Abstract
The mechanism responsible for blood pressure reduction in spontaneously hypertensive rats (SHR) after prolonged cholecalciferol treatment was studied. Two-week treatment of SHR with 0.125 mg cholecalciferol kg−1 body weight per day orally caused significant reductions of systolic blood pressure and of the resting perfusion pressure of the mesenteric vascular bed at constant flow.
In addition, the treated animals presented a normalization of the maximum vasoconstriction response to noradrenaline and a reduction of the maximum effect of the adrenaline concentration-response curves. This latter effect probably was due to recovery of the impaired Ca2+-dependent K+ channels coupled to α2-adrenoceptors since it was prevented by apamin.
The treatment with cholecalciferol also normalized the smooth muscle cell membrane potential of de-endothelialized mesenteric arteries of SHR and their hyperpolarizing responses to α2-adrenergic agonists, which were depressed in untreated SHR.
In mesenteric rings with endothelium, α2-adrenergic agonists caused similar hyperpolarizing responses in the SHR and in normotensive Wistar (NWR) and Wistar Kyoto (WKY). In non cholecalciferol-treated SHR the hyperpolarizing mediator involved in this effect was NO, while in NWR it was the endothelium-derived hyperpolarizing factor (EDHF). After cholecalciferol treatment, the hyperpolarization induced by α2-adrenergic agonists in SHR smooth muscle cells was mediated by EDHF, as in NWR.
Our results indicate that the hypotensive effect of cholecalciferol in the SHR is probably due to the normalization of vascular reactivity, by restoring the functioning of apamin- and ATP-sensitive K+ channels located in the vascular smooth muscle cell membrane, which are impaired in the SHR.
Keywords: SHR, mesenteric arteries, cholecalciferol, membrane potential, alpha-2 adrenoceptors, K+ channels
Introduction
Altered vitamin D3 metabolism has been suggested to be involved in the pathophysiology of essential hypertension (Resnick et al., 1986). Although this could be due to an effect on blood pressure through calcium metabolism, there is evidence that vitamin D3 has a direct effect on vascular smooth muscle cell physiology (Kawashima, 1987; Bukoski et al., 1990; Xue et al., 1991).
Prolonged treatment with vitamin D3 was shown to reduce blood pressure in hypertensive pateints (Lind et al., 1989; Kristal-Boneh et al., 1997), but mechanisms responsible for this effect have not been elucidated.
In the experimental model of the spontaneously hypertensive rat (SHR), lower serum levels of 1,25(OH)2 vitamin D3 (Lucas et al., 1986) and a considerable resistance to vitamin D3 overdose characterize a compromised vitamin D3 status (Matthias et al., 1984), that may play a role in the pathophysiology of the hypertension (Schedl et al., 1984). This hypothesis is supported by the finding that chronic dietary supplementation with cholecalciferol (a vitamin D3 precursor) caused a significant dose-dependent reduction of the systolic blood pressure in the SHR (Vianna et al., 1992). This hypotensive effect was accompanied by recovery of the relaxant effect of bradykinin on the duodenum smooth muscle, due to opening of apamin-sensitive Ca2+-dependent K+ channels, which are impaired in the SHR (Feres et al., 1992).
Among different factors involved in the pathogenesis of hypertension, increased vascular reactivity has been the subject of much attention. In the SHR there is considerable evidence for hyper-responsiveness to vasoconstrictor drugs (Schlegel et al., 1985) and for impaired endothelium-dependent relaxation (Fujii et al., 1992) both factors being probably involved in the maintenance of elevated blood pressure.
Although the involvement of α1- and α2-adrenoceptors in the mechanism of vascular smooth muscle contractions is generally accepted, different authors have reported either contractile or relaxant responses to α2-agonists, depending on the type of vessel studied. In the case of mesenteric arteries from normotensive rats, α2-agonists do not cause a significant contractile effect (Takiguchi et al., 1987). In addition, Silva et al. (1996) demonstrated the presence of α2-adrenoceptors in mesenteric vascular smooth muscles, which appear to be important for the control of muscular tone in resistance vessels. When stimulated by α2-agonists, these receptors cause hyperpolarization of vascular smooth cells. Alpha-2 receptors are coupled to both apamin- and ATP-sensitive K+ channels, which were found to be impaired in mesenteric vessels (Ohya et al., 1996; Feres et al., 1998) as well as in other visceral smooth muscles of the SHR (Feres et al., 1992). Impairment of these channels in resistance vessels could be responsible for the increased tone and peripheral vascular resistance observed in SHR.
Since oral administration of cholecalciferol was shown to normalize the blood pressure (Vianna et al., 1992) and the functioning of Ca2+-dependent K+ channels in SHR visceral smooth muscles (Feres et al., 1992) we have now investigated the effect of that treatment on the reactivity of the isolated mesenteric vascular bed and upon the activity of apamin- and ATP-sensitive K+ channels in mesenteric arteries of SHR.
Methods
Animals
Experiments were carried out using female spontaneously hypertensive rats (SHR) Okamoto & Aoki (1963) and their Wistar-Kyoto normotensive controls (WKY) derived from an original colony supplied by the National Institutes of Health, Bethesda, MD, U.S.A. Normotensive Wistar rats (NWR) from the Wistar Institute, Philadelphia, PA, U.S.A., inbred at Escola Paulista de Medicina, SP, Brazil, were also used. The rats aged 20–30 weeks and weighed 200–220 g. They were fed a standard diet (Labina rat chow, Purina), containing 6600 i.u. vitamin D3 kg−1. After a basal period of 10 days, the treated groups received, by oral gavage, a daily supplementation of 0.125 mg (500 i.u.) cholecalciferol kg−1 body weight, dissolved in 0.3 ml of coconut oil. The duration of treatment was 6 weeks and the control groups receivd only the vehicle. Systolic blood pressure was measured twice weekly from the tail of prewarmed unanaesthetized rats using a pletysmographic method (LE 5650/6, Letica Scientific Instruments). An average of three readings was recorded for each animal. After 6 weeks of treatment, some animals were decapitated to remove their mesenteric vascular bed, which were dissected away from the intestines for perfusion pressure measurements. Others were decapitated to remove the superior mesenteric arteries, which were cut into rings (3–4 mm length) and cleaned of adherent connective tissue for tension and electrophysiological experiments. In some rings, the endothelium was removed by gentle rubbing of the intimal surface with a plastic tube wrapped in cotton. All studies were according to the Ethic Committee for Research of the São Paulo Hospital/Federal University of São Paulo.
Mechanical responses
Mesenteric vascular bed preparations were set up as previously described (McGregor, 1965; Ross, 1972). Under ether anaesthesia, the abdomen was opened and the pancreatic-duodenal, ileo-colic and colic branches of the superior mesenteric artery were tied. The superior mesenteric artery was separated from surrounding tissues in the region of the aorta and a polyethylene cannula (PE 50) was inserted distally into the artery at its origin from the abdominal aorta. The connections of the superior mesenteric plexus to the coeliac ganglia were severed and the intestine was removed by cutting close to the intestinal border of the mesentery. The mesenteric bed was perfused, at a constant flow of 4.0 ml min−1, using a peristaltic pump (Model 2115, LKB-Produkter AB), with Krebs solution of the following composition (in mM): NaCl 137; NaHCO3 5.9; KHCO3 5.9; CaCl2 2.3; MgCl2 1.2 and gluocse 11.8. The solution was bubbled with a 5% CO2-95% O2 gas mixture and maintained at pH 7.4 and 37°C. The perfusion pressure was monitored with pressure transducers (P-1000B, NARCO Bio-Systems) connected to a physiograph (DMP-4B, NARCO) and the pH was monitored continuously with a pH meter (E350B, METROHM), by means of a glass electrode inserted in the perfusion system. After a 20-min period of stabilization, concentration-response curves were obtained by perfusion with Krebs solution containing the agonist (adrenaline or noradrenaline) at 10-min intervals between each concentration.
For the experiments with isolated superior mesenteric artery, rings without endothelium were placed between stainless-steel wires (diameter 50 μm) and suspended in an organ bath chamber (5 ml) containing Krebs solutions (pH 7.4, 37°C, equilibrated with 5% CO2-95% O2). The tension changes of the preparations were measured with an isometric force-displacement transducer (F-60, NARCO) and recorded in a physiograph (DMP-4B, NARCO). The rings were initially equilibrated for 1 h under an optimal resting tension of 1.0 g and washed every 10 min. The absence of endothelium was confirmed by the lack of relaxant response to 1 μM acetylcholine in rings pre-contracted with 1 μM noradrenaline. Then cumulative concentration-response curves for adrenaline were determined both in the absence and in the presence of apamin (100 nmol l−1) in both the mesenteric bed and arterial rings.
Membrane potential
The arterial rings were placed in a perfusion chamber (2 ml) and superfused at a rate of 3 ml min−1 with a Krebs solution (pH 7.4, 37°C, aerated with the mixture 5% CO2-95% O2). The micropipettes (Borosilicate glass capillaries 1B120F-6, World Precision Instruments, WPI), were made by a vertical puller (Pul-100, WPI) and filled with 2 M KCl (tip resistance 20–40 MΩ and tip potential<6 mV). The microelectrodes were mounted in Ag/AgCl half-cells on a micromanipulator (Leitz, Leica) and connected to an electrometer (Intra 767, WPI). The impalements of the smooth muscle cells were made either by the intimal side, in everted rings, or by the adventitial side in rings with intact endothelium. The electrical signals were continuously monitored on an oscilloscope (54645A, HEWLETT PACKARD) and recorded in a potentiometric chart recorder (2210, LKB-Produkter AB). The successful implantation of the electrode was evidenced by a sharp drop in voltage upon entry into a cell, a stable potential (±3 mV) for at least 1 min after impalement, a sharp return to zero upon exit, and minimal change (<10%) in microelectrode resistance after impalement.
Measurements of membrane potential of mesenteric rings were obtained in Krebs solutions before and after stimulation of the vessels with: adrenaline (1 μmol l−1); adrenaline in the presence of prazosin (20 nmol l−1 for 10 min); 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine (UK 14,304; 8 nmol l−1); cromakalim (0.1 μmol l−1). To determine the contribution of endothelium-derived relaxing factor (EDRF) and endothelium-derived hyperpolarizing factor (EDHF) to the hyperpolarizing effect induced by UK 14,304, rings with endothelium were pretreated with the NO-synthesis inhibitor, Nω-nitro-L-arginine (L-NNA, 30 μmol l−1 for 20 min), or with the K+ channel blocker apamin (100 nmol l−1 for 10 min). The time of contact of the agonists with the preparations before the impalements was about 5 min (Feres et al., 1998).
Drugs
The inorganic salts were products of the highest analytical grade from Merck Darmstadt. Vitamin D3 (cholecalciferol), acetylcholine chloride, apamin, cromakalim, adrenaline bitartrate, noradrenaline hydrochloride, Nω-nitro-L-arginine and prazosin, were obtained from Sigma Chemical Co., St. Louis, MO, U.S.A. 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine (UK 14,304) was obtained from Research Biochemicals International, Natick, MA, U.S.A.
Analysis of results
All data are expressed as means±s.e.mean with the number of animals in parentheses. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by the Newman-Keuls test in the case of pairwise comparisons between-groups. When the data consisted of repeated observations at successive time points, ANOVA for repeated measurements was applied to determine differences between groups. Where more than one impalement was made on the same mesenteric ring from the same rat the measurements were averaged and considered as n=1. Differences were considered significant when P<0.05.
Results
Blood pressure
In agreement with previous findings (Vianna et al., 1992), the oral supplementation with 0.125 mg of cholecalciferol kg−1 of body weight per day caused a significant reduction of the systolic blood pressure of SHR, which fell from an initial value of 160±5 (n=36) to 143±6 mmHg (n=30) after 2 weeks of treatment. WKY rats did not present a significant change in blood pressure, which was 130±6 mmHg (n=17) at the beginning of the treatment with cholecalciferol and 135±7 mmHg (n=16) after 6 weeks. No significant difference in body weight was observed between treated and non-treated animals (Table 1). The cholecalciferol treatment caused a rise in serum calcium level in the WKY, but no difference was detected in the SHR (Table 1).
Table 1.
Effect of oral supplementation with cholecalciferol (0.125 mg kg−1 body weight per day) on body weight, systolic blood pressure and serum calcium level
Measurements of mechanical response
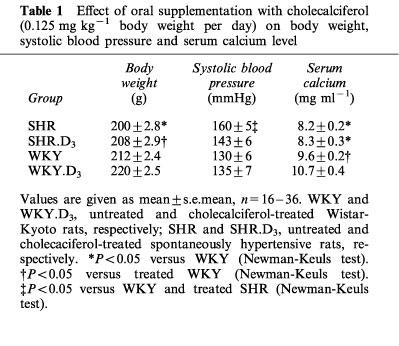
The resting perfusion pressure of the mesenteric vascular bed was higher in SHR (63.9±3.5 mmHg) than in WKY (45±3.9 mmHg) or NWR (44.4±2.7 mmHg). Cholecalciferol treatment reduced perfusion pressure of SHR to 35±3.1 mmHg (Figure 1).
Figure 1.
Effect of oral supplementation with cholecalciferol (0.125 mg kg−1 body weight per day for 2 weeks on the perfusion pressure of the mesenteric arterial bed of NWR, WKY and SHR. The data are means±s.e.mean and the number of experiments is given above the bars. *P<0.05 when compared with the other groups (Newman-Keuls test).
To investigate the responses of the mesenteric vascular bed, adrenaline and noradrenaline were selected because they are physiological agonists and, in addition, adrenaline plus prazosin were previously shown to induce hyperpolarizing responses due to its interaction with α2-adrenoceptors coupled to Ca2+-dependent K+ channel (Silva et al., 1996; Feres et al., 1998).
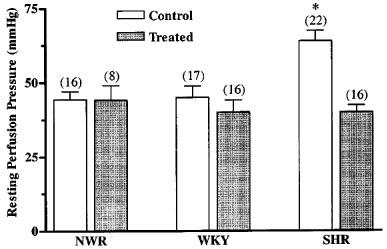
Concentration-response curves to noradrenaline and adrenaline on the mesenteric vascular beds of non-treated animals showed no difference between the pD2 values in the SHR (5.4±0.2, n=10 and 5.9±0.2, n=15, respectively), WKY (5.5±0.2, n=7 and 6.0±0.2, n=8, respectively) and the NWR (5.6±0.2, n=8 and 6.0±0.2, n=10, respectively), but the SHR presented a significantly higher maximum effect for both agonists, when compared to the normotensive controls (Figure 2A and B).
Figure 2.

Concentration-response curves to noradrenaline (A and C) and adrenaline (B and D) on the perfused mesenteric vascular bed from untreated (A and B) and cholecalciferol-treated (C and D) SHR, WKY and NWR. Symbols represent mean increase in pressure ±s.e.mean (n=7–15). *P<0.05 versus other groups (Newman-Keuls test). †P<0.05, ANOVA for repeated measurements.
After the treatment with cholecalciferol no changes of the pD2 values for noradrenaline or adrenaline were observed, either in the NWR, WKY or SHR (Figure 2C and D). However, in SHR, the treatment caused a reduction to normal levels of the maximum response to noradrenaline (Figure 2C), whereas for adrenaline a significant reduction of the maximum response to below normal levels of the concentration-response curves to this agonist was observed (Figure 2D). The latter effect observed with adrenaline was completely reverted in the presence of 100 nmol l−1 apamin (Figure 3A), indicating that the perfusion pressure lowering effect of cholecalciferol treatment is due to a recovery of Ca2+-dependent K+ channels, which have been shown to be defective in SHR (Feres et al., 1998).
Figure 3.
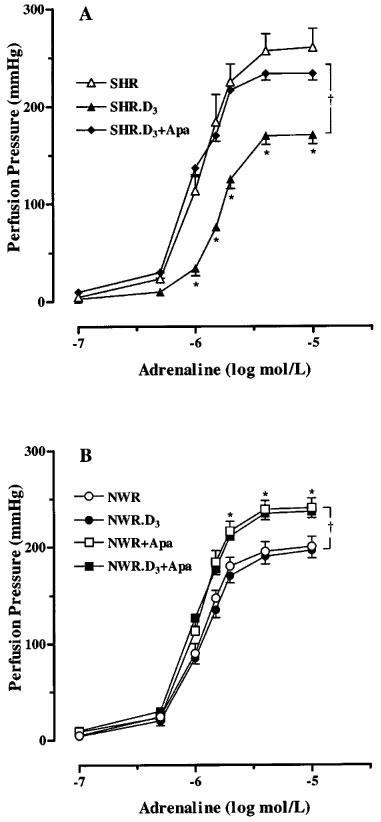
Concentration-response curves to adrenaline on the perfused mesenteric vascular bed from untreated and cholecalciferol-treated (D3) SHR (A) and NWR (B) in the absence and in the presence of apamin (100 nmol l−1, Apa). Symbols represent mean increase in pressure ±s.e.mean (n=7–15). *P<0.05 versus other groups (Newman-Keuls test). †P<0.05, ANOVA for repeated measurements.
In normotensive animals (treated or non-treated) an increase in perfusion pressure response to adrenaline was observed when apamin was present (Figure 3B). Concentration-response curves to adrenaline, in the absence and in the presence of apamin, were also performed in superior mesenteric ring preparations from SHR, with similar results to those observed in vascular mesenteric beds (Figure 4).
Figure 4.
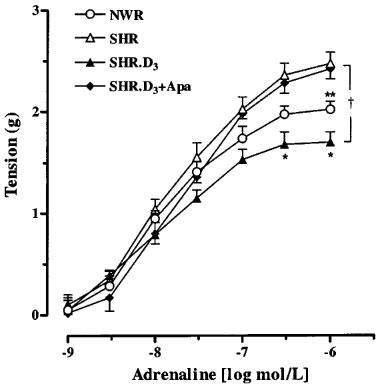
Concentration-response curves to adrenaline on isolated mesenteric arterial rings from untreated NWR, SHR and cholecalciferol-treated (SHR.D3) in the absence and in the presence of apamin 100 nmol l−1 (Apa). Symbols represent mean increase in tension ±s.e.mean (n=7–15). *P<0.05 versus other groups (Newman-Keuls test). **P<0.05 versus untreated SHR and treated SHR in the presence of apamin (Newman-Keuls test). †P<0.05, ANOVA for repeated measurements.
Measurements of membrane potential
To better investigate the recovery of the impaired function of Ca2+-dependent K+ channel by cholecalciferol treatment, we used intracellular recording with microelectrodes to measure membrane potentials in rings from control and treated SHR.
Rings without endothelium
The resting membrane potential (RMP) was measured through impalements from the intimal side of everted rings of de-endothelialized superior mesenteric arteries. Figure 5 shows that the rings from SHR were depolarized (RMP=−33.5±0.7 mV) when compared with those from NWR (RMP=−43.4±1.0 mV) and normotensive rats WKY (RMP=−41.7±3.3 mV, not shown). The hyperpolarizing effects induced by the α2-adrenergic agonists UK 14,304, or adrenaline in the presence of the α1-antagonist prazosin, was significantly higher in arteries from treated than in those from non-treated SHR (Figure 5). UK14,304 was used because it is more selective for α2 receptors. Pretreatment with apamin reduced the membrane potential of NWR arteries but had no effect on those of the SHR. After the treatment with cholecalciferol the SHR resting membrane potential was normalized and a significant depolarization by apamin was observed, similar to that seen in rings from NWR (Figure 5).
Figure 5.
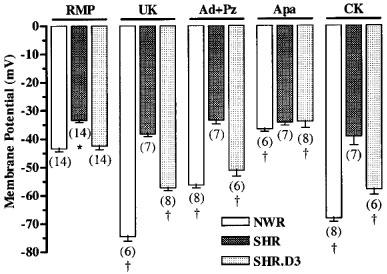
Membrane potential measured in everted rings (without endothelium) of mesenteric arteries form NWR, untreated SHR and cholecalciferol-treated SHR (SHR.D3) in the absence (RMP) and in the presence of 8 nmol l−1 UK 14,304 (UK), 1.0 μmol l−1 adrenaline plus 20 nmol l−1 prazosin (Ad+Pz), 100 nmol l−1 apamin (Apa) and 0.1 μmol l−1 cromakalim (CK). For each mesenteric ring obtained from individual rats (the number of which is shown in parenthesis below the bars) 5–8 cells were impaled and the averages of the respective measurements were used to obtain the means ±s.e.mean. *P<0.05 versus NWR and treated SHR (Newman-Keuls test). †P<0.05 versus respective RMP (Newman-Keuls test).
Since ATP-sensitive K+ channels also couple to α2-adrenoceptors (Silva et al., 1996) and were shown to be impaired in SHR (Ohya et al., 1996) we investigated whether the cholecalciferol treatment was also able to correct their function. Cromakalim (0.1 μmol l−1), an opener of ATP-sensitive K+ channels, induced hyperpolarization in NWR (Figure 5) and WKY (not shown), but not in SHR arteries. After cholecalciferol treatment, the SHR muscle preparations were also hyperpolarized by cromakalim.
Rings with endothelium
Figure 6 shows that the resting membrane potential, measured through impalements from the adventitial side of the arteries, was also less negative in SHR (−36.0±1.2 mV) than in NWR (−47.6±1.8 mV) or in WKY (−44.8±2.7 mV, not shown). Treatment with cholecalciferol also normalized the membrane potential of rings with endothelium.
Figure 6.
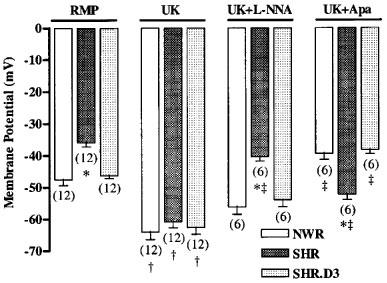
Membrane potential measured in intact rings (with endothelium) of mesenteric arteries from NWR, untreated SHR and cholecalciferol-treated SHR (SHR.D3) in the absence (RMP) and in the presence of 8 nmol l−1 UK 14,304 (UK), UK plus 30 μmol l−1 Nω-nitro-L-arginine (UK+L-NNA) and UK plus 100 nmol l−1 apamin (UK+Apa). For each mesenteric ring obtained from individual rats (the number of which is shown in parenthesis below the bars) 5–8 cells were impaled and the averages of the respective measurements were used to obtain the means±s.e.mean. *P<0.05 versus respective measurements in NWR and treated SHR (Newman-Keuls test). †P<0.05 versus respective RMP (Newman-Keuls test). ‡P<0.05 versus the membrane potential measured in the presence of UK (Newman-Keuls test).
In contrast to the results obtained with de-endothelialized rings, the hyperpolarization induced by UK 14,304 was similar in intact rings from SHR and NWR, and no significant differences were observed after cholecalciferol treatment (Figure 6). However, the factors responsible for the hyperpolarizing effect of UK 14,304 were different in the normotensive and hypertensive animals. In SHR, NO was the major factor responsible for the hyperpolarizing response, since pretreatment with the inhibitor of NO synthase L-NNA abolished this response, whereas the inhibitor of Ca2+-dependent K+ channels, apamin, only partially reduced this effect (Figure 6). On the other hand, in the normotensive rats (NWR) and in the cholecalciferol-treated SHR, the hyperpolarizing effect of UK 14,304 was probably due to the release of EDHF since this effect was abolished by apamin, while L-NNA had no effect (Figure 6).
Discussion
Numerous reports have demonstrated an inverse association between calcium intake and blood pressure, indicating a role of calcium-regulating hormones in hypertension. Spontaneously hypertensive rats are a good model to study this relationship, since an abnormal vitamin D3 metabolism has been reported in these animals (Matthias et al., 1984; Lucas et al., 1986) and calcium supplementation in the diet causes a normalization of their blood pressure (Ayachi, 1979; Lucas et al., 1986).
Our results show that the oral supplementation with cholecalciferol also caused a significant reduction of the SHR systolic blood pressure, already at the second week of treatment, as previously described (Vianna et al., 1992). This contrasts with reports that daily intraperitoneal injections of the active form of vitamin D (1,25(OH)2-vitamin D3) for 6 weeks did not reduce, but rather increased SHR blood pressure (Bukoski et al., 1993). The discrepancy may be due to the form of administration. We believe that the long-term oral administration of the vitamin D3 precursor, cholecalciferol, may be more efficient, since 1,25(OH)2-vitamin D3 is rapidly inactivated by side-chain oxidation in vivo (Kumar et al., 1976). Furthermore, the increased blood pressure observed after long-term treatment with 1,25(OH)2-vitamin D3 could be due to its ionophoric effect on vascular smooth muscle cells (Bukoski et al., 1987). In addition, contrasting effects induced by exogenous administration or endogenous formation of 1,25(OH)2-vitamin D3 described by Goff et al. (1990) reinforce our results.
Since vitamin D3 is closely related with calcium homeostasis, its hypotensive effect might be thought to be due to the increase of serum calcium levels. However, in contrast to what was observed in normotensive rats, no changes in serum calcium levels were seen in the vitamin D3-treated SHR (Gafter et al., 1986; Vianna et al., 1992). This lack of effect on serum calcium levels is probably due to the failure of endogenously produced 1,25(OH)2-vitamin D3 to upregulate the duodenal vitamin D receptor (Goff et al., 1990).
The blood pressure reduction observed in SHR after cholecalciferol treatment might be associated with the normalization of the basal perfusion pressure of the mesenteric vascular bed, which has been shown to contribute substantially to peripheral vascular resistance in rats (Christensen & Mulvany, 1993).
The cholecalciferol treatment also caused a normalization of the contractile response to noradrenaline and a significant reduction of the effect of adrenaline in the SHR mesenteric vascular bed and in mesenteric artery rings. This effect was prevented by apamin, an inhibitor of Ca2+ dependent K+ channels, which suggests that the cholecalciferol treatment corrected the impairment of these channels, that are coupled to α2-adrenoceptors (Feres et al., 1998).
In agreement with our findings, a link between decrease in blood pressure and recovery of K+ channels in arterial smooth muscles from SHR was also reported after anti-hypertensive treatments with hydralazine (Ohya et al., 1996) or ramipril (Rusch & Runnells, 1994). In addition, Ca2+-dependent K+ channels have been shown to regulate transmural pressure in small blood vessels (Wesselman et al., 1997).
Potassium channels regulate smooth muscle resting membrane potential and thereby control muscle tone (Brayden & Nelson, 1992). Although membrane potential measurements are not possible in the mesenteric vascular bed, it is conceivable that the increased response of SHR preparations to adrenergic agonists is due to a less negative smooth muscle cell membrane potential since previous work has shown that smooth muscle cells from SHR caudal (Hermsmeyer et al., 1982) and superior mesenteric (Feres et al., 1998) arteries are less polarized than those of normotensive rats, and this is confirmed in the present work. Cholecalciferol treatment led to total normalization of the resting membrane potential of the intact and de-endothelialized mesenteric rings from SHR. This was probably due to the recovery of Ca2+-dependent K+ channels since apamin, which had no effect on the membrane potential of SHR arteries, inhibited it after the cholecalciferol treatment.
To further investigate this property, the effects of α2-adrenergic agonists on the smooth muscle cell membrane potential were compared in treated and non-treated SHR, since these potassium channels are also mediators of the hyperpolarizing response induced by α2-adrenoceptors (Silva et al., 1996). Our results showed that the small or absent hyperpolarizing effect induced in mesenteric rings from SHR by both UK 14,304 and adrenaline (in the presence of prazosin) was also totally restored by the cholecalciferol treatment. Furthermore, the ATP-sensitive K+ channels, which also couple with α2-adrenoceptors in NWR (Silva et al., 1996), were found to be impaired in SHR, in agreement with previous reports (Rusch & Runnells, 1994; Ohya et al., 1996), and their total recovery was observed after the cholecalciferol treatment.
Both intact and de-endothelialized smooth muscle cells were found to be depolarized in SHR, as compared to WKY and NWR mesenteric arteries. However, α2-adrenergic agonists (UK 14,304 or adrenaline plus prazosin) induced hyperpolarization in NWR and in intact, but not in de-endothelialized mesenteric rings from SHR. Our results with the NO synthase inhibitor L-NNA and the K+ channel inhibitor apamin indicate that the relaxant factor responsible for the hyperpolarizing effect in SHR is NO, while in NWR it is the endothelium-derived hyperpolarizing factor (EDHF). After treatment with cholecalciferol the effect of the α2-adrenergic agonists in SHR rings changed, being mediated by EDHF, similarly to what was seen in the NWR preparations.
In conclusion, our results indicate that the hypotensive effect of cholecalciferol on the SHR is probably due to the normalization of vascular reactivity, by restoring the functioning of both apamin- and ATP-sensitive K+ channels located in the smooth muscle cell membrane, which are impaired in the SHR. We suggest that the membranophilic properties of vitamin D3 are probably responsible for restoring K+ channel function resulting in normalization of the resting membrane potential, of the mesenteric bed perfusion pressure and of the smooth muscle responses mediated by α2-adrenoceptors, which are observed after cholecalciferol treatment.
Acknowledgments
This work was supported by grants and fellowships from the Brazilian National Research Council (CNPq) and by the São Paulo State Research Foundation (FAPESP). The technical assistance of Nelson Alves Mora is gratefully acknowledged.
Abbreviations
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- L-NNA
Nω-nitro-L-arginine
- NWR
normotensive Wistar rats
- RMP
resting membrane potential
- SHR
spontaneously hypertensive rats
- UK 14,304
5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine
- WKY
Wistar-Kyoto rats
References
- AYACHI S. Increased dietary calcium lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1979;28:1234–1238. doi: 10.1016/0026-0495(79)90136-7. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E., NELSON M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- BUKOSKI R.D., LI J., BO J. Effect of long-term administration of 1,25(OH)2 vitamin D3 on blood presssure and resistance artery contractility in the spontaneously hypertensive rat. Am. J. Hypertens. 1993;6:944–950. doi: 10.1093/ajh/6.11.944. [DOI] [PubMed] [Google Scholar]
- BUKOSKI R.D., WANG D., WAGMAN W. Injection of 1,25-(OH)2 vitamin D3 enhances resistance artery contractile properties. Hypertension. 1990;16:523–531. doi: 10.1161/01.hyp.16.5.523. [DOI] [PubMed] [Google Scholar]
- BUKOSKI R.D., XUE H., MCCARRON Effect of 1,25(OH)2 vitamin D3 and ionized Ca2+ on 45Ca uptake by primary cultures of aortic myocytes of spontaneously hypertensive and Wistar Kyoto normotensive rats. Biochem. Biophys. Res. Comm. 1987;146:1330–1335. doi: 10.1016/0006-291x(87)90795-9. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN K.L., MULVANY M.J. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J. Vasc. Res. 1993;30:73–79. doi: 10.1159/000158978. [DOI] [PubMed] [Google Scholar]
- FERES T., BORGES A.C.R., SILVA E.G., PAIVA A.C.M., PAIVA T.B. Impaired function of alpha-2 adrenoceptors in smooth muscle mesenteric arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 1998;125:1144–1149. doi: 10.1038/sj.bjp.0702177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERES T., VIANNA L.M., PAIVA A.C.M., PAIVA T.B. Effect of treatment with vitamin D3 on the responses of the duodenum of spontaneously hypertensive rats to bradykinin and to potassium. Br. J. Pharmacol. 1992;105:881–884. doi: 10.1111/j.1476-5381.1992.tb09072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJII K., TOMINAGA M., OHMORI S., KOBAYASHI K., KOGA T., TAKATA Y., FUJISHIMA M. Decreased endothelium-dependent hyperpolarization to Acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ. Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- GAFTER U., EBY B., MARTIN G., LAU K. Response of spontaneously hypertensive rats to 1,25(OH)2D3 in vivo. Kidney International. 1986;30:497–502. doi: 10.1038/ki.1986.213. [DOI] [PubMed] [Google Scholar]
- GOFF J.P., REINHARDT T.A., BECKMAN M.J., HORST R.L. Contrasting effects of exogenous 1,25-dihydroxyvitamin D [1,25-(OH)2D] versus endogenous 1,25-(OH)2D, induced by dietary calcium restriction, on vitamin D receptors. Endocrinology. 1990;126:1031–1035. doi: 10.1210/endo-126-2-1031. [DOI] [PubMed] [Google Scholar]
- HERMSMEYER K., ABEL P.W., TRAPANI A.J. Noradrenaline sensitivity and membrane potentials of caudal arterial muscle in DOCA-Salt, Dahl, and SHR hypertension in the rat. Hypertension. 1982;4:II-49–II-51. [PubMed] [Google Scholar]
- KAWASHIMA H. Receptor for 1,25-dihydroxyvitamin D in a vascular smooth muscle cell line derived from rat aorta. Biochem. Biophys. Res. Comm. 1987;146:1–6. doi: 10.1016/0006-291x(87)90681-4. [DOI] [PubMed] [Google Scholar]
- KRISTAL-BONEH E., FROOM P., HARARI G., RIBAK J. Association of calcitrol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- KUMAR R., HARNDEN D., DELUCA H. Metabolism of 1,25-dihydroxyvitamin D3: evidence for side-chain oxidation. Biochemistry. 1976;15:2420–2423. doi: 10.1021/bi00656a027. [DOI] [PubMed] [Google Scholar]
- LIND L., WENGLE B., WIDE L., LJUNGHALL S. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am. J. Hypertens. 1989;2:20–25. doi: 10.1093/ajh/2.1.20. [DOI] [PubMed] [Google Scholar]
- LUCAS P.A., BROWN R.C., DRÜEKE T., LACOUR B., METZ J.A., MCCARRON D.A. Abnormal vitamin D metabolism, intestinal calcium transport and bone calcium status in the spontaneously hypertensive rat compared with its genetic control. J. Clin. Invest. 1986;78:221–227. doi: 10.1172/JCI112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHIAS D., BECKER C.H., WOOSSMANN H. Different behaviour of normotonous and spontaneously hypertensive rats with vitamin D intoxication. Biomed. Biochem. Acta. 1984;43:741–748. [PubMed] [Google Scholar]
- MCGREGOR D.D. The effect of sympathetic nerve stimulation on vasoconstrictor responses in perfused mesenteric blood vessels of the rat. J. Physiol. 1965;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYA Y., SETOGUCHI M., FUJII K., NAGAO T., ABE I., FUJISHIMA M. Impaired action of levcromakalim on ATP-sensitive K+ channels in mesenteric artery cells from spontaneously hypertensive rats. Hypertension. 1996;27:1234–1239. doi: 10.1161/01.hyp.27.6.1234. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jap. Circ. 1963;27:282–292. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- RESNICK L.M., NICHOLSON J.P., LARAGH J.H. Calcium metabolism in essential hypertension: relationship to altered renin system activity. Fed. Proc. 1986;45:2739–2745. [PubMed] [Google Scholar]
- ROSS B.D. Oxford, Clarendon Press; 1972. Perfusion Techniques in Biochemistry; pp. 356–394. [Google Scholar]
- RUSCH N.J., RUNNELLS A.M. Remission of high blood pressure reverses arterial potassium channel alterations. Hypertension. 1994;23:941–945. doi: 10.1161/01.hyp.23.6.941. [DOI] [PubMed] [Google Scholar]
- SCHEDL H.P., MILLER D.L., PAPE J.M., HORST R.L., WILSON H.D. Calcium and sodium transport and vitamin D metabolism in the spontaneously hypertensive rat. J. Clin. Invest. 1984;73:980–986. doi: 10.1172/JCI111323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL P.A., MONNEY M., BRUNNER H.R. Isolated perfused mesenteric arteries of hypertensive and normotensive rats; response to noradrenaline, lysine vasopressin and angiotensin II. Clin. Exp. Hypertens. 1985;A7:1583–1596. doi: 10.3109/10641968509073611. [DOI] [PubMed] [Google Scholar]
- SILVA E.G., FERES T., VIANNA L.M., OKUYAMA P., PAIVA T.B. Dual effect of clonidine on mesenteric artery adrenoceptors: agonistic (alpha-2) and antagonistic (alpha-1) J. Pharmacol. Exp. Ther. 1996;277:872–876. [PubMed] [Google Scholar]
- TAKIGUCHI Y., HASHIMOTO H., NAKASHIMA M. Reciprocal potentiation of a vasoconstrictor response between 5-hydroxytryptamine and clonidine in the perfused mesenteric vascular bed of the rat. Arch. Int. Pharmacodyn. 1987;287:16–30. [PubMed] [Google Scholar]
- VIANNA L.M., PAIVA A.C.M., PAIVA T.B. Treatment with vitamin D3 reduces blood pressure of spontaneously hypertensive rats. Genetic Hypertension. 1992;218:589–591. [Google Scholar]
- WESSELMAN J.P.M., SCHUBERT R., VANBAVEL E., NILSSON H., MULVANY M.J. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. Am. J. Physiol. 1997;272:H2241–H2249. doi: 10.1152/ajpheart.1997.272.5.H2241. [DOI] [PubMed] [Google Scholar]
- XUE H., MCCARRON D.A., BUKOSKI R.D. 1,25(OH)2 vitamin D3 attenuates the loss of resistance artery contractile function associated with incubation in culture media. Biochem. Biophys. Res. Comm. 1991;174:11–17. doi: 10.1016/0006-291x(91)90477-o. [DOI] [PubMed] [Google Scholar]


