Abstract
The intracellular transport of leukotriene C4 (LTC4) in hematopoietic cells such as human monocytes is controlled by an ATP dependent carrier encoded by the multidrug resistance protein1 (MRP1) gene whose function can be blocked by the compound MK-571. Since LTs play a major role in control of cytokine expression in monocytes, we questioned whether blocking of the MRP1 mediated function by MK-571 might affect cytokine production.
MK-571 strongly enhanced IL-6 expression at mRNA and protein level in lipopolysaccharide (LPS) and interleukin-1 (IL-1) stimulated human monocytes giving rise to 2.0±0.4 (x±s.d.) and 5.7±3.5 fold induction of IL-6 protein secretion. The increase in IL-6 secretion was accompanied by an enhanced phosphorylation of p38 but not of c-Jun-N terminal kinase.
The involvement of the kinase signalling pathways was further analysed by using SB203580 and PD98059, specific inhibitors of the p38 and ERK1/2 signalling route. MK-571 mediated upregulation of IL-6 in the presence of IL-1 was partially attenuated by SB203580 and PD98059. Electrophoretic mobility shift assays demonstrated that MK-571 did not affect the IL-1 induced DNA binding activity of Activator Protein-1 and Nuclear Factor-κB but rather enhanced the transactivational activity of an IL-6 promoter construct. Finally it was shown that the MK-571 mediated effects on IL-6 secretion could not be inhibited by the LT synthesis inhibitor SB203347 or by the anti-oxidant pyrrolidine dithiocarbamate (PDTC).
These results indicate that the membrane transporter MRP1 is involved in the regulation of IL-6 expression in activated human peripheral blood monocytes.
Keywords: Monocytes, IL-6 secretion, MRP1, MK-571, leukotrienes
Introduction
The leukotrienes (LTs), LTB4 and LTC4 are active biological compounds that function as mediators of various inflammatory processes such as leukocyte chemotaxis, smooth muscle contraction, and cytokine production (Samuelsson et al., 1987). These effects are mediated by binding of the ligand to their specific surface receptor (Yokomizo et al., 1992). The cellular biosynthesis of the LTs are initiated by the release of arachidonic acid that is converted to LTA4 by 5-lipoxygenase. Subsequently LTA4 is catalyzed by LTB4 synthase to LTB4 or by LTC4 synthase that catalyzes the conjugation of LTA4 with reduced glutathione (GSH) to form intracellular LTC4. The biosynthesis of LTC4 is followed by transport across the plasma membrane plus rapid conversion by gamma-glutamyltransferese to the biologically active compound LTD4 (Keppler, 1992).
The membrane transporter involved in this process is the multidrug resistance protein (MRP1), identical to GSH S-conjugate carrier, and belonging to the ATP-binding cassette superfamily of membrane transport proteins (Loe et al., 1996; Müller et al., 1994; 1996; Roelofsen et al., 1997; Leier et al., 1994). To date MRP1 function is especially linked to multidrug resistance since overexpression of the MRP1 gene in malignant cells results in resistance to vinca alkaloids and doxorubicin (Loe et al., 1996; Müller et al., 1994; 1996; Roelofsen et al., 1997; Leier et al., 1994). A specific inhibitor of MRP-mediated transport is MK-571 (Jones et al., 1989). In vitro studies have demonstrated that MK-571 can augment the effects of cytotoxic agents on malignant cells due to a decreased efflux of these compounds as result of an inhibition of the MRP-mediated transport (Jones et al., 1989; Gekeler et al., 1995).
Previous studies have demonstrated that LTs are strongly involved in the production of Interleukin-6 (IL-6) by human monocytic cells (Ferreri et al., 1986; Stankova & Rola-Pleszczynski, 1992; Rola-Pleszczynski & Stankova, 1992; Cuenda et al., 1995). Exogenously added LTB4 induces IL-6 secretion which was accomplished in part at the transcriptional level (Rola-Pleszczynski & Stankova, 1992). Monocytes also produce LTC4 and therefore we questioned whether blocking of MRP1-mediated LTC4 secretion by MK-571 might affect the secretion of this cytokine (Ferreri et al., 1986). Previously it was hypothesized that this approach might be of relevance to inhibit the inflammatory response in disorders in which LTs are strongly involved (Samuelsson et al., 1987; Jones et al., 1989).
In the present study we demonstrate that MK-571 enhances IL-6 secretion by activated monocytes which is, at least partly, mediated through activation of mitogen activated protein kinases (MAPK) and stress activated protein kinases (SAPK) signalling routes.
Methods
Preparation of human peripheral blood monocytes and culture of the human monocytic cell line U937
Peripheral blood cells were obtained from healthy volunteer platelet donors, and mononuclear cell suspensions were prepared by Ficoll-Hypaque density-gradient centrifugation. T lymphocytes were depleted by 2-aminoethylisothiouronium bromide (AET) treated sheep red blood cell (SRBC) rosetting. Monocytes were further enriched by plastic adherence (1 h, 37°C) and demonstrated a purity of >95% as detected by FACS analysis with anti-CD14 antibody. Monocytes were cultured at 37°C at a density of 1–2×106 ml−1 in RPMI 1640 supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 6 ng ml−1 of colistine and the appropriate amount of FBS.
The cell line employed in this study was the U937 cell line, a human monocytic cell line cultured in RPMI 1640 containing 10% FBS.
IL-6 protein determination
Freshly isolated monocytes (1×106 ml−1) were incubated in RPMI I640 with 2% FBS. Sixteen hours after the adherence step, medium was replaced with 1 ml of fresh RPMI 1640 containing 2% FBS and cells were subsequently stimulated. Twenty-four hours after treatment with medium, lipopolysaccharide (1 μg ml−1), IL-1 (100 U ml−1), MK-571 (10 μM) or combinations of these, cell free supernatants were harvested. In addition, blocking experiments were performed with PD98059 (10 μM) and SB203580 (1 μM). In these experiments the cells were pretreated with these agents for 15 min followed by the different activators. A similar experimental design was used with the LT inhibitor SB203347 (10 μM). The cell-free supernatants were collected and analysed for IL-6 protein by ELISA (CLB, Amsterdam, The Netherlands). The used inhibitor appeared to be non-toxic as determined by the trypan blue dye exclusion test tested after 24 h of stimulation. In all cases a viability >95% was observed.
LTC4 determination in cell free supernatant
Freshly isolated monocytes (1×106 ml−1) were cultured in RPMI 1640 with 0.1% FBS. The cells were stimulated with A23187 (1 μM) or pre-treated with SB203347 or MK-571 for 30 min followed by A23187 stimulation over 30 min. Cell free supernatant was collected and stored at −80°C. LTC4 levels were quantified using a commercially available LTC4 Enzyme Immunoassay kit (Cayman Chemical, Ann Arbor, MI, U.S.A.).
ROS determination in human monocytes
Freshly isolated monocytes (1×106 ml−1) were incubated with 5 μg 2′, 7′-dichlorodihydrofluorescein diacetate (DCF, Molecular Probe) per ml. Fluorescence intensity was measured with a fluorescence-activated cell sorter (Facstar, Becton Dickinson, CA, U.S.A.) after stimulation with medium, LPS (1 μg ml−1), MK-571, PDTC or pre-incubated with PDTC followed by stimulation with LPS in the absence or presence of MK-571 during 30 min.
RNA isolation, Northern blotting, and transient transfection assays
RNA was isolated from human monocytes followed by Northern blotting and hybridization with an IL-6 and 28S cDNA probe as previously described (Dokter et al., 1994; 1996). The 600 bp BalI-XhoI IL-6 promoter fragment was cloned into the pGL-2 luciferase reporter plasmid. Subsequent the reporter construct was transfected into U937 cells by means of electroporation. Prior to transfection, cells were cultured for 16 h at a density of 0.5×106 cells ml−1 in the appropriate medium, washed twice and resuspended in RPMI 1640 at a density of 10×106 in 200 μl. Twenty-five micrograms of promoter reporter plasmid DNA was added and the mixture was left at room temperature for 15 min.
Electroporation, in 0.4 cm electroporation cuvettes, was performed at 240 V and 960 μF with a Gene Pulser electroporator (Bio-Rad Laboratories, Richmond, VA, U.S.A.). After several electroporations cells were first pooled and subsequently divided into identical groups and replated in RPMI 1640 containing 2% FBS. Six hours after transfection, cells were stimulated for 24 h with medium, IL-1 or the combination of IL-1 and MK-571. The cells were then harvested and lysed in cell culture lysis reagent (Promega). Luciferase activity was determined using the Authos Lucy I luminometer (Authos Lab Inst, Salzburg, Austria). For the IL-6 promoter construct the luciferase activity of the untreated group was set at 100% and induction observed in the stimulated groups was relative to the unstimulated control.
Bacterial expression of GST-fusion proteins
Bacterial expression of glutathione-S-transferase (GST)-ATF2 and GST-c-Jun has been described previously. Briefly, GST fusion proteins were expressed in Escherichia coli DH5α, induced with 1 mM isopropyl-β-D-thiogalactopyranoside and purified with (GSH)-Sepharose beads.
In vitro kinase assays
JNK and p38 kinase activities were determined by the ability of these enzymes to phosphorylate their respective substrates c-Jun and ATF-2 in the presence of [γ-32P]-ATP. Monocytes were cultured overnight in RPMI 1640 with 1% FBS at a density of 1×106 cells ml−1 and the next day stimulated for 15 min with either LPS (1 μg ml−1), IL1 (100 U ml−1), MK-571 (20 μg ml−1) or the combinations thereof. 15×106 cells were harvested and lysed in 400 μl lysis buffer (HEPES (pH 7.4) 20 mM, EGTA 2 mM, β-glycero-phosphate 50 mM, DTT 1 mM , Na3VO4 1 mM, 1% Triton-X100, 10% glycerol, 10 μg ml−1 Leupeptin and PMSF 0.4 mM) for 15 min on ice. After 10 min centrifugation at 3000 r.p.m. (4°C), the supernatants were normalized for protein content and incubated with either 4 μl anti-JNK or anti-p38 antibody in a total volume of 500 μl for 30 min at 4°C. Twenty-five μl of a slurry of 50% Protein A Sepharose beads was added to the lysate/antibody mixture and left rotating overnight at 4°C. Subsequently, the antibody/beads conjugate was washed three times with lysis buffer, twice with LiCl buffer (LiCl 500 mM, Tris-Cl (pH 7.6) 100 mM, 0.1% Triton-X100 and DTT 1 mM) and finally three times with buffer A (MOPS (pH 7.2) 20 mM, EGTA 2 mM, MgCl2 10 mM, DTT 1 mM and 0.1% Triton-X100). After the supernatant was completely removed, the reaction was initiated by adding 50 μl of reaction mix (43.5 μl buffer A, MgCl2 20 mM, 25 μM ATP, 7.0 μg GST-c-Jun, GST-ATF-2 or 7.5 μg MBP and 10 μCi [γ-32P]-ATP) and incubation took place at 30°C for 20 min. The reaction was terminated by addition of 15 μl SDS-sample buffer (4×). Before examining the phosphorylation of the substrate proteins by SDS–PAGE, the samples were boiled for 5 min. Molecular weight marker (Amersham) was used to asses the correct protein size. The gel was washed in water for 15 min, twice in 5% TCA/1% Na2pyrophosphate and once in water. Gels were dried and phosphorylated proteins were visualized by autoradiography and quantified by Phosphor Imaging (Molecular Dynamics).
Electrophoretic mobility shift assay
Nuclear extracts were prepared from monocytes stimulated with IL-1, MK-571 or combinations over 2 h according to the mini-scale procedure as previously described (Dokter et al., 1994), divided into small aliquots and stored at −80°C. Double-stranded synthetic oligonucleotide probes containing the NF-κB and AP-1 consensus sequences of the IL-6 promoter (NF-κB: 5′-AGCTGCGGGATTTTCCCTG-3′, AP-1: 5′-AGCTCGCGTGACTCAGCTG-3′) were used in the gel retardation assay. Fifty ng of HPLC-purified single-stranded oligonucleotide was labelled with T4-polynucleotide kinase and [γ-32P]-ATP (3000 Ci mmol−1, Amersham), separated from non-incorporated radiolabel by sephadex G50 chromatography, ethanol precipitated, dried, and dissolved in 20 μl of Tris-HCl (pH 7.5) 10 mM, NaCl 50 mM, MgCl2 10 mM, EDTA 1 mM and DTT 1 mM, containing a 4 fold excess of the opposite strand. Annealing of the two strands was performed by heating the mixture for 2 min at 90°C and slow cooling to room temperature. Five μg nuclear extract and 0.2 ng double-stranded labelled oligonucleotide were incubated in HEPES (pH 7.9) 20 mM, KCl 60 mM, EDTA 0.06 mM, DTT 0.6 mM, spermidine 2 mM, 10% glycerol, supplemented with 2 μg poly(dI-dC). The binding reaction was performed at 26°C for 25 min. The samples were loaded on pre-run (30 min, 100 V) 4% (30 : 1) polyacrylamide gels and run for 1 h at 150 V in 0.5×TBE at room temperature. Gels were dried and exposed to Kodak XAR films at −80°C with an intensifying screen.
Materials
RPMI 1640 media was purchased from Biowhittaker, (Verviers, Belgium), foetal bovine serum (FBS) from Hyclone (Logan, UT, U.S.A.) and Lymphoprep was obtained from Nycomed, Oslo, Norway. Radionucleotides were obtained from Amersham (Buckinghamshire, U.K.). Antibodies against JNK1, and p38 kinase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.) and the anti-CD14 was obtained from Becton Dickinson (Sunnyville, CA, U.S.A.). The Mekk1 inhibitor PD98059 was purchased from New England Biolabs, (Beverly, MA, U.S.A.) the p38 inhibitor SB203580, and the LT inhibitor SB203347 were kindly provided by SmithKline Beecham Pharmaceuticals (King of Prussia, PA, U.S.A.) and the anti-oxidant pyrrolidine dithiocarbamate (PDTC) was purchased form Sigma. MK-571 was a generous gift of Dr A.W. Ford-Hutchinson, Merck-Frost, Quebec, Canada. cDNA probe for IL-6 was kindly provided by Dr L. Aarden (CLB, Amsterdam, The Netherlands). cDNA encoding GST-ATF-2 fusion protein was a generous gift from Dr H. van Dam (Molecular Carcinogenesis, University of Leiden, The Netherlands) and cDNA encoding GST-c-Jun was kindly provided by Dr J. Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands). ELISA kits for IL-6 (Pelikine Compac kits) were purchased from CLB, Amsterdam, The Netherlands and the CAT ELISA kit was obtained from Boehringer Mannheim GmbH (Mannheim, Germany). Lipopolysaccharide (LPS), A23187 and IL-1β were obtained respectively from Sigma and Immunex Corporation (Seattle, WA, U.S.A.).
Statistical analysis
Statistical analysis were performed on the secretion and transfection data using the Student's t-test for paired observations. Statistical significance of the data was set at P<0.05.
Results
MK-571 enhances IL-6 production in activated human peripheral blood monocytes
Freshly isolated monocytes were stimulated with medium, LPS (1 ng ml−1, MK-571 (10 μM), and the combination of both during 24 h. In response to LPS stimulation a distinct increase in IL-6 secretion was observed compared to unstimulated cells (3.9±2.2 ng ml−1, x±s.d. vs 74±10 pg ml−1, n=5). The LPS induced IL-6 secretion was further augmented by co-stimulating the cells with 10 μM MK-571 (2.0±0.4 fold increase, P<0.01). MK-571 alone did not induce IL-6 secretion (<60 pg ml−1) when used at the same concentration. Dose response curves with variable concentrations of MK-571 (1, 3, 5, 10 μM) in the presence of 1 μg ml−1 LPS demonstrated that the IL-6 secretion increased 1.3, 1.7, 3.0 and 4.3 fold. We therefore used 10 μM MK-571 in the following experiments. The upregulation of IL-6 expression was not only observed at protein level but also at mRNA level. A 1.6 fold increase in IL-6 mRNA expression was observed by the combination of LPS and MK-571 compared to the effects of LPS alone, as determined by Northern blotting and quantified by phosphoimager analysis. Moreover, it appeared that the stimulatory effect of MK-571 was not restricted to LPS. Monocytes stimulated with IL-1 also showed an enhanced production of IL-6 in the presence of MK-571 (5.7±3.5 fold enhancement, P<0.01).
Regulatory pathways involved in the MK-571 mediated IL-6 upregulation
Previous studies have demonstrated that IL-6 production is strongly regulated by stress activating protein (SAPK) in response to LPS and tumour necrosis factor (TNF-α) stimulation (Beyaert et al., 1996). In order to determine whether MK-571 affects these pathways, we studied the effect of MK-571 on the LPS-induced phosphorylation of p38 and JNK. MK-571 enhanced the phosphorylation of p38 in two independent experiments (1.4–1.6 fold induction) if the cells were co-stimulated with LPS, whereby MK-571 alone enhanced the activity of these kinases only slightly. A representative experiment is shown in Figure 1. In contrast no effect of MK-571 was observed on the phosphorylation of JNK in the presence of LPS.
Figure 1.
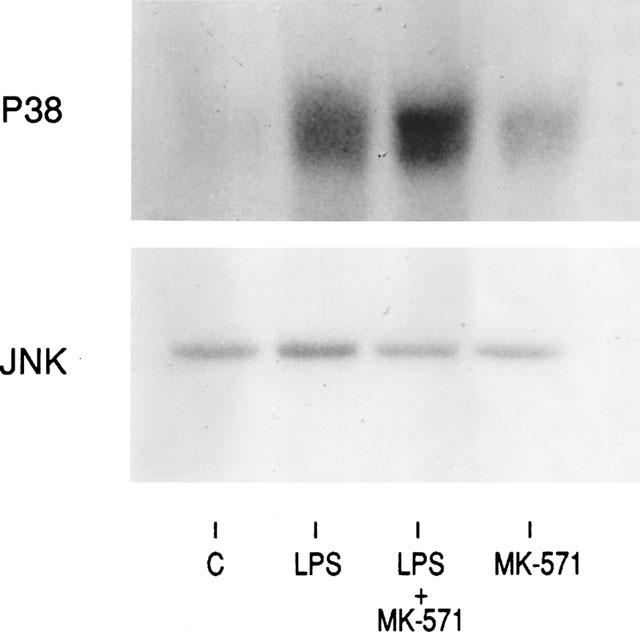
Synergistic effect of MK-571 and LPS co-stimulation on p38 but not JNK phosphorylation in peripheral blood monocytes. Monocytes were stimulated with LPS (1 ng ml−1) in the absence and presence of MK-571 (10 μM) The phosphorylation of p38 and JNK was studied by kinase assays as described in Methods. The presented data is one of the two experiments performed.
To confirm the involvement of p38 pathway in the MK-571 mediated effects, monocytes were also activated with IL-1 and MK-571 in the absence and presence of a specific inhibitor of the p38 route, e.g. SB203580 (Cuenda et al., 1995). As depicted in Figure 2A SB203580 (24±10% vs 100%) inhibited the IL-1 mediated secretion of IL-6. However, for IL-1 the p38 signalling was not the only signalling route involved in the IL-6 regulation since PD98059 a specific inhibitor of the MKK1,2 route (Alessi et al., 1995) inhibited the IL-1 mediated IL-6 secretion (37±16% vs 100%) The combination of PD98059 and SB203580 was most suppressive (7±3%). Similar experiments were performed in the presence of MK-571 and demonstrated that the MK-571 mediated up regulation in the presence of IL-1 was partially attenuated by each inhibitor applied separately (Figure 2B). However, the IL-1 induced IL-6 secretion could still be up regulated to a certain degree by MK-571 if the monocytes were treated with PD98059 and SB203580 (2.5±1.4 fold induction vs 9.0±3.0, Figure 2B) suggesting that other signalling pathways than p38 and ERK might also contribute to the MK-571 mediated upregulation of IL-6 expression.
Figure 2.
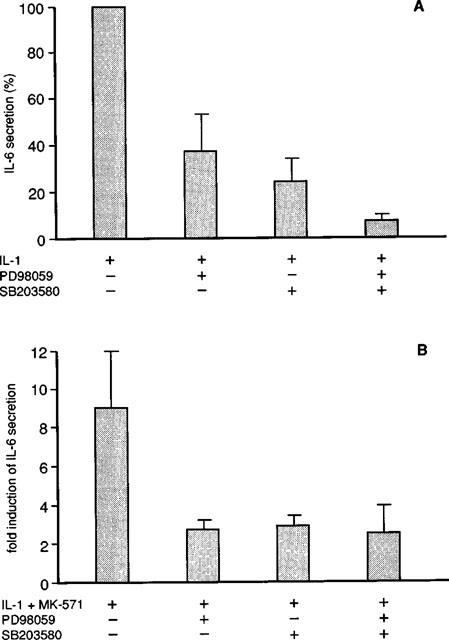
The inhibitory effects of PD98059 and SB203580 on the IL-1 and IL-1 plus MK-571 mediated IL-6 secretion from human monocytes. (A) Percentage increase in IL-6 secretion in response to IL-1 in the absence or presence of PD98059 (10 μM) and SB203580 (1 μM) or combinations. in unstimulated monocytes IL-6 protein was <60 pg ml−1 in the presence of IL-1 (0.9±0.7 ng ml−1, x±s.d.). This was set at 100%. The following results were obtained: IL-1 plus PD98059: 37±16% (x±s.d., n=3); IL-1 plus SB 203580 24±10% and IL-1 plus PD 98059 and SB203580: 7±3%. (B) Fold induction of IL-6 secretion compared to IL-1 stimulation alone; IL-1 plus MK-571: 9.0±3.0 (x±s.d., n=3), IL-1 plus MK-571 and PD98059 2.7±0.5; IL-1 plus MK-571 and SB203580: 2.9±0.5; IL-1 plus MK-571 and PD98059/SB203580: 2.5±1.4.
The data suggest that the activity of different transcription factors which are located downstream of p38 and Erk's are affected by MK-571. We and others previously demonstrated that the transcription factors AP-1 and NF-κB are very important for the regulation of IL-6 transcription (Dokter et al., 1994; 1996; Beyaert et al., 1996; Alessi et al., 1995; Libermann & Baltimore, 1990; Matsusaka et al., 1993). Therefore the DNA binding activity of AP-1 and NF-κB was studied by means of electrophoretic mobility shift assay. Optimal IL-1 or LPS induced binding activity of AP-1 or NF-κB was observed after 2 h of stimulation (Dokter et al., 1994) As depicted in Figure 3, AP-1 and NF-κB were distinctly up regulated by IL-1; the addition of MK-571 did not modulate the DNA binding activity of either of the transcription factors. Phospho-imaging analysis of two independent experiments showed that the IL-1 induced increase of NF-κB varied between 1.4–1.5, whereas the combination of IL-1 plus MK-571 resulted in 1.2–1.5 fold increase. With regard to AP-1, the values were 1.4–1.9 fold and 1.3–1.9 fold increase respectively. Similar results were obtained with LPS and MK-571 (1.5–1.8 fold increase irrespective of the presence of MK-571) (Figure 4).
Figure 3.
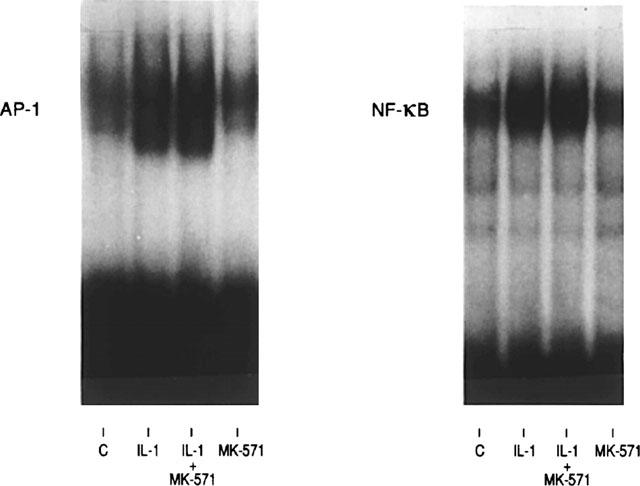
The effects of MK-571 on the DNA binding activity of NF-κB and AP-1 in response to IL-1 stimulation. Monocytes were stimulated with medium (C), MK-571, and IL-1 (100 U ml−1) in the absence and presence of MK-571 (10 μM). The DNA binding activity of NF-κB and AP1 were studied by means of electrophoretic mobility shift assay as described in Methods. The presented data is one of the two experiments performed.
Figure 4.
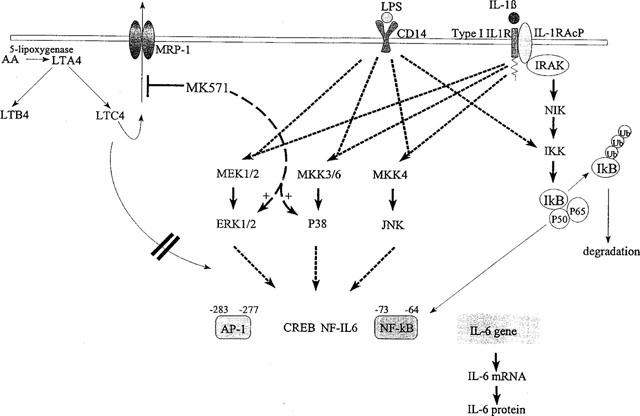
Schematic representation of the effects of LPS, IL-1 and MK-571 on IL-6 gene regulation in human monocytes. Both LPS and IL-1 activate the three depicted MAP kinase cascades resulting in the phosphorylation and activation of ERK1/2, p38 and JNK. In addition, both LPS and IL-1 activate the IκB kinase IKK, upon which IκB is phosphorylated and targeted for ubiquitination and degradation. NF-κB is then released from the inhibitor IκB and translocates to the nucleus. Activation of these pathways leads to enhanced IL-6 gene transcription and protein secretion, which is, at least partly, mediated by increased binding and transcriptional activity of the transcription factors NF-κB and AP-1. Blocking the function of the MRP1 pump by the compound MK-571 results in enhanced phosphorylation of ERK1/2 and p38, but not JNK, when monocytes are costimulated with either LPS or IL1. MK-571-enhanced activity of ERK and p38 is accompanied by an increased IL-6 transcription and IL-6 protein secretion. MK-571-mediated effects on IL-6 gene regulation are not inhibited when leukotriene-synthesis is inhibited by SB203347, suggesting that leukotrienes appear to play no role in the MK-571-stimulated IL-6 production.
In view of these data we questioned whether the transactivational activity of these transcription factors might be influenced by MK-571. Transient transfection studies were performed with a 300 bp IL-6 promoter construct containing binding sites for AP-1 and NF-κB fused to a luciferase reporter. For these experiments we used the monocytic cell line U-937 since we were unable to transfect freshly isolated human monocytes. U-937 cells were transfected with the 300 bp IL-6 promoter construct and activated with IL-1 (100 U ml−1), MK-571 (10 μM), or the combination of both. The addition of MK-571 resulted in a significant increase in IL-6 promoter activity compared to the effects of IL-1 alone: 3.9±1.0 fold induction vs 1.58±0.33, P<0.05), whereby MK-571 alone had no effect.
Blocking of the endogenous LT production does not modulate MK-571 mediated effects
The next set of experiments was aimed at discriminating between effects of exogenous or endogenous LTs. Firstly we tested whether exogenously added LTD4 might increase the IL-6 secretion by human monocytes. LTD4 used alone at a concentration of 0.25 and 2.5 μM did not induce IL-6 protein secretion (<60 pg ml−1, n=4). However, in combination with LPS a significant increase in IL-6 production was observed (1.5±0.3 fold increase, P<0.01) compared to the effects of LPS alone (10.3±4 ng ml−1). Recently it has been demonstrated that the compound SB203347 is a selective blocker of the 5-lipoxygenase pathway without effects on the prostaglandin production in activated human monocytes (Marshall et al., 1997). These results were confirmed in our experiments in which SB203347 suppressed the LTC4 secretion by human monocytes in response to stimulation with the calcium ionophore A23187 (1 μM), the most potent LT stimulator (2.84 ng ml−1±1.5 vs 13 pg ml−1±7 (n=3)). Moreover it appeared that SB203347 did not interfere with the MRP1 function since the MRP1 mediated efflux of carboxyfluorescein was not modulated by SB203347 (Van der Kolk et al., 1998) (data not shown). Subsequently we tested whether SB203347 could block the IL-6 production. The addition of SB203347 to LPS stimulated cells resulted in all cases in a slight decrease of the IL-6 production; 83±6% (x±s.d., n=3) of the LPS stimulated cells. However, when SB203347 was added to the LPS plus MK-571 stimulated cells no effect was observed on the IL-6 secretion. A similar pattern was observed when IL-1 was used instead of LPS, suggesting that the activation of signalling pathways and increase of IL-6 production by MK-571 is not mediated through effects on endogenous produced LTs.
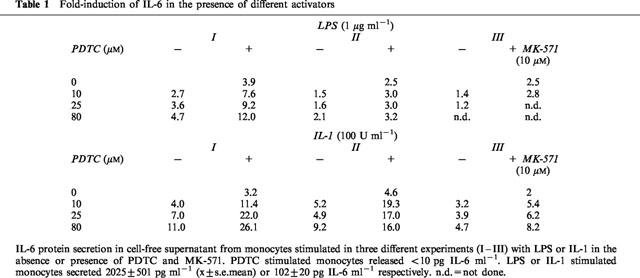
Recently it has been demonstrated that intracellular reactive oxygen species (ROS) promote NF-κB transactivation in response to IL-1 stimulation (Sulciner et al., 1996). Since ROS are effluxed in a GSH-dependent fashion by MRP1 we questioned whether inhibition of the MRP1 function by MK-571 might affect intracellular ROS levels and subsequent affect the IL-6 secretion. To study this aspect in more detail experiments were performed using the anti-oxidant PDTC in the absence and presence of MK-571 and LPS or IL-1. As depicted in Table 1, PDTC (10–80 μM) increased the IL-6 secretion especially when the cells were stimulated by IL-1 plus MK-571 whereby PDTC alone did not induce IL-6 secretion (<10 pg IL-6 ml−1, n=3) suggesting that ROS mediated signalling pathway is not involved in the MK-571 mediated effect. Eighty μM PDTC inhibited the basal (50%) and the LPS mediated increase of ROS production by a factor of 2.5 or more.
Table 1.
Fold-induction of IL-6 in the presence of different activators
Discussion
LTs are important mediators in the inflammatory response in which LTB4, LTC4 and LTD4 seem to have the most important functions. Previous studies have demonstrated that LTB4 is a potent stimulator of the IL-6 production by human monocytes (Ferreri et al., 1986; Stankova et al., 1992; Rola-Pleszczynski & Stankova, 1992). The present study shows that the biological activity of LTD4 is different from LTB4 since LTD4 enhanced the IL-6 secretion only when the cells were co-activated by LPS or IL-1. Additional differences that have been noted between LTC4, LTD4 vs LTB4 are differences in cellular metabolism and conjugation with GSH followed by transport across the plasma membrane by the ATP dependent transporter MRP1 (Keppler, 1992); Loe et al., 1996; Roelofsen et al., 1997; Leier et al., 1994). This is supported by the findings that photolabelling of the 190-kDa protein MRP1 with LTC4 in MRP1-expressing cells is inhibited by MK-571 (Loe et al., 1996). Moreover evidence has been obtained that LTC4 binds directly to MRP1 at a site within or near the epitope that is detected by the McAb QCRL-3.
Different down-stream kinases have been identified that are activated in response to LPS and IL-1 stimulation like the P38 and JNK kinases that are phosphorylated by their respective upstream kinases MKK-3 and MKK-4 (Schaub et al., 1991; Minden et al., 1994; Kyriakis et al., 1994; Rosette & Karin, 1996; Marshall, 1995; Sulciner et al., 1996). The results of the blocking studies confirm that p38 is involved since SB203580, a specific inhibitor of the p38 pathway, suppresses the IL-6 secretion in human monocytes. However, in contrast to the regulation of IL-6 in HeLa cells (Beyaert et al., 1996), the MKK-1/ERK cascade is also involved in the IL-1 mediated effect because PD98059 also inhibited the IL-1 mediated secretion of IL-6. The most pronounced inhibition was observed with the combination of both the p38 and ERK kinase inhibitors and this implies that p38 and ERK pathways are the most dominant routes involved in IL-1 mediated IL-6 production.
The observed stimulatory effect of MK-571 is also in part mediated by these signalling routes because MK571 further enhanced the phosphorylation of these kinases. Moreover the blocking studies demonstrated that PD98059 and SB203580 inhibited the MK-571 mediated secretion of IL-6. However, it is most likely that additional kinases are involved since PD98059 and SB203580 did not totally block the MK-571 mediated IL-6 upregulation. Of interest was the observation that MK-571 did not modulate the DNA binding of transcription factors AP-1 and NF-κB but rather enhanced the transactivational activity. Comparable results were recently observed for IL-6 activity in the HeLa cell line in which the blockers of the p38 signalling route suppressed IL-6 promoter activity in response to TNF stimulation without altering the DNA binding activity of NF-κB (Beyaert et al., 1996).
The stimulatory effect of MK-571 could not be inhibited by SB203347, a specific inhibitor of the LT production that does not affect the prostaglandin synthesis (Marshall et al., 1997). This observation implies that LT's appear to play no role in the MK571 stimulated IL-6 production, either by inhibiting MRP1-mediated LT secretion or by modulating yet unknown intracellular leukotriene-dependent pathways. This view is also supported by the data demonstrating that intracellular synthesized LTs have limited impact on the IL-6 production. The addition of SB203347 to the LPS or IL-1 stimulated monocytes resulted only in a 20% reduction of IL-6 production. These data suggest that additional mechanisms are involved in the MK-571 effects on IL-6 secretion. One possibility could be an effect on intracellular redox regulation. Recently it has been demonstrated that reactive oxygen species (ROS) are involved in NF-κB activation which is mediated by the small GTP-binding protein Rac1 (Sulciner et al., 1996). Cytokines, UV light and phorbol esters are capable of increasing intracellular ROS levels that are effluxed in a GSH-dependent fashion by MRP1 (Sulciner et al., 1996). Inhibition of the MRP1 transporter by MK-571 can affect the regulation of the redox state of the cell which in turn might influence the cytokine production by these cells. However experiments with the anti-oxidant PDTC demonstrated that PDTC did not inhibit but enhanced the IL-6 secretion even in the presence of MK-571. Alternatively the stimulatory effect might be related to an upregulation the transcription factor nuclear factor IL-6 (NF-IL-6) which has a prominent function in IL-6 promoter activity as recently described (Chinery et al., 1997).
MK-571 have been used in the past to inhibit inflammatory disorders especially whereby LT's are involved (Samuelsson et al., 1987; Jones et al., 1989). The present study demonstrates that the use of these agents might have detrimental effects especially in cases where the cells are triggered by cytokines.
Acknowledgments
This study was supported by a grant of the Dutch Cancer Foundation (RUG 94-788).
Abbreviations
- AET
2-aminoethylisothiouroniumbromide
- AP-1
activator protein-1
- DCF
dichlorodihydrofluorescein diacetate
- FBS
foetal bovine serum
- GSH
glutathione
- GST
glutathione-S-transferase
- IL
interleukin
- LPS
lipopolysaccharide
- LTC4
leukotriene C4
- MAPK
mitogen activated protein kinases
- MRP1
multidrug resistance protein1
- NF-κB
nuclear factor κB
- PDTC
pyrrolidine dithiocarbamate
- ROS
reactive oxygen species
- SAPK
stress activated protein kinases
- SRBC
sheep red blood cells
- TNF-α
tumour necrosis factor-α
References
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- BEYAERT R., CUENDA A., VANDEN BERGHE W., PLAISANCE S., LEE J.C., HAEGEMAN G., COHEN P., FIERS W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis in response to tumour necrosis factor. EMBO J. 1996;15:1914–1917. [PMC free article] [PubMed] [Google Scholar]
- CHINERY R., BROCKMAN J., PEELER M.O., SHYR Y., BEAUCHAMP R.D., COFFEY R.J. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: A p53-independent induction of p21waf1/CIP1 via C/EBPβ. Nature Medicine. 1997;3:1233–1239. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- CUENDA A., ROUSE J., DOZA Y.N., MEIER R., COHEN P., GALLAGHER T.F., YOUNG P.R., LEE J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–234. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- DOKTER W.HA., DIJKSTRA A.J., KOOPMANS S.B., STULP B.K., KECK W., HALIE M.R., VELLENGA E. G(Anh)MTetra, a natural bacterial cell wall breakdown product, induces interleukin-1 beta and interleukin-6 expression in human monocytes. A study of the molecular mechanisms involved in inflammatory cytokine expression. J. Biol. Chem. 1994;269:4201–4207. [PubMed] [Google Scholar]
- DOKTER W.H.A., KOOPMANS S.B., VELLENGA E. Effects of IL-10 and IL-4 on LPS-2 induced transcription factors (AP-1, NF-IL6 and NF-kappa B) which are involved in IL-6 regulation. Leukemia. 1996;10:1308–1313. [PubMed] [Google Scholar]
- FERRERI N.R., HOWLAND W.C., SPIEGELBERG H.L. Release of leukotrienes C4 and B4 and prostaglandin E2 from human monocytes stimulated with aggreated IgG, IgA and IgE. J. Immunology. 1986;136:4188–4195. [PubMed] [Google Scholar]
- GEKELER V., ISE W., SANDERS K.H., ULRICH W.R., BECK J. The leukotriene LTD4 receptor antagonist MK-571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995;208:345–349. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- JONES R.T., ZAMBONI R., BELLEY M., CHAMPION E., CHARETTE L., FORD-HUTSCHINSON A.W., FRENETT R., GAUTHIER J-Y., LEGER S., MASSON P., MCFARLANDE C.S., PEICHUTA H., ROKACH J., WILLIAMS H., YOUNG R.N., DEHAVEN R.N., PONG S.S. Pharmacology L-660, 711 (MK-571): a novel potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1989;67:17–31. doi: 10.1139/y89-004. [DOI] [PubMed] [Google Scholar]
- KEPPLER D. Leukotrienes: biosynthesis, transport, inactivation, and analysis. Rev. Physiol. Biochem. Pharmacol. 1992;121:1–12. doi: 10.1007/BFb0033192. [DOI] [PubMed] [Google Scholar]
- KYRIAKIS J.M., BANERJEE P., NIKOLAKAKI E., DAI T., RUBIE E.A., AHMAD M.F., AVRUCH J., WOODGETT J.R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–161. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- LEIER J., JEDLITSCHKY G., BUCHHOLZ U., COLE S.P.C., DEELEY R.G., KEPPLER D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugtes. J. Biol. Chem. 1994;269:27807–27811. [PubMed] [Google Scholar]
- LIBERMANN T.A., BALTIMORE D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990;10:2327–2331. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE D.W., ALMQUIST K.C., DEELEY G., COLE S.P.C. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. J. Biol. Chem. 1996;271:9675–9677. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- MARSHALL C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–187. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- MARSHALL L.A., BOLOGNESE B., WINKLER J.D., ROSHAK A. Depletion of human monocyte 85-kDa phospholipase A2 does not alter leukotriene formation. J. Biol. Chem. 1997;72:759–765. doi: 10.1074/jbc.272.2.759. [DOI] [PubMed] [Google Scholar]
- MATSUSAKA T., FUJIKAWA K., NISHIO Y., MUKAIDA N., MATSUSHIMA K., KISHIMOTO T., AKIRA S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINDEN A., LIN A., MCMAHON M., LANGE-CARTER C., DÉRIJARD B., DAVIS R.J., JOHNSON G.L., KARIN M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- MÜLLER M., MEIJER C., ZAMAN G.J.R., BORST P., SCHEPER R.J., MULDER N.H., DE VRIES E.G.E., JANSEN P.L.M. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc. Natl. Acad. Sci. U.S.A. 1994;91:13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜLLER M., DE VRIES E.G.E., JANSEN P.L.M. Role of multidrug resistance protein (MRP) in glutathione s-conjugate transport in mammalian cells. J. Hepatology. 1996;24:1–6. [PubMed] [Google Scholar]
- ROELOFSEN H., VOS T.A., SCHIPPERS I.J., KUIPERS F., KONING H., MOSHAGE H., JANSEN P.L.M., MÜLLER M. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells. Gastroenterology. 1997;112:511–516. doi: 10.1053/gast.1997.v112.pm9024305. [DOI] [PubMed] [Google Scholar]
- ROLA-PLESZCZYNSKI M., STANKOVA J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood. 1992;80:1004–1008. [PubMed] [Google Scholar]
- ROSETTE C., KARIN M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1199. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B, , DAHLEN S.E., LINDGREN C.A., ROUZER C., SERHAN C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1175. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- SCHAUB T., ISHIKAWA T., KEPPLER D. ATP-dependent leukotriene export form mastocytoma cells. FEBS Lett. 1991;279:83–94. doi: 10.1016/0014-5793(91)80256-3. [DOI] [PubMed] [Google Scholar]
- STANKOVA J., ROLA-LESZCZYNSKI M. Interleukin 6 production by mononuclear phagocytes can be stimulated by leukotrienes. Archivum. Imm. et Th. 1992;40:17–25. [PubMed] [Google Scholar]
- SULCINER D.J., IRANI K., YU Z-X., FERRANS V.J., GOLDSMIDT-CLERMONT P., FINKEL T. RAC1 regulates a cytokine-stimulated redox-dependent pathway necessary for NF-κB activation. Mol. Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER KOLK D.M., DE VRIES E.G.E., KONING J.A., VAN DEN BERG E., MÜLLER M., VELLENGA E. Activity and expression of the multidrug resistance protein MRP1 and MRP2 in acute myeloid leukemia (AML) cells, tumor cell lines and normal hematopoietic CD34+ peripheral blood cells. Clin. Cancer Res. 1998;4:1727–1736. [PubMed] [Google Scholar]
- YOKOMIZO T., IZUMI T., CHANG K., TAKUWA Y., SHIMIZU T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1992;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]


