Abstract
Northern blotting experiments have been performed with RNA extracted from several cell lines derived from the human lung in order to detect P2Y1, P2Y2, P2Y4 and P2Y6 mRNA. We have investigated the 1HAEo− and 16HBE14o− epithelial cell lines derived from the airway epithelium, the A549 cell line displaying properties of type II alveolar epithelial cells, the CALU-3 serous cells, the 6CFSMEo− submucosal cells and the HASMSC1 airway smooth muscle cells. We have also evaluated one pancreatic epithelial cell line called CFPAC-1. These experiments revealed that P2Y2 and P2Y6 mRNA are co-expressed in the 1HAEo−, 16HBE14o− and A549 epithelial cell lines. The CFPAC-1 pancreatic cell line was strongly positive for the P2Y2 receptor. No signal was obtained for the P2Y1 and P2Y4 receptors.
We have then performed RT–PCR experiments with specific oligonucleotides of these last two P2Y receptors with the RNA used for the Northern blotting experiments. P2Y4 mRNA was detected in five cell lines: 1HAEo−, 16HBE14o−, 6CFSMEo−, HASMSC1 and CFPAC-1. P2Y1 mRNA was only detected in the CALU-3 cell line.
Inositol trisphosphates assays have identified a response typical of the P2Y2 receptor in the 1HAEo− and the 16HBE14o− airway epithelial cell lines which co-express P2Y2 and P2Y6 mRNA. By contrast, the 6CFSMEo− submucosal cells expressed a UTP-specific response which displayed pharmacological characteristics compatible with the human P2Y4 receptor: in particular, there was no response to UDP or ATP and the UTP effect was totally inhibited by pertussis toxin.
Keywords: P2Y receptors, P2Y1 receptors, P2Y2 receptors, P2Y4 receptors, P2Y6 receptors, lung, epithelial cells
Introduction
One important effect of nucleotides on the airway epithelium is an increase in the apical permeability to Cl−. This observation has been made both in vitro on human nasal epithelial cells, and in vivo: indeed superfusion of nucleotides, in the presence of the sodium channel blocker amiloride, decreased the nasal transepithelial potential difference both in normal subjects and in cystic fibrosis patients (Knowles et al., 1991). These results suggested a potential therapeutic role of aerosolized nucleotides in the treatment of cystic fibrosis. As UTP and ATP were equipotent, pyrimidine nucleotides seem preferable, since they are not degraded to adenosine which induces bronchoconstriction, and a clinical assessment of UTP is currently underway. The action of nucleotides seems to involve more than one mechanism (Stutts et al., 1994). On the one hand, single-channel patch-clamp studies on human airway epithelial cells have revealed a direct effect of ATP on the open probability of outward rectifying chloride channels, requiring no soluble second messenger (Stutts et al., 1992). On the other hand, ATP increased the formation of inositol phosphates and the intracellular calcium concentration in human nasal epithelial cells, the effect being more pronounced when the nucleotide was added at the serosal side (Paradiso et al., 1995). The equipotency of ATP and UTP suggests that the effects of nucleotides on airway epithelium are mediated by P2U/P2Y2 receptors. The human P2Y2 receptor was indeed cloned from the airway epithelial cell line CF/T43 (Parr et al., 1994). However, Northern blotting revealed the presence in human lungs of mRNA of the P2Y1 (Janssens et al., 1996), P2Y4 (Communi, unpublished data) and P2Y6 (Communi et al., 1996a) receptors in addition to the P2Y2 subtype (Parr et al., 1994); it is only for the recently cloned P2Y11 receptor that no signal could be detected in the lung by Northern blotting (Communi et al., 1997). Furthermore, there is pharmacological evidence that in addition to the P2Y2 receptor, a receptor selectively responsive to UDP (presumably the P2Y6 subtype) is present on the apical surface of human nasal epithelial cells (Lazarowski et al., 1997). The aim of this paper was to characterize the expression of four human P2Y receptor subtypes (P2Y1, P2Y2, P2Y4 and P2Y6) in a variety of cell lines derived from human lung, using a combination of Northern blotting, RT–PCR (reverse-transcriptase-polymerase chain reaction) and inositol phosphates assays.
Methods
Materials
Trypsin was from Flow Laboratories (Bioggio, Switzerland). Culture media, foetal bovine serum (FBS), restriction enzymes and Taq polymerase were purchased from GIBCO BRL (Grand Island, NY, U.S.A.). The radioactive compounds myo-D-[2-3H]-inositol (17.7 Ci mmol−1) and [α32P]-ATP (800 Ci mmol−1) were from Amersham (Ghent, Belgium). Dowex AG1X8 (formate form) was from Bio-Rad Laboratories (Richmond, CA, U.S.A.). ATP, ADP, UTP and UDP were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Human CALU-3, A549 and CFPAC-1 cell lines were obtained from American Type Culture Collection (ATCC) (Rockville, MD, U.S.A.). The 16HBE14o−, 1HAEo− and 6CFSMEo− cell lines were obtained from Dr Dieter Gruenert, University of California, San Francisco. The smooth muscle cells HASMSC1 were obtained from Dr Graham Place (Bayer, Slough, U.K.) and prepared as described in Watson et al. (1998).
Cell culture
The 1HAEo−, 16HBE14o− and 6CFSMEo− cells were grown in MEM (minimum essential medium) with Earle's salts supplemented with 100 units ml−1 penicillin, 100 μg ml−1 streptomycin, 2.5 μg ml−1 amphotericin B, 10% FBS and 2 mM L-glutamine, at 37°C with 5% CO2. The CALU-3 cell line was cultured in MEM supplemented with MEM non-essential amino acids, 10% FBS, 1 mM pyruvate and the antibiotics. The A549 cell line was cultured in MEM supplemented with 10% FBS and the antibiotics. The CFPAC-1 cell line was cultured in Iscove's MDM (modified Dulbecco's medium), 10% FBS, 2 mM L-glutamine and the antibiotics. The HASMSC1 cell line was cultured in DMEM (Dulbecco's modified Eagle's medium) (with 25 mM HEPES, sodium pyruvate) supplemented with 10% FBS, 2 mM L-glutamine and 50 μg ml−1 gentamycin.
Northern blot analysis
The RNA from the different cell lines were prepared with the RNeasy kit (Quiagen). The RNA from lung and placenta were obtained from Clontech (Palo Alto, CA, U.S.A.). The polyA RNA was prepared with the polyATtract mRNA isolation system IV (Promega). The blots were prehybridized 8 h at 42°C in a 50% formamide, 0.3% SDS solution and hybridized for 18 h in the same solution supplemented with 10% dextran sulphate and the α32P-labelled probe. The final washing conditions were 0.1×SSC and 0.1% SDS at 65°C. The blots were exposed during 6 days and visualized using the PhosphorImager SI (Molecular Dynamics).
RT–PCR
The reverse-transcription was performed with 2 μg of total RNA from the different cell lines using the Superscript kit (Gibco BRL). In order to amplify P2Y receptor cDNAs, sets of specific oligonucleotide primers were synthesized on the basis of the published sequences of the P2Y1 and P2Y4 genes (see Table 1) (Ayyanathan et al., 1996; Communi et al., 1995a). The PCR amplification conditions were as follows: 93°C, 1 min, 50°C, 2 min, 72°C, 3 min; 25 cycles.
Table 1.
P2Y receptor primers used for PCR on the human cell lines derived from the lung and the pancreas

Inositol trisphosphate (IP3) measurements
Cells (4×105 per 35 mm-dish) were labelled for 24 h with 10 μCi ml−1 [3H]-inositol in inositol free-DMEM containing 5% FCS (foetal calf serum) and antibiotics. Cells were directly incubated without wash steps in Krebs-Ringer HEPES (KRH) buffer of the following composition (in mM: NaCl 124, KCl 5, MgSO4 1.25, CaCl2 1.45, KH2PO4 1.25, HEPES (pH:7.4) 25 and glucose 8) for 30 min. The cells were then challenged by various nucleotides for 30 s. The incubation was stopped by the addition of an ice cold 3% perchloric acid solution. IP3 were extracted and separated on Dowex columns as previously described (Communi et al., 1995b).
Results
We have performed Northern blotting experiments with polyA RNA isolated from several human cell lines: most of these cell lines were lung-derived, one was from pancreas. The cells were all epithelial except HASMSC1 cells which are derived from smooth muscle. Placenta, in which the expression of P2Y1, P2Y2, P2Y4 and P2Y6 receptors was previously demonstrated by Northern blotting experiments (Janssens et al., 1996; Parr et al., 1994; Communi et al., 1995a; 1996a) was used as positive control. Two blots were hybridized respectively with a P2Y2 probe (Figure 1A) and a P2Y6 probe (Figure 1B). The HASMSC1 cell line is only present in the second blot due to the poor yield of RNA extracted from this cell line. P2Y2 and P2Y6 mRNA were detected in the A549, 16HBE14o− and 1HAEo− cell lines. In the CFPAC-1 cell line, there was a significant P2Y2 signal while P2Y6 mRNA was barely detectable. The placenta was clearly positive for the two receptors. We have then tested P2Y1 and P2Y4 probes on these two blots. There was no signal for any of these two receptors on the blots, except for the human placenta (data not shown). After these experiments, a hybridization of these two blots with a GAPDH (glyceraldehyde phosphodehydrogenase) probe, which corresponds to the ubiquitous glycerophosphate dehydrogenase gene, revealed that the RNA material was still present even after three rounds of hybridization (Figure 1C).
Figure 1.
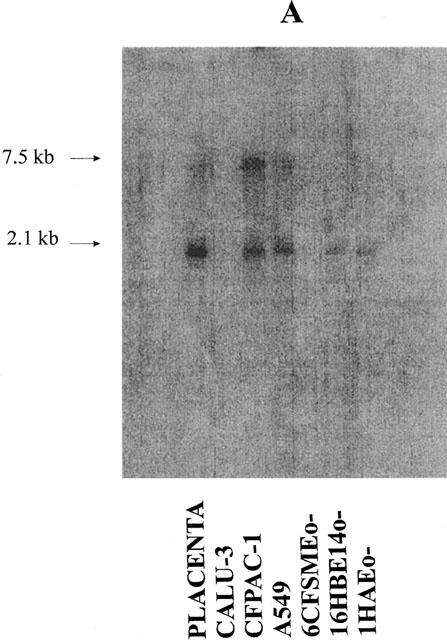
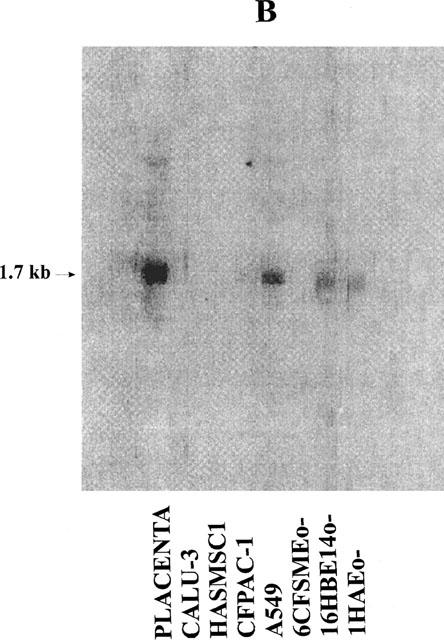
Detection of P2Y2 and P2Y6 mRNA in human cell lines derived from the human lung and pancreas by Northern blotting experiments. Two blots containing polyA RNA (2 μg lane−1) (from human placenta (positive control) and the cell lines CALU-3, HASMSC1 (present only in the second blot (B)), CFPAC-1, A549, 6CFSMEo−, 16HBE14o− and 1HAEo−) were performed and hybridized respectively with a P2Y2 probe (A) or a P2Y6 probe (B). The hybridization of the first blot with a GAPDH probe is shown on panel (C). The RNA from placenta were obtained from Clontech (Palo Alto, CA, U.S.A.). The probes correspond to the full coding region of the P2Y2, P2Y6 and GAPDH genes. The pictures were obtained from a PhosphorImager SI (Molecular Dynamics).
In view of the absence of signal for the P2Y1 and P2Y4 receptors in our Northern blotting experiments, we have then performed RT–PCR experiments with specific oligonucleotides of these two genes (Table 1). We have used the same RNA as for the Northern blotting experiments. In contrast to Northern blotting, RT–PCR revealed mRNA of these two receptors in several lines (Figure 2). For the P2Y1 receptor, a 1.3 kb-PCR product was amplified in the positive control (10 ng of pcDNA3-P2Y1 recombinant construction), the CALU-3 cell line and the lung. This band was sequenced and corresponded to the P2Y1 gene sequence. A 1.1 kb PCR product was also amplified in the CALU-3 cell line and the lung: its sequence did not correspond to the P2Y1 receptor but to a non coding sequence. P2Y4 receptor mRNA was strongly detected in five cell lines (1HAEo−, 16HBE14o−, 6CFSMEo−, HASMSC1 and CFPAC-1) and the positive control (10 ng of pcDNA3-P2Y4 recombinant construction) (Figure 2). The 128 bp band was sequenced and corresponded to the P2Y4 gene sequence. A weak signal was visualized in the other lanes and corresponds to the oligonucleotides used in the PCR experiments. As positive controls, we have reproduced the Northern blot data for the P2Y2 and P2Y6 receptors by RT–PCR experiments (data not shown).
Figure 2.
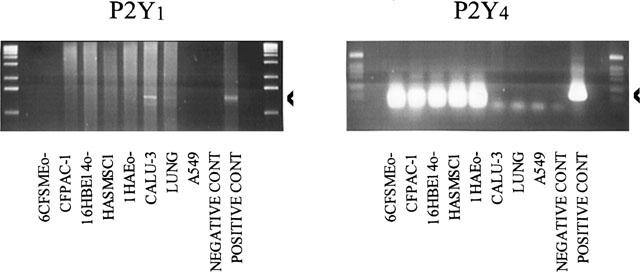
Detection of P2Y1 and P2Y4 mRNA in cell lines derived from human lung and pancreas by RT–PCR experiments. The extraction of RNA and the reverse-transcription were performed as described under ‘Methods'. The RNA from lung and placenta were obtained from Clontech (Palo Alto, CA, U.S.A.). PCR products (1.3 kb for the P2Y1 receptor and 128 bp for the P2Y4 receptor) are visualized after electrophoresis on a 1.5% agarose gel and ethidium bromide coloration and are indicated by an arrow.
We have then studied the effect of several nucleotides on IP3 formation in three particular cell lines: the 1HAEo− and the 16HBE14o− cell lines derived from the airway surface epithelium and the 6CFSMEo− cell line derived from the submucosal glands. We have chosen these three cell lines in view of the expression of P2Y6 transcripts in the 1HAEo− and 16HBE14o− cell lines and the detection of P2Y4 transcripts by RT–PCR experiments in the 6CFSMEo− cell line. These two pyrimidinoceptors have not been studied at this time in the human lung.
In the 1HAEo− and 16HBE14o− cell lines, ATP and UTP increased IP3 to the same extent whereas there was no significant effect of ADP nor of UDP (Figure 3A and B). By contrast, only UTP had a significant effect in the 6CFSMEo− cell line (Figure 3C). At a concentration of 100 μM, UDP, ATP and ADP were without any effect. These results were reproduced with HPLC-purified nucleotides (data not shown). More particularly, no response was obtained with HPLC-purified UDP at a concentration of 300 μM suggesting that only UTP is really active on this receptor (data not shown). The response to UTP was completely inhibited by an 18 h pretreatment of the cells with pertussis toxin (50 ng ml−1) (Figure 4). The responses to nucleotides were lost after three passages for the 6CFSMEo− and the 1HAEo− cell lines, although the mRNA of respectively P2Y4 and P2Y2 receptors was still detectable by RT–PCR (data not shown).
Figure 3.
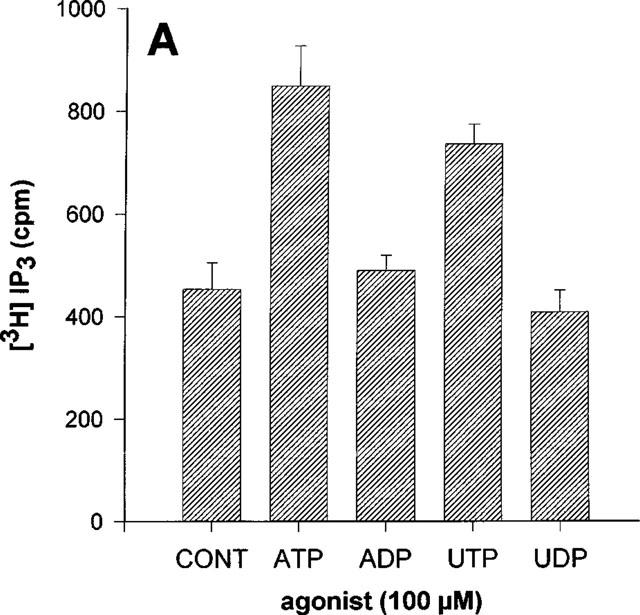
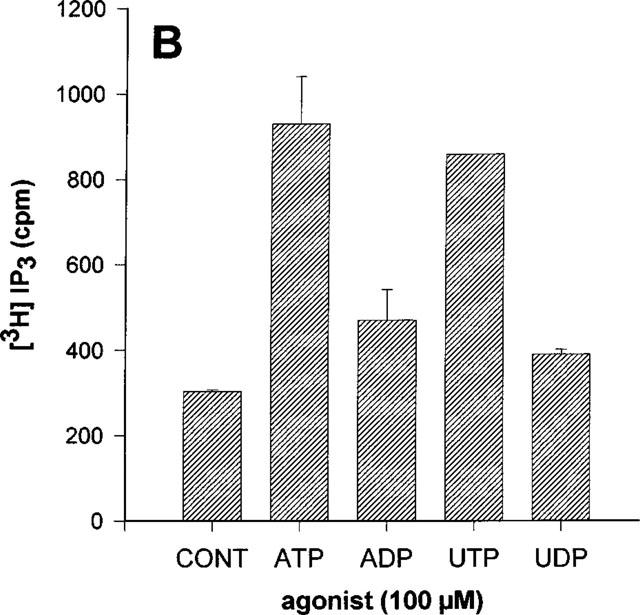
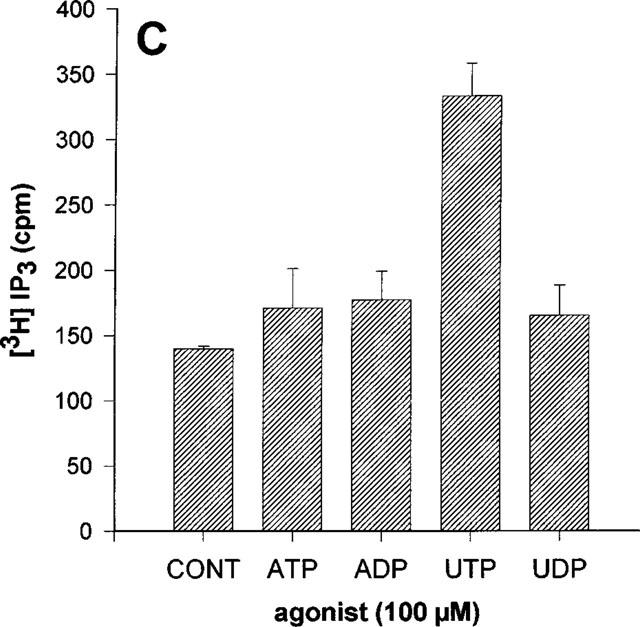
Effect of various nucleotides on the IP3 accumulation in 1HAEo− (A), 16HBE14o− (B) and 6 CFSMEo− cells (C). The cells were incubated with ATP, ADP, UTP and UDP at the same concentration of 100 μM or without agonist (CONT) for 30 s. The data represent the mean±s.d. of triplicate points and are representative of two independent experiments.
Figure 4.
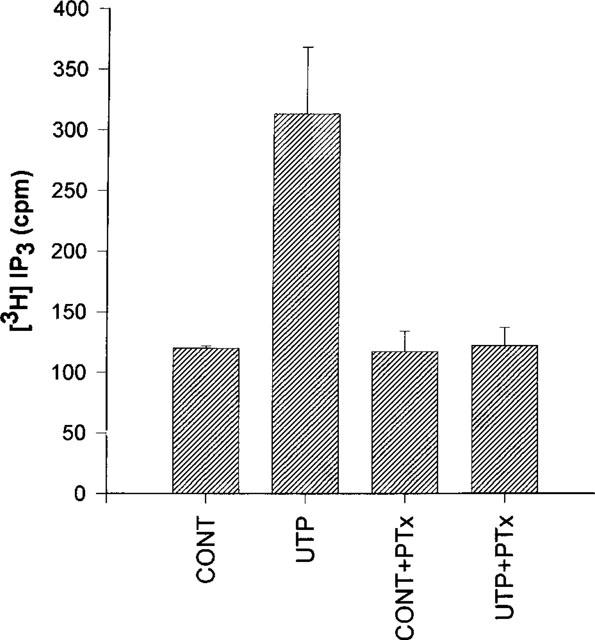
Effect of pertussis toxin on the UTP-induced accumulation of IP3 in 6CFSMEo− cells. The cells were pre-incubated for 18 h in the presence or the absence of 50 ng ml−1 pertussis toxin (PTx). The cells were then incubated with or without UTP 100 μM for 30 s. The data represent the mean±s.d. of triplicate points and are representative of two independent experiments.
Discussion
The deficiency in cyclic AMP-dependent Cl− transport via CFTR in airway epithelial cells (Widdecombe, 1986; Boucher et al., 1989) and pancreatic duct cells (Gray et al., 1994) can be compensated by the extracellular nucleotide-induced activation of alternative channels, either directly or as a result of increased intracellular calcium concentration (Mason et al., 1991; Knowles et al., 1991). There is thus a particular interest in the identification of the P2Y subtypes expressed in the human lung or pancreas.
We have used epithelial cell lines derived from the human lung, except for the CFPAC-1 cell line which is of pancreatic origin. The 1HAEo− and 16HBE14o− cell lines are derived from the airway surface epithelium (Cozens et al., 1991; 1994). CALU-3 cells exhibit some phenotypic properties typical of serous cells (Finkbeiner et al., 1993). The 6CFSMEo− cell line is an epithelial cell line derived from submucosal glands (Cozens et al., 1992). The A549 cell line displays properties of type II alveolar epithelial cells (Lieber et al., 1976) and the HASMSC1 cells are airway smooth muscle cells. The CFPAC-1 is an epithelial cell line exhibiting the basic CF defect (Schoumacher et al., 1990). Of course, caution must be taken in assigning a physiological or therapeutic value to the observations made with the cells since they are immortalized cell lines. Furthermore the surface (mucosal versus basolateral) selectivity of receptor expression was not addressed in this study.
As concerns the P2Y1 receptor, our results were essentially negative, except for the CALU-3 cells (positive in RT–PCR experiments). Not unexpectedly, P2Y2 receptor transcripts were detected in four out of the seven cell lines (1HAEo−, 16HBE14o−, A549 and CFPAC-1). P2Y6 mRNA was co-expressed in the same cells, except in the CFPAC-1 cell line where the signal was barely detectable. Expression of P2Y6 receptors in 1HAEo− and 16HBE14o− cells, which exhibit properties of airway surface epithelial cells, would be consistent with the observation of a UDP response, presumably mediated by P2Y6 receptors in human nasal epithelial cells, in addition to the P2Y2-mediated response to ATP and UTP (Lazarowski et al., 1997). However, the inositol phosphate response of 1HAEo− and 16HBE14o− cells was characterized by a biochemical response typical of P2Y2 receptors and no significant response to UDP was detectable. The same results have been obtained by another group in the CFPAC-1 pancreatic cell line (O'Reilly et al., 1998) in which we have also detected P2Y2 and P2Y6 mRNA. It is possible that the level of expression of P2Y6 receptors is too low in these cells to be detected by a biochemical approach.
P2Y4 mRNA was detected by RT–PCR in five out of seven cell lines (1HAEo−, 16HBE14o−, 6CFSMEo−, HASMSC1 and CFPAC-1), though Northern blotting experiments were negative. These discrepancies between the Northern blotting and the RT–PCR data is likely to be due to the higher sensitivity of the RT–PCR technique. This is reminiscent of the results of Webb et al. (1998), who observed a widespread distribution of the P2Y4 receptor transcripts in rat tissues using RT–PCR while Northern blots were negative. They concluded that the P2Y4 receptor is expressed at low level in adult organs. Surprisingly, we did not amplify any PCR product in the human lung for the P2Y4 receptor whereas we obtained a weak signal for this receptor in this tissue by Northern blotting experiments (data not shown). Interestingly, a functional response typical of P2Y4 receptors was observed in the 6CFSMEo− cells, where no other P2Y transcripts could be detected by either RT–PCR or Northern blotting. UTP rapidly stimulated the accumulation of IP3 in these cells. As expected for the human P2Y4 receptor (Nicholas et al., 1996), only UTP had an effect. No response was obtained with HPLC-purified UDP. Furthermore, the UTP effect was abolished in pertussis toxin-pretreated cells. We have previously reported that in 1321N1 astrocytoma cells stably expressing the human P2Y4 receptor, the stimulation of phospholipase C by UTP is sensitive to pertussis toxin inhibition (Communi et al., 1996b).
The response of the 1HAEo− and 6CFSMEo− cell lines to nucleotides was lost after three passages in our laboratory. However these cells had been passaged respectively 73 and 49 times when we received them. We have directly extracted RNA from these cells to perform the Northern blotting and RT–PCR experiments. Clearly, P2Y2, P2Y4 and P2Y6 mRNA were still present in 1HAEo− cells and P2Y4 mRNA in 6CFSMEo− cells after a substantial number of passages. Therefore the loss of responsiveness in our hands is likely to be related to the retrieval from cryostorage: apparently, when the cells are placed in culture, they initially express P2Y receptors but on passaging they start to down-regulate this expression, though mRNA was still detectable. Changes in receptor expression during cell culture are not uncommon: for instance, this has been shown for the P2Y1 receptor in endothelial cells from adrenal medulla, the P2Y2 receptor in rat salivary gland cells and for muscarinic receptors (Mateo et al., 1996; Turner et al., 1997; Tracey et al., 1992).
In conclusion, the 6CFSMEo− cells constitute a unique model of cells natively and selectively expressing functional P2Y4 receptors. 6CFSMEo− cells are representative of submucosal gland epithelial cells, which are the predominant site of CFTR expression in the human bronchi (Engelhardt et al., 1992). Although caution must be taken in interpreting results obtained with an immortalized cell line, it might be speculated that the P2Y4 receptor is a target for cystic fibrosis treatment by uridine nucleotides, in addition to P2Y2 and possibly P2Y6 receptors.
Acknowledgments
This work was supported by an Action de Recherche Concertée of the Communauté Française de Belgique, by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Federal Service for Science, Technology and Culture, by grants of the Fonds de la Recherche Scientifique Médicale, the Fonds Médical Reine Elisabeth and Boehringer Ingelheim. We thank Dr J.E. Dumont and Dr G. Vassart for helpful advice and discussions.
Abbreviations
- bp
base pair(s)
- DMEM
Dulbecco's modified Eagle's medium
- FBS
foetal bovine serum
- FCS
foetal calf serum
- GAPDH
glyceraldehyde phosphodehydrogenase
- IP3
inositol trisphosphate
- kb
kilobase(s)
- MEM
minimum essential medium
- RT–PCR
reverse-transcription-polymerase chain reaction
References
- AYYANATHAN K., WEBB T.E., SANDHU A.K., ATHWAL R.S., BARNARD E.A., KUNAPULI S.P. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem. Biophys. Res. Commun. 1996;218:783–788. doi: 10.1006/bbrc.1996.0139. [DOI] [PubMed] [Google Scholar]
- BOUCHER R.C., CHENG E.H.C., PARADISO A.M., STUTTS M.J., KNOWLES W.R., EARP H.S. Chloride secretory response of cystic fibrosis human airway epithelia. J. Clin. Invest. 1989;84:1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., GOVAERTS C., PARMENTIER M., BOEYNAEMS J.M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., MOTTE S., BOEYNAEMS J.M., PIROTTON S. Pharmacological characterization of the human P2Y4 receptor. Eur. J. Pharmacol. 1996b;317:383–389. doi: 10.1016/s0014-2999(96)00740-6. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., PARMENTIER M., BOEYNAEMS J.M. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem. Biophys. Res. Commun. 1996a;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., PIROTTON S., PARMENTIER M., BOEYNAEMS J.M. Molecular cloning and functional expression of a human uridine nucleotide receptor. J. Biol. Chem. 1995a;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., RASPE E., PIROTTON S., BOEYNAEMS J.M. Coexpression of P2Y and P2U receptors on aortic endothelial cells. Circ. Res. 1995b;76:191–198. doi: 10.1161/01.res.76.2.191. [DOI] [PubMed] [Google Scholar]
- COZENS A.L., YEZZI M.J., CHIN L., SIMON E.M., FINKBEINER W.E., WAGNER J.A., GRUENERT D.C. Characterisation of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5171–5175. doi: 10.1073/pnas.89.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COZENS A.L., YEZZI M.J., CHIN L., SIMON E.M., FRIEND D.S., GRUENERT D.C. Chloride ion transport in transformed normal and cystic fibrosis epithelial cells. Adv. Exp. Med. Biol. 1991;290:187–196. doi: 10.1007/978-1-4684-5934-0_19. [DOI] [PubMed] [Google Scholar]
- COZENS A.L., YEZZI M.J., KUNZELMANN K., OHRUI T., CHIN L., ENG K., FINKBEINER W.E., WIDDICOMBE J.H., GRUENERT D.C. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- ENGELHARDT J.F., YANKASKAS J.R., ERNST S.A., YANG Y., MARINO C.R., BOUCHER R.C., COHN J.A., WILSON J.M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nature Genetics. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- FINKBEINER W.E., CARRIER S.D., TERESI C.E. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Phenotypic Analysis of Cell Cultures of Human Tracheal Epithelium, Tracheobronchial Glands, and Lung Carcinomas. Am. J. Respir. Cell Mol. Biol. 1993;9:547–556. doi: 10.1165/ajrcmb/9.5.547. [DOI] [PubMed] [Google Scholar]
- GRAY M.A., WINPENNY J.P., PORTEOUS D.J., DORIN J.R., ARGENT B.E. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am. J. Physiol. 1994;266:C213–C221. doi: 10.1152/ajpcell.1994.266.1.C213. [DOI] [PubMed] [Google Scholar]
- JANSSENS R., COMMUNI D., PIROTTON S., SAMSON M., PARMENTIER M., BOEYNAEMS J.M. Cloning and tissue distribution of the human P2Y1 receptor. Biochem. Biophys. Res. Commun. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- KNOWLES M.R., CLARKE L.L., BOUCHER R.C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., PARADISO A.M., WATT W.C., HARDEN T.K., BOUCHER R.C. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBER M., SMITH B., SZAKAL A., NELSON-REES W., TODARO G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- MASON S.J., PARADISO A.M., BOUCHER R.C. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br. J. Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATEO J., MIRAS-PORTUGAL M.T., CASTRO E. Co-existence of P2Y- and PPADS-insensitive P2U-purinoceptors in endothelial cells from adrenal medulla. Br. J. Pharmacol. 1996;119:1223–1232. doi: 10.1111/j.1476-5381.1996.tb16026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- O'REILLY C.M., O'FARRELL A.M., RYAN M.P. Purinoceptor activation of chloride transport in cystic fibrosis and CFTR-transfected pancreatic cell lines. Br. J. Pharmacol. 1998;124:1597–1606. doi: 10.1038/sj.bjp.0701990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARADISO A.M., MASON S.J., LAZAROWSKI E.R., BOUCHER R.C. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature. 1995;377:643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- PARR C.E., SULLIVAN D.M., PARADISO A.M., LAZAROWSKI E.R., BURCH L.H., OLSEN J.C., ERB L., WEISMAN G.A., BOUCHER R.C., TURNER J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOUMACHER R.A., RAM J., IANUZZI M.C., BRADBURY N.A., WALLACE R.W., HON C.T., KELLY D.R., SCHMID S.M., GELDER F.B., RADO T.A., FRIZZELL R.A. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUTTS M.J., CHINET T.C., MASON S.J., FULLTON J.M., CLARKE L.L., BOUCHER R.C. Regulation of Cl− channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUTTS M.J., FITZ J.G., PARADISO A.M., BOUCHER R.C. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. Am. J. Physiol. 1994;267:C1442–1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- TRACEY W.R., PEACH M.J. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circ. Res. 1992;70:234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- TURNER J.T., WEISMAN G.A., CAMDEN J.M. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am. J. Physiol. 1997;273:C1100–1107. doi: 10.1152/ajpcell.1997.273.3.C1100. [DOI] [PubMed] [Google Scholar]
- WATSON M.L., GRIX S.P., JORDAN N.J., PLACE G.A., DODD S., LEITHEAD J., POLL C.T., YOSHIMURA T., WESTWICK J. Interleukin 8 and monocyte chemoattractant protein 1 production by cultured human airway smooth muscle cells. Cytokine. 1998;10:346–352. doi: 10.1006/cyto.1997.0350. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., HENDERSON D.J., ROBERTS J.A., BARNARD E.A. Molecular cloning and characterization of the rat P2Y4 receptor. J. Neurochem. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- WIDDECOMBE J.H. Cystic fibrosis and β-adrenergic response of airway epithelial cell cultures. Am. J. Physiol. 1986;251:R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]


