Abstract
To delineate the mechanism by which cyclic AMP (cAMP) suppresses interleukin (IL)-5 synthesis, the effects of prostaglandin (PG) E2, forskolin, dibutyryl (db)-cAMP and the Ca2+ ionophore, ionomycin on cytokine synthesis, proliferation and CD25 expression of human T cells were investigated. Further studies were performed by measurement of the intracellular concentrations of cyclic AMP ([cAMP]i) and Ca2+ ([Ca2+]i) and by electrophoretic mobility shift analysis (EMSA).
PGE2, forskolin and db-cAMP suppressed IL-5 production by human T cell line following T cell receptor (TCR)-stimulation. PGE2 suppressed TCR-induced messenger RNA (mRNA) expression of IL-2, IL-4 and IL-5, as well as proliferation and CD25 expression.
Cyclic AMP-mediated suppression of cytokine synthesis, proliferation and CD25 expression in human T cells were attenuated by ionomycin.
[cAMP]i was increased by PGE2 and forskolin. PGE2 suppressed the TCR-induced biphasic increase in [Ca2+]i. EMSA revealed that four specific protein-DNA binding complexes related to NF-AT were detected at the IL-5 promoter sequence located from −119 to −90 relative to the transcription initiation site. The slowest migrating complex induced by TCR stimulation was enhanced by PGE2 and further upregulated by ionomycin. Another binding which did not compete with cold AP-1 oligonucleotides, was constitutively present and was unaffected by PGE2 but enhanced by ionomycin.
The suppressive effect of cyclic AMP on human IL-5 synthesis is mediated by interference with intracellular Ca2+ mobilization but distinct from the NF-AT-related pathway.
Keywords: Ca2+, cyclic AMP, helper T cells, interleukin-5, NF-AT
Introduction
Cyclic AMP (cAMP) has been recognized as an important second messenger regulating immune and inflammatory responses. Agents that elevate intracellular cyclic AMP levels possess immunosuppressive and anti-inflammatory properties (Goodwin & Ceuppens, 1983; Moore & Willoughby, 1995). It has been demonstrated that these effects are in part caused by the inhibition of various T cell functions including cytokine production (Bastin et al., 1990; Chouaib et al., 1985; Mary et al., 1987), proliferation (Lingk et al., 1990; Minakuchi et al., 1990) and expression of activation markers on the cell surface (Anastassiou et al., 1992; Krause & Deutsch, 1991; Rincon et al., 1988).
Among T cell responses, there have been conflicting reports of regulation of human interleukin (IL)-5 synthesis by cyclic AMP. We have demonstrated that prostaglandin (PG) E2, forskolin and dibutyryl (db)-cAMP suppressed concanavalin A-induced IL-5 production by human peripheral blood mononuclear cells (PBMC) (Kaminuma et al., 1996). Inhibitors of phosphodiesterase, a cyclic nucleotide-decomposing enzyme, suppressed IL-5 messenger RNA (mRNA) expression and protein production by human PBMC stimulated by specific antigens (Essayan et al., 1995; Kaminuma et al., 1996). In contrast, Snijdewint et al. (1993) reported that PGE2 enhanced IL-5 production by human T cells stimulated with anti-CD2 and anti-CD28 antibodies plus phorbol ester. Watanabe et al. (1994) also reported that PGE2 enhanced production of IL-5 by human T cell clones stimulated with phorbol ester plus Ca2+ ionophore. Therefore, it seems that the effect of cyclic AMP on IL-5 production by human helper T cells differ depending on the nature of the activation signals.
Accumulating evidence has indicated that IL-5 is the key cytokine involved in allergic diseases associated with eosinophilic inflammation such as asthma and atopic dermatitis. IL-5 is produced primarily by activated T cells and enhances the proliferation, differentiation and survival of eosinophils (Sanderson, 1992; Sanderson et al., 1988). Activated T cells expressing IL-5 mRNA were found in increased numbers in the bronchial mucosa of asthmatic patients (Hamid et al., 1991) and further increased upon antigen challenge (Robinson et al., 1993). Administration of anti-IL-5 neutralizing antibody abrogated eosinophilic inflammation in experimental asthma models (Chand et al., 1992; Kaminuma et al., 1997a; Van Oosterhout et al., 1993). Therefore, as a step towards the development of a novel therapeutic intervention for allergic disorders, it seems very important to define precisely the action of cyclic AMP on human IL-5 synthesis.
Several cellular targets for cyclic AMP action on T cell functions have been reported (Bismuth et al., 1988; Hagiwara et al., 1993; Hsueh & Lai, 1995). c-Jun N-terminal kinase (JNK) but not mitogen-activated protein kinase was inhibited by cyclic AMP in parallel with the inhibition of IL-2 production (Hsueh & Lai, 1995). Proliferation of T cells was inhibited by cyclic AMP which effect was accompanied by the suppression of phosphatidylinositol turnover (Bismuth et al., 1988), tyrosine phosphorylation of phospholipase C (PLC)γl and its enzymatic activity (Granja et al., 1991), and phosphorylation of the γ and ε TCR polypeptides (Patel et al., 1987). In addition, cyclic AMP stimulates transcription of a number of genes that contain cyclic AMP-responsive element (CRE) in their promoter/enhancer region. Cyclic AMP binds to the regulatory subunits of protein kinase A (PKA) and thereby induces the dissociation of two catalytic subunits which are translocated to the nucleus (Hagiwara et al., 1993; Nigg et al., 1985). The catalytic subunit then phosphorylates the CRE-binding transcription factor CREB, leading to transcriptional induction (Meyer & Habener, 1993). These findings indicate that cyclic AMP may enhance several T cell responses at the level of gene transcription.
We have previously reported that the Ca2+ ionophore, ionomycin, in cooperation with phorbol ester, induces IL-5 synthesis by human T cells (Mori et al., 1995a). In addition, suppression of Ca2+ signaling by cyclic AMP-elevating agents has been reported (Choudhry & Sayeed, 1996; Lerner et al., 1988), suggesting that Ca2+ mobilization is the target of the action of cyclic AMP on IL-5 synthesis. In the present study, we explored the role of the Ca2+-dependent signaling pathway in the regulation of human IL-5 synthesis by cyclic AMP.
Methods
Human T cell line
A Dermatophagoides farinae mite extract (mite)-reactive T cell line was established from PBMC of allergic individuals as described previously (Mori et al., 1995b). Briefly, PBMC (2×106 ml−1) were cultured with mite antigen (10 μg ml−1) in AIM-V medium for 10 days, and non-adherent cells were recovered. Then 2×105 live cells were cultured in 24 well culture plates with antigen (10 μg ml−1) and 2500 rad-irradiated autologous PBMC (2×106 ml−1). Fresh medium containing 10 u ml−1 recombinant human (rh)IL-2 was added once a week. The antigenic stimulation was repeated every 2–3 weeks. Cells to be used for the experiments were passaged at least five times and harvested at least 10 days after the last antigenic stimulatioan. These cells were layered onto Ficoll-Paque and centrifuged. The interface was recovered, washed twice, and resuspended in fresh medium. The resulting preparation usually consisted of more than 98% CD3+CD4+ cells, as determined by flow cytometry.
Stimulation of T cell line
Cells (105 ml−1) were stimulated via the T cell receptor (TCR). For stimulation, wells of culture plates were preincubated with 10 μg ml−1 monoclonal anti-human CD3 antibody (OKT3) in 0.05 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight and washed with fresh medium three times before use. After the designated culture periods, the supernatants were collected and kept frozen at −70°C until assay. IL-5 was measured by EIA using purified rat anti-mouse/human IL-5 monoclonal antibody as the capture antibody and biotinylated rat anti-human IL-5 monoclonal antibody as the detecting antibody as described previously (Kaminuma et al., 1996). The range of detection of the assay system was 0.02–10 ng ml−1. IL-2 and IL-4 were measured by specific ELISA kits (Duo Set®, Genzyme, Cambridge, MA, U.S.A.), according to the manufacturer's instruction. The minimum detection concentration of these ELISA systems were 10 pg ml−1.
Measurement of intracellular cyclic AMP concentration ([cAMP]i)
Cells (107 ml−1) were incubated with each test compound at 37°C for 2 h, at which time, [cAMP]i had stabilized following transient fluctuation due to the addition of compounds (data not shown). The reaction was stopped by adding a 2 fold volume of ice-cold 100% ethanol and the fluid was transferred to a centrifuge tube. The remaining precipitate in culture wells was further washed with ice-cold 65% v v−1 ethanol and also included in the corresponding tube. After drying these extracts in a vacuum oven, cyclic AMP was measured by enzyme immunoassay with the Biotrak cAMP EIA system (Amersham) according to the manufacturer's protocol. The range of detection of the assay system was 0.125–32 pmol ml−1.
Cytokine mRNA expression
Gene expression of IL-2, IL-4 and IL-5 were analysed by the reverse transcription-polymerase chain reaction (RT–PCR) method, as reported previously (Mori et al., 1995a). Briefly, RNA was extracted from the pelleted cells essentially following the one-step acid guanidinium isothiocyanate/phenol chloroform extraction method (Chomczynski & Sacchi, 1987) using ISOGEN. cDNA was synthesized from 1 μg cytoplasmic RNA using oligo dT primers and murine Moloney leukaemia virus reverse transcriptase. PCR was performed using the following RT–PCR amplimer sets (Clontech, Palo Alto, CA, U.S.A.). IL-2: 5′-CATGCACTAAGTCTTGCACTTGTCA-3′; 5′-CGTTGATATTGCTGATTAAGTCCCTG-3′; IL-4: 5′-ATGGGTCTCACCTCCCAACTGCT-3′; 5′-CGAACACTTTGAATATTTCTCTCTCAT-3′; IL-5: 5′-GCTTCTGCATTTGAGTTTGCTAGCT-3′; 5′-TGGCCGTCAATGTATTTCTTTATTAAG-3′; β-actin: 5′-ATGGATGATGATATCGCCGCG-3′; 5′-CTAGAAGCATTTGCGGTGGACGATGGGGGCC-3′.
To 50 μl (final volume) amplification solution (mM: KCl 50, Tris-HCl (pH 8.3) 10, MgCl2 2, 0.01% w v−1 gelatin, 0.2 mM each deoxynucleotide triphosphate), 2 μl cDNA (corresponding to about 250 ng starting RNA material), 0.4 μM each primer, and 2 U GeneAmp® DNA polymerase were added. The mixture was heated at 95°C for 2 min, followed by 20 cycles, each consisting of incubation for 30 s at 95°C, 30 s at 60°C and 90 s at 73°C. The PCR products were analysed by 2% w v−1 agarose gel electrophoresis in the presence of ethidium bromide. Expected sizes of PCR amplification products were 305, 456, 294, and 838 bp for IL-2, IL-4, IL-5 and β-actin, respectively.
Cell proliferation assay
Cell proliferation was assessed by the bioreduction of tetrazolium salt into formazan as described by Roehm et al. (1991) using a Cell Titer 96™ Aqueous Nonradioactive Cell Proliferation Assay kit (Promega, Madison, WI, U.S.A.) according to the manufacturer's protocol. Briefly, 20 μl tetrazolium assay solution was added to 100 μl each well culture. After incubation for 4 h at 37°C, the absorbance of each well at 515 nm was measured. Stimulation Index was calculated as the ratio of values in the stimulated cultures to those in the control cultures.
Flow cytometric analysis of CD25 expression of human T cell line
Cells (2×106) were washed and resuspended in staining buffer (PBS supplemented with 0.25% w/v−1 BSA and 0.1% w v−1 NaN3). After blocking with murine IgG (100 μg ml−1) for 30 min at 4°C, these cells were incubated with phycoerythrin (PE)-labelled anti-CD25 antibody (5 μg ml−1) or its isotype-matched control antibody (5 μg ml−1) for 30 min at 4°C. Thereafter, cells were washed twice and analysed using a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA, U.S.A.). Dead cells were gated out by their forward and angle light scatter profile. Data were analysed using the CellQuest® program.
Measurement of intracellular Ca2+ concentration ([Ca2+]i)
[Ca2+]i in the human T cell line was measured by the method described by Grynkievicz et al. (1985) using the Ca2+ chelator, Fura-2. In brief, cells were stained by incubation with 2 μM acetoxymethyl-Fura-2 for 30 min at room temperature in RPMI1640 medium containing 10% w v−1 foetal bovine serum without phenol red. After incubation, the cells were washed twice and resuspended in Hank's balanced salt solution (HBSS) at a concentration of 5×106 ml−1. All measurements of [Ca2+]i were carried out at 37°C in fluorometer cuvettes in a Calcium-Ion Analyzer FS-100 (Kowa, Tokyo, Japan) with stirring, at excitation wavelengths of 340 and 380 nm and emission at 540 nm. Graphic representation of [Ca2+]i was made according to the equation (Grnykiewicz et al., 1985):
where KD=224 nM, R=ratio of fluorescence (F), i.e., F340/F380, Rmax=F340/F380 ratio after the addition of Triton X-100, Rmin=F340/F380 ratio after addition of EGTA.
Preparation of nuclear extracts
Crude nuclear and cytoplasmic extracts were prepared from unstimulated and stimulated cells as described by Schreiber et al. (1989) with modifications. Cells were washed in ice-cold PBS, suspended at 5×107 cells ml−1 in ice-cold buffer A (in mM: HEPES-KOH (pH 7.9) 10, KCl 10, EDTA 0.1, EGTA 0.1, dithiothreitol (DTT) 1, phenylmethylsulphonyl fluoride (PMSF) 0.5) and kept on ice for 15 min. Then, 1/16 volume of 10% Nonidet P-40 was added and the mixture was vigorously vortexed. After centrifugation at 12,000 r.p.m. for 30 s at 4°C, the cytoplasmic supernatant was retained on ice and the nuclear pellet was washed with the same buffer (buffer A containing 0.6% v v−1 Nonidet P-40). The pellet was then incubated with three volumes of ice-cold buffer C (in mM: HEPES-KOH (pH 7.9) 10, NaCl 400, EDTA 10, EGTA 1, DTT 1, PMSF 1) at 5×108 nuclei ml−1 for 15 min and thereafter centrifuged at 15,000 r.p.m. for 15 min at 4°C. The nuclear and cytoplasmic supernatants were kept frozen in aliquots at −70°C. Protein concentrations in nuclear extracts were determined using BCA protein assay reagent® (Pierce, Rockford, IL, U.S.A.) according to the manufacturer's directions. For some experiments, the nuclear extracts were dialyzed against 500 volumes of dialysis buffer (in mM: HEPES-KOH (pH 7.9) 10, NaCl 50, 50% glycerol (v v−1), DTT 1, MgCl2 1) for 12 h at 4°C.
Electrophoretic mobility shift assay (EMSA)
The oligonucleotides corresponding to the human IL-5 promoter sequence from −119 to −90 (−119/−90) and the distal NF-AT site of human IL-2 gene (NF-AT) were purchased from Sawady Technology (Tokyo, Japan): −119/−90 (5′-GCATTGGAAACATTTAGTTTCACGATATGC-3′), NF-AT (5′ - GGAGGAAA AAC TGTTTCATAC AGAAGGCGT - 3′. AP-1 and AP-2 oligonucleotides used for competition assays were purchased from Promega: AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′), AP-2 (5′-GATCGAACTGACCGCCCGCGGCCCGT-3′). Pairs of synthetic high-performance liquid chromatography-purified oligonucleotides containing complementary sequences were annealed by boiling equimolar concentrations of each strand for 10 min and allowing the mixture to slowly cool in a water bath to room temperature. Then, 3.5 pmol annealed oligonucleotide was incubated in a 10 μl reaction mixture containing Tris-HCl (pH 7.6) 70 mM, MgCl2 10 mM, DTT 5 mM, and 10 μCi [γ-32P]-ATP (3000 Ci mmol−1) with 10 U T4 polynucleotide kinase for 30 min at 37°C. The reaction was stopped by adding 1 μl 0.5 mM EDTA and 89 μl Tris-EDTA (TE) buffer (Tris-HCl (pH 8.0) 10 mM, EDTA 1 mM). Gel shift analysis was performed using the Gel Shift Assay Systems (Promega) according to the manufacturer's protocol with slight modifications. 32P end-labelled oligonucleotides (35 fmol) were incubated in a 10 μl reaction mixture containing (in mM) Tris-HCl (pH 7.5) 10 EDTA 0.5, DTT 0.5, NaCl 50, MgCl2 1, 4% glycerol, 0.5 μg, poly(dI-dC)·poly(dI-dC) with 4 μg nuclear extract for 30 min at room temperature. After incubation, bromophenol blue and xylene cyanol were added to 0.02% and the resulting complexes were resolved on 8% polyacrylamide gel (acrylamide/bisacrylamide, 30 : 1 w w−1) by electrophoresis at 100 V in 0.5×TBE buffer (1×TBE: Tris-HCl (pH 8.0) 89 mM, boric acid 89 mM, EDTA 2 mM) at room temperature. The gel was subsequently dried and exposed to RX film at −70°C.
Statistics
Data are presented as mean±standard error of mean (s.e.mean). The number of samples in each experiment is shown by n. Statistical analysis was performed by Student's t-test for comparison between two groups and one-way ANOVA with Bonferroni's method for three groups or more. Values of P<0.05 were considered to be statistically significant.
Drugs
Mite was purchased from Torii Pharmaceutical Co. (Tokyo, Japan). PGE2, forskolin and dibutyryl (db)-cAMP were from Sigma (St. Louis, MO, U.S.A.), rhIL-2 was from Peprotech (London, U.K.), OKT3 was from Ortho (Raritan, NJ, U.S.A.), purified rat anti-mouse/human IL-5 monoclonal antibody, biotinylated rat anti-human IL-5 monoclonal antibody, PE-labelled anti-CD25 antibody, and its isotype-matched control antibody were from Pharmingen (San Diego, CA, U.S.A.), anti-mouse IgG antibody was from Organon Technica (Durham, NC, U.S.A.), [γ-32P]-ATP was from Amersham (Buckinghamshire, U.K.), acetoxymethyl-Fura-2 was from Dojindo (Kumamoto, Japan), ISOGEN was from Nippongene (Tokyo, Japan), murine Moloney leukaemia virus reverse transcriptase and GeneAmp® DNA polymerase were from Perkin-Elmer Cetus (Norwalk, CT, U.S.A.), T4 polynucleotide kinase was from Takara (Otsu, Japan), RX film was from Fuji Photo Film (Tokyo, Japan), Ficoll-Paque was from Pharmacia (Uppsala, Sweden), and AIM-V and RPMI1640 medium were from Gibco BRL (Gaithersburg, MD, U.S.A.).
Results
Ionomycin prevented cyclic AMP-mediated suppression of IL-5 production by human T cell line
The first experiment was carried out to examine the mutual effects of intracellular cyclic AMP and Ca2+ on cytokine production by human T cells. The mite-reactive human T cell line was stimulated via TCR by incubation in OKT3-precoated culture plates, and the resulting supernatants were assayed for IL-5. No detectable IL-5 was produced without stimulation (<20 pg ml−1). A significant increase in IL-5 production was detected upon TCR stimulation for 24 h (4.47±0.61 ng ml−1, n=3). Neither IL-2 nor IL-4 were detectable in the culture supernatants of T cells with or without TCR stimulation (<10 pg ml−1). As shown in Figure 1, PGE2, forskolin and db-cAMP concentration-dependently suppressed TCR-stimulated IL-5 production. In parallel with the inhibition of IL-5 production, PGE2 and forskolin increased [cAMP]i of T cells (Figure 2), indicating that the effects of PGE2 and forskolin were mediated through increase of intracellular cyclic AMP. The Ca2+ ionophore, ionomycin concentration-dependently attenuated the suppression of IL-5 production mediated by PGE2 (Figure 3). Forskolin (10 μM)- and db-cAMP (100 μM)-mediated inhibition of IL-5 production were also competed by ionomycin (0.1 μM). Ionomycin alone did not significantly affect IL-5 production by T cells stimulated through TCR (4.30±0.59 ng ml−1, n=3). There was no difference in the cell concentration between before (1.0×105 ml−1) and after (0.96–1.05×105 ml−1) incubation in all culture groups, excluding the possibility that the effects of cyclic AMP-modulating agents and ionomycin on cytokine production were mediated through effects on cell proliferation.
Figure 1.

TCR-induced IL-5 production by human T cells was suppressed by PGE2, forskolin and db-cAMP. Cells (105 ml−1) were incubated in culture plates pretreated with OKT3 (10 μg ml−1). PGE2, forskolin or db-cAMP at designated concentrations was included from the start of some cultures. Culture supernatants were harvested after 24 h and assayed for Il-5 by EIA. The data are the mean±s.e.mean of the per cent inhibition of TCR-induced IL-5 production (4.47±0.61 ng ml−1) form three separate experiments.
Figure 2.
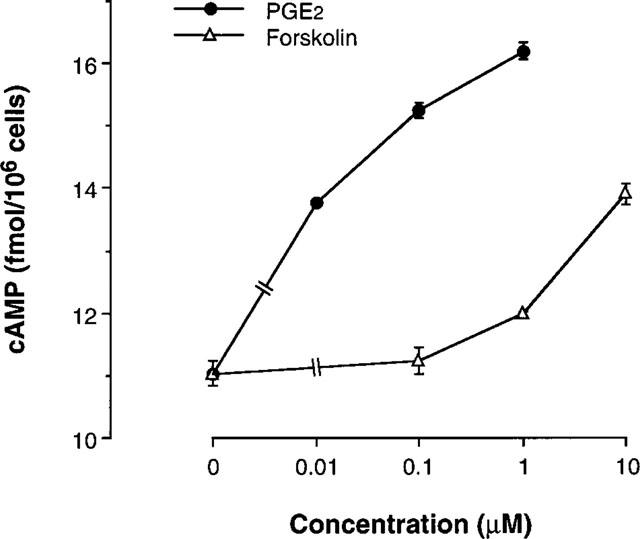
PGE2 and forskolin increased intracellular cyclic AMP level of human T cells. Cells (107 ml−1) were incubated with various concentrations of PGE2 forskolin. Two hours later, intracellular cyclic AMP concentration was measured with the Biotrak cAMP EIA system. The data are the mean±s.e.mean from three separate experiments.
Figure 3.
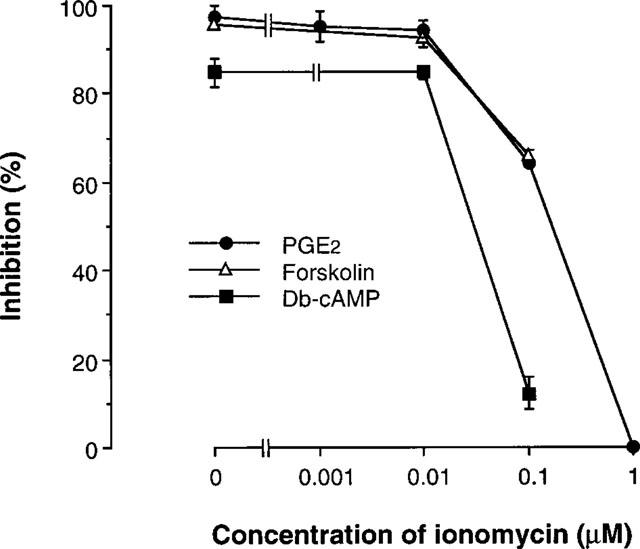
Ionomycin reduced cyclic AMP-mediate inhibition of IL-5 production. Cells (105 ml−1) were stimulated with immobilized OKT3 (10 μg ml−1) in the presence of 1 μM PGE2, 10 μM forskolin or 100 μM db-cAMP. Ionomycin at designated concentrations was included from the start of some cultures. Culture supernatants were harvested after 24 h and assayed for IL-5 by EIA. The data are the mean±s.e.mean of the per cent inhibition of TCR-induced IL-5 production from four separate experiments.
PGE2-mediated suppression of cytokine mRNA expression was attenuated by ionomycin
To confirm the effects of intracellular cyclic AMP and Ca2+ concentration on cytokine production at the level of gene expression, we examined the effects of PGE2 and ionomycin on IL-2, IL-4 and IL-5 mRNA expression in a human T cell line. Representative results are shown in Figure 4. As we showed in previous reports (Mori et al., 1996), significant increases in IL-2, IL-4 and IL-5 mRNA expression were detected 3–24 h after TCR stimulation, and expression reached maximum levels at 6 h (Figure 4). mRNA expression of IL-2, IL-4 and IL-5 was significantly suppressed by PGE2 (lane 3). PGE2-mediated suppression of cytokine mRNA expression was clearly abrogated by the addition of ionomycin (lane 4).
Figure 4.
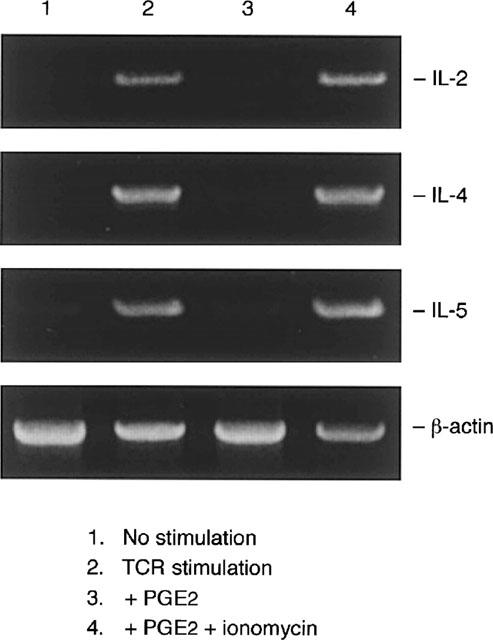
Ionomycin reduced PGE2-mediated inhibition of cytokine gene expression. Cells (106 ml−1) were stimulated with immobilized OKT3 (10 μg ml−1) in the presence or absence of PGE2 (1 μM) and ionomycin (1 μM). Six hours later cells were harvested. Total RNA was extracted, reverse transcribed, and amplified by PCR. The 305, 456, 294, and 838 bp products correspond to the expected size of IL-2, IL-4, IL-5 and β-actin amplification products, respectively. Shown is a representative result out of three experiments with similar results.
Ionomycin prevented cyclic AMP-mediated suppression of proliferation and CD25 expression of human T cell line
We also examined another T cell response i.e. proliferation. Because the proliferative response of the human T cell line following TCR stimulation was detectable on day 2 (2 days after stimulation) and reached the maximum on day 4 (Stimulation Index=8.9±0.3, n=4), the effect of PGE2 and ionomycin on proliferation was asssessed on day 4. As shown in Figure 5, proliferation of TCR-stimulated T cells was clearly suppressed by PGE2. Addition of ionomycin abrogated the suppressive effect of PGE2 on T cell proliferation (Figure 5). Essentially the same results were obtained using three other T cell lines (data not shown). Ionomycin (1 μM) also attenuated the forskolin (10 μM)- and db-cAMP (100 μM)-mediated inhibition of T cell proliferation.
Figure 5.
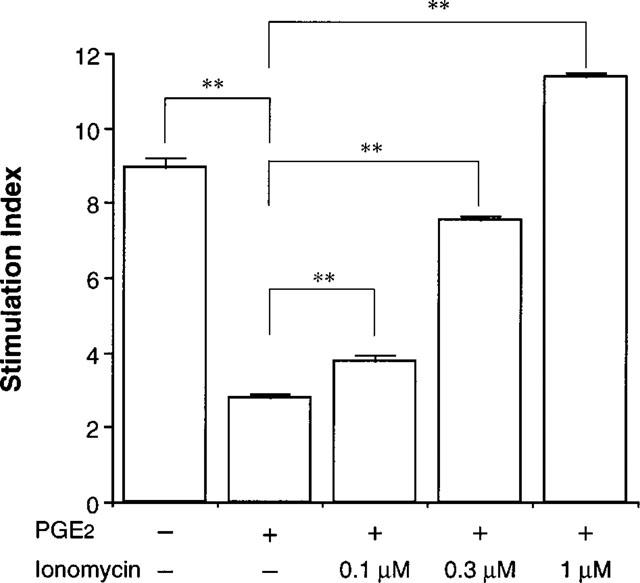
Ionomycin reduced PGE2-mediated inhibition of human T cell proliferation. Cells (106 ml−1) were stimulated with immobilized OKT3 (10 μg ml−1) in the presence or absence of PGE2 (1 μM) and ionomycin. After 4 days, the proliferation of T cells was measured by non-radioactive cell proliferation assay system. The data are the mean±s.e.mean of the Stimulation Index from four separate experiments. *P<0.05, **P<0.01; compared with PGE2+/Ionomycin–(Bonferroni's test).
T cell proliferation is regulated by the production of T cell growth factors, such as IL-2 and IL-4, and by the expression of receptors for these cytokines. We therefore, examined the effects of PGE2 and ionomycin on the expression of CD25, IL-2 receptor α-chain, present on the T cell surface membrane. Upon TCR stimulation, an increase in CD25 expression was detectable on day 1 (data not shown), and the expression reached the maximum on day 3 (Figure 6). The TCR-stimulated CD25 expression was clearly suppressed by PGE2. Again, the PGE2-mediated inhibition of CD25 expression was clearly attenuated by ionomycin (Figure 6). Inhibition of CD25 expression mediated by forskolin (10 μM) or db-cAMP (100 μM) was also prevented by ionomycin (1 μM). Ionomycin did not significantly affect the proliferation and CD25 expression of TCR-stimulated T cells (data not shown).
Figure 6.
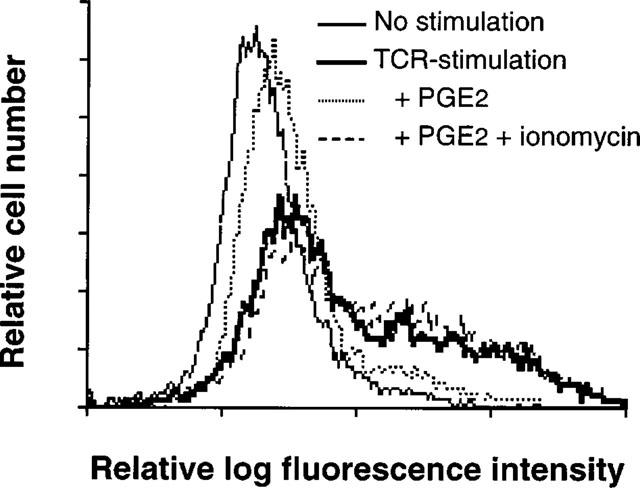
Ionomycin reduced PGE2-mediated inhibition of CD25 expression of human T cells. Cells (106 ml−1) were incubated with immobilized OKT3 in the presence or absence of PGE2 (1 μM) and ionomycin (1 μM). After 3 days, cells were prepared for cytofluorometric analysis using PE-labelled anti-CD25 monoclonal antibody. A representative result of more than three experiments is shown.
Effect of PGE2 on Ca2+ mobilization in human T cell line
The finding that cyclic AMP-mediated suppression of IL-5 synthesis as well as other cytokine mRNA expression, proliferation, and CD25 expression of the human T cell line was attenuated by ionomycin strongly suggests the possibility that the elevation in [cAMP]i affects the Ca2+ mobilization pathway. Suppression of Ca2+ mobilization by cyclic AMP-elevating agents has been reported using murine and rat T cells (Choudhry & Sayeed, 1996; Lerner et al., 1988). Thus, we next examined the effect of PGE2 on intracellular Ca2+ level in TCR-stimulated human helper T cells.
The time course of the change in [Ca2+]i of the human T cell line in response to TCR stimulation was investigated. After preincubation with OKT3, cells were stimulated through TCR, by crosslinking of pretreated OKT3 using anti-mouse IgG antibody (Figure 7). Addition of OKT3 elicited a slight increase in [Ca2+]i in T cells. [Ca2+]i was dramatically elevated by the addition of cross-linking secondary antibody, peaked 1 min after the addition of secondary antibody, and then declined. Thereafter, a sustained increase of [Ca2+]i was observed, which remained for greater than 9 min after stimulation. As shown in Figure 8, PGE2 suppressed both the peak and sustained increase in [Ca2+]i measured at 1 and 5 min after the addition of secondary antibody, respectively. The effect of PGE2 on the sustained increase in [Ca2+]i was significantly greater than that on the peak [Ca2+]i (Figure 8).
Figure 7.

TCR-stimulated mobilization of [Ca2+]i in human T cells. Fura-2-loaded cells (5×106 ml−1) were stimulated by crosslinking pretreated OKT3 using anti-mouse IgG antibody. OKT3 (2 μg ml−1) and crosslinking secondary antibody (20 μg ml−1) were added at −2 and 0 min, respectively. The mean±s.e.mean of [Ca2+]i during the time course are shown (n=4 separate experiments). Some s.e.mean bars are within the symbol. The peak and sustained phase increase in [Ca2+]i were assessed at 1 and 5 min after the addition of secondary antibody respectively in the following experiments.
Figure 8.
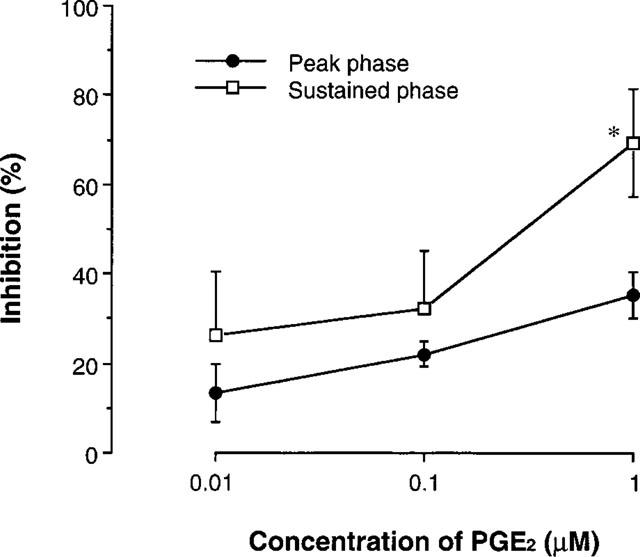
PGE2 suppressed TCR-stimulated [Ca2+]i mobilization. PGE2 at various concentrations was added in the cell suspension 5 min before the addition of crosslinking secondary antibody. The effects on the peak and sustained phase increase in [Ca2+]i were assessed at 1 and 5 min after the addition of secondary antibody, respectively (n=4 separate experiments). *P<0.05, compared with the inhibition of peak [Ca2+]i (Student's t-test).
EMSA analysis of PGE2 and ionomycin-treated human T cell line
The above findings clearly indicate that [Ca2+]i is involved in the cyclic AMP-mediated modulation of T cell responses and prompted us to examine whether NF-AT, a Ca2+-dependent transcription factor, was affected by PGE2 and ionomycin. Synthetic oligonucleotides corresponding to the human IL-5 promoter sequence from −119 to −90 (−119/−90), which was previously reported as a functional NF-AT binding site (Lu-Hesselmann et al., 1997; Prieschl et al., 1995; Stranick et al., 1997), and the distal NF-AT site of human IL-2 gene (NF-AT) were used for the gel shift analysis. As shown in Figure 9, at least four binding activities to the labelled −119/−90 oligonucleotides were detected using nuclear extracts of the stimulated human T cell line. Binding was reduced by excess amounts of the corresponding unlabelled oligonucleotides as well as the NF-AT oligonucleotides, suggesting that the binding complex consisted of NF-AT family proteins. In addition, three of these binding complexes were reduced by excess amounts of unlabelled AP-1 oligonucleotides, though one complex was not affected (Figure 9a, II), suggesting that NF-AT could bind to this sequence either with or without AP-1. A single specific binding complex was detected using the NF-AT probe, which binding was reduced with both cold −119/−90 and AP-1 oligonucleotides (Figure 9a, I). Cold AP-2 oligonucleotides did not retard any of these binding activities to −119/−90 or NF-AT oligonucleotides, clearly indicating the specific binding of the complexes.
Figure 9.
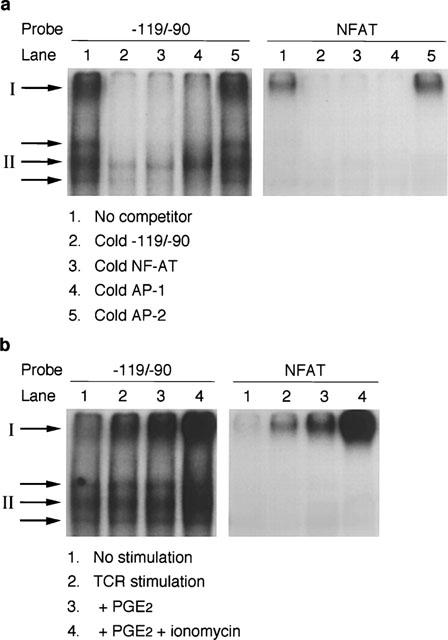
Electrophoretic mobility shift assay using human T cell extracts. Each binding reaction contained 4 μg nuclear protein and −119/−90 or NF-AT probe. The protein-DNA complexes were resolved on 8% polyacrylamide gel. (a) Nuclear protein extracts were prepared from TCR-stimulated human T cell line. A 50 fold excess of unlabelled oligonucleotides was included in some binding reactions as competitors. (b) Effects of PGE2 and ionomycin on −119/−90 and NF-AT binding activity. Cells were stimulated with immobilized OKT3 for 4 h. PGE2 (1 μM) and/or ionomycin (1 μM) were included throughout the culture period. The slowest migrating binding complex and the complex that did not compete with cold AP-1 are indicated by I and II, respectively. A representative result of three similar experiments is shown.
The effects of PGE2 and ionomycin on these binding activities were next examined. As shown in Figure 9b, the binding activity to NF-AT probe was induced upon TCR stimulation, enhanced by the addition of PGE2 and further upregulated by ionomycin. The same response was observed using the −119/−90 probe (I). Another binding activity to −119/−90, which did not compete with cold AP-1, was constitutively present and was not affected by PGE2 but was enhanced by ionomycin (II). The other two binding complexes were constitutive and were not affected by either PGE2 or ionomycin.
Discussion
Our present findings clearly demonstrate that protein production of IL-5, gene expression of IL-2, IL-4 and IL-5, proliferative response and CD25 expression of TCR-stimulated human T cells were suppressed by PGE2, and that all of these effects were attenuated by the addition of ionomycin. PGE2 elevated intracellular cyclic AMP level, and suppressed the TCR-induced increase in [Ca2+]i, suggesting that the Ca2+ mobilization pathway is a critical target of the action of cyclic AMP to suppress T cell responses.
We have previously reported that cyclic AMP-affecting agents such as PGE2, forskolin, db-cAMP and a type 4 phosphodiesterase inhibitor (T-440) suppressed IL-5 production by TCR-stimulated human PBMC (Kaminuma et al., 1996). PBMC comprise several cell types such as T cells, B cells, macrophages and monocytes. Our present findings support the previous results and further show that the target of the ability of cyclic AMP to suppress IL-5 synthesis was actually helper T cells. Several investigators have obtained conflicting results concerning the effect of cyclic AMP on IL-5 production by human T cells. IL-5 production stimulated by anti-CD2 and anti-CD28 antibodies plus phorbol ester, or Ca2+ ionophore plus phorbol ester was enhanced by cyclic AMP (Snijdewint et al., 1993; Watanabe et al., 1994). IL-5 production stimulated by IL-2 was also enhanced by cAMP (Kaminuma et al., 1997b). These findings suggest that the effect of cyclic AMP on IL-5 production may differ depending on the nature of the activation signals and that the target of cyclic AMP to cause suppression of IL-5 production is located downstream of the signal cascade initiated by the TCR-CD3 complex.
Stimulation of T cells through TCR results in the activation of PLCγl which hydrolyzes the membrane phospholipid, phosphatidylinositol 4, 5-biphosphate (PIP2), into inositol 1, 4, 5-trisphosphate (IP3) and 1, 2-diacylglycerol (Isakov et al., 1987; Kikkawa & Nishizuka, 1986). IP3 induced the release of Ca2+ from intracellular stores after binding to its receptor. It has been reported that the IP3 receptor was the substrate of the cyclic AMP-activated protein kinase, PKA (Ferris et al., 1991; Quinton & Dean, 1992). PKA-mediated phosphorylation of the IP3 receptor significantly reduces the ability of IP3 to release Ca2+ from membrane vesicles (Quinton & Dean, 1992; Supattapone et al., 1988). In addition, cyclic AMP abrogated phosphatidylinositol turnover as well as PLCγl tyrosine phosphorylation (Bismuth et al., 1988; Granja et al., 1991). Our present experiment suggested that cyclic AMP downregulated both peak and sustained [Ca2+]i mobilization in activated human helper T cells (Figure 8). The findings are consistent with those of previous reports and directly demonstrate that interference with [Ca2+]i mobilization is most likely the crucial mechanism involved in the modulation of T cell responses by cyclic AMP.
Activation of cell surface receptors that are coupled to phosphatidylinositol metabolism evokes a biphasic rise in [Ca2+]i, due to Ca2+ release from intracellular stores followed by Ca2+ influx across the plasma membrane (Berridge & Irvine, 1989 ). The peak and sustained increase in [Ca2+]i after TCR stimulation seem to be comprised mainly of Ca2+ release from intracellular stores and Ca2+ influx from outside of the cells, respectively. Depletion of intracellular Ca2+ stores can elicit Ca2+ influx in human T cells without the action of IP3 on the Ca2+ channels present in the plasma membrane (Zweifach & Lewis, 1993), via the release of an unknown messenger that stimulates Ca2+ influx in human T cells (Randriamampita & Tsien, 1993). Our present findings that the inhibitory effect of PGE2 on the sustained increase in [Ca2+]i was more potent than that on the peak [Ca2+]i is consistent with the view that the target of cyclic AMP in its inhibition of Ca2+ mobilization also exists independent of IP3 receptor phosphorylation.
mRNA for IL-2 and IL-4 in human T cells was clearly detected upon TCR stimulation (Figure 4), although neither IL-2 nor IL-4 was detectable in the culture supernatants (<10 pg ml−1). The apparent discrepancy may be explained by the possibility that T cell-derived IL-2 and IL-4 proteins were captured by their respective receptors present on the T cell surface, and accordingly, no detectable amonts of IL-2 and IL-4 were present in the culture supernatants. We have previously shown that IL-5 synthesis by human T cells was totally dependent on the autocrine production of IL-2 (Kaminuma et al., 1997b; Mori et al., 1996). TCR-stimulated IL-2 mRNA expression was significantly suppressed by PGE2, suggesting that inhibition of IL-2 production was involved in the effect of cyclic AMP on IL-5 production. However even 6 h after stimulation, when secretion of IL-2 protein was minimal, the suppression of IL-5 mRNA expression by PGE2 was observed, suggesting that cyclic AMP directly downregulates protein production of IL-5 via the suppression of its gene expression.
The finding obtained by EMSA analysis that NF-AT-related proteins specifically bound to the human IL-5 promoter region between −119 and −90 is consistent with several previous reports (Lu-Hesselmann et al., 1997; Prieschl et al., 1995; Stranick et al., 1997). Stranick et al. (1997) reported that the transcription factors reactive with anti-NF-ATc and anti-c-Jun antibodies bound to this element which was critical for the induction of IL-5 promoter activity in the murine T cell clone, D10.G4.1. Essentially the same results were obtained using a murine mast cell line (Prieschl et al., 1995), and Jurkat cells, a human T cell leukaemia cell line (Lu-Hesselmann et al., 1997). In this study, we further demonstrate that multiple NF-AT-related binding complexes are formed on −119/−90 element with or without AP-1.
PGE2 did not inhibit any binding activity at −119/−90, although the slowest migrating binding complex and the complex that did not compete with cold AP-1 were upregulated by ionomycin (Figure 9, I; II). It seems that the binding of NF-AT-related proteins at −119/−90 was not involved in the mechanism by which cyclic AMP suppressed IL-5 synthesis via interference with Ca2+ mobilization. Our present findings that cyclic AMP did not suppress the binding activity of NF-AT-related factors to the human IL-5 promoter/enhancer region seems to conflict with previous reports that cyclic AMP inhibited NF-AT activation (Li & Handschumacher, 1996; Paliogianni et al., 1993). Li & Handschumacher (1996) reported that the activation of NF-AT in human T cells was inhibited by cyclic AMP-elevating agents. A decrease in cyclic AMP-mediated suppression of human IL-2 production by overexpression of calcineurin was reported by Paliogianni et al. (1993). Nevertheless, consistent with our results, a cyclic AMP-mediated increase of the binding at the distal NF-AT site of human IL-2 gene in human T cells was reported by Watanabe et al. (1994). The discrepancy between these previous reports and the present results may relate to the difference of cell types used. The cell type that Li & Handschumacher (1996) and Paliogianni et al. (1993) examined was Jurkat, whereas we and Watanabe et al. (1994) employed untransformed human T cells. Other NF-AT-homologous elements have also been reported in the human IL-5 promoter/enhancer region (Karlen et al., 1996; Okudaira et al., 1994). As the function of transcription factors is context-dependent, it is necessary to determine whether NF-AT elements in human IL-5 gene, including −119/−90, are really functional in untransformed human T cells, and regulated by cyclic AMP via Ca2+ related mechanisms.
In conclusion, we demonstrate here for the first time, using untransformed human helper T cells, that the Ca2+ mobilization pathway is crucially involved in the mechanism of cyclic AMP modulation of TCR-stimulated IL-5 synthesis. Thorough elucidation of the mechanisms by which cyclic AMP interferes with Ca2+ influx would improve our understanding of T cell activation and might facilitate the development of a novel treatment involving immune regulation.
Acknowledgments
The authors thank Ms Rieko Kameda for technical assistance and Dr Wendy A. Gray for reviewing this manuscript.
Abbreviations
- AM
acetoxymethyl
- [Ca2+]i
intracellular Ca2+ concentration
- cAMP
cyclic AMP
- CRE
cyclic AMP responsive element
- CREB
CRE binding protein
- db-cAMP
dibutyryl-cyclic AMP
- DTT
dithiothreitol
- HBSS
Hank's balanced salt solution
- IP3
inositol 1, 4, 5-trisphosphate
- JNK
c-Jun N-terminal kinase
- NF-AT
nuclear factor of activated T cells
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A, PLC, phospholipase C
- PMSF
phenylmethylsulphonyl fluoride
- TE
Tris-EDTA
References
- ANASTASSIOU E.D., PALIOGIANNI F., BALOW J.P., YAMADA H., BOUMPAS D.T. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J. Immunol. 1992;148:2845–2852. [PubMed] [Google Scholar]
- BASTIN B., PAYET M.D., DUPUIS G. Effects of modulators of adenylyl cyclase on interleukin-2 production, cytosolic Ca2+ elevation, and K+ channel activity in Jurkat cells. Cell. Immunol. 1990;128:385–399. doi: 10.1016/0008-8749(90)90035-p. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., IRVINE R.F. Inositol phosphates and cell signaling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- BISMUTH G., THEODOROU I., GOUY H., LE GOUVELLO S., BERNARD A., DEBRE P. Cyclic AMP-mediated alteration of the CD2 activation process in human T lymphocytes. Preferential inhibition of the phosphoinositide cycle-related transduction pathway. Eur. J. Immunol. 1988;18:1351–1357. doi: 10.1002/eji.1830180908. [DOI] [PubMed] [Google Scholar]
- CHAND N., HARRISON J.E., ROONEY S., PILLAR J., JAKUBICKI R., NOLAN K., DIAMANTIS W., SOFIA R.D. Anti-IL-5 monoclonal antibody inhibits allergic late phase bronchial eosinophilia in guinea pigs: A therapeutic approach. Eur. J. Pharmacol. 1992;211:121–123. doi: 10.1016/0014-2999(92)90273-7. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol chloroform extraction. Anal. Biochem. 1987;162:156–160. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHOUAIB S., WELTE K., MERTELSMANN R., DUPONT B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: Inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J. Immunol. 1985;135:1172–1179. [PubMed] [Google Scholar]
- CHOUDHRY M.A., SAYEED M.M. Calcium signaling restitution prevents T-cell proliferative suppression by prostaglandin E2. Shock. 1996;6:101–105. doi: 10.1097/00024382-199608000-00004. [DOI] [PubMed] [Google Scholar]
- ESSAYAN D.M., HUANG S., KAGEY-SOBOTKA A., LICHTENSTEIN L.M. Effects of nonselective and isozyme selective cyclic nucleotide phosphodiesterase inhibitors on antigen-induced cytokine gene expression in peripheral blood mononuclear cells. Am. J. Respir. Cell Mol. Biol. 1995;13:692–702. doi: 10.1165/ajrcmb.13.6.7576707. [DOI] [PubMed] [Google Scholar]
- FERRIS C.D., CAMERON A.M., BREDT D.S., HUGANIR R.L., SNYDER S.H. Inositol 1,4,5-trisphosphate receptor is phosphorylated by cyclic AMP-dependent protein kinase at serines 1755 and 1589. Biochem. Biophys. Res. Commun. 1991;175:192–198. doi: 10.1016/s0006-291x(05)81219-7. [DOI] [PubMed] [Google Scholar]
- GOODWIN J.S., CEUPPENS J. Regulation of immune response by prostaglandins. J. Clin. Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- GRANJA C., LIN L.L., YUNIS E.J., RELIAS V., DASGUPTA J.D. PLC gamma 1, a possible mediator of T cell receptor function. J. Biol. Chem. 1991;266:16277–16280. [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN T.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HAGIWARA M., BRINDLE P., HAROOTUNIAN A., ARMSTRONG R., RIVIER J., VALE W., TSIEN R., MONTMINY M.R. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol. Cell. Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMID Q., AZZAWI M., YING S., MOQBEL R., WARDLAW A.J., CORRIGAN C.J., BRADLEY B., DURHAM S.R., COLLINS J.V., JEFFERY P.K., QUINT J., KAY A.B. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J. Clin. Invest. 1991;87:1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSUEH Y.P., LAI M.Z. c-Jun N-terminal kinase but not mitogen-activated protein kinase is sensitive to cAMP inhibition in T lymphocytes. J. Biol. Chem. 1995;270:18094–18098. doi: 10.1074/jbc.270.30.18094. [DOI] [PubMed] [Google Scholar]
- ISAKOV N., MALLY M.I., SCHOLZ W., ALTMAN A. T-lymphocyte activation: The role of protein kinase C and the bifurcating inositol phospholipid signal transduction pathway. Immunol. Rev. 1987;95:89–111. doi: 10.1111/j.1600-065x.1987.tb00501.x. [DOI] [PubMed] [Google Scholar]
- KAMINUMA O., MORI A., OGAWA K., NAKATA A., KIKKAWA H., NAITO K., SUKO M., OKUDAIRA H. Successful transfer of late phase eosinophil infiltration in the lung by infusion of helper T cell clones. Am. J. Respir. Cell Mol. Biol. 1997a;16:448–454. doi: 10.1165/ajrcmb.16.4.9115756. [DOI] [PubMed] [Google Scholar]
- KAMINUMA O., MORI A., OGAWA K., WADA K., KIKKAWA H., NAITO K., SUKO M., OKUDAIRA H. Two distinct effects of cyclic AMP on IL-5 production by antigen-specific human T cell line. J. Pharmacol. Exp. Ther. 1997b;283:345–349. [PubMed] [Google Scholar]
- KAMINUMA O., MORI A., SUKO M., KIKKAWA H., IKEZAWA K., OKUDAIRA H. Interleukin-5 production by peripheral blood mononuclear cells of asthmatic patients is suppressed by T-440: Relation to phosphodiesterase inhibition. J. Pharmacol. Exp. Ther. 1996;279:240–246. [PubMed] [Google Scholar]
- KARLEN S., MORDVINOV V.A., SANDERSON C.J. How is expression of the interleukin-5 gene regulated. Immunol. Cell Biol. 1996;74:218–223. doi: 10.1038/icb.1996.31. [DOI] [PubMed] [Google Scholar]
- KIKKAWA U., NISHIZUKA Y. The role of protein kinase C in transmembrane signaling. Ann. Rev. Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- KRAUSE D.S., DEUTSCH C. Cyclic AMP directly inhibits IL-2 receptor expression in human T cells: Expression of both p55 and p75 subunits is affected. J. Immunol. 1991;146:2285–2296. [PubMed] [Google Scholar]
- LERNER A., JACOBSON B., MILLER R.A. Cyclic AMP concentrations modulate both calcium influx and hydrolysis of phosphotidylinositol phosphates in mouse T lymphocytes. J. Immunol. 1988;140:936–940. [PubMed] [Google Scholar]
- LI W., HANDSCHUMACHER R.E. Regulation of the nuclear factor of activated T cells in stably transfected Jurkat cell clones. Biochem. Biophys. Res. Commun. 1996;219:96–99. doi: 10.1006/bbrc.1996.0187. [DOI] [PubMed] [Google Scholar]
- LINGK D.S., CHAN M.A., GELFAND E.W. Increased cyclic adenosine monophosphate levels blocks progression but not initiation of human T cell proliferation. J. Immunol. 1990;145:449–455. [PubMed] [Google Scholar]
- LU-HESSELMANN J., MESSER G., VAN BEUNINGEN D., KIND P., PETER R.U. Transcriptional regulation of the human IL-5 gene by ionizing radiation in Jurkat cells: Evidence for repression by an NF-AT-like element. Radiat. Res. 1997;148:531–542. [PubMed] [Google Scholar]
- MARY D., AUSSEL C., FERRUA B., FEHLMANN M. Regulation of interleukin 2 synthesis by cAMP in human T cells. J. Immunol. 1987;139:1179–1184. [PubMed] [Google Scholar]
- MEYER T.E., HABENER J.F. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid binding protein. Endocr. Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- MINAKUCHI R., WACHOLTZ M.C., DAVIS L.S., LIPSKY P.E. Delineation of the mechanism of inhibition of human T cell activation by PGE2. J. Immunol. 1990;145:2616–2625. [PubMed] [Google Scholar]
- MOORE A.R., WILLOUGHBY D.A. The role of cAMP regulation in controlling inflammation. Clin. Exp. Immunol. 1995;101:387–389. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORI A., SUKO M., KAMINUMA O., NISHIZAKI Y., MIKAMI T., OHMURA T., HOSHINO A., INOUE S., TSURUOKA N., OKUMURA Y., ITO K., OKUDAIRA H. A critical role of IL-2 for the production and gene transcription of IL-5 in allergen-specific human T cell clones. Int. Immunol. 1996;8:1889–1895. doi: 10.1093/intimm/8.12.1889. [DOI] [PubMed] [Google Scholar]
- MORI A., SUKO M., NISHIZAKI Y., KAMINUMA O., KOBAYASHI S., MATSUZAKI G., YAMAMOTO K., ITO K., TSURUOKA N., OKUDAIRA H. IL-5 production by CD4+ T cells of asthmatic patients is suppressed by glucocorticoids and the immunosuppressants FK506 and cyclosporin A. Int. Immunol. 1995a;7:449–457. doi: 10.1093/intimm/7.3.449. [DOI] [PubMed] [Google Scholar]
- MORI A., SUKO M., TSURUOKA N., KAMINUMA O., OHMURA T., NISHIZAKI Y., ITO K., OKUDAIRA H. Allergen-specific human T cell clones produce interleukin-5 upon stimulation with the Th1 cytokine interleukin-2. Int. Arch. Allergy Immunol. 1995b;107:220–222. doi: 10.1159/000236983. [DOI] [PubMed] [Google Scholar]
- NIGG E.A., HILZ H., EPPENBERGER H.M., DUTLY F. Rapid and reversible translocation of the catalytic subunit of cAMP-dependent protein kinase type II from the Golgi complex to the nucleus. EMBO J. 1985;4:2801–2806. doi: 10.1002/j.1460-2075.1985.tb04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUDAIRA H., MORI A., SUKO M., ETOH T., NAKAGAWA H., ITO K. Enhanced production and gene expression of IL-5 in bronchial asthma Management of atopic diseases with agents that downregulate IL-5 gene transcription. ACI News. 1994;6:19–25. [Google Scholar]
- PALIOGIANNI F., KINCAID R.L., BOUMPAS D.T. Prostaglandin E2 and other cyclic AMP elevating agents inhibit interleukin 2 gene transcription by counteracting calcineurin-dependent pathways. J. Exp. Med. 1993;178:1813–1817. doi: 10.1084/jem.178.5.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL M.D., SAMELSON L.E., KLAUSNER R.D. Multiple kinases and signal transduction. Phosphorylation of the T cell antigen receptor complex. J. Biol. Chem. 1987;262:5831–5838. [PubMed] [Google Scholar]
- PRIESCHL E.E., GOUILLEUX GRUART V., WALKER C., HARRER N.E., BAUMRUKER T. A nuclear factor of activated T cell-like transcription factor in mast cells is involved in IL-5 gene regulation after IgE plus antigen stimulation. J. Immunol. 1995;154:6112–6119. [PubMed] [Google Scholar]
- QUNITON T.M., DEAN W.L. Cyclic AMP-dependent phosphorylation of the inositol-1,4,5-trisphosphate receptor inhibits Ca+ release from platelet membranes. Biochem. Biophys. Res. Commun. 1992;184:893–899. doi: 10.1016/0006-291x(92)90675-b. [DOI] [PubMed] [Google Scholar]
- RANDRIAMAMPITA C., TSIEN R.Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- RINCON M., TUGORES A., LOPEZ RIVAS A., SILVA A., ALONSO M., DE LANDAZURI M.O., LOPEZ BOTET M. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur. J. Immunol. 1988;18:1791–1796. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- ROBINSON D., HAMID Q., BENTLEY A., YING S., KAY A.B., DURHAM S.R. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J. Allergy Clin. Immunol. 1993;92:313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- ROEHM N.W., RODGERS G.H., HATFIELD S.M., GLASEBROOK A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- SANDERSON C.J. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- SANDERSON C.J., CAMPBELL H.D., YOUNG I.G. Molecular and cellular biology of eosinophil differentiation factor (interleukin-5) and its effects on human and mouse B cells. Immunol. Rev. 1988;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- SCHREIBER E., MATTHIAS P., MULLER M.M., SCHAFFNER W. Rapid detection of octamer binding proteins with ‘mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNIJDEWINT F.G.M., KALINSKI P., WIERENGA E.A., BOS J.D., KAPSENBERG M.L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- STRANICK K.S., ZAMBAS D.N., USS A.S., EGAN R.W., BILLAH M.M., UMLAND S.P. Identification of transcription factor binding sites important in the regulation of the human interleukin-5 gene. J. Biol. Chem. 1997;272:16453–16465. doi: 10.1074/jbc.272.26.16453. [DOI] [PubMed] [Google Scholar]
- SUPATTAPONE S., DANOFF S.K., THEIBERT A., JOSEPH S.K., STEINER J., SNYDER S.H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc. Natl. Acad. Sci. U.S.A. 1988;85:8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN OOSTERHOUT A.J., LADENIUS A.R., SAVELKOUL H.F., VAN ARK I., DELSMAN K.C., NIJKAMP F.P. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am. Rev. Respir. Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- WATANABE S., YSSEL H., HARADA Y., ARAKI K. Effects of prostaglandin E2 on Th0-type human T cell clones: Modulation of functions of nuclear proteins involved in cytokine production. Int. Immunol. 1994;6:523–532. doi: 10.1093/intimm/6.4.523. [DOI] [PubMed] [Google Scholar]
- ZWEIFACH A., LEWIS R.S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


