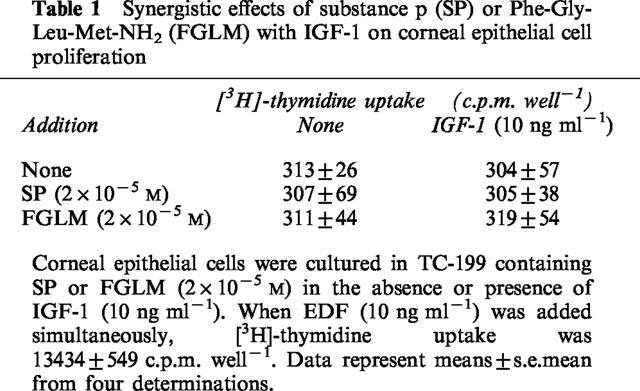
We then examined the synergistic effects of Phe-Gly-Leu-Met-NH2 and IGF-1 on the attachment of corneal epithelial cells to the fibronectin matrix (Figure 5). Whether the cells were cultured in the absence or presence of substance P or Phe-Gly-Leu-Met-NH2 and/or IGF-1, the number of cells attached to the control plates coated with BSA remained low, and there was no significant difference in the number of cells attached (Figure 5B). When the cells were cultured with or without substance P, Phe-Gly-Leu-Met-NH2, or IGF-1 alone, no significant effect of fibronectin-coated plates on cellular attachment was observed. However, when the cells were cultured in the presence of substance P or Phe-Gly-Leu-Met-NH2 and IGF-1, the number of cells attached on fibronectin-coated plates increased significantly (Figure 5A). These results demonstrate that substance P, Phe-Gly-Leu-Met-NH2, or IGF-1 alone did not affect the attachment of corneal epithelial cells to a fibronectin matrix, but they act synergistically to promote attachment of the cells to a fibronectin matrix.
Figure 5.
Synergistic effects of substance P (SP) or Phe-Gly-Leu-Met-NH2 (FGLM) with IGF-1 on corneal epithelial cell attachment to the fibronectin matrix. Corneal epithelial cells were cultured for 24 h in TC-199 containing SP or FGLM (2×10−5 M) in the absence or presence of IGF-1 (10 ng ml−1). The dissociated epithelial cells were then plated on the fibronectin (coated at 10 μg ml−1) plus BSA (1 mg ml-1) (A) or BSA matrix (B), and incubated for 45 min. Error bars represent the s.e.mean from three determinations. *P<0.01 versus the corneal epithelial cells cultured without IGF-1.
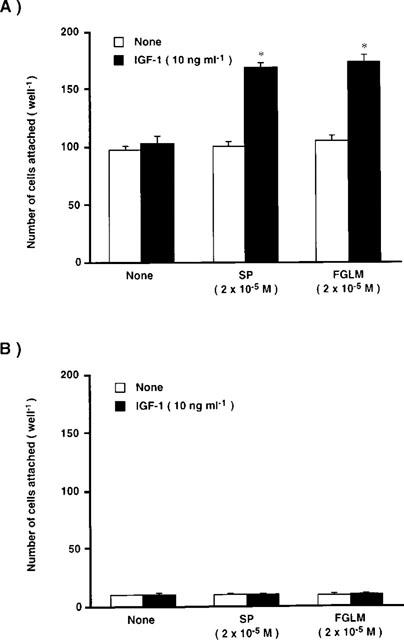
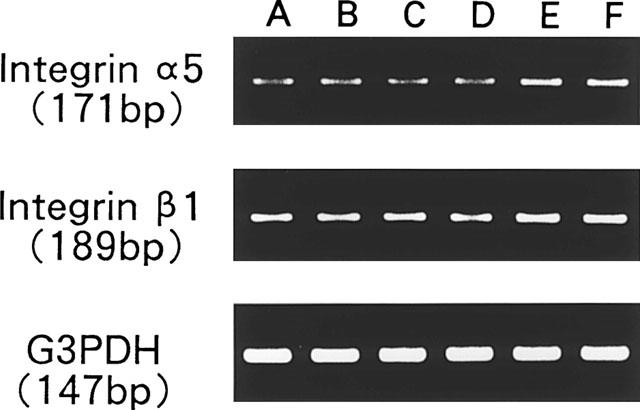
Using a RT–PCR, we then investigated whether the combination of Phe-Gly-Leu-Met-NH2 and IGF-1 up-regulate the expression of integrin α5β1, a fibronectin receptor on corneal epithelial cells (Figure 6). When the cells were treated with or without substance P, Phe-Gly-Leu-Met-NH2, or IGF-1 alone for 2 h, no significant change was observed in the levels of transcripts of either α5 or β1 integrin. When the cells were treated in the presence of both substance P or Phe-Gly-Leu-Met-NH2 and IGF-1, however, the levels of both α5 and β1 integrin mRNA increased significantly, resulting in about 3 and 1.5 fold increases, respectively (Figure 7A and B). The level of G3PDH mRNA did not affect any treatments, indicating that the expression of α5 and β1 integrin were specifically up-regulated by the combination of substnace P or Phe-Gly-Leu-Met-NH2 and IGF-1.
Figure 6.
RT–PCR transcription analysis of integrin α5 and β1 expression in corneal epithelial cells after treatment with substance P (SP) or Phe-Gly-Leu-Met-NH2 (FGLM) and/or IGF-1. Ethidium bromide-staining gels of PCR amplified products of reverse-transcribed epithelial cell RNA. A: None, B: SP (2×10−5 M), C: FGLM (2×10−5 M), D: IGF-1 (10 ng ml−1), E: SP plus IGF-1, F: FGLM plus IGF-1.
Figure 7.
Effects of substance P (SP) or Phe-Gly-Leu-Met-NH2 (FGLM) and/or IGF-1 on the gene expression of integrin α5 (A) and integrin β1 (B) in cultured cornea epithelial cells using an RT–PCR. Corneal epithelial cells were cultured for 2 h in TC-199 containing SP or FGLM (2×10−5 M) and/or IGF-1 (10 ng ml−1), and then RT–PCR was performed. Error bars represent the s.e.mean from three determinations. *P<0.05 versus the corneal epithelial cells cultured without SP, FGLM, or IGF-1.
We next, investigated the combined effects of Phe-Gly-Leu-Met-NH2 and IGF-1 on corneal epithelial wound closure in vivo. No significant difference in the size of the wound area was observed among the control (PBS) and experimental groups immediately after debridement (Figure 8). During the first 6 h after debridement, healing was rather slow, and no differences were observed between the control group and the groups treated with substance P or Phe-Gly-Leu-Met-NH2 and/or IGF-1. Twelve to 30 h after debridement, the rate of wound closure accelerated and was almost linear. The administration of substance P, Phe-Gly-Leu-Met-NH2, or IGF-1 alone did not change the rate of closure compared with that of the control group. However, the combination of substance P or Phe-Gly-Leu-Met-NH2 and IGF-1 significantly increased this rate compared with that of the control group. After 36 h, the healing rate slowed in both the control and experimental groups.
Figure 8.
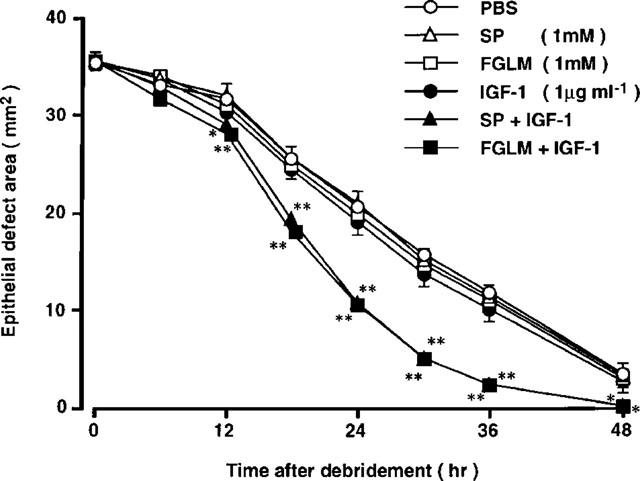
Effects of substances P (SP), Phe-Gly-Leu-Met-NH2 (FGLM), and/or IGF-1 on the area of the cornea epithelial defect. There were eight eyes per treatment group. Data are expressed as means±s.e.mean. *P<0.05; **P<0.01 compared with control group.
The mean healing rates (±s.e.mean, in square millimeters per hour) were 0.87±0.03 (PBS eye drops alone), 0.92±0.06 (substance P alone), 0.90±0.06 (Phe-Gly-Leu-Met-NH2 alone), 0.92±0.06 (IGF-1 alone), 1.34±0.04 (substance P and IGF-1), and 1.28±0.03 (Phe-Gly-Leu-Met-NH2 and IGF-1). No significant difference was observed between the control group receiving only PBS and the group receiving substance P, Phe-Gly-Leu-Met-NH2, or IGF-1. The mean healing rate in the group treated with substance P or Phe-Gly-Leu-Met-NH2 and IGF-1 was significantly higher than in control eyes (P<0.01).
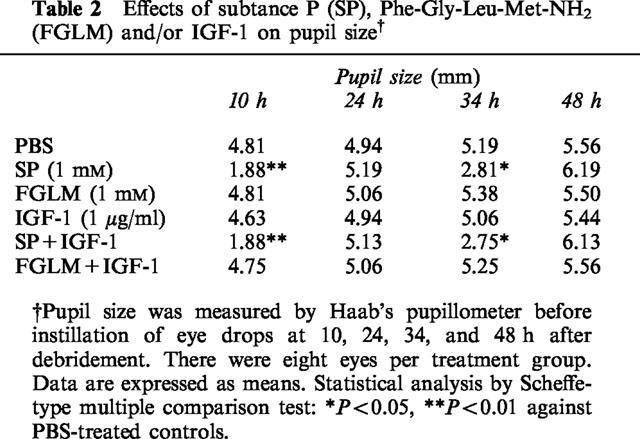
In contrast, instillation of substance P significantly decreased pupil size, whether or not IGF-1 was present. However, Phe-Gly-Leu-Met-NH2 and/or IGF-1 did not affect pupil size (Table 2).
Table 2.
Effects of subtance P (SP), Phe-Gly-Leu-Met-NH2 (FGLM) and/or IGF-1 on pupil size†
Discussion
The pathophysiological role of innervation in the cornea is not yet fully understood. However, loss of corneal sensation often leads to the destruction of normal integrity of the cornea. Persistent corneal epithelial defects or delayed epithelial wound healing often occur in patients whose corneal sensation is diminished as a result of diseases such as herpes simplex, herpes zoster, and diabetes mellitus. Furthermore, corneal ulceration may often develop in the anaesthetized eye (Dawson & Togni, 1976; Mackie, 1978; Groos, 1997). On the basis of these clinical observations, we believe that corneal innervation may be important for maintaining normal integrity of the cornea. Nonetheless, the definitive role of the neural function in corneal pathophysiology has not yet been clarified.
We recently demonstrated that substance P and IGF-1 acting alone do not influence corneal epithelial wound healing but that they act synergistically to stimulate epithelial wound healing in vitro and in vivo (Nishida et al., 1996; Nakamura et al., 1997b). Furthermore, this synergistic effect of substance P and IGF-1 was found to be mediated through the NK-1 receptor for substance P on corneal epithelial cells (Nakamura et al., 1997c). We tried to identify the minimum essential amino-acid sequence of substance P to develop the clinical use of substance P for the treatment of neurotrophic keratopathy, because substance P is easily degraded and inactivated by peptidases in the body (Guyon et al., 1979; Matsas et al., 1983; 1984; LeBien & McCormack, 1989). The present results demonstrated that Phe-Gly-Leu-Met-NH2, the sequence of 4 amino-acids from the C-terminal of substance P, is the minimum sequence necessary to generate a synergistic effect with IGF-1 on corneal epithelial migration in vitro and epithelial wound closure in vivo.
Tachykinins have a common C-terminal sequence of 5 amino-acids: Phe-X-Gly-Leu-Met-NH2 (FXGLM). Among the various amino-acids at position 8 (marked by an X in the sequence above) in the tachykinins examined, only Phe-Gly-Leu-Met-NH2 exhibited a synergistic effect with IGF-1 on corneal epithelial migration. Furthermore, unlike substance P or Phe-Gly-Leu-Met-NH2, Gly-Leu-Met-NH2, the tachykinin C-terminal sequence of 3 amino-acids, did not demonstrate any synergistic effect with IGF-1. On the other hand, most of the biological effects of substance P have been thought to be mediated by the C-terminal of substance P, but the N-terminal of substance P, such as substance P (1–7), also has been shown to produce biological effects and often has produced effects opposite from those produced by the C-terminal of substance P (Hall & Stewart, 1984; Igwe et al., 1990; Larson & Sun, 1992; Yukhanaov & Larson, 1994). Substance P (1–7) did not, however, demonstrate any synergistic effect with IGF-1. Therefore, this C-terminal sequence of 4 amino-acids would appear to be essential for expressing the synergistic effect with IGF-1. Although we cannot explain why the C-terminal sequence of 4 amino-acids of tachykinins other than substance P does not produce a synergistic effect with IGF-1, it could be that the amino acid at position 8 is critical for producing the synergistic effect with IGF-1 on corneal epithelial wound healing.
An intact epithelium is very important to the survival of a multicellular organism. The epithelium serves as the first defense against the external environment, maintaining the internal homeostasis. Continuous renewal through an active repair system is one of the most important mechanisms for the maintenance of epithelial integrity. There are three phases involved in the process of corneal epithelial wound healing: migration or mobilization, proliferation or mitosis, and differentiation of the epithelial cell (Nishida, 1993; Dua et al., 1994; Gipson & Inatomo, 1995; Woodley, 1996). Among these events in epithelial wound healing, epithelial cell migration is the initial step in successful and complete resurfacing of a defect. During this step, attachment of the corneal epithelial cells to the underlying provisional fibronectin matrix is required for spreading and migration. Fibronectin and fibronectin receptor (integrin α5β1) play significant roles in this process. As we reported in this study, both Phe-Gly-Leu-Met-NH2 and substance P act synergistically with IGF-1 on cell migration, attachment to a fibronectin matrix, and expression of integrin α5β1. Furthermore, in an in vivo study, we observed the synergistic effect of Phe-Gly-Leu-Met-NH2 and IGF-1 during the initial phase of healing 12 h after debridement. However, no synergistic effect of Phe-Gly-Leu-Met-NH2 and IGF-1 on epithelial cell proliferation was observed. Therefore, these results demonstrate that the synergistic effect of Phe-Gly-Leu-Met-NH2 and IGF-1 on corneal epithelial wound healing results from an effect on corneal epithelial migration rather than on proliferation. This synergism would appear to represent an important cooperative action of neural and humoral regulation on corneal epithelial wound healing.
Substance P is thought to be a neurotransmitter mediating functions such as neurogenic inflammation and the transmission of pain in various tissues (Payan, 1989; Otsuka & Yoshioka, 1993), but neurotransmitters were inactivated by enzymatic degradation and re-uptake. In the case of substance P, enzymatic degradation is the main mechanism of inactivation. Substance P is degraded by cell-surface and soluble peptidases, especially cell-surface peptidases, such as aminopeptidases, carboxypeptidases and endopeptidases, which have been investigated thoroughly (Krause, 1985; Kenny & Hooper, 1991). Indeed, ocular tissues and fluids showed various types of peptidase activity (Pahlitzsch & Sinha, 1985; Stratford & Lee, 1985; Kashi & Lee, 1986; Sharma & Ortwerth, 1987; Igic, 1993; Coupland et al., 1994), and substance P was degraded and inactivated by these peptidases. Among these peptidases, neural endopeptidase (NEP) has been purified and well characterized about attack sites of substance P (Matsas et al., 1983; 1984; Skidgel et al., 1984; Roques et al., 1993). In general, NEP is hydrolyzed at bonds Gln-Phe (positions 6 and 7), Phe-Phe (positions 7 and 8), and Gly-Leu (positions 9 and 10) of substance P. However, Lee and others (Lee et al., 1981) reported that no attack was observed at the bond at positions 9 and 10, and substrate specificity of NEP might have species or regional variations. As we reported in this paper, 4 amino-acid sequences at the C-terminal or substance P might not be degraded by enzymatic cleave. Thus, Phe-Gly-Leu-Met-NH2 might be stable, when it is given to the eye in place of a whole molecule of substance P.
Our present study also shows that topical application of substance P induces mitosis, one of the pharmacological actions of substance P, but application of Phe-Gly-Leu-Met-NH2 did not. Therefore, the use of Phe-Gly-Leu-Met-NH2 may be excluded from some adverse effects of substance P because mitosis induces darkness. Further studies are needed to understand the stability and pharmacological actions of Phe-Gly-Leu-Met-NH2 in the corneal epithelium.
Substance P has been reported to stimulate the migration and proliferation of skin fibroblasts through NK-1 receptors (Ziche et al., 1990; Parenti et al., 1996). Although we did not examine the effect of substance P on corneal stromal cells, the same stromal cells are able to produce growth factors, which in turn could stimulate epithelial cells. In the present studies, however, substance P and Phe-Gly-Leu-Met-NH2 acting alone did not affect the migration and proliferation of epithelial cells. As shown in Figure 1, the cells used in our experiments were corneal epithelial cells, not corneal fibroblasts. Therefore, the mechanisms that trigger the action of substance P might be different in corneal epithelial cells and fibroblasts.
The corneas contain sensory nerve fibres containing substance P immunoreactivity (Miller et al., 1981; Tervo et al., 1981; 1982; Stone & Kuwayama, 1985). Previous studies have demonstrated a correlation between reduced substance P levels in the cornea and denervation of the trigeminal nerve to the eye (Butler et al., 1980; Unger et al., 1981; Keen et al., 1982). However, like previous studies, the present study has demonstrated that adding only substance P did not influence corneal epithelial wound healing (Nishida et al., 1996; Nakamura et al., 1997b). Kingsley & Murfurt (1997) also reported that substance P alone had no significant effect on corneal epithelial wound healing. Cooperative action of the neural and humoral factors in the corneal epithelium may be important in improving the corneal disorder resulting from corneral denervation. Our present study strongly suggests the possibility of using Phe-Gly-Leu-Met-NH2 and IGF-1 to treat pathological conditions such as herpetic keratitis or diabetic keratopathy, which are due to delayed wound healing following corneal desensitization.
Acknowledgments
This research was supported in part by a Grant-in-Aid for Scientific Research (B9470381) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank Miss Michiyo Suetomi for her secretarial assistance during the preparation of the manuscript. The technical assistance of Miss Megumi Kawahara is acknowledged.
Abbreviations
- BSA
bovine serum albumin fraction V
- EGF
epidermal growth factor
- FBS
foetal bovine serum
- GLM
Gly-Leu-Met-NH2
- IGLM
Ile-Gly-Leu-Met-NH2
- IGF-1
insulin-like growth factor-1
- FGLM
Phe-Gly-Leu-Met-NH2
- RT–PCR
reverse transcription-polymerase chain reaction
- SP
substance P
- YGLM
Tyr-Gly-Leu-Met-NH2
- VGLM
Val-Gly-Leu-Met-NH2
References
- ARAKI K., OHASHI Y., KINOSHITA S., HAYASHI K., KUWAYAMA Y., TANO Y. Epithelial wound healing in the denervated cornea. Curr. Eye Res. 1994;13:203–211. doi: 10.3109/02713689408995778. [DOI] [PubMed] [Google Scholar]
- BAKER K.S., ANDERSON S.C., ROMANOWSKI E.G., THOFT R.A., SUNDARRAJ N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Invest. Ophthalmol. Vis. Sci. 1993;34:137–144. [PubMed] [Google Scholar]
- BROWN S.M., LAMBERTS D.W., REID T.W., NISHIDA T., MURPHY C.J. Neurotrophic and anhidrotic keratopathy treated with substance P and insulinlike growth factor 1. Arch. Ophthalmol. 1997;115:926–927. doi: 10.1001/archopht.1997.01100160096021. [DOI] [PubMed] [Google Scholar]
- BUTLER J.M., POWELL D., UNGER W.G. Substance P levels in normal and sensorily denervated rabbit eyes. Exp. Eye Res. 1980;30:311–313. doi: 10.1016/0014-4835(80)90012-3. [DOI] [PubMed] [Google Scholar]
- CINTRON C., HASSINGER L., KUBLIN C.L., FRIEND J. A simple method for the removal of rabbit corneal epithelium utilizing n-heptanol. Ophthalmic Res. 1979;11:90–96. [Google Scholar]
- COUPLAND S.E., PENFOLD P.L., BILLSON F.A. Hydrolases of anterior segment tissues in the normal human, pig and rat eye: a comparative study. Graef. Arch. Clin. Exp. Ophthalmol. 1994;232:182–191. doi: 10.1007/BF00176789. [DOI] [PubMed] [Google Scholar]
- DAWSON C.R., TOGNI B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv. Ophthalmol. 1976;21:121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- DUA H.S., GOMES J.A., SINGH A. Corneal epithelial wound healing. Br. J. Ophthalmol. 1994;78:401–408. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINI M.E., GIRARD M.T., MATSUBARA M., BARTLETT J.D. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest. Ophthalmol. Vis. Sci. 1995;36:622–633. [PubMed] [Google Scholar]
- GIPSON I.K., INATOMI T. Extracellular matrix and growth factors in corneal wound healing. Curr. Opin. Ophthalmol. 1995;6:3–10. doi: 10.1097/00055735-199508000-00002. [DOI] [PubMed] [Google Scholar]
- GROOS E.B.Neurotrophic keratitis Cornea 1997Vol. 2St. Louis, Mosby; 1339–1347.ed. Krachmer J.H., Mannis M.J. & Holland E.J. pp [Google Scholar]
- GUYON A., ROQUES B.P., GUYON F., FOUCAULT A., PERDRISOT R., SWERTS J.P., SCHWARTZ J.C. Enkephalin degradation in mouse brain studied by a new H.P.L.C. method: further evidence for the involvement of carboxydipeptidase. Life Sci. 1979;25:1605–1612. doi: 10.1016/0024-3205(79)90444-2. [DOI] [PubMed] [Google Scholar]
- HALL M.E., STEWART J.M. Modulation of isolation-induced fighting by N-and C-terminal analogs of substance P: Evidence for multiple recognition sites. Peptides. 1984;5:85–89. doi: 10.1016/0196-9781(84)90056-1. [DOI] [PubMed] [Google Scholar]
- IGIC R. Substance P inactivation by aqueous humor. Exp. Eye Res. 1993;57:415–417. doi: 10.1006/exer.1993.1142. [DOI] [PubMed] [Google Scholar]
- IGWE O.J., KIM D.C., SEYBOLD V.S., LARSON A.A. Specific binding of substance P aminoterminal heptapeptide [SP(1-7)] to mouse brain and spinal cord membranes. J. Neurosci. 1990;10:3653–3663. doi: 10.1523/JNEUROSCI.10-11-03653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHI S.D., LEE V.H. Hydrolysis of enkephalins in homogenates of anterior segment tissues of the albino rabbit eye. Invest. Ophthalmol. Vis. Sci. 1986;27:1300–1303. [PubMed] [Google Scholar]
- KEEN P., TULLO A.B., BLYTH W.A., HILL T.J. Substance P in the mouse cornea: Effects of chemical and surgical denervation. Neurosci. Lett. 1982;29:231–235. doi: 10.1016/0304-3940(82)90322-6. [DOI] [PubMed] [Google Scholar]
- KENNY A., HOOPER N.Peptidases involved in the metabolism of bioactive peptides 1991CRC Press, Boco Raton, FL; 47–49.J.H. Henriksen, editor [Google Scholar]
- KINGSLEY R.E., MARFURT C.F. Topical substance P and corneal epithelial wound closure in the rabbit. Invest. Ophthalmol. Vis. Sci. 1997;38:388–395. [PubMed] [Google Scholar]
- KRAUSE J.E.On the physiological metabolism of substance P Substance P metabolism and biological actions 1985London: Taylor & Francis; 13–31.eds. Jordan C.C. & Ohme P. pp [Google Scholar]
- LARSON A.A., SUN X. Amino terminus of substance P potentiates kainic acid-induced activity in the mouse spinal cord. J. Neurosci. 1992;12:4905–4910. doi: 10.1523/JNEUROSCI.12-12-04905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBIEN T.W., MCCORMACK R.T. The common acute lymphoblastic leukemia antigen (CD10)–emancipation from a functional enigma. Blood. 1989;73:625–635. [PubMed] [Google Scholar]
- LEE C.M., SANDBERG B.E., HANLEY M.R., IVERSEN L.L. Purification and characterisation of a membrane-bound substance P-degrading enzyme from human brain. Eur. J. Biochem. 1981;114:315–327. doi: 10.1111/j.1432-1033.1981.tb05151.x. [DOI] [PubMed] [Google Scholar]
- MACKIE I.A. Role of the corneal nerves in destructive disease of the cornea. Trans. Ophthalmol. Soc. U.K. 1978;98:343–347. [PubMed] [Google Scholar]
- MATSAS R., FULCHER I.S., KENNY A.J., TURNER A.J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSAS R., KENNY A.J., TURNER A.J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem. J. 1984;223:433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER A., COSTA M., FURNESS J.B., CHUBB I.W. Substance P immunoreactive sensory nerves supply the rat iris and cornea. Neurosci. Lett. 1981;23:243–249. doi: 10.1016/0304-3940(81)90005-7. [DOI] [PubMed] [Google Scholar]
- MISHIMA S. The effects of the denervation and the stimulatioan of the sympathetic and the trigeminal nerve on the mitotic rate of the corneal epithelium in the rabbit. Jpn. J. Ophthalmol. 1957;1:65–73. [Google Scholar]
- NAKAMURA M., NISHIDA T., OFUJI K., REID T.W., MANNIS M.J., MURPHY C.J. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp. Eye Res. 1997a;65:321–329. doi: 10.1006/exer.1997.0345. [DOI] [PubMed] [Google Scholar]
- NAKAMURA M., OFUJI K., CHIKAMA T., NISHIDA T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr. Eye Res. 1997b;16:275–278. doi: 10.1076/ceyr.16.3.275.15409. [DOI] [PubMed] [Google Scholar]
- NAKAMURA M., OFUJI K., CHIKAMA T., NISHIDA T. The NK1 receptor and its participatioan in the synergistic enhancement of corneal epithelial migration by substance P and insulin-like growth factor-1. Br. J. Pharmacol. 1997c;120:547–552. doi: 10.1038/sj.bjp.0700923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIDA T. Extracellular matrix and growth factors in corneal wound healing. Curr. Opin. Ophthalmol. 1993;4:4–13. doi: 10.1097/00055735-199608000-00002. [DOI] [PubMed] [Google Scholar]
- NISHIDA T., NAKAGAWA S., AWATA T., OHASHI Y., WATANABE K., MANABE R. Fibronectin promotes epithelial migration of cultured rabbit cornea in situ. J. Cell Biol. 1983;97:1653–1657. doi: 10.1083/jcb.97.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIDA T., NAKAGAWA S., WATANABE K., YAMADA K.M., OTORI T., BERMAN M.B. A peptide from fibronectin cell-binding domain inhibits attachment of epithelial cells. Invest. Ophthalmol. Vis. Sci. 1988;29:1820–1825. [PubMed] [Google Scholar]
- NISHIDA T., NAKAMURA M., OFUJI K., REID T.W., MANNIS M.J., MURPHY C.J. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J. Cell Physiol. 1996;169:159–166. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- OHASHI H., MAEDA T., MISHIMA H., OTORI T., NISHIDA T., SEKIGUCHI K. Up-regulation of integrin α5β1 expression by interleukin-6 in rabbit corneal epithelial cells. Exp. Cell Res. 1995;218:418–423. doi: 10.1006/excr.1995.1174. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- PAHLITZSCH T., SINHA P. The alkali burned cornea: electron microscopical, enzyme histochemical, and biochemical observations. Graef. Arch. Clin. Exp. Ophthalmol. 1985;223:278–286. doi: 10.1007/BF02153659. [DOI] [PubMed] [Google Scholar]
- PARENTI A., AMERINI S., LEDDA F., MAGGI C.A., ZICHE M. The tachykinin NK-1 receptor mediates the migration-promoting effect of substance P on human skin fibroblasts in culture. Naunyn. Schmiedebergs. Arch. Pharmacol. 1996;353:475–481. doi: 10.1007/BF00169165. [DOI] [PubMed] [Google Scholar]
- PAYAN D.G. Neuropeptides and inflammation: the role of substance P. Annu. Rev. Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- PERNOW B. Substance P. Pharmacol. Rev. 1983;35:85–141. [PubMed] [Google Scholar]
- ROQUES B.P., NOBLE F., DAUGE V., FOURNIE-ZALUSKI M.C., BEAUMONT A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- SCHULTZ G.S., DAVIS J.B., EIFERMAN R.A. Growth factors and corneal epithelium. Cornea. 1988;7:96–101. [PubMed] [Google Scholar]
- SHARMA K.K., ORTWERTH B.J. Purification and characterization of an aminopeptidase from bovine cornea. Exp. Eye Res. 1987;45:117–126. doi: 10.1016/s0014-4835(87)80083-0. [DOI] [PubMed] [Google Scholar]
- SKIDGEL R.A., ENGELBRECHT S., JOHNSON A.R., ERDOS E.G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- SOTOZONO C., INATOMI T., NAKAMURA M., KINOSHITA S. Keratinocyte growth factor accelerates corneal epithelial wound healing in vivo. Invest. Ophthalmol. Vis. Sci. 1995;36:1524–1529. [PubMed] [Google Scholar]
- STONE R.A., KUWAYAMA Y. Substance P-like immunoreactive nerves in the human eye. Arch. Ophthalmol. 1985;103:1207–1211. doi: 10.1001/archopht.1985.01050080119031. [DOI] [PubMed] [Google Scholar]
- STRATFORD R.E., JR, LEE V.H. Ocular aminopeptidase activity and distribution in the albino rabbit. Curr. Eye Res. 1985;4:995–999. doi: 10.3109/02713689509000007. [DOI] [PubMed] [Google Scholar]
- TAKAGI H., REINACH P.S., YOSHIMURA N., HONDA Y. Endothelin-1 promotes corneal epithelial wound healing in rabbits. Curr. Eye Res. 1994;13:625–628. doi: 10.3109/02713689408999897. [DOI] [PubMed] [Google Scholar]
- TERVO K., TERVO T., ERANKO L., ERANKO O. Substance P immunoreactive nerves in the rodent cornea. Neurosci. Lett. 1981;25:95–97. doi: 10.1016/0304-3940(81)90107-5. [DOI] [PubMed] [Google Scholar]
- TERVO K., TERVO T., ERANKO L., VANNAS A., CUELLO A.C., ERANKO O. Substance P-immunoreactive nerves in the human cornea and iris. Invest. Ophthalmol. Vis. Sci. 1982;23:671–674. [PubMed] [Google Scholar]
- UNGER W.G., BUTLER J.M., COLE D.F., BLOOM S.R., MCGREGOR G.P. Substance P, vasoactive intestinal polypeptide (VIP) and somatostatin levels in ocular tissue of normal and sensorily denervated rabbit eyes. Exp. Eye Res. 1981;32:797–801. doi: 10.1016/0014-4835(81)90030-0. [DOI] [PubMed] [Google Scholar]
- WOODLEY D.T.Reepithelialization The molecular and cellular biology of wound repair 1996New York: Plenum Press; 339–354.ed. Clark R.A.F. pp [Google Scholar]
- YUKHANAOV R.Y., LARSON A.A. An N-terminal fragment of substance P, substance P (1-7), down-regulates neurokinin-1 binding in the mouse spinal cord. Neurosci. Lett. 1994;178:163–166. doi: 10.1016/0304-3940(94)90315-8. [DOI] [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI L., PACINI M., DOLARA P., MAGGI C.A. NK-1 receptors mediate the proliferative response of human fibroblasts to tachykinins. Br. J. Pharmacol. 1990;100:11–14. doi: 10.1111/j.1476-5381.1990.tb12043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


