Abstract
We investigated in the present study whether 5-HT is able to exert direct relaxant responses in canine basilar and middle cerebral arteries via the 5-HT7 receptor.
In arterial rings deprived of endothelium and pre-contracted with prostaglandin F2α (2 μM), 5-HT, 5-carboxamidotryptamine (5-CT), 5-methoxytryptamine, sumatriptan or α-methyl-5-HT produced further increase in tone and/or slight relaxation. Blockade of 5-HT1B/1D and 5-HT2A receptors with GR127935 (1 μM) and ketanserin (0.1 μM), respectively, antagonized the vasoconstrictor component of the response and unmasked a concentration-dependent relaxation to 5-HT, 5-CT and 5-methoxytryptamine; sumatriptan and α-methyl-5-HT remained inactive as relaxant agonists. The rank order of agonist potency in both arteries was 5-CT>5-HT>5-methoxytryptamine>>sumatriptan⩾α-methyl-5-HT.
In dog basilar artery, pre-incubated with GR127935 (1 μM) and ketanserin (0.1 μM) and pre-contracted with prostaglandin F2α (2 μM), the 5-HT7 ligands, clozapine (1 μM), mesulergine (0.3 μM), methiothepin (3 nM), risperidone (3 nM), spiperone (1 μM) and LY215840 (10–100 nM), produced significant rightward shifts of the concentration-response curves for 5-HT and 5-CT. Only methiothepin and risperidone reduced significantly the maximum relaxant response (Emax), whilst the other drugs behaved as competitive antagonists with affinity values (pKB) that significantly correlated with their binding affinity (pKi) at recombinant 5-HT7 receptors.
These data disclosing the involvement of the 5-HT7 receptor in cerebrovascular relaxation may be strongly relevant in the light of : (1) the involvement of 5-HT in migraine; (2) the putative linkage between cephalovascular vasodilatation and migraine headache; and (3) the relatively high 5-HT7 receptor affinity of migraine prophylactic 5-HT antagonists.
Keywords: Basilar artery, 5-hydroxytryptamine, middle cerebral artery, migraine, 5-HT7 receptor, relaxation, vascular smooth muscle
Introduction
Serotonin (5-hydroxytryptamine; 5-HT) has long been implicated in the pathophysiology of migraine (Fozard, 1982; Kimball et al., 1960; Sicuteri et al., 1961; Sjaastad, 1975; Silberstein, 1994) though the precise mechanisms involved are still a matter of debate. From the standpoint of the ‘vascular' hypothesis of migraine, very early data suggested that vasodilatation of the extra-cerebral branches of the external carotid artery (Ostfeld & Wolff, 1958; Saxena, 1972; Saxena & De Vlaam-Schluter, 1974; Tunis & Wolff, 1952) and to some extent also in the cerebral vessels (O'Brien, 1971; Skinhöj & Paulson, 1969) could be associated with the headache phase. Although new concepts have emerged from extensive research in this field (Fozard & Kalkman, 1994; Moskowitz, 1992), in the context of this vascular link (Humphrey & Feniuk, 1991; Saxena & Ferrari, 1989) the mechanisms underlying cerebrovascular vasodilatation in migraine and the possible role of 5-HT in its aetiology, remain to be determined.
More recently, given the evidence that nitric oxide (NO) may be involved in migraine headaches (Olesen et al., 1994), it was suggested that endogenous 5-HT, released perhaps from platelets, but most likely from perivascular 5-HT containing neurons in response to stress, stimulates endothelial 5-HT2B receptors in cerebral blood vessels to release NO (Fozard, 1995; Fozard & Kalkman, 1994; Schmuck et al., 1996). This latter, in addition to causing vasodilatation, would produce the ‘sterile inflammatory response' in the cerebral vasculature which, according to the ‘neurogenic' hypothesis of migraine, is believed to be the key step in the development of this disorder (Fozard, 1995; Fozard & Kalkman, 1994; Moskowitz, 1992). These observations notwithstanding, it should be noted that no convincing evidence for an endothelium-dependent NO-mediated relaxant effect by 5-HT in cerebral blood vessels has yet been provided (Schmuck et al., 1996; Connor & Feniuk, 1989). If cerebrovascular vasodilatation does underlie the development of headache, as suggested by the ‘vascular' hypothesis of migraine (Humphrey & Feniuk, 1991; Saxena & Ferrari, 1989), and 5-HT is involved in this process, one could expect from the above observations that another mechanism e.g. 5-HT7 receptor, may be involved in a putative vasodilator effect induced by 5-HT in the cerebral vasculature. In fact, it is very interesting to note that most of the migraine prophylactic drugs, including amitriptyline, chlorpromazine, cyproheptadine, lisuride, LY215840, metergoline, methysergide, mianserin, and sergolexole, display moderate to high affinity for the recombinant 5-HT7 receptor (see Terrón, 1998a for review). On this basis, the present study was aimed to investigate whether, as previously reported in the dog coronary artery (Terrón, 1996), treatment with the potent and selective 5-HT1B/1D receptor antagonist, GR127935 (Skingle et al., 1996), could unmask a direct relaxing mechanism probably related to the 5-HT7 receptor in canine cerebral arteries. Since large intracranial arterial dilatation has been associated with migraine headache in patients (Friberg et al., 1991), we selected the basilar and middle cerebral arteries of a model system, i.e. the dog, for the study. The results reveal that 5-HT does produce direct relaxation in the above vessels through a receptor that displays close pharmacological alignment to the 5-HT7 type. Preliminary accounts of this investigation have been published previously in abstract form (Terrón, 1997a; 1998b).
Methods
Tissue preparation
A total of 28 mongrel dogs (15–25 kg) of either sex were anaesthetized with sodium pentobarbitone (30 mg kg−1) and killed by rapid exsanguination from the carotid arteries. Brains were quickly removed and basilar and middle cerebral arteries were dissected and placed in Krebs bicarbonate solution and stored overnight at 4°C. The vessels were cleaned of adherent tissue and cut into ring segments 3–4 mm in length. Up to eight (basilar) or four (middle cerebral) adjacent rings from the same vessel were used as experimental and control rings. The middle cerebral artery of both sides was employed. In order to remove the endothelium, the vessels were perfused intraluminally with Triton X-100 (0.1%, 0.5 ml min−1 for 1 min), as previously reported (Verrechia et al., 1986).
Organ chamber studies
Rings were suspended horizontally in an organ bath by two L-shaped stainless-nikrom wire hooks (0.2 mm diameter). The lower hook was attached by a clamp to a tissue holder, whilst the upper hook was connected directly to an isometric force displacement transducer (Grass FT03C) so that isometric changes in force could be recorded on a Grass model 7D Polygraph. The rings were mounted in organ chambers containing 10 ml of Krebs bicarbonate solution of the following composition (mM concentrations): NaCl, 118; KCl, 4.8; CaCl2, 1.15; MgSO4, 1.2; NaHCO3, 24; KH2PO4, 1.2; dextrose, 11; and Ca2EDTA, 0.026. This solution was maintained at 37°C and aerated continuously with 95% O2 and 5% CO2 to give pH 7.4. Tissues were gradually stretched over a period of 30 min to a basal tension of 3 g; this amount of resting tension has been shown to maximize the contractile response to 5-HT and other spasmogens, including prostaglandin F2α (Allen et al., 1974). The preparations were allowed to equilibrate for 2 h with elicitation of contractile responses every 30 min by depolarization with 30 mM potassium chloride. Then, two or three contractile responses to prostaglandin F2α (2 μM) were obtained. This procedure ensured strong and sustained contractile responses to prostaglandin F2α for analysis of agonist-induced relaxation. The absence of functional endothelium was verified by exposure to oxytocin (1 μM) under pre-contraction with prostaglandin F2α (2 μM). Those rings that relaxed to oxytocin were not used for the study.
Experimental protocols
After completion of the above procedures, two protocols were followed. The first protocol was designed to evaluate the vasomotor activity of 5-HT (10 nM–100 μM), 5-carboxamidotryptamine (5-CT; 1 nM–10 μM), 5-methoxytryptamine (100 nM–100 μM), sumatriptan (100 nM–100 μM) and α-methyl-5-HT (100 nM–100 μM) in basilar and middle cerebral artery rings pre-incubated with either vehicle or GR127935 (1 μM) to block 5-HT1B/1D receptors. Since 5-HT2A receptors have been suggested to partly mediate the contractile response to 5-HT in the dog basilar artery (Connor et al., 1989), the experiments were conducted in the presence of ketanserin (0.1 μM) as well. Thus, after 30 min of equilibration with either vehicle or GR127935 and ketanserin, preparations were contracted with prostaglandin F2α (2 μM). In the plateau phase of the contraction, which became stable in about 15 min, the agonists were cumulatively added to obtain full concentration-response (C-R) curves. Therefore, the incubation time with vehicle or GR127935 and ketanserin was approximately 45 min.
The second protocol explored the effects of the high-affinity 5-HT7 receptor ligands, clozapine (1 μM), mesulergine (0.3 μM), methiothepin (3 nM), risperidone (3 nM), spiperone (1 μM) and LY215840 (10–100 nM), on the relaxation induced by 5-HT and 5-CT in the canine basilar artery. These experiments were conducted in the presence of GR127935 (1 μM) and ketanserin (0.1 μM). Then, cumulative C-R curves for 5-HT and 5-CT were generated in tissues incubated with either vehicle (controls) or antagonist for 1 h. Each concentration of agonist (spaced by a factor of 10½) was added only after the maximum response to the previous concentration had been attained. Responses to 5-HT and 5-CT in vehicle- and antagonist-treated tissues were elicited in separate rings so that only one C-R curve was obtained in each tissue.
Data presentation and statistical evaluation
All data in the text and figures are expressed as the mean±s.e.mean, where n represents the number of dogs from which the vessels were taken. In order to restrict the number of dogs used in the present study, no more than one tissue was used from each animal for any given treatment. Changes in tension are expressed as percentage of the contraction to prostaglandin F2α (2 μM). Comparisons between vehicle- and antagonist-treated rings obtained from the same animal were performed in separate tissues and no tissue was used to generate more than one agonist C-R curve. The pD2 values (negative logarithm of EC50, the agonist concentration producing 50% of the maximum relaxant response, calculated by nonlinear regression analysis) and the maximum relaxant response (Emax) were determined from individual C-R curves. Apparent antagonist dissociation constants (KB), expressed as the negative logarithm (−log KB=pKB), were determined according to the equation KB=B/(dose ratio-1) where B is the concentration of the antagonist and dose ratio is the EC50 of the agonist in the presence of the antagonist divided by the EC50 of the agonist in vehicle-treated preparations.
Comparisons of the relaxant responses obtained in vehicle- and antagonist-treated rings excised from the same animal were performed in separate tissues using one-way analysis of variance, followed by a Newman-Keuls' test. For comparison of the agonist potency (pD2) and efficacy (Emax) in the basilar and middle cerebral arteries, and the antagonist affinity estimates (pKB) obtained against 5-HT and 5-CT in the basilar artery, t-test was applied. Statistical significance was defined at P<0.05. The data for LY215840 were also analysed in accordance with the procedure of Arunlakshana & Schild (1959). The dose-ratios were determined at various concentrations of LY215840. If blockade is competitive under equilibrium conditions, then a plot of the logarithm of (dose ratio−1) against the negative logarithm of the molar concentration of the antagonist should yield a straight line whose slope is not different from unity and whose intercept on the abscissa is the pA2 which is generally considered to be equivalent to −log KB.
Drugs
The drugs used in the present study (obtained from the sources indicated) were the following: 5-hydroxytryptamine creatinine sulphate, oxytocin, prostaglandin F2α (Sigma Chemical Company, St. Louis, MO, U.S.A.); 5-methoxytryptamine hydrochloride and α-methyl-5-HT (Research Biochemicals Int., Natick, MA, U.S.A.); 5-carboxamidotryptamine maleate, GR127935 and sumatriptan succinate (gift: Glaxo Group Research, Ware, U.K.); clozapine and mesulergine (gift: Sandoz A.G., Basel, Switzerland); ketanserin tartrate, risperidone and spiperone (gift: Janssen Pharmaceutica, Beerse, Belgium); LY215840 (gift: Eli Lilly, Indianapolis, IN, U.S.A.); and methiothepin maleate (gift: Hoffman-La Roche Ltd., Basel, Switzerland).
All compounds were dissolved in distilled water. When needed, 4% ascorbic acid (clozapine, LY215840 and spiperone) or 5% (v v−1) DMSO (mesulergine, methiothepin and risperidone) was employed to prepare stock solutions from which aqueous dilutions were made. Fresh solutions were prepared for each experiment and vehicles had no effect on baseline tension or agonist-induced responses.
Results
As shown in Figure 1, 5-HT, 5-CT, 5-methoxytryptamine, sumatriptan and α-methyl-5-HT had no effects or produced further increase in tone and/or slight relaxation in control pre-contracted vessels. However, in the presence of GR127935 (1 μM) and ketanserin (0.1 μM), 5-HT, 5-CT and 5-methoxytryptamine, but not sumatriptan or α-methyl-5-HT, produced concentration-dependent relaxant responses in both the basilar and middle cerebral artery (Figure 1). Based upon the corresponding pD2 values, the rank order of agonist potency in both arteries was 5-CT>5-HT>5-methoxytryptamine>>sumatriptan⩾α-methyl-5-HT. Agonist affinity estimates were not significantly different when comparing the basilar and the middle cerebral artery and only small differences in agonist efficacy were observed (Table 1; Figure 1). For this reason, we decided to perform the interaction experiments in the basilar artery only.
Figure 1.
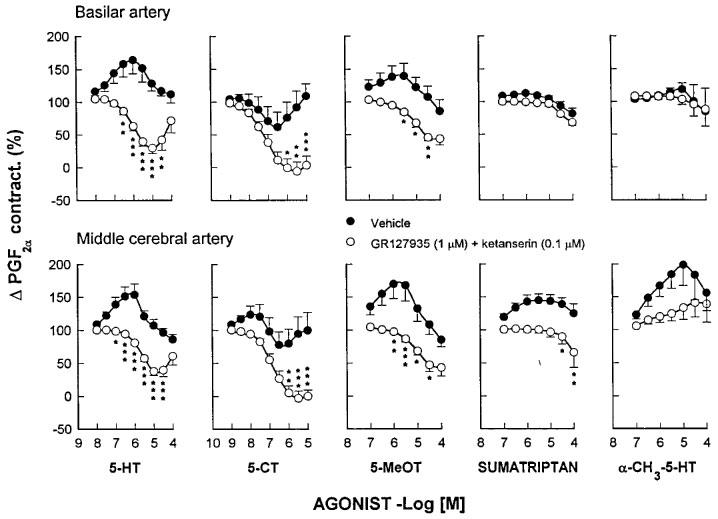
Effect of ketanserin and GR127935, as compared to that of vehicle, on cumulative concentration-response curves for 5-HT, 5-CT, 5-methoxytryptamine (5-MeOT), sumatriptan and α-methyl-5-HT (α-CH3-5-HT) in endothelium-denuded canine basilar and middle cerebral artery rings taken from the same animal. Changes in tension are expressed as percentage of the contraction to prostaglandin F2α (2 μM). Points are the mean, and vertical bars denote the s.e.mean of 3–6 observations. Contraction to prostaglandin F2α in the basilar artery was 2.06±0.13 and 1.83±0.1 g while in the middle cerebral artery was 1.49±0.17 and 1.33±0.11 g in vehicle- and antagonist-treated rings, respectively. *P<0.05, **P<0.01, ***P<0.001.
Table 1.
Relaxing potency (−log EC50; pD2) and efficacy (Emax) of 5-HT receptor agonists in endothelium-denuded canine basilar and middle cerebral arteries
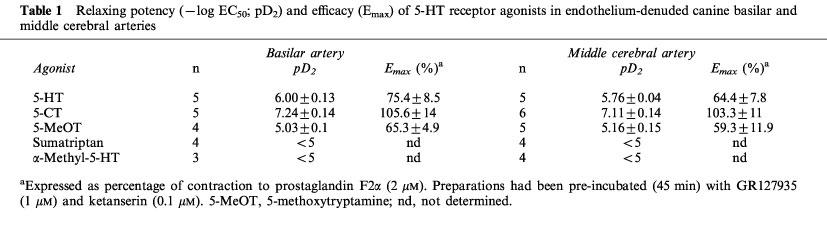
Thus, incubation of basilar artery ring segments with clozapine (1 μM), mesulergine (0.3 μM), methiothepin (3 nM), risperidone (3 nM) or spiperone (1 μM), produced significant rightward shifts of the C-R curves for 5-HT and 5-CT (Figure 2). As previously noticed in other vascular preparations (Martin & Wilson, 1995; Terrón, 1996; 1997b), methiothepin and risperidone behaved as potent unsurmountable antagonists as both caused a significant reduction in the Emax of 5-HT and 5-CT, while clozapine, mesulergine and spiperone were simple competitive antagonists (Figure 2). In addition, LY215840 produced a parallel concentration-dependent rightward shift of the C-R curves for 5-HT and 5-CT (Figure 3A). Schild analysis yielded straight lines with pA2 values (7.58 and 7.67 against 5-HT and 5-CT, respectively) that matched the affinity of LY215840 for the human 5-HT7 receptor (pKi=7.83; Cushing et al., 1996); the slope of the Schild plot (0.95 and 1.26 against 5-HT and 5-CT, respectively) was not significantly different from unity (Figure 3B).
Figure 2.
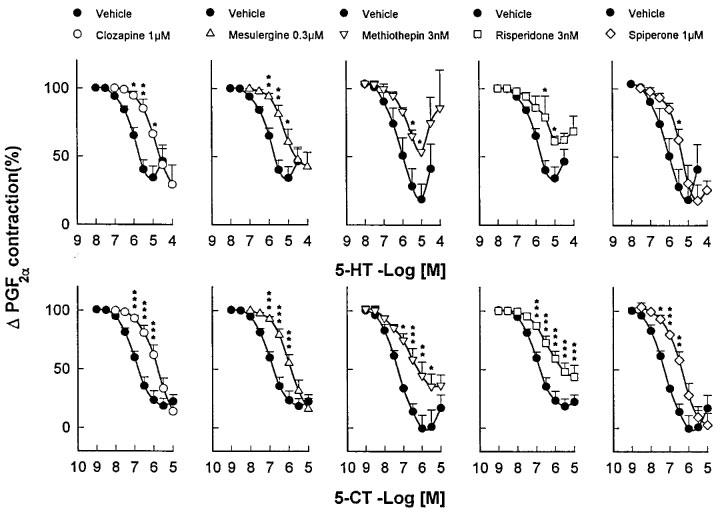
Effect of clozapine, mesulergine, methiothepin, risperidone or spiperone, as compared to that of vehicle, on cumulative concentration-response curves for 5-HT and 5-CT in endothelium-denuded canine basilar artery rings taken from the same animal. The vessels had been pre-incubated with GR127935 (1 μM) and ketanserin (0.1 μM). Changes in tension are expressed as percentage of the contraction to prostaglandin F2α (2 μM). Points are the mean and vertical bars denote the s.e.mean of 3–8 observations. Contraction to prostaglandin F2α was 2.45±0.31 and 2.75±0.25 g (5-HT set) and 2.45±0.27 and 2.24±0.2 g (5-CT set) in vehicle- and antagonist-treated rings, respectively. *P<0.05, **P<0.01, ***P<0.001.
Figure 3.
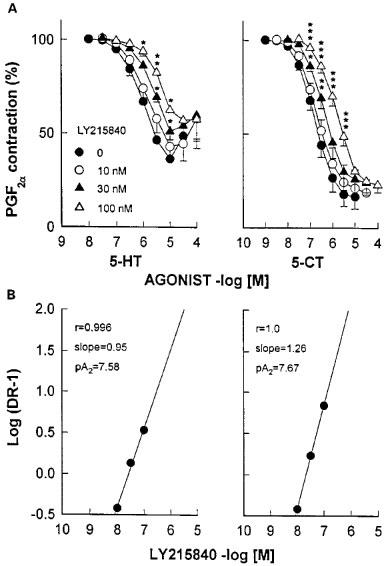
(A) Effect of increasing concentrations of LY215840 on cumulative concentration-response curves for 5-HT and 5-CT in endothelium-denuded canine basilar artery ring segments taken from the same animal. The vessels had been pre-incubated with GR127935 (1 μM) and ketanserin (0.1 μM). Changes in tension are expressed as percentage of the contraction to prostaglandin F2α (2 μM). Points are the mean and vertical bars denote the s.e.mean of 4–5 observations. Contraction to prostaglandin F2α was 3.46±0.35 and 2.95±0.23 g (5-HT set) and 3.36±0.25 and 3.19±0.21 g (5-CT set) in vehicle- and antagonist-treated rings, respectively. (B) Schild plots for the antagonist effects of LY215840. *P<0.05, **P<0.01, ***P<0.001.
Discussion
The present study demonstrates, for the first time, that 5-HT does produce endothelium-independent relaxant responses in major conduit cerebral arteries. On the basis of operational criteria, the 5-HT receptor involved can be classified within the 5-HT7 type. Despite the role of the 5-HT7 receptor in peripheral vascular and nonvascular smooth muscle relaxation has been elucidated (Eglen et al., 1997; Terrón, 1998a), its potential implication in the regulation of cerebroarterial tone may be strongly relevant in the light of: (1) the involvement of 5-HT in the pathogenesis of migraine; (2) the putative linkage between cephalovascular vasodilatation and migraine headache; and (3) the relatively high affinity of several migraine prophylactic drugs for the 5-HT7 receptor (see Terrón, 1998c for review). Apart from the implications discussed below, these findings may provide new insights into the regulatory role of 5-HT in cerebrovascular tone and perhaps also in the pathophysiological mechanisms of migraine.
Direct relaxant effects of 5-HT receptor agonists in dog basilar and middle cerebral arteries
It seems clear, from the use of GR127935 in the present experiments, that blockade of 5-HT1B/1D receptors is a requisite for the direct relaxant activity of 5-HT and some 5-HT receptor agonists to be manifest. Thus, the previously reported inability of 5-HT and 5-CT to cause relaxation in canine endothelium-intact basilar artery rings pre-contracted with prostaglandin F2α, U46619, uridine triphosphate or potassium chloride, even in the presence of a high concentration of ketanserin (1 μM) and phenoxybenzamine (30 μM) (Connor & Feniuk, 1989), most likely reflects a predominant role of vasoconstrictor mechanisms over those mediating relaxation due to the inability of these blockers to abolish 5-HT1B/1D receptor-mediated vasoconstriction. Since endothelium-denuded preparations were employed in the present study, the relaxant effect of 5-HT is most likely due to an action on smooth muscle cells. In fact, the relaxant response induced by 5-HT and 5-CT in endothelium-denuded GR127935- and ketanserin-treated preparations of basilar and middle cerebral artery could not be inhibited by pre-treatment with the NO synthase and cyclo-oxygenase inhibitors, Nω-nitro-L-arginine methyl ester (L-NAME; 100 μM) and indomethacin (10 μM), respectively (unpublished). On the other hand, the rather selective 5-HT2B receptor agonist, α-methyl-5-HT (Baxter et al., 1995), which is well-documented to produce strong and potent endothelium-dependent 5-HT2B receptor-mediated relaxant responses in peripheral vessels (Bodelson et al., 1993; Glusa & Richter, 1993; Leff et al., 1987; Sumner, 1991), failed to relax both endothelium-intact (not shown) and endothelium-free dog basilar and middle cerebral artery ring segments (Figure 1) pre-incubated with GR127935 (1 μM) and ketanserin (0.1 μM). These observations in dog cerebral arteries may therefore argue against the mechanistic concept in migraine involving a 5-HT-induced release of NO, via activation of endothelial 5-HT2B receptors, in cerebral blood vessels (Fozard & Kalkman, 1994; Fozard, 1995; Schmuck et al., 1996).
Pharmacological profile at the relaxant 5-HT receptor in canine cerebral arteries
As observed in other vascular preparations in which 5-HT mediates smooth muscle relaxation through the 5-HT7 receptor (Leung et al., 1996; Martin & Wilson, 1995; Terrón, 1996; 1997b), the rank order of agonist potency obtained in the canine basilar and middle cerebral arteries i.e. 5-CT>5-HT>5-methoxytryptamine>>sumatriptan⩾α-methyl-5-HT, is consistent with the binding profile reported at recombinant 5-HT7 receptors (Bard et al., 1993; Lovenberg et al., 1993; Plassat et al., 1993; Ruat et al., 1993; Shen et al., 1993). Since agonist pD2 values obtained in the basilar and middle cerebral arteries were not significantly different (Table 1), it is likely that a closely related or even an identical receptor mediates the relaxant response to 5-HT in both tissues.
On the other hand, the 5-HT7 receptor ligands, clozapine, mesulergine, methiothepin, risperidone and spiperone, which are well-documented antagonists at vascular 5-HT7 receptors (Leung et al., 1996; Martin & Wilson, 1995; Sumner et al., 1989; Terrón, 1996; 1997a,1997b; Villalón et al., 1997), significantly blocked the relaxant response to 5-HT and 5-CT in the basilar artery (Figure 2). Importantly, these effects were achieved at concentrations consistent with the affinity of the antagonists at the 5-HT7 receptor (Table 2; see Bard et al., 1993; Ruat et al., 1993; Shen et al., 1993). In order to further characterize the relaxant cerebrovascular 5-HT receptor, we decided to evaluate the effects of LY215840 (Cushing et al., 1996), on 5-HT and 5-CT-induced relaxation in the basilar artery. In addition to having high affinity for 5-HT2 receptor subtypes, this ergoline was recently demonstrated to display high affinity for a transiently expressed human 5-HT7 receptor and to behave as potent competitive antagonist at the relaxant 5-HT7 receptor in the canine coronary artery smooth muscle (Cushing et al., 1996). As expected, LY215840 exerted a concentration-dependent rightward displacement of the C-R curves for 5-HT and 5-CT with no significant reduction in the maximum relaxant response to the agonists (Figure 3A). Both pKB values calculated from a single concentration of LY215840 (Table 2) and pA2 values obtained from the Schild plots depicted in Figure 3B are in close agreement with the binding affinity of LY215840 at the human 5-HT7 receptor (pKi=7.83; Cushing et al., 1996). In view that the antagonist dissociation constants (pKB values) for all the above drugs were similar regardless of whether 5-HT or 5-CT was used as relaxant agonist (Table 2), the involvement of a common receptor site can be suggested.
Table 2.
Affinity estimates for antagonists against 5-HT- and 5-CT-induced relaxation in endothelium-denuded canine basilar artery
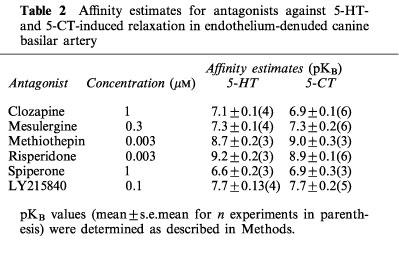
Although the antagonist drugs used in the present study display moderate to high affinity for other 5-HT receptors (see Terrón, 1998a for review), their affinity estimates at the relaxant 5-HT receptor significantly (P<0.01) correlated with their binding affinity at the recombinant 5-HT7 receptor (r=0.972 and r=0.981 against 5-HT and 5-CT, respectively, Figure 4). Correlation with other 5-HT receptors, including the 5-HT1A subtype (Figure 4), which is targeted by some agonists (e.g, 8-OH-DPAT and 5-CT) that also stimulate the 5-HT7 receptor (Eglen et al., 1997; Terrón, 1998a), and the 5-HT2B receptor (Figure 4), which has been associated with migraine pathogenesis (see below), were not only much lower than those obtained with the 5-HT7 type, but also a theoretical straight line connecting the correlation points could be rejected (P>0.05). Similarly, non-significant correlations were obtained with 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2C and 5-ht6 receptors (not shown).
Figure 4.
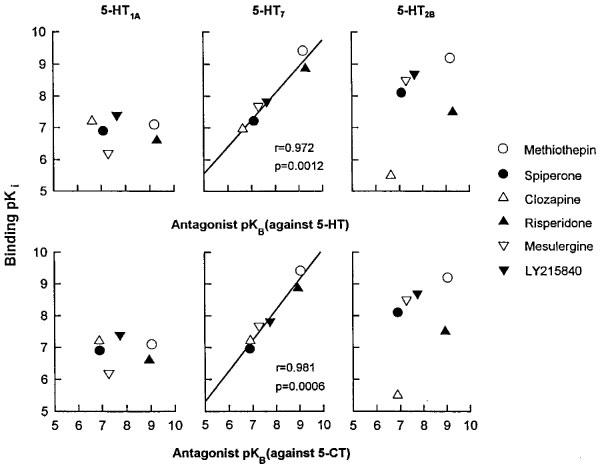
Correlation of antagonist affinity estimates (pKB values) against 5-HT and 5-CT at the relaxant 5-HT receptor in the canine basilar artery smooth muscle and binding affinity (pKi values) at 5-HT receptor subtypes.
Finally, it is worth mentioning that a high concentration (100 μM) of the Rp diastereoisomer of adenosine cyclic 3′,5′-monophosphothioate (Rp-cAMPS), an antagonist of the cyclic AMP-dependent protein kinase (PKA; Van Haastert et al., 1984), partially – but significantly – inhibited the relaxant response induced by 5-CT in the dog basilar artery pre-incubated with GR127935 (1 μM) and ketanserin (0.1 μM) (Terrón, unpublished observation). Although other transduction mechanisms could be involved, this finding suggests that, like native (Trevethick et al., 1986; Sumner et al., 1989) and cloned (Bard et al., 1993; Lovenberg et al., 1993; Plassat et al., 1993; Ruat et al., 1993; Shen et al., 1993) 5-HT7 receptors, the cerebrovascular 5-HT7 receptor may be positively coupled to the adenylyl cyclase system. This possibility could be further supported by an earlier study showing that 5-HT-induced inhibition of spontaneous rhythmic contraction of porcine pial veins, an effect displaying a 5-HT7-like receptor pharmacology, was (1) enhanced by a cyclic AMP phosphodiesterase inhibitor; (2) diminished by a PKA inhibitor; and (3) accompanied by an increase in cyclic AMP, but not cyclic GMP synthesis (Ueno et al., 1995). Although additional experiments are required to further elucidate the transductional pathway(s) linked to the cerebrovascular 5-HT7 receptor, the above observations are in agreement with the transductional criterion under which the 5-HT7 receptor was classified by the International Union of Pharmacology (IUPHAR) serotonin receptor classification committee (Hoyer et al., 1994).
Potential impact of the 5-HT7 receptor in migraine
An interesting observation prompting us to search for a relaxant 5-HT7-like receptor mechanism in cerebral vessels was the fact that, with the exception of pizotifen and propranolol for which no binding data at the 5-HT7 receptor have been reported thus far, most of the migraine prophylactic drugs such as amitriptyline, cyproheptadine, lisuride, methysergide and mianserin, display relatively high affinity (pKi values between 9 and 7.1) for the recombinant 5-HT7 receptor (Bard et al., 1993; Ruat et al., 1993; Shen et al., 1993). The same applies to the antimigraine drugs, sergolexole and LY215840, two ergoline-derivatives which are in phases II and III of clinical development, respectively, as well as to the recently launched antimigraine compound, metergoline (see Terrón, 1998a for review). In fact, some of the above drugs i.e. lisuride, LY215840, metergoline, methysergide, mianserin and sergolexole (the others have not been tested thus far), have been shown to antagonize functional 5-HT7 receptors mediating vasorelaxation in several vascular smooth muscle preparations (Cushing et al., 1996; Leung et al., 1996; Martin & Wilson, 1995; Sumner et al., 1989; Terrón, 1996; 1997a,1997b; Ueno et al., 1995), including the external carotid circulation (Villalón et al., 1997) which was long suggested to be involved in the pathophysiology of migraine (Ostfeld & Wolf, 1958; Saxena, 1972; Saxena & de Vlaam-Schluter, 1974; Tunis & Wolff, 1952). Most interesting is the observation that the average – pharmaceutically-active – doses of several migraine prophylactic drugs, including amitriptyline, chlorpromazine, cyproheptadine, lisuride, methysergide and mianserin, significantly correlate (r=0.989; P<0.001) with their reported affinity at the recombinant 5-HT7 receptor (Terrón, unpublished observation). On these bases, it seems reasonable to hypothesize that the migraine prophylactic efficacy of 5-HT2B/5-HT2C (and 5-HT7) receptor antagonists is due, at least in part, to blockade of craniovascular 5-HT7 receptors. In the case of propranolol, i.e. another major migraine prophylactic drug, it should be recalled its early-reported ability to potently antagonize 5-HT-induced relaxant responses in human pial vessels and temporal artery (pA2=8.29 and 8.50, respectively; Edvinsson et al., 1978). If such an interaction is confirmed to involve the 5-HT7 receptor, a similar mechanism of anti-migraine action for this drug could be speculated.
The potential involvement of the relaxant 5-HT7 receptor in the regulation of cerebroarterial tone and perhaps in migraine is further supported by recent molecular biological studies showing high expression of 5-HT7 receptor transcripts in both pig cerebral blood vessels (Ullmer et al., 1995) and several human meningeal tissues, including the internal carotid and middle meningeal artery (Schmuck et al., 1996). Although a consistent expression of the 5-HT2B message in these human tissues was observed, no convincing evidence for a functional role of the 5-HT2B receptor in the pig cerebral artery was provided i.e. the nonselective 5-HT2 receptor agonist, DOI, hardly relaxed the vessel by about 15% of the spasmogen-induced contraction (Schmuck et al., 1996). This is in marked contrast to the profound and potent 5-HT2B receptor-mediated endothelium-dependent relaxant responses produced by 5-HT and α-methyl-5-HT in various peripheral blood vessels, such as the rabbit jugular vein (Leff et al., 1987; Martin et al., 1987), pig vena cava (Sumner, 1991), pig pulmonary artery (Glusa & Richter, 1993) and rat jugular vein (Bodelsson et al., 1993). It must be recalled that the hypothesis suggesting a link between 5-HT and endothelial NO in the pathogenesis of migraine was primarily based on the unsubstantiated assumption that an endothelial 5-HT2B receptor-mediated mechanism, similar to that observed in peripheral vessels, would promote release of NO in the cerebral vasculature (Fozard, 1995; Fozard & Kalkman, 1994). However, the weak endothelium-dependent cerebroarterial relaxation referred to above may argue against this hypothesis. It follows that other mechanisms, such as the one mediating cerebrovascular dilatation through the 5-HT7 receptor (present results), could be involved in the pathophysiological events that occur in migraine.
Finally, another implication arising from the present study is concerned with the controversy as to whether it is depletion or mobilization of 5-HT that predisposes to migraine. If the relaxant mechanism shown here and elsewhere (Ueno et al., 1995; Villalón et al., 1997) is actually involved in cephalovascular vasodilatation and migraine, the proposed excess of 5-HT as a key event in the initiation of migraine (Fozard, 1992; 1995; Fozard & Kalkman, 1994) is supported. Interestingly, the 5-HT7 receptor, being located in craniovascular smooth muscle, would be best targeted by neuronal 5-HT released from perivascular 5-HT-containing neurons so that smooth muscle relaxation is produced without a need of interacting with the endothelial compartment. In this context, and relevant to the present observations in canine basilar and middle cerebral arteries, previous transcranial Doppler sonography studies showed a significant decrease in middle cerebral artery blood velocity, and its reversal by sumatriptan, on the headache side in migraine patients; in contrast, blood flow velocity was unchanged in the non-headache side (Friberg et al., 1991).
In conclusion, the present study demonstrates that 5-HT elicits direct relaxation in canine basilar and middle cerebral arteries through a receptor highly resembling the 5-HT7 type. These findings, along with those reported in the canine external carotid circulation (Villalón et al., 1997) and the porcine pial vein (Ueno et al., 1995), may be strongly relevant in the context of the role of 5-HT in cerebrovascular vasodilatation and migraine. This contention does gain weight when considering the relatively high affinity of several migraine prophylactic 5-HT receptor antagonists for the 5-HT7 receptor and its highly significant correlation with their orally-active clinical doses. Provided that this mechanism operates in the human cerebral vasculature, the potential implication of the 5-HT7 receptor in migraine and other vascular headaches deserves a serious consideration.
Acknowledgments
The skilful technical assistance of Juan J. López-Guerrero (B.Sc.) is gratefully acknowledged. The authors thank the pharmaceutical companies for their generous gifts.
Abbreviations
- C-R
concentration-response
- IUPHAR
International Union of Pharmacology
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- PKA
cyclic AMP-dependent protein kinase
- Rp-cAMPS
Rp diastereoisomer of adenosine cyclic 3′,5′-monophosphothioate
References
- ALLEN G.S., HENDERSON M.L., CHOU S.N., FRENCH L.A. Cerebral arterial spasm Part 1: In vitro contractile activity of vasoactive agents on canine basilar and middle cerebral arteries. J. Neurosurg. 1974;40:433–441. doi: 10.3171/jns.1974.40.4.0433. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SHILD H.D. Some quantitative uses of drugs antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T.A., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- BAXTER G., KENNET G., BLANEY F., BLACKBURN T. 5-HT2 receptor subtypes: a family re-united. Trends Pharmacol. Sci. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- BODELSSON M., TORNEBRANDT K., ARNEKLO-NOBIN B. Endothelial relaxing 5-hydroxytryptamine receptors in the rat jugular vein; similarity with the 5-hydroxytryptamine1C receptor. J. Pharmacol. Exp. Ther. 1993;264:709–716. [PubMed] [Google Scholar]
- CONNOR H.E., FENIUK W. Influence of the endothelium on contractile effects of 5-hydroxytryptamine and selective 5-HT agonists in canine basilar artery. Br. J. Pharmacol. 1989;96:170–178. doi: 10.1111/j.1476-5381.1989.tb11797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR H.E., FENIUK W., HUMPHREY P.P.A. Characterization of 5-HT receptors mediating contraction of canine and primate basilar artery by use of GR43175, a selective 5-HT1-like receptor agonist. Br. J. Pharmacol. 1989;96:379–387. doi: 10.1111/j.1476-5381.1989.tb11828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSHING D.J., ZGOMBICK J.M., NELSON D.L., COHEN M.L. LY215840, a high-affinity 5-HT7 receptor ligand, blocks serotonin-induced relaxation in canine coronary artery. J. Pharmacol. Exp. Ther. 1996;277:1560–1566. [PubMed] [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. Trend Pharmacol. Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., HARDEBO J.E., OWMAN C. Pharmacological analysis of 5-hydroxytryptamine receptors in isolated intracranial vessels of cat and man. Circ. Res. 1978;42:143–151. doi: 10.1161/01.res.42.1.143. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R.Serotonin, migraine and platelets Drugs and platelets 1982New York: Gustav Fischer Verlag; 135–146.eds. Van Zwieten, P.A. & Schönbaum, E. pp [Google Scholar]
- FOZARD J.R.5-HT1C receptor agonism as an initiating event in migraine 5-Hydroxytryptamine mechanisms in primary headache 1992New York: Raven Press; 200–212.eds. Olesen, J. & Saxena, P.R. pp [Google Scholar]
- FOZARD J.R. The 5-hydroxytryptamine-nitric oxide connection: the key link in the initiation of migraine. Arch. Int. Pharmacodyn. Ther. 1995;329:111–119. [PubMed] [Google Scholar]
- FOZARD J.R., KALKMAN H.O. 5-Hydroxytryptamine (5-HT) and the initiation of migraine: new perspectives. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:225–229. doi: 10.1007/BF00175026. [DOI] [PubMed] [Google Scholar]
- FRIBERG L., OLESEN J., IVERSEN H.K., SPERLING B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- GLUSA E., RICHTER M. Endothelium-dependent relaxation of porcine pulmonary arteries via 5-HT1C-like receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:471–477. doi: 10.1007/BF00166737. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HUMPHREY P.P.A., FENIUK W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol. Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- KIMBALL R.W., FRIEDMAN A.P., VALLEJO E. Effect of serotonin in migraine patients. Neurology (Minneap.) 1960;10:107–111. doi: 10.1212/wnl.10.2.107. [DOI] [PubMed] [Google Scholar]
- LEFF P., MARTIN G.R., MORSE J.M. Differential classification of vascular smooth muscle and endothelial cell 5-HT receptors by use of tryptamine analogues. Br. J. Pharmacol. 1987;91:321–331. doi: 10.1111/j.1476-5381.1987.tb10287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG E., WALSH L.K., PULIDO-RIOS M.T., EGLEN R.M. Characterization of putative 5-ht7 receptors mediating direct relaxation in Cynomolgus monkey isolated jugular vein. Br. J. Pharmacol. 1996;117:926–930. doi: 10.1111/j.1476-5381.1996.tb15282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG T.W., BARON B.M., DE LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANIELSON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- MARTIN G.R., LEFF P., CAMBRIDGE D., BARRET V.J. Comparative analysis of two types of 5-hydroxytryptamine receptor mediating vasorelaxation: differential classification using tryptamines. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:365–373. doi: 10.1007/BF00164867. [DOI] [PubMed] [Google Scholar]
- MARTIN G.R., WILSON R.J. Operational characteristics of a 5-HT receptor mediating direct vascular relaxation: identity with the 5-ht7 receptor. Br. J. Pharmacol. 1995;114:383P. [Google Scholar]
- MOSKOWITZ M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- O'BRIEN M.D. Cerebral blood flow changes in the migraine headache. Headache. 1971;10:139–143. doi: 10.1111/j.1365-2524.1971.hed1004139.x. [DOI] [PubMed] [Google Scholar]
- OLESEN J., THOMSEN L.L., IVERSEN H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol. Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- OSTFELD A.M., WOLFF H.G. Identification mechanisms and managements of the migraine syndrome. Med. Clin. N. Amer. 1958;42:1497–1509. doi: 10.1016/s0025-7125(16)34201-8. [DOI] [PubMed] [Google Scholar]
- PLASSAT J.L., AMLAIKY N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.-M., SCHWARTZ J.-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAXENA P.R. The effects of antimigraine drugs on the vascular responses by 5-hydroxytryptamine and related biogenic substances on the external carotid bed of dogs: Possible pharmacological implications to their antimigraine action. Headache. 1972;12:44–53. doi: 10.1111/j.1526-4610.1972.hed1202044.x. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R., DE VLAAM-SCHLUTER G.M. Role of some biogenic substances in migraine and relevant mechanism in antimigraine action of ergotamine – Studies in an experimental model for migraine. Headache. 1974;13:142–163. doi: 10.1111/j.1526-4610.1974.hed1304142.x. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R., FERRARI M.D. 5-HT1-like receptor agonists and the pathophysiology of migraine. Trends Pharmacol. Sci. 1989;10:200–204. doi: 10.1016/0165-6147(89)90238-1. [DOI] [PubMed] [Google Scholar]
- SCHMUCK K., ULLMER C., KALKMAN H.O., PROBST A., LÜBBERT H. Activation of meningeal 5-HT2B receptors: An early step in the generation of migraine headache. Eur. J. Neurosci. 1996;8:959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J., METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- SICUTERI F., TESTI A., ANSELMI B. Biochemical investigations in headache: increase in the hydroxyindoleacetic acid excretion during migraine attacks. Int. Arch. Allergy. 1961;19:55–58. [Google Scholar]
- SILBERSTEIN S.D. Serotonin (5-HT) and migraine. Headache. 1994;34:408–417. doi: 10.1111/j.1526-4610.1994.hed3407408.x. [DOI] [PubMed] [Google Scholar]
- SJAASTAD O. The significance of blood serotonin levels in migraine. A critical review. Acta Neurol. Scand. 1975;51:200–210. doi: 10.1111/j.1600-0404.1975.tb07601.x. [DOI] [PubMed] [Google Scholar]
- SKINGLE M., BEATTIE D.T., SCOPES D.I.C., STARKEY S.J., CONNOR H.E., FENIUK W., TYERS M.B. GR127935: a potent and selective 5-HT1D receptor antagonist. Behav. Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- SKINHÖJ E., PAULSON O.B. Regional blood flow in internal carotid distribution during migraine attack. Br. Med. J. 1969;3:569–570. doi: 10.1136/bmj.3.5670.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMNER M.J. Characterization of the 5-HT receptor mediating endothelium-dependent relaxation in porcine vena cava. Br. J. Pharmacol. 1991;97:292–300. doi: 10.1111/j.1476-5381.1991.tb12280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMNER M.J., FENIUK W., HUMPHREY P.P.A. Further characterization of the 5-HT receptor mediating vascular relaxation and elevation of cyclic AMP in porcine isolated vena cava. Br. J. Pharmacol. 1989;97:292–300. doi: 10.1111/j.1476-5381.1989.tb11953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRÓN J.A. The relaxant 5-HT receptor in the dog coronary artery smooth muscle: pharmacological resemblance to the cloned 5-ht7 receptor subtype. Br. J. Pharmacol. 1996;118:1421–1428. doi: 10.1111/j.1476-5381.1996.tb15555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRÓN J.A. Evidence for the putative 5-HT7 receptor mediating direct relaxation to 5-hydroxytryptamine in canine cerebral blood vessels. Ann. N.Y. Acad. Sci. 1997a;861:283. doi: 10.1111/j.1749-6632.1998.tb10226.x. [DOI] [PubMed] [Google Scholar]
- TERRÓN J.A. Role of 5-ht7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br. J. Pharmacol. 1997b;121:563–571. doi: 10.1038/sj.bjp.0701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRÓN J.A. The 5-HT7 receptor: a target for novel therapeutic avenues. I. Drugs. 1998a;1:302–310. [PubMed] [Google Scholar]
- TERRÓN J.A. Antagonism of the relaxant 5-HT receptor in the dog basilar artery by the high-affinity 5-ht7 receptor ligand, LY215840. Proc. West. Pharmacol. Soc. 1998b;41:129–130. [PubMed] [Google Scholar]
- TERRÓN J. A. Involvement of the 5-HT7 receptor in cerebrovascular vasodilation: potential impact in migraine. Proc. West. Pharmacol. Soc. 1998c;41:247–251. [PubMed] [Google Scholar]
- TREVETHICK M.A., FENIUK W., HUMPHREY P.P.A. 5-Carboxamidotryptamine: a potent agonist mediating relaxation and elevation of cyclic AMP in the isolated neonatal porcine vena cava. Life Sci. 1986;38:1521–1528. doi: 10.1016/0024-3205(86)90566-7. [DOI] [PubMed] [Google Scholar]
- TUNIS M.M., WOLFF H.G. Analysis of cranial artery pulse waves in patients with vascular headaches of migraine type. Amer. J. Med. Sci. 1952;224:565–568. doi: 10.1097/00000441-195211000-00013. [DOI] [PubMed] [Google Scholar]
- UENO M., ISHINE T., LEE T.J.F. A novel 5-HT1-like receptor subtype mediates cAMP synthesis in porcine pial vein. Am. J. Physiol. 1995;268:H1383–H1389. doi: 10.1152/ajpheart.1995.268.4.H1383. [DOI] [PubMed] [Google Scholar]
- ULLMER C., SCHMUCK K., KALKMAN H.O., LUBERT H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- VAN HAASTERT P.J., VAN DRIEL R., JASTORFF B., BARANIAK J., STEC W.J., DE WIT R.J. Competitive cAMP antagonists for cAMP-receptor proteins. J. Biol. Chem. 1984;259:10020–10024. [PubMed] [Google Scholar]
- VERRECHIA C., SERCOMBE R., PHILIPSON V., SEYLAZ J. Functional destruction of cerebral vascular endothelium by Triton X-100. Blood Vessels. 1986;23:106. doi: 10.1159/000158632. [DOI] [PubMed] [Google Scholar]
- VILLALÓN C.M., CENTURIÓN D., LUJÁN-ESTRADA M., TERRÓN J.A., SÁNCHEZ-LÓPEZ A. Mediation of 5-HT-induced external carotid vasodilatation in GR127935-pretreated vagosympathectomized dogs by the putative 5-HT7 receptor. Br. J. Pharmacol. 1997;120:1319–1327. doi: 10.1038/sj.bjp.0701020. [DOI] [PMC free article] [PubMed] [Google Scholar]


