Abstract
We examined the effects of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[f]quinoxaline-7-sulphonamide (NBQX), the kainate receptor antagonists γ-(R-)-glutamylaminomethanesulphonic acid (GAMS) and 6,7,8,9-tetrahydro-5-nitro-1H-benz[g]indole-2,3-dione-3-oxime (NS-102), and the group III metabotropic glutamate receptor (mGluR) agonist 2-amino-4-phosphono-S-butanoic acid (L-AP4) on c-fos-like immunoreactivity (c-fos LI) in trigeminal caudalis (Sp5C), lateral reticular (LRt), medullary reticular (Md) and solitary tract (Sol) nuclei, after intracisternal injection of capsaicin in urethane anaesthetized Sprague-Dawley rats.
Few c-fos labelled cells were observed within Sp5C in capsaicin-vehicle treated animals. The number of positive c-fos cells increased by 17 fold after intracisternal capsaicin (5 nmol) administration.
Pretreatment with CNQX (0.02, 0.1, 0.6, 3 and 15 mg kg−1) or NBQX (0.01, 0.1 and 1 mg kg−1), administered intraperitoneally 15 min before capsaicin, significantly reduced labelled cells within Sp5C by a maximum of 45 and 34%, respectively. The number of c-fos LI cells within LRt, Md and Sol was not affected. Pretreatment with L-AP4 (1, 3 and 10 mg kg−1) decreased the number of Sp5C c-fos LI cells by a maximum of 30%, whereas GAMS (1 and 10 mg kg−1) and NS-102 (1 and 5 mg kg−1) did not show any significant effect.
These results suggest that blockade of AMPA receptors, but not kainate receptors, or the activation of group III mGluRs, decrease the response of Sp5C neurons to trigeminovascular activation. Thus, in addition to NMDA receptors, mGluRs and AMPA receptors may modulate cephalic pain and may provide a potential therapeutic target for antimigraine drugs.
Keywords: AMPA/kainate receptors, metabotropic glutamate receptors, meninges, trigeminal system, pain, migraine, headache
Introduction
The trigeminal nerve transmits nociceptive information from the meninges to the brain stem trigeminal nucleus caudalis (Sp5C) in part via capsaicin sensitive fibres (Strassman et al., 1986; Bove & Moskowitz, 1997). These fibres contain neuropeptides such as substance P (Liu-Chen et al., 1986), calcitonin gene related peptide (CGRP; Uddman et al., 1985) and neurokinin A (Saito et al., 1987). As shown in several species, activated trigeminal fibres release neuropeptides from peripheral and central axons and transmit impulses to synaptic endings within Sp5C (Buzzi et al., 1991; Nozaki et al., 1992a; Strassmann et al., 1986; Goadsby & Hoskin 1997).
Glutamate (Glu) is the major excitatory amino acid in the mammalian central nervous system acting both at ligand-gated ion channels (ionotropic) and G-protein coupled metabotropic receptors. Several studies have implicated glutamate in nociception. It has been identified in peripheral and central terminals of primary afferent neurons, co-existing with substance P in some central nerve endings (Battaglia & Rustioni, 1988; Wanaka et al., 1988; Smullin et al., 1990; Baranauskas & Histri, 1998). Presynaptic Glu-immunoreactive terminals are found within lamina II of Sp5C (Iliakis et al., 1996). High densities of N-methyl-D-aspartate (NMDA), α - amino - 3 - hydroxy - 5 - methyl - 4 -isoxazolepropionic acid (AMPA), kainate and metabotropic Glu receptor (mGluR) binding sites are present within lamina I, II of Sp5C (Tallaksen-Greene et al., 1992) and mRNA expression for NMDA receptors was found within trigeminal ganglion (TG) cells (Watanabe et al., 1994). At the spinal cord level, NMDA, kainate and AMPA receptors are expressed within both the dorsal horn (Furuyama et al., 1993) and the dorsal root ganglion (Sato et al., 1993; Huettner, 1990). Using microdialysis techniques Bereiter & Benetti (1996) showed that noxious stimulation to the face excite small trigeminal C-fibres and release acutely Glu and aspartate within Sp5C. Administration of Glu, or its receptor agonists NMDA, AMPA, and kainate results in mechanical or thermal allodynia and hyperalgesia (Zhou et al., 1996; Jackson et al., 1995; Lawand et al., 1997), while blockade of Glu receptors antagonizes the nociceptive effects (Raigorodsky & Urca, 1990; Birder & Groat, 1992; Eisenberg et al., 1993; Bereiter et al., 1996).
Expression of the immediate early gene protein product c-fos has been used as a marker of neuronal activity (Abbadie et al., 1997; Carrive & Meyer-Carrive, 1997). Treatment with Glu, NMDA, and AMPA induce c-fos expression within neurons both in vitro (Figiel & Kaczmarek, 1997; Lauritzen et al., 1997; Griffiths et al., 1997) and in vivo (Sharp et al., 1990; Berretta et al., 1992). Neurons within Sp5C express c-fos in response to noxious meningeal stimulation by the irritant capsaicin or autologous blood (Nozaki et al., 1992a; Cutrer et al., 1995b). This expression is attenuated in animals pretreated with drugs effective in the treatment of migraine headache, such as sumatriptan, dihydroergotamine or valproate (Nozaki et al., 1992b; Hoskin et al., 1996a, 1996b; Cutrer et al., 1995a).
We recently showed that the potent and selective NMDA receptor antagonist MK-801 – (5R, 10S)-(+)-5-methyl-10,11-dihydro - 5H - dibenzo[a,d]cyclo - hepten - 5,10-imine hydrogen maleate – blocks capsaicin induced c-fos LI within Sp5C in rats (Mitsikostas et al., 1998). In addition, NMDA as well as non-NMDA receptor antagonists reduces c-fos LI within Sp5C after corneal or facial stimulation (Eisenberg et al., 1993; Bereiter et al., 1996; Bereiter & Bereiter, 1996). In this report we investigate the effects of non-NMDA receptor agonists and antagonists on capsaicin induced c-fos response within brain stem nuclei.
Methods
Animal preparation and c-fos immunohistochemistry
Male Sprague-Dawley rats (250–300 g, Charles River Laboratories, Wilmington, MA, U.S.A.) were anaesthetized with intraperitoneal (i.p.) urethane (1.2 g kg−1) and maintained with 0.2 g kg−1 urethane i.p., every 2 h as needed to suppress the withdrawal response to hindpaw stimulation. A soft catheter (PE-10, 0.28 mm internal diameter; Intramedic, Clay Adams, Parsippany, NJ, U.S.A.) was introduced into the cisterna magna and after 45 min either the vehicle or drug was administered i.p. Fifteen minutes later, a capsaicin solution (0.1 ml; 50 μM) was injected into the cisterna magna via the catheter. Capsaicin was diluted in artificial CSF (see drugs). Animals were euthanized by an overdose of pentobarbitone (80 mg kg−1, i.p.) 2 h after capsaicin administration and perfused immediately via the ascending aorta with 0.9% saline (200 ml), followed by 4% formaldehyde (500 ml) in 0.1 M phosphate buffer (PB). Brain stems with attached cervical cords were stored overnight in the same fixative and then placed in a cryoprotectant (20% sucrose, 30% ethylene glycol in 0.1 M PB) until sectioning (50 μm thick; from 3 mm rostral to obex to the C2 level) with a freezing microtome (Reichert-Jung, 2000 Leica, Deerfield, IL, U.S.A.). Every third tissue section was saved for immunohistochemistry. We used the free floating, avidin-biotin procedure, as has been previously described (Mitsikostas et al., 1998). The primary c-fos antibody (Oncogene Research Products, Cambridge, MA, U.S.A.) was diluted in 0.1 M PB (1 : 8000). Biotinylated goat antirabbit serum (Vector, Burlingame, CA, U.S.A.) was used as a secondary antibody (1 : 600).
Cell counting
C-fos positive nuclei were counted by an observer naive to the treatment groups (D.D. Mitsikostas) and confirmed (in randomly selected sections) by another investigator (M. Sanchez del Rio) under similar conditions. C-fos LI cells were counted in laminae I, II of Sp5C using the weighted average method, previously described and validated in guinea-pigs (Cutrer et al., 1995b) and rats (Mitsikostas et al., 1998). Briefly, based on the observation that c-fos LI was maximal at the level −2.00 to −2.30 mm and decreased linearly both rostrally and caudally, six 50 μm sections (every third section) were counted at each of three levels from 0 (obex) to −0.90 mm (mid-point −0.45 mm), −1.80 to −2.30 (mid-point −2.05 mm) and −6.00 to −6.50 (mid-point −6.25 mm). The mean number of labelled cells at these three levels was determined (x1, x2 and x3, respectively). The trapezoid area under the curve was 8.5·x1+22.5·x2+15·x3. The weighted average was calculated by dividing this area by 45 (i.e. the number of 50 μm sections counted every 150 μm from obex −0.45 to obex −6.25). This value reflects the total c-fos expression within the entire Sp5C. An assessment of the extent of c-fos LI in solitary tract nucleus (Sol; visible in six serial sections), lateral reticular nucleus (LRt; six sections) and medullary reticular nucleus (Md; six sections) was also performed. In these nuclei the average number of labelled cells per section was calculated.
Effect of catheter placement on c-fos expression
Since mechanical and chemical (blood within the subarachnoid space) stimulation of C-fibres can occur as a result of surgery and induce c-fos expression within Sp5C, preliminary experiments investigated the effect of catheter placement into the cisterna magna on c-fos LI within Sp5C. A total number of 28 urethane anaesthetized animals were studied in three groups. Three intact animals were anaesthetized and euthanized 3 h later. A second group of 22 animals were euthanized 2 (n=3), 3 (n=4), 4 (n=3), 5 (n=3), 6 (n=3), 7 (n=3) and 8 h (n=3) after i.c. catheter placement (no capsaicin treatment). And a third group of animals (n=3) was treated with i.c. capsaicin (5 nmol) 1 h after catheter placement and sacrificed 2 h later. Brain stems from all animals were sectioned as described above and c-fos stained cells were counted in Sp5C, at the obex level (six sections for each animal).
Drug treatment
Dose ranges of drugs tested in the present study were chosen based on the previously observed ratios between potency in the c-fos paradigm and in vitro affinity of drugs at the presumed target. Previously tested agents include the 5-HT1 receptor agonist CP-93,129 (Nozaki et al., 1992b), the NK1 receptor antagonist RPR-100893 (Cutrer et al., 1995b), the GABAA receptor antagonist bicuculline (Cutrer et al., 1995a) and the NMDA receptor antagonist MK-801 (Mitsikostas et al., 1998) and the 5-hydroxytryptamine1B/1D/1F receptor agonist sumatriptan (Mitsiko et al., 1999 in press). Each drug was first tested at a relatively high dose. If a significant activity was observed, the dose was gradually decreased until the drug lost significant efficacy. Because day to day variations were observed in the c-fos response to intracisternal capsaicin, a separate control group was used for each drug-treatment. Drug vehicle (normal saline, i.p., n=10) and the selective AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) 15 mg kg−1 (n=8), 3 mg kg−1 (n=8), 0.6 mg kg−1 (n=8), 0.1 mg kg−1 (n=6), or 0.02 mg kg−1 (n=6) were injected i.p. in urethane anaesthetized rats. The selective AMPA receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulphonamide disodium (NBQX) 1 mg kg−1 (n=7), 0.1 mg kg−1 (n=7), and 0.01 mg kg−1 (n=7), or drug vehicle (normal saline, n=9) were administered in another set of animals. The kainate receptor antagonists γ-(R-)-glutamylaminomethane-sulphonic acid (GAMS) 1 mg kg−1 (n=7) and 10 mg kg−1 (n=8) and the selective agonist of group III mGluRs 2-amino-4-phosphono-S-butanoic acid (L-AP4) 3 mg kg−1 (n=5) and 10 mg kg−1 i.p., (n=12) were injected i.p. in a separate group of animals, a control group for GAMS and L-AP4 treatments received drug-vehicle (normal saline; n=11). The competitive Glu receptor antagonist with high selectivity for the low-affinity [3H]-kainate binding site 6,7,8,9-tetrahydro-5-nitro-1H-benz[g]indole-2,3-dione-3-oxime (NS-102) 1 mg kg−1 (n=5) and 5 mg kg−1 (n=6), or drug-vehicle (dimethyl sulphoxide (DMSO), n=6), were also administered in another set of animals. Because CNQX and NBQX are short acting drugs (Chizh et al., 1994; Birder & de Groat, 1992; Shinozaki et al., 1990), they were administered 15 min before i.c. capsaicin treatment; GAMS, NS-102 and L-AP4 were injected 30 min before capsaicin.
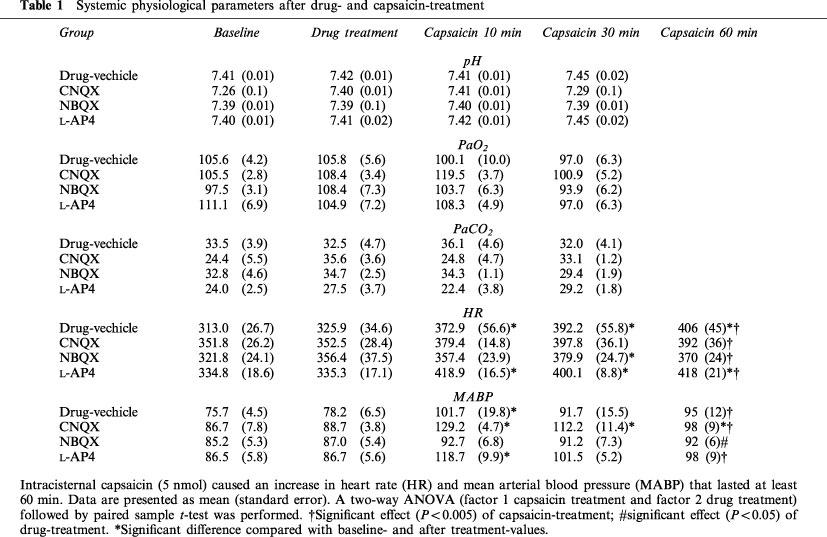
Systemic physiological parameters
Physiological monitoring was carried out in 16 animals. After anaesthesia with i.p. urethane and placement of the intracisternal catheter, a catheter (PE-50, internal diameter 0.58 mm; Becton Dickinson Co., Sparks, MD, U.S.A.) was placed in the left femoral artery. The effects of i.c. capsaicin injection on arterial pH, PaCO2 and PaO2, mean arterial blood pressure (MABP) and heart rate (HR) were measured before drug administration, 15 min after drug treatment, 10 and 30 min after capsaicin administration. HR and MABP were monitored continuously for 60 min in animals pretreated with i.p. CNQX 1 mg kg−1 (n=4), NBQX 1 mg kg−1 (n=4), L-AP4 10 mg kg−1 (n=4) and drug vehicle (n=4). Blood gases and pH measurements were performed four times in each animal (15 min after the placement of the catheter, i.e. baseline; 10 min after i.p. drug treatment; 10 and 30 min after i.c. capsaicin injection) using a blood gas/pH analyzer (Corning 178; Ciba-Corning Diagnostics, Medford, MA, U.S.A.). MABP and HR were monitored using Mac/Lab8 data acquisition system (AD Instruments, Medford, MA, U.S.A.) equipped with ETH 400 transducer amplifier. Core temperature was maintained at 36–37°C by a homeothermic blanket (Harvard Apparatus No. 551, South Natick, MA, U.S.A.).
Drugs
A fresh capsaicin solution (8-methyl-N-vanillyl-6-nonenamide, Sigma, St. Louis, MO, U.S.A) was made 2 h before use. Capsaicin (1.5 mg) was diluted in 1 ml of saline : ethanol : Tween 80 (8 : 1 : 1) and sonicated for 30 min. The solution was further diluted into artificial CSF (in mM): NaCl 132, KCl 3, MgCl2 0.6, CaCl2 1.5, NaHCO3 49, urea 6.6, D(+)-glucose 7.4, HEPES 5, pH adjusted to 7.4) to a final concentration of 50 μM. CNQX disodium, NBQX disodium, GAMS and L-AP4 (Research Biochemical International, Natick, MA, U.S.A.) were dissolved in normal saline. NS-102 (Research Biochemical International) was dissolved in DMSO (Sigma). Urethane (ethyl carbamate, Sigma) was diluted in water (3.4 M). Sodium pentobarbitone was obtained from Abbott Laboratories (Chicago, IL, U.S.A.).
Statistics
Data are expressed as a weighted average±standard error of cells per 50 μm section. The weighted averages derived from the cell counting method (see Methods) were compared by one-way ANOVA followed by Tukey's post hoc procedure. Student's two-tailed t-test was used when appropriate for simple comparisons between means. Data from physiological parameters were compared by two-way ANOVA (factor 1, capsaicin-treatment and factor 2, drug-treatment), followed by paired sample t-test. P values of 0.05 or less were considered significant.
Results
Physiological parameters
Capsaicin and drug treatment had no significant effect on pH, PaO2 and PaCO2 (Table 1). Capsaicin caused a significant increase in HR and MABP, that lasted at least 30 min. An effect of capsaicin on HR was also observed in animals pretreated with CNQX, NBQX, or L-AP4. In the presence of NBQX the effect of capsaicin on MABP was not observed. No significant interaction of two factors (capsaicin and drug treatment) was revealed (Table 1).
Table 1.
Systemic physiological parameters after drug- and capsaicin-treatment
Distribution of c-fos-LI positive brain stem neurons after capsaicin
Capsaicin increased c-fos LI bilaterally in lamina I, II of Sp5C (Figure 1) and was more intensely expressed within dorsal aspects at each sampled level. C-fos expression was greatest at −2.05 mm. Cells in Sol, AP, LRt and Md were also labelled, as were in the leptomeninges (Figure 1). Staining was also found in association with arachnoid and pial blood vessels and was most prominent in that portion of meninges overlying the Sp5C or the dorsal horn of the spinal cord; the identity of this immunostaining was not determined. No evidence of subarachnoid haemorrhage was present.
Figure 1.

Camera lucida drawings showing the location of c-fos antigen immunostained cells (dots) within superficial laminae of trigeminal nucleus caudalis (Sp5C) and the deeper laminae of solitary tract nucleus (Sol), lateral reticular nucleus (LRt) and medullary reticular nucleus (Md), in coronal brain stem sections taken from two representative animals treated with intracisternal capsaicin (5 nmol). C-fos expression was detected bilaterally and most intensely within dorsal than ventral aspects of laminae I and II, being greatest at −2.05 mm (2a), as previously has been reported (a images, drug-vehicle treated animal). Pretreatment with CNQX (15 mg kg−1; b images) reduced the c-fos positive cells per 50 μm section at each level of Sp5C, from dorsal (obex, 1b) to caudal (obex−6.45 mm, 3b), but not in Sol, LRt and Md. These nuclei are also labelled in capsaicin-vehicle treated animals, or intact anaesthetized animals, suggesting that intraperitoneal injection, or urethane, or both, could be the primary trigger. Sections 1, obex; sections 2, −2.05 mm; and sections 3, spinal cord (−6.25 mm). All the drawings have the same magnification.
There was no statistically significant change in the number of c-fos positive neurons between 2 and 8 h post surgery (data not shown). In capsaicin treated animals the average number of c-fos LI neurons within Sp5C was at least 17 times higher than in capsaicin-vehicle treated rats (3 h after surgery) (Table 2). Thus, surgical catheter placement does not significantly contribute to the estimated number of capsaicin induced c-fos LI neurons within Sp5C. AP, Sol, LRt and Md were also labelled even in intact (only anaesthetized) animals, and showed no changes with time (data not shown). Based on these findings, c-fos expression was studied 3 h after surgery in all subsequent experiments.
Table 2.
Capsaicin-induced c-fos immunoreactive cells within brain stem nuclei after drug treatment

Drug treatment
Both AMPA receptor antagonists CNQX and NBQX reduced c-fos LI within Sp5C, with ID50 values of 0.07±0.03 and 0.03±0.01 mg kg−1, respectively (0.25±0.1 μmol and 0.08±0.02 μmol kg−1, Figures 1 and 2). The effect of both CNQX and NBQX was dose-dependent, with a threshold at 0.1 mg kg−1. At obex, −2.05 mm and −6.45 mm, CNQX (15 mg kg−1) reduced c-fos LI by 44, 43 and 45%, respectively. The maximum reduction caused by NBQX at these levels was 38, 36 and 2% respectively. CNQX, or NBQX did not change the average number of c-fos positive neurons per section in Sol, LRt and Md (Table 2).
Figure 2.

Pretreatment with CNQX and NBQX dose-dependently decreased capsaicin-induced c-fos immunoreactivity (c-fos LI) within trigeminal nucleus caudalis (laminae I, II). Drugs were given intraperitoneally followed by intracisternal injection of capsaicin (5 nmol). Animals were euthanized 2 h after menigeal irritation with capsaicin. Data are presented as percentage of decrease of c-fos LI cells per section (weighted average, see methods).
The metabotropic receptor agonist L-AP4 (10 mg kg−1) reduced the weighted average of c-fos LI within the entire Sp5C by 30% (Table 2). The reduction was significant only at obex −2.05 mm level. At lower dose of L-AP4 (3 mg kg−1) did not change the c-fos LI (only at −6.25 mm level a significant decrease was seen). Pretreatment with the putative kainate receptor antagonist GAMS (1 and 10 mg kg−1) did not significantly affect expression within laminae I, II nor did treatment with the selective antagonist of low affinity kainate receptors NS-102 (1 and 5 mg kg−1). Pretreatment with L-AP4, GAMS or NS-102 did not change the c-fos expression within Md, LRt and Sol (Table 2).
Discussion
AMPA/kainate receptor antagonists
We used a panel of Glu receptor antagonists to determine the importance of two Glu receptor subtypes to induce c-fos expression in Sp5C after administering the irritant capsaicin into the subarachnoid space. Treatment with the AMPA/kainate receptor antagonists CNQX, NBQX significantly and dose-dependently decreased the number of capsaicin induced c-fos LI neurons within Sp5C. GAMS and NS-102, two antagonists at low-affinity kainate receptor did not reduce the number of c-fos labelled neurons within Sp5C. None of the above treatments blocked capsaicin induced c-fos expression within Md, LRt and Sol nuclei. Drug-treatment did not change the animals' respiratory function, at variance with previous reports (Engberg et al., 1993; Foutz et al., 1994; Pierrefiche et al., 1994). It is possible that the doses used in our study were too low or that simultaneous blockade of multiple glutamate receptor subtype is required to affect respiratory function. It is unlikely that the effect on c-fos are due to cardiovascular changes since none of the tested drugs had any effect on c-fos LI within Sol, which is the most important nucleus involved in baroreceptor afferent integration (Zhang & Mifflin, 1998). Taken together these data suggest that AMPA receptors can modulate c-fos expression and possibly neurotransmission within the trigeminovascular pain system after noxious meningeal stimulation by capsaicin. Kainate receptors do not seem to participate in this modulation. Interestingly, none of the compounds tested was able to decrease c-fos expression to background levels (i.e. as observed in animals receiving an intracisternal injection of capsaicin vehicle alone). Similar findings have been reported previously for other drugs attenuating c-fos expression in Sp5C, e.g., valproate (Cutrer, 1995a), the NK-1 receptor antagonist RPR 100893 (Cutrer et al., 1995b), the NMDA receptor antagonist MK-801 (Mitsikostas et al., 1998) and the 5-hydroxytryptamine1B/1D/1F receptor agonist sumatriptan (Mitsikostas et al., 1999 in press), suggesting that multiple neurotransmitter systems activate second order Sp5C neurons.
Both CNQX and NBQX are potent and selective AMPA receptor antagonist (Ki values 0.27 and 0.06 μM, respectively) and show low affinity for NMDA (Ki values 25 and >100 μM) and kainate receptors (Ki values 1.8 and 4.1 μM, respectively) (Shimizu-Sasamata et al., 1996; Dev et al., 1996). Thus, while CNQX shows only limited selectivity for AMPA versus kainate receptors (affinity ratio ≈7), NBQX is a more potent AMPA receptor antagonist (affinity ratio≈70). NBQX is estimated to be three times more effective than CNQX in our model. Thus, it seems that AMPA more than kainate receptors can modulate capsaicin-induced c-fos expression within Sp5C.
The importance of kainate receptors was examined by administering GAMS. In most studies GAMS showed a preferential antagonistic action at kainate-type receptors in spinal cord neurons (Davies & Watkins, 1983; 1985), or in seizures induced by excitatory amino acids (1 μmol; Turski et al., 1985). Additionally, GAMS discriminates between kainate and AMPA receptors in vitro (Zhou et al., 1993; Zhou & Parks, 1992). However, GAMS showed only limited selectivity for the kainate-preferring receptor subtype expressed by rat dorsal root ganglion neurons (KB value 360 μM) and the AMPA preferring subtype expressed by neurons from rat cerebral cortex (KB value 750 μM) (Wilding & Huettner, 1996). Because GAMS also blocks NMDA receptors at >1 mM (Raigorodsky & Urca, 1990), we did not use doses higher than 10 mg kg−1 (40 μmol kg−1).
To further investigate the importance of kainate receptors in capsaicin-induced c-fos LI within Sp5C, we administered NS-102, a non-NMDA receptor antagonist that selectively displaces low-affinity [3H]-kainate binding (Johansen et al., 1993; Verdoorn et al., 1994). Wilding & Huettner (1996) found that the KB value of NS-102 was 115 μM and 6 μM for neurons within the rat cerebral cortex (AMPA receptor) and the dorsal root ganglion (kainate receptor), respectively, suggesting that NS-102 is about ten times more potent than GAMS at kainate receptors. Similar to GAMS, NS-102 did not significantly change c-fos LI within Sp5C. The maximum dose of NS-102 used in the present study is 50 times higher than the threshold dose for CNQX. However, the affinity of NS-102 at kainate receptors is only five times smaller than that of CNQX for AMPA receptors (Wilding & Heuttner, 1996), suggesting than kainate receptors are unlikely to be involved in the c-fos response.
It is worth mentioning that urethane weakly blocks kainate and NMDA receptors (Dalo & Larson, 1990). However, we previously reported reduction of c-fos response when an NMDA receptor antagonist was given in the presence of urethane anaesthesia (Mitsikostas et al., 1998). It is thus unlikely that a partial attenuation of the c-fos response by the anaesthetic accounts for the lack of effect of kainate receptor antagonists in our study.
We cannot determine the exact location of the relevant AMPA receptors mediating the c-fos response. Current evidence favours an action on brain stem neurons because expression of AMPA receptors within primary trigeminal afferents has not been documented (Iida 1997; Sahara et al., 1997).
Metabotropic glutamate receptors
L-AP4 was administered to examine the potential importance of the group III mGluRs to capsaicin-induced c-fos response. L-AP4 inhibits glutamate release (Roberts 1995; Pin & Duvoisin, 1995; Pisani et al., 1997). It binds with high affinity to mGluR4 (0.4 μM), mGluR6 (0.9 μM) and mGluR8 (0.4 μM), whereas the affinities for the other mGluR subunits and ionotropic Glu receptors are very low (Conn & Pin, 1997; Eriksen & Thomsen, 1995). These receptors are probably pre-synaptic auto-receptors that fine-tune synaptic transmission by reducing calcium flux (Herrero et al., 1992; Thomsen, 1997). The mGluR4 and mGluR7 subtypes are present within rat TG and Sp5C neurons (Ohishi et al., 1995; Li et al., 1996). L-AP4 also modulates GABAergic activity within neo-cortex in other pain models (Thomsen, 1997). Thus, L-AP4 may modulate nociceptive pathways at many levels from the primary afferent neurons to the somatosensory cortex.
In conclusion the presented data suggest that AMPA and group III mGlu receptors modulate noxious responses within second order trigeminal neurons after meningeal irritation by capsaicin. Based on the lack of effect of GAMS and NS-102, it seems that kainate receptors are not involved. Since this model may be predictive for treatment of vascular headaches (Moskowitz & MacFarlane, 1993), AMPA and MGluR4 receptors may also serve as potential targets for the development of new anti-migraine drugs.
Acknowledgments
This study was supported in part by the Hellenic Navy, General Staff (D.D. Mitsikostas); by the International Headache Society research fellowship award 1997 (M. Sanchez del Rio); and by NS 01803 (F.M. Cutrer) and NS 35611 (C. Waeber and M.A. Moskowitz) of the National Institutes of Health.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- c-fos LI
c-fos-like immunoreactivity
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione disodium
- GAMS
γ-(R-)-glutamylaminomethanesulphonic acid
- Glu
glutamate
- HR
heart rate
- L-AP4
2-amino-4-phosphonono-S-butanoic acid
- LRt
lateral reticular nucleus
- MABP
mean arterial blood pressure
- Md
medullary reticular nucleus
- mGluR
metabotropic glutamate receptor
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulphonamide disodium
- NMDA
N-methyl-D-aspartate
- NS-102
6,7,8,9-tetrahydro-5-nitro-1H-benz[g]indole-2,3-dione-3-oxime
- Sol
solitary tract nucleus
- Sp5C
trigeminal nucleus caudalis
- TG
trigeminal ganglion
References
- ABBADIE C., TAYLOR K.B., PETERSON M.A., BASBAUM A.I. Differential contribution of two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- BARANAUSKAS G., NISTRI A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog. Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- BATTAGLIA G., RUSTIONI A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J. Comp. Neurol. 1988;277:302–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- BEREITER D.A., BENETTI A.P. Excitatory amino release within spinal trigeminal nucleus after mustard oil injection into the temporomandibular joint region of the rat. Pain. 1996;67:451–459. doi: 10.1016/0304-3959(96)03156-9. [DOI] [PubMed] [Google Scholar]
- BEREITER D.A., BEREITER D.F. N-methyl-D-aspartate receptor antagonism reduces FOS-like immunoreactivity in central trigeminal neurons after corneal stimulation in the rat. Neuroscience. 1996;73:249–258. doi: 10.1016/0306-4522(96)00038-3. [DOI] [PubMed] [Google Scholar]
- BEREITER D.A., BEREITER D.F., HATHAWAY C.B. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity in central trigeminal neurons and blocks select endocrine and autonomic responses to corneal stimulation in the rat. Pain. 1996;64:179–189. doi: 10.1016/0304-3959(95)00095-X. [DOI] [PubMed] [Google Scholar]
- BERRETTA S., ROBERTSON H.A., GRAYBIEL A.M. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J. Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., DE GROAT W.C. The effect of glutamate antagonists on c-fos expression induced in spinal neurons by irritation of the lower urinary tract. Brain Res. 1992;580:115–120. doi: 10.1016/0006-8993(92)90934-2. [DOI] [PubMed] [Google Scholar]
- BOVE M.G., MOSKOWITZ M.A. Primary afferent neurons innervating guinea pig dura. J. Neurophysiol. 1997;77:299–308. doi: 10.1152/jn.1997.77.1.299. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., CARTER W.B., SHIMIZU T., HEATH H., III, MORKOWITZ M.A. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat auperior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30:1193–1200. doi: 10.1016/0028-3908(91)90165-8. [DOI] [PubMed] [Google Scholar]
- CARRIVE P., MEYER-CARRIVE I. Changes in formalin-evoked spinal Fos expression and nociceptive behaviour after oral administration of Bufferin A (aspirin) and L-5409709 (ibuprofen + caffeine + paracetamol) Pain. 1997;70:253–266. doi: 10.1016/s0304-3959(97)03325-8. [DOI] [PubMed] [Google Scholar]
- CHIZH B.A., COMBERBATCH M.J., HEADLEY P.M. A comparison of intravenous NBQX and GYKI53655 as AMPA antagonists in the rat spinal cord. Br J. Pharmacol. 1994;112:843–846. doi: 10.1111/j.1476-5381.1994.tb13156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- CUTRER F.M., LIMMROTH V., AYATA G., MOSKOWITZ M.A. Attenuation by valproate of c-fos immunoreactivity in trigeminal nucleus caudalis induced by intracisternal capsaicin. Br. J. Pharmacol. 1995a;116:3199–3204. doi: 10.1111/j.1476-5381.1995.tb15124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTRER F.M., MOUSSAOUI S., GARRETT C., MOSKOWITZ M.A. The non-peptide neurokinin 1 antagonist, RPR 100893 decreases c-fos expression in trigeminal nucleus caudalis following chemical meningeal stimulation. Neuroscience. 1995b;64:741–750. doi: 10.1016/0306-4522(94)00428-8. [DOI] [PubMed] [Google Scholar]
- DALO N.L., LARSON A.A. Effects of urethane and ketamine on substance P- and excitatory amino acid-induced behavior in mice. Eur. J. Pharmacol. 1990;184:173–177. doi: 10.1016/0014-2999(90)90679-z. [DOI] [PubMed] [Google Scholar]
- DAVIES J., WATKINS J.C. Role of excitatory amino acids in mono- and polysynaptic excitation in the cat spinal cord. Exp. Brain Res. 1983;49:280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- DAVIES J., WATKINS J.C. Depressant actions of γ-D-glutamylaminomethyl sulphonate (GAMS) on amino acid-induced and synaptic excitation in the cat spinal cord. Brain Res. 1985;327:113–120. doi: 10.1016/0006-8993(85)91505-7. [DOI] [PubMed] [Google Scholar]
- DEV K.K., PETERSEN V., HONORE T., HENLEY J.M. Pharmacology and regional distribution of the binding of 6-[3H]nitro-7-sulphamoylbenzo[f]-quinoxaline-2,3-dione to rat brain. J. Neurochem. 1996;67:2609–2612. doi: 10.1046/j.1471-4159.1996.67062609.x. [DOI] [PubMed] [Google Scholar]
- EISENBERG E., VOS B.P., STRASSMAN A.M. The NMDA antagonist memantine blocks pain behavior in a rat model of formalin-induced facial pain. Pain. 1993;54:301–307. doi: 10.1016/0304-3959(93)90029-O. [DOI] [PubMed] [Google Scholar]
- ENGBERG I., TARNAWA I., DURAND J., OUARDOUZ M. An analysis of synaptic transmission to motoneurones in the cat spinal cord using a new selective receptor blocker. Acta Physiol. Scand. 1993;148:97–100. doi: 10.1111/j.1748-1716.1993.tb09537.x. [DOI] [PubMed] [Google Scholar]
- ERIKSEN L., THOMSEN C. [3H]-L-2-Amino-4-phosphonobutyrate labels a metabotropic glutamate receptor, mGluR4a. Br. J. Pharmacol. 1995;116:3279–3287. doi: 10.1111/j.1476-5381.1995.tb15136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIGIEL I., KACZMAREK L. Cellular and molecular correlates of glutamate-evoked neuronal programmed cell death in the in vitro cultures of rat hippocampal dentate gyrus. Neurochem. Int. 1997;31:229–240. doi: 10.1016/s0197-0186(96)00152-0. [DOI] [PubMed] [Google Scholar]
- FOUTZ A.S., PIERREFICHE O., DENAVIT-SAUBIE M. Combined blockade of NMDA and non-NMDA receptors produces respiratory arrest in the adult cat. Neuroreport. 1994;5:481–484. doi: 10.1097/00001756-199401120-00028. [DOI] [PubMed] [Google Scholar]
- FURUYAMA T., KIYAMA H., SATO K., PARK H.T., MAENO H., TAKAGI H., TOHYAMA M. Region-specific expression of subunits of ionotropic glutamate receptors (AMPA-type, KA-type, and NMDA receptors) in the rat spinal cord with special reference to nociception. Mol. Brain Res. 1993;18:141–151. doi: 10.1016/0169-328x(93)90183-p. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN H.L. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)13/10 receptor agonist zolmitriptan (311C90): are brain stem sites therapeutic target in migraine. Pain. 1996;67:355–359. doi: 10.1016/0304-3959(96)03118-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. The distribution of trigeminovascular afferents in the non-human primate brain Macaca nemestrina: a c-fos immunocytochemical study. J. Anat. 1997;190:367–375. doi: 10.1046/j.1469-7580.1997.19030367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITHS R., MALCOLM C., RITCHIE L., FRANDSEN A., SCHOUSBOE A., SCOTT M., RUMSBY P., MEREDITH C. Association of c-fos mRNA and excitoxicity in primary cultures of mouse neocortical and cerebellar neurons. J. Neurosci. Res. 1997;15:533–542. doi: 10.1002/(sici)1097-4547(19970615)48:6<533::aid-jnr6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- HERRERO I., MIRAS-PORTUGAL T., SANCHEZ-PIETRO J. Positive feedback of glutamate exocytocis by metabotropic presynaptic receptor stimulation. Nature. 1992;360:163–166. doi: 10.1038/360163a0. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine: a c-fos and electrophysiological study. Brain. 1996a;119:249–256. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Sumatriptan can inhibit trigeminal afferents by an exclusively neural mechanism. Brain. 1996b;119:1419–1428. doi: 10.1093/brain/119.5.1419. [DOI] [PubMed] [Google Scholar]
- HUETTNER J.E. Glutamate receptor channels in rat dorsal root ganglion neurons: activation by kainate and quisqualate, and blockade of desensitization by concanavalin A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- IIDA Y. Functional properties of receptors for glutamate and GABA in cultured trigeminal ganglion neurons. Kokubyo Gakkai Zasshi. 1997;64:121–132. doi: 10.5357/koubyou.64.121. [DOI] [PubMed] [Google Scholar]
- ILIAKIS B., ANDERSON N.L., IRISH P.S., HENRY M.A., WESTRUM L.E. Electron microscopy of immunoreactivity patterns for glutamate and gamma-aminobutyric acid in synaptic glomeruli of the feline spinal trigeminal nucleus (Subnucleus Caudalis) J. Comp. Neurol. 1996;366:465–477. doi: 10.1002/(SICI)1096-9861(19960311)366:3<465::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- JACKSON D.L., GRAFF C.B., RICHARDSON J.D., HARGREAVES K.M. Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur. J. Pharmacol. 1995;284:321–325. doi: 10.1016/0014-2999(95)00449-u. [DOI] [PubMed] [Google Scholar]
- JOHANSEN T.H., DREJER J., WATJEN F., NIELSEN E.O. A novel non-NMDA receptor antagonist shows selective displacement of low-affinity [3H]kainate binding. Eur. J. Pharmacol. 1993;246:195–204. doi: 10.1016/0922-4106(93)90031-4. [DOI] [PubMed] [Google Scholar]
- LAURITZEN I., DE WEILLE J.R., LAZDUNSKI M. The potassium channel opener (−)-cromakalim prevents glutamate-induced cell death in hippocampal neurons. J. Neurochem. 1997;69:1570–1579. doi: 10.1046/j.1471-4159.1997.69041570.x. [DOI] [PubMed] [Google Scholar]
- LAWAND N.B., WILLIS W.D., WESTLUND K.N. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur. J. Pharmacol. 1997;324:169–177. doi: 10.1016/s0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- LI J.L., OHISHI H., KANEKO T., SHIGEMOTO R., NEKI A., NAKANISHI S., MIZUNO N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR7, in ganglion neurons of the rat; with special reference to the presence in glutamatergic ganglion neurons. Neurosci. Lett. 1996;204:9–12. doi: 10.1016/0304-3940(95)12299-0. [DOI] [PubMed] [Google Scholar]
- LIU-CHEN L.Y., LISZCZAK T.M., KING J.C., MOSKOWITZ M.A. Immunoelectron microscopic study of substance P-containing fibers in feline cerebral arteries. Brain Res. 1986;369:12–20. doi: 10.1016/0006-8993(86)90508-1. [DOI] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., SANCHEZ DEL RIO M., WAEBER C., MOSKOWITZ MA., CUTRER F.M. The NMDA receptor antagonist MK-801 reduces capsaicin induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76:239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., SANCHEZ DEL RIO M., MOSKOWITZ M.A., WAEBER C.Both 5-hydroxytryptamine1B and 5-hydroxytryptamine1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis Eur. J. Pharmacol. 1999. in press [DOI] [PubMed]
- MOSKOWITZ M.A., MACFARLANE R. Neurovascular and molecular mechanisms in migraine headaches. Cereb. Brain Metab. Rev. 1993;5:159–177. [PubMed] [Google Scholar]
- NOZAKI K., BOCCALINI P., MOSKOWITZ M.A. Expression of c-fos-like immunoreactivity in brain stem after meningeal irritation by blood in the subarachnoid space. Neuroscience. 1992a;49:669–680. doi: 10.1016/0306-4522(92)90235-t. [DOI] [PubMed] [Google Scholar]
- NOZAKI K., BOCCALINI P., MOSKOWITZ M.A. CP-93, 129, sumatriptan, dihydroergotamine block c-fos expression within rat trigeminal nucleus caudalis caused by chemical stimulation of the meninges. Br. J. Pharmacol. 1992b;106:409–415. doi: 10.1111/j.1476-5381.1992.tb14348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHISHI H., AKAZAWA C., SHIGEMOTO R., NAKANISHI S., MIZUNO N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J. Comp. Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- PIERREFICHE O, , FOUTZ A.S., CHAMPAGNAT J., DENAVIT-SAUBIE M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. J. Physiol. 1994;47:509–523. doi: 10.1113/jphysiol.1994.sp020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIN J.P., DUVOISIN R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- PISANI A., CALABRESI P., CENTONZE D., BERNARDI G. Activation of group III metabotropic glutamate receptors depress glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36:845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- RAIGORODSKY G., URCA G. Spinal antinociceptive effects of excitatory amino acids antagonists: quisqualate modulates the action of N-methyl-D-aspartate. Eur. J. Pharmacol. 1990;182:37–47. doi: 10.1016/0014-2999(90)90491-n. [DOI] [PubMed] [Google Scholar]
- ROBERTS P.J. Pharmacological tools for the investigation of metabotropic glutamate receptors (mGluRs): Phenylglycine derivatives and other selective antagonists - an update. Neuropharmacology. 1995;34:813–819. doi: 10.1016/0028-3908(95)00094-m. [DOI] [PubMed] [Google Scholar]
- SAHARA Y., NORO N., IIDA Y., SOMA K., NAKAMURA Y. Glutamate receptor subunits GluR5 and KA-2 are co-expressed in rat trigeminal ganglion neurons. J. Neurosci. 1997;17:6611–6620. doi: 10.1523/JNEUROSCI.17-17-06611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO K., GREENBERG S., MOSKOWITZ M.A. Trigeminal origin of beta-preprotachykinin products in feline pial blood vessels. Neurosci. Lett. 1987;76:69–73. doi: 10.1016/0304-3940(87)90194-7. [DOI] [PubMed] [Google Scholar]
- SATO K., KIYAMA H., PARK H.T., SHARP F.R. The NMDA receptor mediates cortical induction of fos and fos-related antigens following cortical injury. Exp. Neurol. 1990;109:323–332. doi: 10.1016/s0014-4886(05)80023-8. [DOI] [PubMed] [Google Scholar]
- SHIMIZU-SASAMATA M., KAWASAKI-YATSUGI S., OKADA M., SAKAMOTO S., YATSUGI S.I., TOGAMI J., HATANAKA K.I., OHMORI J., KOSHIYA K., USUDA S., MURASE K. YM90K: pharmacological characterization as a selective and potent a-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor antagonist. J. Pharmacol. Exper. Ther. 1996;276:84–92. [PubMed] [Google Scholar]
- SHINOZAKI H., GOTOH Y., ISHIDA M. Selective N-methyl-D-asparate (NMDA) antagonists increase gastric motility in the rat. Neurosci. Lett. 1990;113:56–61. doi: 10.1016/0304-3940(90)90494-t. [DOI] [PubMed] [Google Scholar]
- SMULLIN D.H., SKILLING S.R., LARSON A.A. Interactions between substance P, calcitonin gene-related peptide, taurine and excitatory amino acids in the spinal cord. Pain. 1990;42:93–101. doi: 10.1016/0304-3959(90)91095-Z. [DOI] [PubMed] [Google Scholar]
- STRASSMAN A.M., MASON P., MOSKOWITZ M.A., MACIEWICZ R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain. Res. 1986;379:242–250. doi: 10.1016/0006-8993(86)90777-8. [DOI] [PubMed] [Google Scholar]
- TALLAKSEN-GREENE S.J., YOUNG A.B., PENNEY J.B., BEITZ A.J. Excitatory amino acid bindings sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci. Lett. 1992;141:79–83. doi: 10.1016/0304-3940(92)90339-9. [DOI] [PubMed] [Google Scholar]
- THOMSEN C. The L-AP4 receptor. Gen. Pharmacol. 1997;29:151–158. doi: 10.1016/s0306-3623(96)00417-x. [DOI] [PubMed] [Google Scholar]
- TURSKI L., MELDRUM B.S., JONES A.W., WATKINS J.C. Anticonvulsant action of stereoisomers of gamma-glutamylaminomethylsulphonic acid in mice. Eur. J. Pharmacol. 1985;111:279–283. doi: 10.1016/0014-2999(85)90769-1. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., EDVINSSON L., EKMAN R., KINGMAN T., MCCULLOCH J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide. Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985;62:131–136. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- VERDOORN T.A., JOHANSEN T.H., DREJER J., NIELSEN E.O. Selective block of recombinant glu6 receptors by NS-102, a novel non-NMDA receptor antagonist. Eur. J. Pharmacol. 1994;269:43–49. doi: 10.1016/0922-4106(94)90024-8. [DOI] [PubMed] [Google Scholar]
- WANAKA A., SHIOTANI Y., KIYAMA H., MATSUYAMA T., KAMADA T., SHIOSAKA S., TOHYAMA M. Glutamate-like immunoreactive structures in primary sensory neurons in the rat detected by specific antiserum against glutamate. Exp. Brain Res. 1988;65:691–694. doi: 10.1007/BF00235995. [DOI] [PubMed] [Google Scholar]
- WATANABE M.M., MISHINA M., INOUE Y. Distinct gene expression of the N-methyl-D-aspartate receptor channel subunit in peripheral neurons of the mouse sensory ganglia and adrenal gland. Neurosci. Lett. 1994;165:183–186. doi: 10.1016/0304-3940(94)90740-4. [DOI] [PubMed] [Google Scholar]
- WILDING T.J., HEUTTNER J.E. Antagonist pharmacology of kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring receptors. Mol. Pharmacol. 1996;49:540–546. [PubMed] [Google Scholar]
- ZHANG J., MIFFLIN S.W. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of rat. J. Physiol. 1998;511:733–745. doi: 10.1111/j.1469-7793.1998.733bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU S., BONASERA L., CARLTON S.M. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;22:895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]
- ZHOU N., PARKS T.N. γ-d-Glutamylaminomethyl sulfonic acid (GAMS) distinguishes subtypes of glutamate receptor in the chick cochlear nucleus (nuc. Magnocellularis) Hearing Res. 1992;60:20–26. doi: 10.1016/0378-5955(92)90054-q. [DOI] [PubMed] [Google Scholar]
- ZHOU N., HAMMERLAND L.G., PARKS T.N. γ-D-Glutamylaminomethyl sulfonic acid (GAMS) distinguishes kainic acid- from AMPA-induced responses in Xenopus oocytes expressinc chick brain glutamate receptors. Neuropharmacology. 1993;32:767–775. doi: 10.1016/0028-3908(93)90185-6. [DOI] [PubMed] [Google Scholar]


