Abstract
In this study we have evaluated the pharmacological profile of the muscarinic antagonist glycopyrrolate in guinea-pig and human airways in comparison with the commonly used antagonist ipratropium bromide.
Glycopyrrolate and ipratropium bromide inhibited EFS-induced contraction of guinea-pig trachea and human airways in a concentration-dependent manner. Glycopyrrolate was more potent than ipratropium bromide.
The onset of action (time to attainment of 50% of maximum response) of glycopyrrolate was similar to that obtained with ipratropium bromide in both preparations. In guinea-pig trachea, the offset of action (time taken for response to return to 50% recovery after wash out of the test antagonist) for glycopyrrolate (t1/2 [offset]=26.4±0.5 min) was less than that obtained with ipratropium bromide (81.2±3.7 min). In human airways, however, the duration of action of glycopyrrolate (t1/2 [offset]>96 min) was significantly more prolonged compared to ipratropium bromide (t1/2 [offset]=59.2±17.8 min).
In competition studies, glycopyrrolate and ipratropium bromide bind human peripheral lung and human airway smooth muscle (HASM) muscarinic receptors with affinities in the nanomolar range (Ki values 0.5–3.6 nM). Similar to ipratropium bromide, glycopyrrolate showed no selectivity in its binding to the M1–M3 receptors. Kinetics studies, however, showed that glycopyrrolate dissociates slowly from HASM muscarinic receptors (60% protection against [3H]-NMS binding at 30 nM) compared to ipratropium bromide.
These results suggest that glycopyrrolate bind human and guinea-pig airway muscarinic receptors with high affinity. Furthermore, we suggest that the slow dissociation profile of glycopyrrolate might be the underlying mechanism by which this drug accomplishes its long duration of action.
Keywords: Human peripheral lung, human airway smooth muscle, glycopyrrolate, ipratropium bromide, muscarinic receptor antagonists, guinea-pig trachea
Introduction
In mammalian airways, the dominant neural bronchoconstrictor mechanism is provided by the cholinergic nerves (Barnes, 1986). Of the five cloned muscarinic receptor subtypes, mammalian airways express four receptor subtypes (M1, M2, M3 and M4) that are differentially distributed in the airways (Lazareno et al., 1990; Haddad et al., 1991; 1994a,b; 1996; Mak et al., 1992; Ramnarine et al., 1996). M1-receptors are found in parasympathetic ganglia where they facilitate neurotransmission and to alveolar walls where their function remains unclear. M2-receptors are localized to the post-ganglionic cholinergic nerve terminals and provide a functional negative feedback modulation of acetylcholine release (autoreceptors) (Patel et al., 1995). Muscarinic M2 receptors are also found in high proportion in airway and non-airway smooth muscle where they may be involved in functional antagonism, although this does not seem to be the case in human airways (Eglen et al., 1996; Zaagsma et al., 1997). Excitatory M3-receptors localized to airway smooth muscle and mucosal glands mediate bronchoconstriction and mucus secretion respectively (Barnes et al., 1995; Haddad & Rousell, 1998). In rabbit lung, the occurrence of M4-receptors has been demonstrated but its role in airway function remains to be elucidated (Lazareno et al., 1990; Mak et al., 1993).
Cholinergic neural tone is the major reversible component in the bronchoconstiction observed in chronic obstructive pulmonary disease (COPD) (Gross & Skorodin, 1984a,1984b; Gross, 1988) and, through the circadian variation of vagal tone, also contributes to nocturnal asthma (Coe & Barnes, 1986; Morrison et al., 1988). Anticholinergics are not very effective in the treatment of chronic asthma, but are more effective in the treatment of asthma exacerbations, suggesting that cholinergic mechanisms may become more important during acute exacerbations (O'Driscoll et al., 1989). The duration of action of the present anticholinergic drugs is sometimes insufficient to provide convenient maintenance therapy for patients or to control nocturnal asthma. Therefore, there is a clinical requirement to develop new drugs of this class.
Glycopyrrolate, a quaternary ammonium compound, is a muscarinic receptor antagonist which has been used in pre-anaesthetic medication to reduce gastric acid and salivary secretions or to reverse neuromuscular blockade (Norgaard et al., 1970; Mirakhur & Dundee, 1983). Recently, glycopyrrolate has been used as a bronchodilator in patients with asthma and COPD (Walker et al., 1987; Gilman et al., 1990; Tzelepis et al., 1996). In this study we have investigated the potency and duration of action of glycopyrrolate in inhibiting cholinergic contractile responses evoked by electrical field stimulation (EFS) in guinea-pig, and human airways. Parallel studies have investigated the antagonist affinity and the time taken for dissociation of the drug from muscarinic receptors in order to evaluate its possible clinical role as a bronchodilator for the treatment of reversible airways obstruction in patients with COPD and in acute exacerbations of asthma. The pharmacological profile of glycopyrrolate was also determined in rat cerebral cortex using [3H]-telenzepine to label M1-receptors and in rat heart and salivary glands using [N-methyl-3H]-scopolamine ([3H]-NMS) to label M2 and M3-receptors, respectively. Furthermore the binding characteristics of glycopyrrolate in human peripheral lung and human airway smooth muscle preparations have also been evaluated. In all experiments, the commonly used muscarinic receptor antagonist ipratropium bromide was included as a reference drug.
Methods
Guinea-pig trachea
Tissue preparation
Male Dunkin-Hartley guinea-pigs (250–500 g) were killed by cervical dislocation. The trachea and lungs were rapidly excized and the trachea placed in oxygenated Krebs-Henseleit solution of the following composition (mM): NaCl 118, KCl 5.9, MgSO4 1.2, CaCl2 2.5, NaH2PO4 1.2, NaHCO3 25.5 and glucose 5.6. The trachea was open longitudinally by cutting through the cartilage. The epithelium was removed by careful rubbing, minimizing damage to the smooth muscle. Indomethacin (10 μM) was present throughout the studies to inhibit the production of endogenous prostanoids.
Method
Eight transverse segments, each containing three to four cartilaginous strips, were prepared and suspended between parallel wire field electrodes in 10 ml organ baths containing Krebs-Henseleit solution (pH 7.4), which continually gassed by a 95% O2/5% CO2 mixture and maintained at 37°C. The tissues were allowed to equilibrate for 1 h with frequent washing, under a resting tension of 1.0 g for tracheal strips, which was found to be optimal for determining changes in tension. Before the experiment, capsaicin (10 μM) was introduced and washed out 30 min after the pre-treatment to deplete endogenous tachykinins. Tissues were also pre-treated with propranolol (1 μM) 10 min before the experiment to prevent any effects of endogenous catecholamines. Isometric tension responses were measured using force-displacement transducers (Model FT-03; Grass Instruments Co., Quincy, MA, U.S.A.) connected to a polygraphy (Model 7D; Grass Instruments Co.). Electrical field stimulation (EFS) was delivered via two platinum wire field electrodes inserted in parallel (10 mm apart) with the tissue sample between them. A stimulator (Model D345; Digitimer Ltd., Welwyn Garden City, Hertfordshire, U.K.) provided biphasic square wave impulses with a supramaximal voltage of 40 V at source and 0.5 ms duration.
Protocol
In order to elicit cholinergic contractile responses EFS was applied for 15 s periods every 4 min at a frequency of 4 Hz which produced approximately 50% of the maximal neural contraction. After at least four stable responses of equal magnitude were obtained test antagonists were introduced and further stimulations were delivered until the maximal effects of drugs were observed. Appropriate time controls were carried out for all kinetic studies. Some preparations were incubated with the minimal concentration of antagonist that gave maximal inhibition, and then the antagonist was washed out. Further stimulations were then applied until the responses returned to 50% of the original response.
Human tissue
Tissue preparation
We studied tracheal strips and bronchial rings (5–8 mm diameter) from 15 recipients and 11 donor patients for heart and heart-lung transplantation (15–58 years, eight males). The lung tissues were immediately placed into oxygenated Krebs-Henseleit solution, cooled to 4°C and transported to the laboratory. The airways were dissected from the parenchyma, with removal of all bronchial blood vessels and the epithelial layer. Tissues were suspended in organ baths as with the guinea-pig tissues and allowed to equilibrate for 2 h with washing every 20 min. The resting tension was adjusted to 2 g, which was found to be optimal for measuring changes in tension in these airways. Indomethacin (10 μM) and propranolol (1 μM) were present throughout.
Protocol
EFS was delivered with 40 V, 0.5 ms duration and a frequency of 8 Hz for 15 s in every 4 min (which produced approximately 50% of the maximal neural contraction). After at least four stable responses of equal magnitude were obtained, test antagonists were introduced, and further stimulations were delivered until the maximal effects of the drugs were observed. In some preparations, the concentration of antagonist used was the minimum concentration required to induce complete inhibition. When complete inhibition was achieved, the antagonist was washed out, and then further stimulations were applied until the response returned to 50% of the baseline values. Appropriate time controls in the absence of antagonists were also studied. Contractile responses obtained to EFS under these conditions were completely blocked by both atropine (1 μM) and tetrodotoxin (1 μM) indicating that the responses were evoked by stimulation of cholinergic nerves.
Radioligand binding studies
Membrane preparation
All membrane preparation procedures were performed at 4°C. Human peripheral lung and airway smooth muscle, and Wistar rat heart, cerebral cortex and salivary glands were finely minced with scissors and homogenized in 10–20 volumes (w v−1 of ice-cold 0.35 M sucrose–25 mM Tris-HCl with an Ultra-Turax homogenizer (10–15 bursts). After centrifugation at 800 ×g for 10 min at 4°C, the pellets were suspended, homogenized and resedimented at the same speed. The supernatants from these two stages were pooled, diluted with 50 volumes of ice-cold 25 mM Tris buffer (pH 7.4). The membranes were pelted by a centrifugation at 40,000 ×g for 20 min. The resulting pellets were resuspended in an appropriate volume of buffer. The membrane suspension was then stored as aliquots at −80°C. Protein concentration was determined according to the method of Lowry et al. (1951).
Binding assays
All binding studies were performed in 1 ml of 25 mM Tris buffer (pH 7.4) containing the following tissue concentrations (μg protein ml−1: cerebral cortex, 100–200; heart, 200–400; salivary glands, 100–200; human peripheral lung 400–600 and human airway smooth muscle, 250–350. In competition experiments using [3H]-NMS, a fixed concentration of 0.3 nM was used while in studies involving [3H]-telenzepine, a final radioligand concentration of 0.4 nM was used. [3H]-antagonist saturation curves were performed using a concentration range varying from 0.04 to 6 nM. Non-specific binding was defined as the binding in the presence of 1 μM atropine. Incubations were performed at 30°C for 2.5–3 h and terminated by rapid vacuum filtration over 0.2% polyethyleneimine pre-treated Whatman GF/C glass fibre filters using a Brandel cell harvester. The filters were washed twice with 4 ml of ice-cold Tris buffer and placed in vials with 4 ml of scintillation cocktail (Filtron X, National Diagnostics, Manville, NJ, U.S.A.) and counted in a liquid scintillation counter (Packard 2200 CA model, Meriden, CT, U.S.A.).
Kinetics studies
To investigate the reversibility of the muscarinic antagonist binding, aliquots of human airway smooth muscle membranes were treated for 3 h at 30°C with assay buffer (control), glycopyrrolate (30 nM) or ipratropium bromide (30 nM). The concentration of the antagonists used in this set of experiments was calculated to occupy 80–90% of receptors. After this preincubation period, membranes were diluted (1 : 70) with assay buffer containing [3H]-NMS (final concentration of 0.5 nM) in order to induce dissociation of the antagonists. [3H]-NMS binding was measured at various time intervals. Non-specific binding was defined as the binding in the presence of 1 μM atropine. Specific [3H]-NMS binding in the presence of the antagonists was corrected for protein concentration and expressed as a percentage of that obtained for binding to control membranes.
Statistical analysis
Contractile responses were expressed as absolute changes in tension. In kinetic studies the relevant time control in the absence of antagonist was used to control for decreased responses with time. Potency values for the test antagonists were expressed as the concentration required to inhibit cholinergic neural responses by 50% (IC50) and calculated by a non-linear regression iterative curve fitting using Graphpad Inplot (Graphpad Inc., San Diego, CA, U.S.A.). The time of onset (t1/2 [onset]) was defined as the time from starting the administration of the test antagonist to the attainment of 50% inhibition of cholinergic neural responses. The time for offset of action (t1/2 [offset]) was defined as the time from washout of the test antagonist to attainment of 50% recovery of cholinergic responses. Values of (t1/2 [onset]) and (t1/2 [offset]) were determined by interpolation from each time-effect curve and expressed as mean±s.e.mean.
Data for the saturation and inhibition binding studies were analysed using the non-linear regression analysis (LIGAND program) as previously described (Haddad et al., 1994a,1994b). The precision of fit to a one- or two-site model was determined with an F test (P<0.01), by comparing the residual sum of squares for fitting data to a one- or two-site model.
Drugs and chemicals
The following drugs were used: indomethacin, tetrodotoxin, atropine sulphate, ipratropium bromide, capasaicin (Sigma Chemical Co., Poole, U.K.); propranolol hydrochloride (Imperial Chemical Industries, plc., Alderley Edge, U.K.); glycopyrrolate a gift from Muro Pharmaceuticals (Muro Pharmaceuticals Inc., Tewksbury, MA, U.S.A.). [3H]-NMS (specific activity 84 Ci mmol−1) and [3H]-telenzepine (specific activity 76.4 Ci mmol−1 were purchased from NEN (DuPont New England Nuclear, Stevenage, Herts, U.K.). Krebs-Henseleit solution was made up fresh every day. Indomethacin was made up in alkaline phosphate buffer (pH 7.8) of the following composition (mM): KH2PO4 20 and Na2HPO4 120. Drug additions did not exceed 1% of the bath volume. All concentrations refer to the final bath concentrations.
Results
Functional characteristics of glycopyrrolate in guinea-pig trachea
Potency of antagonists in inhibiting contractile responses to EFS
Glycopyrrolate inhibited contraction induced by EFS (40 V, 0.5 ms, 4 Hz for 15 s every 4 min) in guinea-pig trachea in a concentration-dependent manner (IC50=0.15 nM, n=5–12). Glycopyrrolate was more potent than ipratropium bromide (IC50=0.58 nM, n=6) (Figure 1).
Figure 1.
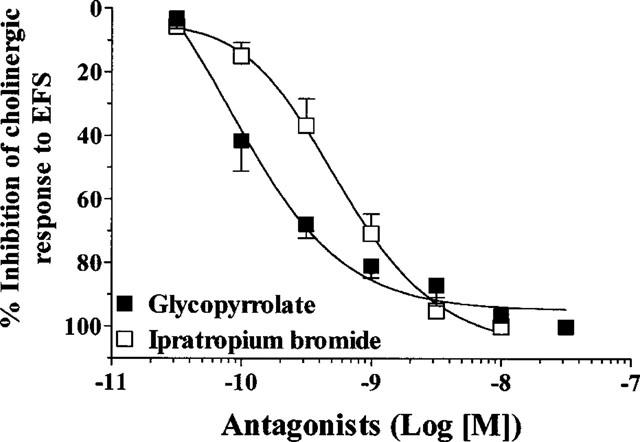
Effects of glycopyrrolate and ipratropium bromide on constrictor responses induced by electrical field stimulation (4 Hz, 40 V, 0.5 ms for 15 s every 4 min) in guinea-pig trachea. Each value is the mean±s.e.mean of 5–12 animals.
Time course for antagonists for inhibition of cholinergic contractile responses
Glycopyrrolate and ipratropium bromide had a very similar onset of action. The t1/2 (onset) for glycopyrrolate (10 nM) was 9.0±1.7 min (n=8) compared to that for ipratropium bromide (10 nM), 7.6±1.2 min (n=5) (Figure 2a). After washout, the inhibitory effect of ipratropium bromide (10 nM) on cholinergic responses was significantly pronounced (t1/2 [offset]=81.2±3.7 min, n=5) compared to glycopyrrolate (10 nM) (t1/2 [offset]=26.4±0.5 min, n=3) (Figure 2b).
Figure 2.
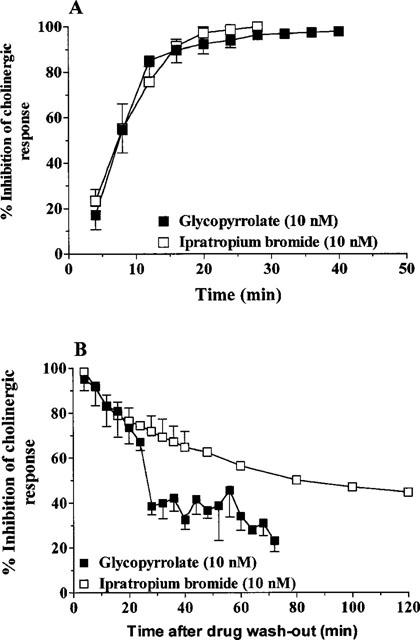
(A) Time course of onset of action of glycopyrrolate and ipratropium bromide in guinea-pig trachea. Each value is the mean±s.e.mean of 5–8 animals. (B) Time course of the effect of glycopyrrolate and ipratropium bromide after washout of the test drugs in guinea-pig trachea. Each value is the mean±s.e.mean of 3–5 animals.
Functional characteristics of glycopyrrolate in human airways
Potency of antagonists in inhibiting contractile responses to EFS
Glycopyrrolate inhibited contraction induced by EFS (40 V, 0.5 ms, 8 Hz for 15 s every 4 min) in human trachea in a concentration-dependent manner (IC50=0.44 nM, n=3–8). Glycopyrrolate was more potent than ipratropium bromide (IC50=1.36 nM, n=4–7) (Figure 3).
Figure 3.
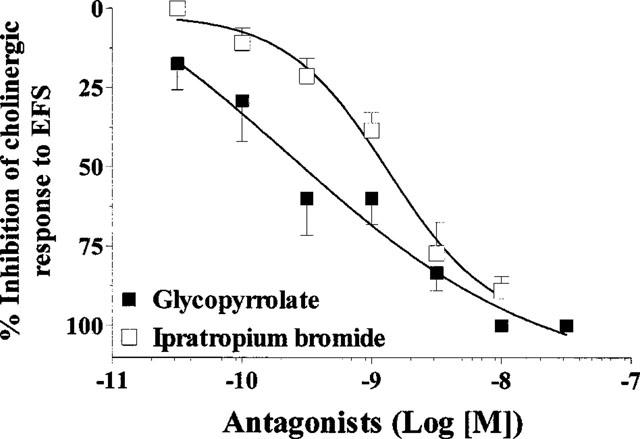
Effects of glycopyrrolate and ipratropium bromide on constrictor responses induced by electrical field stimulation (8 Hz, 40 V, 0.5 ms for 15 s every 4 min) in human airways. Each value is the mean±s.e.mean of 3–8 patients.
Time course for antagonists for inhibition of cholinergic contractile responses
Glycopyrrolate and ipratropium bromide had a very similar onset of action. The t1/2 (onset) for glycopyrrolate (3 nM) was 19.7±3.5 min (n=9) compared to that for ipratropium bromide (10 nM), 14.9±2.8 min (n=6) (Figure 4a). After washout, the inhibitory effect of glycopyrrolate (3 nM) on cholinergic responses was significantly prolonged (t1/2 [offset]<96 min, n=6) compared to ipratropium bromide (10 nM) Tt1/2 [offset]=59.1±17.8 min, n=3) (Figure 4b).
Figure 4.
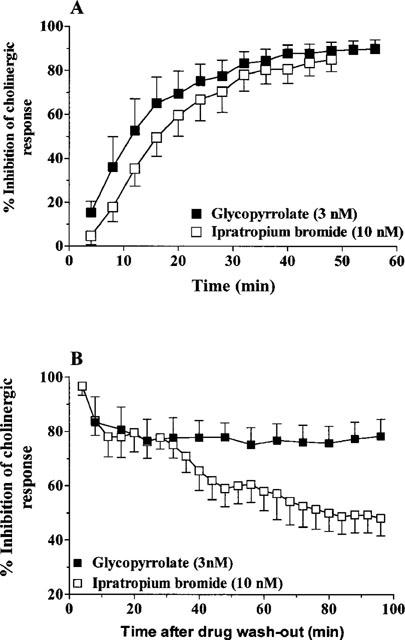
(A) Time course of onset of action of glycopyrrolate and ipratropium bromide in human airways. Each value is the mean±s.e.mean of 6–9 patients. (B) Time course of effects of glycopyrrolate and ipratropium bromide after washout of the test drugs in human airways. Each value is the mean±s.e.mean of 3–6 patients.
Binding characteristics of glycopyrrolate
Selectivity of glycopyrrolate for M1–M3 receptors
The pharmacological profile of glycopyrrolate was determined in rat cerebral cortex using [3H]-telenzepine to label M1-receptors and in rat heart and salivary glands using [N-methyl-3H]-scopolamine ([3H]-NMS) to label M2- and M3-receptors, respectively. Specific [3H]-NMS binding to muscarinic receptors in rat heart and salivary glands was saturable and best described by the interaction of the radioligand with a single population of high affinity binding sites. The affinities (KD in nM) were 0.09±0.02 and 0.35±0.04 in rat salivary glands and heart, respectively (data not shown). In rat cerebral cortex, [3H]-telenzepine, at a concentration of 0.4 nM, labelled exclusively the M1-receptors (Schudt et al., 1989). [3H]-Telenzepine/telenzepine displacement studies revealed a KD value of 0.62 nM. Ipratropium bromide bound to all the receptor subtype studied with almost the same high affinity in agreement with published data. Similar to ipratropium bromide, glycopyrrolate showed no selectivity in its interaction with M1, M2 and M3-receptors, although a 3–5-fold higher affinity at M3-receptors compared to M1 and M2-receptors was observed (Figure 5, Table 1).
Figure 5.
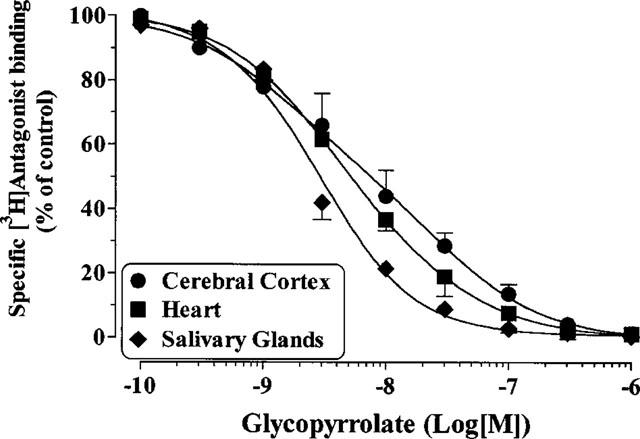
Inhibition of [3H]-NMS (rat heart and salivary glands) and [3H]-telenzepine (rat cerebral cortex) binding by glycopyrrolate. Values are means±s.e.mean from 4–6 independent experiments performed in duplicate on separate membrane preparations.
Table 1.
Inhibition of [3H]-telenzepine (cerebral cortex) and [3H]-NMS (heart and salivary glands) binding by glycopyrrolate and ipratropium bromide
Affinity of glycopyrrolate and ipratropium bromide in human airways
The non-selective and hydrophilic muscarinic antagonist [3H]-NMS was used to label muscarinic receptors present in human peripheral lung and airway smooth muscle homogenates. Specific [3H]-NMS binding was saturable and best described by the interaction of the radioligand with a single and homogeneous population of high affinity binding sites. The affinites (KD in nM) were 0.21±0.02 and 0.34±0.08 in human long and human airway smooth muscle, respectively. In competition studies, glycopyrrolate and ipratropium bromide fully inhibited the specific binding of [3H]-NMS in a concentration dependent manner in both preparations (Figure 6a, b). The results of the competition studies are shown in Table 2.
Figure 6.
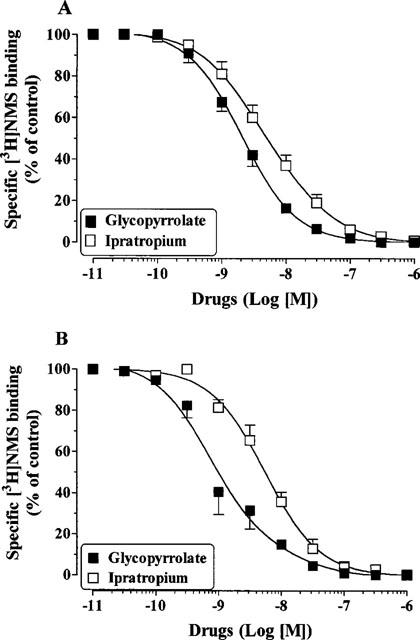
Inhibition of [3H]-NMS binding by glycopyrrolate and ipratropium bromide in human peripheral lung (A) and human airway smooth muscle (B). Values are means±s.e.mean from 3–6 independent experiments performed in duplicate on separate membrane preparations.
Table 2.
Binding characteristics of glycoprrolate and ipratropium bromide in human airways
Kinetics profile of glycopyrrolate and ipratropium bromide in human airway smooth muscle
Wash-out experiments suggest that glycopyrrolate possesses a longer duration of action when compared to ipratropium bromide (Figure 4b). To investigate the potential mechanisms by which glycopyrrolate achieves its duration of action in vitro, we assessed its rate of dissociation from human airway smooth muscle muscarinic receptors indirectly by monitoring the rate of [3H]-NMS association. Human airway smooth muscle membranes were preincubated for 3 h with glycopyrrolate (30 nM) or ipratropium bromide (30 nM) in order to occupy 80–90% of the receptors. To induce antagonist dissociation, the volume of the membrane's suspension was then increased 70 fold with the assay buffer containing [3H]-NMS, thereby reducing the concentration of both antagonists. The time course of [3H]-NMS association in buffer- and ipratropium-pre-treated membranes was rapid and reached equilibrium within 30–40 min of incubation (t1/2 ∼7 min). In glycopyrrolate pre-treated membranes, the rate of [3H]-NMS association (shown in Figure 7 as percentage of glycopyrrolate-occupied receptors) was considerably slower. This result suggests that [3H]-NMS binding to human lung muscarinic receptors was probably limited by the slow dissociation rate of glycopyrrolate.
Figure 7.
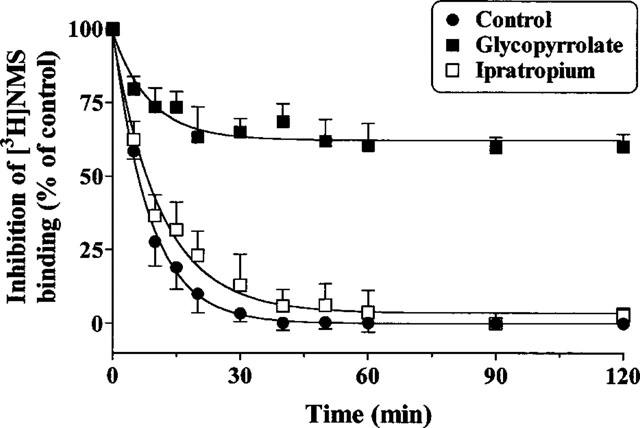
[3H]-NMS binding to human airway smooth muscle muscarinic receptors occupied by glycopyrrolate and ipratropium bromide. Human airway membranes were treated with the assay buffer (Control) or the test antagonists (each at 30 nM) for 3 h at 30°C. After this preincubation period, the volume of membrane suspension was then diluted (in order to induce antagonist dissociation) with the assay buffer containing final [3H]-NMS concentration of 0.5 nM. The association of [3H]-NMS (expressed as percentage inhibition of [3H]-NMS binding which reflect the percentage of glycopyrrolate or ipratropium occupied receptors) was measured at appropriate time intervals. Specific [3H]-NMS binding in the presence of the antagonists was corrected for protein concentration and expressed as a percentage of that obtained for binding to control membranes. Values are means±s.e.mean from three independent experiments performed in duplicate on separate membrane preparations.
Discussion
In this study we examined the effect of glycopyrrolate, a muscarinic receptor antagonist, on EFS-induced cholinergic neurotransmission, but not on exogenous ACh-induced bronchoconstriction, because EFS-induced bronchoconstriction may be more representative of an increase in vagal tone than exogenous ACh-induced bronchoconstriction. We have demonstrated that glycopyrrolate was more potent (3–4 fold) than the anti-cholinergic bronchodilator commonly used in the clinic, ipratropium bromide, in inhibiting cholinergic neural responses in guinea-pig and human airways.
In guinea-pig trachea and human airways, the onset of action of glycopyrrolate (t1/2 [onset] of 9–19.8 min) was similar to that obtained with ipratropium bromide and faster compared to tiotropium bromide (Ba 679 BR) (t1/2 [onset] of 34–43 min) (Takahashi et al., 1994). However, there is a marked difference in the dissociation profile of glycopyrrolate and ipratropium bromide in guinea-pig and human airways. In this set of experiments, airway tissue was incubated with the minimal concentration of the test antagonist that produced complete inhibition of the cholinergic neural response. When complete inhibition was achieved, the antagonist was washed out, and then further stimulations were applied until the response returned to 50% of the baseline values. The time for offset of action (t1/2 [offset]) was defined as the time from washout of the test antagonist to attainment of 50% recovery of cholinergic responses. In guinea-pig trachea, the inhibitory effect of glycopyrrolate (10 nM) and ipratropium bromide (10 nM) on cholinergic responses was similar with time, although the time taken for the offset of action of glycopyrrolate was less than that obtained with ipratropium bromide. After wash-out, the inhibitory effect of glycopyrrolate (3 nM) was significantly more prolonged (over 70% inhibition of the neural response) compared to ipratropium bromide (10 nM) in human tissues. In fact, a t1/2 [offset] could not be obtained for glycopyrrolate within the time our preparations were maintained. Therefore, our results suggest differences in the dissociation kinetics of these antagonists with muscarinic receptors in different species. The longer duration of action of glycopyrrolate in inhibiting cholinergic neurotransmission in human airways suggests that it may have a therapeutic advantage over existing drugs such as ipratropium bromide. This property is also shared with the recently developed muscarinic antagonist tiotropium bromide. We have previously shown that this antagonist, in addition to its powerful inhibitory effect of cholinergic nerve-induced contraction of guinea-pig and human airways, dissociated extremely slowly from post-junctional muscarinic receptors when compared to atropine or ipratropium bromide (Takahashi et al., 1994).
We have also determined the binding characteristics of glycopyrrolate. We first investigated the selectivity of glycopyrrolate and ipratropium bromide in their interaction with the rat cerebrocortical M1-receptors labelled with 3H-telenzepine and heart M2- and salivary glands M3-receptors labelled with 3H-NMS. Ipratropium bromide bound to all the receptor subtypes studied with almost the same high affinity in agreement with published data. Similar to ipratropium bromide and tiotropium bromide (Ba 679 BR), glycopyrrolate showed no selectivity in its binding to the M1–M3-receptors (Haddad et al., 1994a; Gomez et al., 1995), although a 3–5 fold higher affinity at M3-receptors compared to M1 and M2 receptors was observed. It should be noted that functional affinity derived from Schild analysis has shown that glycopyrrolate possesses a high affinity at M1/M3 (pKB values of 10.31–11) receptors compared to M2 receptors (pA2 value of 8.16–9.09) (Lau & Szilagyi, 1992; Fuder & Meincke, 1993).
The affinity of glycopyrrolate and ipratropium bromide for muscarinic receptors in human peripheral lung and airway smooth muscle membranes was also evaluated. 3H-NMS labelled homogeneous binding sites in these preparations were not discriminated by the drugs used. Indeed, both of the antagonists examined produced mass action competition curves compatible with the existence of a single receptor subtype in the different membrane preparations used. It should be noted that a 5 fold higher affinity at human airway smooth muscle muscarinic receptors was observed for glycopyrrolate compared to ipratropium bromide.
Experiments have also been performed to determine the dissociation profile of glycopyrrolate from human airway muscarinic receptors. Unlike ipratropium bromide (30 nM) which dissociated so quickly that there was little difference in the [3H]-NMS association to vehicle treated membranes, glycopyrrolate (30 nM) has a strong protective effect against [3H]-NMS binding (>60%) which lasted for 2 h. These results suggest that the slow dissociation profile of glycopyrrolate from human airway smooth muscle muscarinic receptors provide a mechanistic explanation for the long duration of action of this drug observed in functional experiments. Similar data were obtained in human peripheral lung with the new muscarinic antagonist tiotropium bromide (Haddad et al., 1994a). The slow dissociation profile of tiotropium bromide was in agreement with functional and clinical studies confirming its long lasting bronchodilator action in patients with asthma and COPD (O'Connor et al., 1996; Barnes et al., 1995).
Prolonged inhibitory effects of glycopyrrolate have also been observed in functional tests in vitro (this report) and in vivo in humans. In normal subjects, glycopyrrolate produces significant bronchodilation of long duration (Gal & Suratt, 1981; Gal et al., 1984). In patients with chronic obstructive pulmonary disease, inhaled nebulized glycopyrrolate (1 mg) produced a bronchodilating effect that lasted for 8 h (Tzelepis et al., 1996). Similarly, in patients with asthma, when compared to placebo, metered-dose aerosols of glycopyrrolate (doses ranging from 100–1200 mg) elicited significantly greater bronchodilation that was noted within 30 min of dosing and was sustained for at least 8–12 h (Walker et al., 1987; Schroeckenstein et al., 1988). Thus, there seems to be a close correlation between the binding experiments obtained in human airway smooth muscle and the functional data regarding the antagonist dissociation profile and the duration of action in humans.
Anticholinergic drugs have been found to be as effective and, in some cases more effective that β-agonists as bronchodilator agents in the treatment of airways obstruction in patients with COPD (Gross, 1988) and in some patients with nocturnal asthma (Coe & Barnes, 1986; Morrison et al., 1988). However, the duration of action of the present anti-cholinergic drugs is insufficient to provide convenient maintenance therapy for the patients or to control nocturnal asthma. These results suggest, that at least in human airways, glycopyrrolate had a longer duration of action than ipratropium bromide and that it may prove a more efficient therapy for these conditions than the drugs commonly used today.
Acknowledgments
This work was supported by Muro Pharmaceuticals (Tewksbury, MA, U.S.A.). We thank the Wellcome Trust (U.K.) and the European Union for support.
Abbreviations
- [3H]-NMS
[N-methyl-3H]-scopolamine
- COPD
chronic obstructive pulmonary disease
- EFS
electrical field stimulation
- HASM
human airway smooth muscle
References
- BARNES P.J. Neural control of human airways in health and disease. Am. Rev. Resp. Dis. 1986;134:1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., BELVISI M.G., MAK J.C.W., HADDAD E.-B., O'CONNOR B.J. Tiotropium bromide (Ba 679 BR), a novel long-acting muscarinic antagonist for the treatment of obstructive airways disease. Life Sci. 1995;56:853. doi: 10.1016/0024-3205(95)00020-7. [DOI] [PubMed] [Google Scholar]
- COE C.I., BARNES P.J. Reduction of nocturnal asthma by an inhaled anticholinergic drug. Chest. 1986;90:485. doi: 10.1378/chest.90.4.485. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- FUDER H., MEINCKE M. Glycopyrronium bromide blocks differentially responses mediated by muscarinic receptor subtypes. Naunyn Schmiedebergs Arch. Pharmacol. 1993;347:591–595. doi: 10.1007/BF00166941. [DOI] [PubMed] [Google Scholar]
- GAL T.J., SURATT P.M. Atropine and glycopyrrolate effects on lung mechanics in normal man. Anesth. Analg. 1981;60:85–90. [PubMed] [Google Scholar]
- GAL T.J., SURATT P.M., LU J.Y. Glycopyrrolate and atropine inhalation: comparative effects on normal airway function. Am. Rev. Respir. Dis. 1984;129:871–873. doi: 10.1164/arrd.1984.129.5.871. [DOI] [PubMed] [Google Scholar]
- GILMAN M.J., MEYER L., CATER J., SLOVIS C. Comparison of aerosolized glycopyrrolate and metaproterenol in acute asthma. Chest. 1990;98:1095–1098. doi: 10.1378/chest.98.5.1095. [DOI] [PubMed] [Google Scholar]
- GOMEZ A., BELLIDO I., SANCHEZ DE LA CUESTA F. Atropine and glycopyrronium show similar binding patterns to M2 (cardiac) and M3 (submandibular gland) muscarinic receptor subtypes in the rat. Br. J. Anaesth. 1995;74:549–552. doi: 10.1093/bja/74.5.549. [DOI] [PubMed] [Google Scholar]
- GROSS N.J. Ipratropium bromide. New Engl. J. Med. 1988;319:486–494. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- GROSS N.J., SKORODIN M.S. Anticholinergic antimuscarinic bronchodilators. Am. Rev. Respir. Dis. 1984a;129:856–870. doi: 10.1164/arrd.1984.129.5.856. [DOI] [PubMed] [Google Scholar]
- GROSS N.J., SKORODIN M.S. Role of parasympathetic system in airway obstruction due to emphysema. N. Eng. J. Med. 1984b;311:421–425. doi: 10.1056/NEJM198408163110701. [DOI] [PubMed] [Google Scholar]
- HADDAD E-B., LANDRY Y., GIES J.-P. Muscarinic receptors subtypes in guinea pig airways. Am. J. Physiol. 1991;261:L327–L333. doi: 10.1152/ajplung.1991.261.4.L327. [DOI] [PubMed] [Google Scholar]
- HADDAD E.-B., MAK J.C.W., BARNES P.J. Characterization of [3H]Ba 679 BR, a slowly dissociating muscarinic antagonist, in human lung: Radioligand binding and autoradiographic mapping. Mol. Pharmacol. 1994a;45:899–907. [PubMed] [Google Scholar]
- HADDAD E.-B., MAK J.C.W., HISLOP A., HAWORTH S.G., BARNES P.J. Characterization of muscarinic receptor subtypes in pig airways: radioligand binding and Northern blotting studies. Am. J. Physiol. 1994b;266:L642–L648. doi: 10.1152/ajplung.1994.266.6.L642. [DOI] [PubMed] [Google Scholar]
- HADDAD E.-B., MAK J.C.W., NISHIKAWA M., BELVISI M.G., ROUSELL J., BARNES P.J. Muscarinic and β-adrenergic receptor expression in peripheral lung from normal and asthmatic patients. Am. J. Physiol. 1996;270:L947–L953. doi: 10.1152/ajplung.1996.270.6.L947. [DOI] [PubMed] [Google Scholar]
- HADDAD E.-B., ROUSELL J. Regulation of the expression and function of the M2 muscarinic receptor. Trends Pharmacol. Sci. 1998;19:322–327. doi: 10.1016/s0165-6147(98)01231-0. [DOI] [PubMed] [Google Scholar]
- LAU W.M., SZILAGYI M. A pharmacological profile of glycopyrrolate: interactions at the muscarinic acetylcholine receptor. Gen. Pharmacol. 1992;23:1165–1170. doi: 10.1016/0306-3623(92)90306-5. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., BUCKLEY N.J., ROBERTS F.F. Characterization of muscarinic M4 binding sites in rabbit lung, chicken heart, and NG108-15 cells. Mol. Pharmacol. 1990;38:805–815. [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAK J.C.W., BARANIUK J.N., BARNES P.J. Localization of muscarinic receptor subtype mRNAs in human lung. Am. J. Resp. Cell Mol. Biol. 1992;7:344–348. doi: 10.1165/ajrcmb/7.3.344. [DOI] [PubMed] [Google Scholar]
- MAK J.C.W., HADDAD E.-B., BUCKLEY N.J., BARNES P.J. Visualization of muscarinic m4 mRNA and M4 receptor subtype in rabbit lung. Life Sci. 1993;53:1501–1508. doi: 10.1016/0024-3205(93)90624-c. [DOI] [PubMed] [Google Scholar]
- MIRAKHUR R.K., DUNDEE J.W. Glycopyrrolate: pharmacology and clinical use. Anaesthesia. 1983;38:1195–1204. doi: 10.1111/j.1365-2044.1983.tb12525.x. [DOI] [PubMed] [Google Scholar]
- MORRISON J.F.L., PEARSON S.B., DEAN H.G. Parasympathetic nervous system in nocturnal asthma. Br. Med. J. 1988;296:1427. doi: 10.1136/bmj.296.6634.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORGAARD R.P., POLTER D.E., WHEELER J.W., FORDTRAN J.S. Effect of long term anticholinergic therapy on gastric acid secretion, with observations on the serial measurement of peak histalog response. Gastroenterology. 1970;58:750–755. [PubMed] [Google Scholar]
- O'CONNOR B.J., TOWSE L.J., BARNES P.J. Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am. J. Respir. Crit. Care Med. 1996;154:876–880. doi: 10.1164/ajrccm.154.4.8887578. [DOI] [PubMed] [Google Scholar]
- O'DRISCOLL B.R., TAYLOR R.J., HORSLEY M.G., CHAMBERS D.U., BERNSTEIN A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet. 1989;1:1418. doi: 10.1016/s0140-6736(89)90126-8. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., BARNES P.J., TAKAHASHI T., TADJKARIMI S., YACOUB M.H., BELVISI M.G. Evidence for prejunctional muscarinic autoreceptors in human and guinea pig trachea. Am. J. Resp. Crit. Care Med. 1995;152:872–878. doi: 10.1164/ajrccm.152.3.7663798. [DOI] [PubMed] [Google Scholar]
- RAMNARINE S.I., HADDAD E.-B., KHAWAJA A.M., MAK J.C.W., ROGERS D.F. On muscarinic control of neurogenic mucus secretion in ferret trachea. J. Physiol. 1996;494:577–586. doi: 10.1113/jphysiol.1996.sp021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHROECKENSTEIN D.C., BUSH R.K., CHERVINSKY P., BUSSE W.W. Twelve-hour bronchodilation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolate. J. Allergy Clin. Immunol. 1988;82:115–119. doi: 10.1016/0091-6749(88)90060-7. [DOI] [PubMed] [Google Scholar]
- SCHUDT C., BOER R., ELTZE M., RIEDEL R., GRUNDLER G., BIRDSALL N.J.M. The affinity, selectivity and biological activity of telenzepine enantiomers. Eur. J. Pharmacol. 1989;165:87–96. doi: 10.1016/0014-2999(89)90773-5. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T., BELVISI M.G., PATEL H., WARD J.K., TADJKARIMI S., YACOUB M.H., BARNES P.J. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am. J. Respir. Crit. Care Med. 1994;150:1640–1645. doi: 10.1164/ajrccm.150.6.7952627. [DOI] [PubMed] [Google Scholar]
- TZELEPIS G., KOMANAPOLLI S., TYLER D., VEGA D., FULAMBARKER A. Comparison of nebulized glycopyrrolate and metaproterenol in chronic obstructive pulmonary disease. Eur. Respir. J. 1996;9:100–103. doi: 10.1183/09031936.96.09010100. [DOI] [PubMed] [Google Scholar]
- WALKER F.B., KAISER D.L., KOWAL M.B., SURATT P.M. Prolonged effect of inhaled glycopyrrolate in asthma. Chest. 1987;91:49–51. doi: 10.1378/chest.91.1.49. [DOI] [PubMed] [Google Scholar]
- ZAAGSMA J., ROFFEL A.F., MEURS H. Muscarinic control of airway function. Life Sci. 1997;60:1061–1068. doi: 10.1016/s0024-3205(97)00048-9. [DOI] [PubMed] [Google Scholar]


