Abstract
Treatment with acetylcholine (ACh) of a β-escin-permeabilized intrapulmonary bronchial smooth muscle of the rat induced force when the Ca2+ concentration was clamped at 1 μM. The ACh-induced Ca2+ sensitization of myofilaments was significantly greater in antigen-induced airway hyperresponsive rats than in control rats. The ACh-induced Ca2+ sensitization was completely blocked by treatment with Clostridium botulinum C3 exoenzyme, an inactivator of Rho family of proteins. Moreover, the protein level of RhoA in the intrapulmonary bronchi was significantly increased in the airway hyperresponsive rats. Thus, increased airway smooth muscle contractility observed in asthmatics may be related to augmented agonist-induced, Rho-mediated Ca2+ sensitization of myofilaments.
Keywords: Asthma, airway hyperresponsiveness, intrapulmonary bronchial smooth muscle, Ca2+ sensitization, Rho, β-escin
Introduction
In general, smooth muscle contraction has been thought to be induced by an increase in cytosolic Ca2+ via the activation of plasma membrane Ca2+ channels and/or Ca2+ release from sarcoplasmic reticulum. However, additional mechanisms have been suggested in agonist-induced smooth muscle contraction by studies which used the simultaneous measurements of force development and intracellular Ca2+ concentration (Sato et al., 1988), and chemically permeabilized preparations (Fujita et al., 1995) in various types of smooth muscles including airways (Ozaki et al., 1990). It has been demonstrated that agonist stimulation increases myofilament Ca2+ sensitivity in β-escin-permeabilized smooth muscles of the rat coronary artery (Satoh et al., 1994), guinea-pig vas deferens (Fujita et al., 1995), canine trachea (Bremerich et al., 1997), and so on. Although the detailed mechanism is not fully understood, a participation of Rho protein, a monomeric GTP binding protein, in the agonist-induced Ca2+ sensitization has been suggested by many investigators (e.g., Fujita et al., 1995; Otto et al., 1996; Gong et al., 1997).
Asthmatic patients have an increased contractility of airway smooth muscle (Roberts et al., 1984), which might be a major cause of airway hyperresponsiveness (AHR). Similarly, an increased responsiveness of bronchial smooth muscle has been demonstrated in a rat model of AHR induced by repeated antigen inhalation (Misawa & Chiba, 1993; Chiba & Misawa, 1995a,1995b). In this animal model of AHR, the bronchial smooth muscle contraction induced by receptor agonists such as acetylcholine (ACh), but not by high K+ depolarization, is markedly augmented (Misawa & Chiba, 1993; Chiba & Misawa, 1995a,1995b). Moreover, it has also been demonstrated that muscarinic receptor density and antagonist affinity of airway smooth muscle are normal (Chiba & Misawa, 1995a). Thus, it is possible that the mechanisms responsible for the AHR exist, at least in part, in the downstream pathway of muscarinic receptor signaling, including ACh-mediated Ca2+ sensitization.
In the present study, we examined participation of ACh-induced Ca2+ sensitization in the augmented contraction of airway smooth muscle at the antigen-induced AHR by using β-escin-permeabilized muscle strips.
Methods
Male Wistar rats (170–190 g, specific pathogen-free) were used. The induction of AHR was performed as described previously (Chiba & Misawa, 1995a,1995b). Briefly, the rats were sensitized with 2,4-dinitrophenylated Ascaris suum extract (DNP-Asc) together with Bordetella pertussis and were boosted 5 days later. Eight days after the first immunization, the rats were challenged by inhaling DNP-Asc for 20 min under conscious state. Then the animals were subjected to totally three times repeated antigen challenge every 48 h with the same inhalational challenge method.
Twenty-four hours after the last antigen challenge, the third branch of intrapulmonary bronchus was isolated, carefully cleaned of lung parenchyma and adhering connective tissue, and then cut into ring strips (about 200 μm width, 500 μm diameter). The epithelium was removed by gently rubbing with keen-edged tweezers. The ring strips were then permeabilized by a 30-min treatment with 10 μM β-escin (Sigma) at room temperature in relaxing solution.
Relaxing solution contained: (in mM): PIPES 20, Mg2+-dimethanesulphonate 7.1, K+-methanesulphonate 108, EGTA 2, Na2ATP 5.875, creatine phosphate 2, creatine pohphokinase 4 u ml−1, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) 1 μM and E-64 (pH 6.8) 1 μg ml−1 containing 10 μM A23187. Free Ca2+ concentration was changed by adding an appropriate amount of CaCl2. The apparent binding constant of EGTA for Ca2+ was considered to be 106 M−1 (Hori et al., 1993). The permeabilized muscle strip was then suspended in a 400 μl organ bath at room temperature. The contractile force developed was measured by an isometric transducer under a resting tension of 50 mg. To determine the involvement of Rho in the ACh-induced myofilament Ca2+ sensitization, the β-escin-permeabilized muscle strips were treated wtih Clostridium botulinum C3 exoenzyme (1 μg ml−1; Calbiochem) in the presence of 100 μM NAD for 20 min at room temperature.
To quantify the expression of Rho proteins, Western blot was performed in the homogenates of intrapulmonary bronchi that were dissected from from lung parenchyma. Briefly, the samples (10 μg of total protein per lane) were subjected to 12% SDS–PAGE and the proteins were then electrophoretically transferred to a nitrocellulose membrane. After blocking with 3% gelatin, the nitrocellulose membrane was incubated with primary antibody (polyclonal rabbit anti-human RhoA [amino acids 119–132] or anti-human RhoB [amino acids 119–132]; 1 : 2500 dilution, respectively; Santa Cruz). Then the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1 : 2500 dilution; Amersham), detected by an enhanced chemiluminescent system (Amersham) and analysed by a densitometry system. Thereafter, the primary and secondary antibodies were stripped and the membrane was reprobed by using monoclonal mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1 : 1000 dilution; Chemicon) to confirm the same amount of proteins loaded. Rat whole brain was used as a positive control for RhoA and RhoB (Olenic et al., 1997).
All the data are expressed as the mean±s.e.mean. Statistical significance of difference was determined by Dunnett's multiple analysis.
Results
Our previous study revealed that the sensitization procedure to antigen used in the present study had no significant effect by itself on the ACh responsiveness of the bronchial muscle and muscarinic receptors property (Chiba & Misawa, 1995a) in rats. So in the present study, the age-matched nonsensitized normal rats were used as control.
In all tissue preparations treated wtih 10 μM β-escin (for 30 min), the application of free Ca2+ (pCa=6.5, 6.0, 5.5 and 5.0) induced a concentration-dependent reproducible contractile response, indicating successful permeabilization. In the β-escin-permeabilized intrapulmonary bronchial smooth muscle, no significant difference between groups was observed in the Ca2+ responsiveness and the maximal contractile response induced by pCa=5.0 (Table 1). When the Ca2+ concentration was clamped at pCa=6.0, the application of 100 μM ACh in the presence of 100 μM GTP caused a further contraction (i.e. ACh-induced Ca2+ sensitization) in both the control and AHR groups (Figure 1). However, ACh-induced Ca2+ sensitization was significantly greater in the AHR group (Figure 1). The ACh-induced Ca2+ sensitization was completely antagonized by 10−6 M atropine (data not shown).
Table 1.
Summary of the Ca2+-induced contractile responses of the permeabilized bronchial smooth muscle in rats

Figure 1.
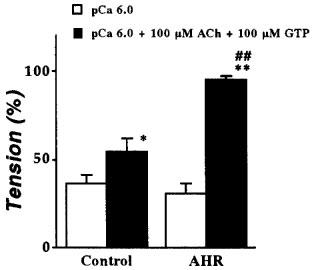
Comparison of ACh-induced Ca2+ sensitization in β-escin-permeabilized intrapulmonary bronchial smooth muscle from normal rats (Control; n=6) and the antigen-induced airway hyperresponsiveness rats (AHR; n=5). The contractile responses induced by 10−6 M Ca2+ in the presence (closed column) and absence (open column) of 100 μM ACh and 100 μM GTP are expressed as percentage of maximal contraction induced by 10−5 M Ca2+. Data represent the mean±s.e.mean. *P<0.05 and **P<0.01 vs respective Ca2+-induced contraction in the absence of ACh and GTP. ##P<0.01 vs ACh-induced Ca2+ sensitization in the control group.
To determine the involvement of Rho proteins in the ACh-induced Ca2+ sensitization, the effect of pretreatment with C3 exoenzyme, an inactivator of Rho family of proteins (Fujita et al., 1995), on the contractile responses of the β-escin-permeabilized muscle was estimated. In both groups, the C3 treatment had no effect on the contractile response induced by pCa=6.0 (typical recordings being shown in Figure 2). However, the ACh-induced Ca2+ sensitizing effect was completely blocked after the treatment with C3 both in the AHR and normal rats (Figure 2). These findings indicate that the ACh-induced Ca2+ sensitization might be mediated by Rho proteins and that the Rho-mediated Ca2+ sensitizing effect might be augmented at the AHR.
Figure 2.

Effect of Clostridium botulinum C3 exoenzyme on the ACh-induced Ca2+ sensitization of the β-escin-permeabilized intrapulmonary bronchial smooth muscle from the antigen-induced airway hyperresponsive rats. Left panel: typical recordings. After the permeabilization with 10 μM β-escin, the Ca2+ (10−6 M)-induced contractile responses in the presence and absence of 100 μM ACh and 100 μM GTP were observed as indicated (before C3). Then the strip was incubated with C3 exoenzyme (1 μg ml−1, for 20 min) in relaxing solution (see Methods), and the contractile responses were re-estimated (After C3). Right panel: summary of the inhibition of ACh-induced Ca2+ sensitization by C3 exoenzyme. The data are expressed as percentage increase in tension induced by ACh (in the presence of Ca2+ and GTP) from the sustained contraction induced by Ca2+ (10−6 M). Data represent the mean±s.e.mean from four experiments. **P<0.01 vs before C3.
Although Rho family proteins comprise RhoA, RhoB, Rac, Cdc42, and so on, a strong involvement of RhoA in agonist-induced smooth muscle Ca2+ sensitization has been reported (Otto et al., 1996; Gong et al., 1997). Therefore, we assessed the expression of RhoA in the homogenates of the intrapulmonary bronchi by using immunoblotting. As shown in Figure 3, immunoblotting with the antibody against RhoA gave a single 21 kD band, indicating the existence of RhoA protein in the intrapulmonary bronchi of rats. The level of RhoA in samples from the antigen-induced AHR rats was significantly increased compared with that from control rats (Figure 3). On the other hand, RhoB was not detected in the samples from either group (data not shown).
Figure 3.

The levels of RhoA protein in intrapulmonary bronchi from normal rats (Control) and the antigen-induced airway hyperresponsive rats (AHR). Upper panel: typical immunoblot. Lane 1; control, Lane 2; AHR, markers; protein molecular weight markers, and GAPDH; glyceraldehyde-3-phosphate dehydrogenase as a tissue marker. The bands were analysed by a densitometer and normalized by loading protein, and the data are summarized as shown in the lower panel. The data represent the mean±s.e.mean from four individual experiments, respectively. **P<0.01 vs control.
Discussion
In the present study, the Ca2+-induced contractile responses (in the absence of ACh and GTP) of β-escin-permeabilized intrapulmonary bronchial smooth muscles from the AHR rats were normal (Table 1, Figure 1). This finding is in agreement with our previous study that the contractile response of intact (non-permeabilized) bronchial smooth muscle induced by high K+ depolarization was normal in AHR (Chiba & Misawa, 1995b). Taken together, the baseline Ca2+ sensitivity (no receptor stimulation) of contractile elements is unlikely to change in AHR.
In the presence of GTP, ACh elicited a further contraction of the β-escin-permeabilized intrapulmonary bronchial smooth muscles from control rats, even though the Ca2+ concentration was clamped (Figure 1). This effect was antagonized by atropine, indicating existence of a muscarinic receptor-mediated Ca2+ sensitizing mechanism. Likewise, muscarinic receptor-mediated Ca2+ sensitization has also been reported in permeabilized smooth muscles of the canine (Bremerich et al., 1997) and porcine trachea (Croxton et al., 1998). So, it is possible that the agonist-induced Ca2+ sensitization might occur in airway smooth muscles of other species. In the current study, the ACh-induced Ca2+ sensitization was completely blocked by treatment with C3 exoenzyme, a selective inactivator of Rho family proteins (Fujita et al., 1995). Although the signal transduction pathways involved in the regulation of Ca2+ sensitization appear complex and, partly smooth muscle type specific, participation of Rho proteins has been reported in various types of smooth muscle including airways (Croxton et al., 1998). A part of the mechanism by which Rho mediated Ca2+ sensitization has been demonstrated as the Rho-associated kinase phosphorylates the 20 kD myosin light chain (Amano et al., 1996) and the application of Rho-associated kinase to permeabilized smooth muscle induces contraction (Kureishi et al., 1997).
ACh-induced Ca2+ sensitizing effect was augmented in bronchial smooth muscle from the AHR rats, the effect was blocked by C3 exoenzyme, and the protein level of RhoA in AHR was increased. This is the first study, to our knowledge, that demonstrates an augmentation of ACh-induced, Rho (probably RhoA)-mediated Ca2+ sensitization, which coincides with enhanced RhoA protein expression. An increase in responsiveness to muscarinic agonists of airway smooth muscle has been reported in animal models of AHR (Misawa & Chiba, 1993; Gavett et al., 1993; Lee et al., 1994; Chiba & Misawa, 1995a,1995b) and asthmatic patients (Roberts et al., 1984), although no change in the levels of muscarinic receptors was observed (Gavett et al., 1993; Lee et al., 1994; Chiba & Misawa, 1995a). Thus, it is likely that the enhanced contractility to muscarinic agonists reflects the augmentation of muscarinic receptor- and Rho-mediated Ca2+ sensitization. The mechanism(s) for the activation of Rho proteins by ACh is currently unclear. If Rho proteins are activated via receptors other than muscarinic receptor, it might account for the ‘non-specific' AHR, which is a common feature of asthmatics.
In conclusion, we demonstrated the existence of ACh-induced, Rho-mediated Ca2+ sensitization in the rat intrapulmonary bronchial smooth muscle contraction and suggest that enhancement of this Ca2+ sensitizing effect, probably by upregulated expression of the RhoA protein, might be involved in the augmented contractility of airway smooth muscle at the antigen-induced AHR.
Acknowledgments
We thank Ms Kaori Matsukawa, Mr Isao Nakayama and Ms Kyoko Akiyama for their help in technical assistance. This work was supported by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- ACh
acetylcholine
- AHR
airway hyperresponsivness
- DNP-Asc
2,4-dinitrophenylated Ascaris suum extract
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- pCa
−log [Ca2+]
References
- AMANO M., ITO M., KIMURA K., FUKATA Y., CHIHARA K., NAKANO T., MATSUURA Y., KAIBUCHI K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- BREMERICH D.H., WARNER D.O., LORENZ R.R., SHUMWAY R., JONES K.A. Role of protein kinase C in calcium sensitization during muscarinic stimulation in airway smooth muscle. Am. J. Physiol. 1997;273:L775–L781. doi: 10.1152/ajplung.1997.273.4.L775. [DOI] [PubMed] [Google Scholar]
- CHIBA Y., MISAWA M. Characteristics of muscarinic cholinoceptors in airways of antigen-induced airway hyperresponsive rats. Comp. Biochem. Physiol. 1995a;111C:351–357. doi: 10.1016/0742-8413(95)00061-5. [DOI] [PubMed] [Google Scholar]
- CHIBA Y., MISAWA M. Alteration in Ca2+ availability involved in antigen-induced airway hyperresponsiveness in rats. Eur. J. Pharmacol. 1995b;278:79–82. doi: 10.1016/0014-2999(95)00132-5. [DOI] [PubMed] [Google Scholar]
- CROXTON T.L., LANDE B., HIRSHMAN C.A. Role of G proteins in agonist-induced Ca2+ sensitization of tracheal smooth muscle. Am. J. Physiol. 1998;275:L748–L755. doi: 10.1152/ajplung.1998.275.4.L748. [DOI] [PubMed] [Google Scholar]
- FUJITA A., TAKEUCHI T., NAKAJIMA H., NISHIO H., HATA F. Involvement of heterotrimeric GTP-binding protein and rho protein, but not protein kinase C, in agonist-induced Ca2+ sensitization of skinned muscle of guinea pig vas deferens. J. Pharmacol. Exp. Ther. 1995;274:555–561. [PubMed] [Google Scholar]
- GAVETT S.H., WILLS-KARP M. Elevated lung G protein levels and muscarinic receptor affinity in a mouse model of airway hyperreactivity. Am. J. Physiol. 1993;265:L493–L500. doi: 10.1152/ajplung.1993.265.5.L493. [DOI] [PubMed] [Google Scholar]
- GONG M.C., FUJIHARA H., SOMLYO A.V., SOMLYO A.P. Translocation of rhoA associated wtih Ca2+ sensitization of smooth muscle. J. Biol. Chem. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- HORI M., SATO K., MIYAMOTO S., OZAKI H., KARAKI H. Different pathways of calcium sensitization activated by receptor agonists and phorbol esters in vascular smooth muscle. Br. J. Pharmacol. 1993;110:1527–1531. doi: 10.1111/j.1476-5381.1993.tb13996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUREISHI Y., KOBAYASHI S., AMANO M., KIMURA K., KANAIDE H., NAKANO T., KAIBUCHI K., ITO M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- LEE J.Y., UCHIDA Y., SAKAMOTO T., HIRATA A., HASEGAWA S., HIRATA F. Alteration of G protein levels in antigen-challenged guinea pigs. J. Pharmacol. Exp. Ther. 1994;271:1713–1720. [PubMed] [Google Scholar]
- MISAWA M., CHIBA Y. Repeated antigenic challenge-induced airway hyperresponsiveness and airway inflammation in actively sensitized rats. Jpn. J. Pharmacol. 1993;61:41–50. doi: 10.1254/jjp.61.41. [DOI] [PubMed] [Google Scholar]
- OLENIC C., BARTH H., JUST I., AKTORIES K., MEYER D.K. Gene expression of the small GTP-binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult rat brain. Brain Res. Mol. Brain Res. 1997;15:263–269. doi: 10.1016/s0169-328x(97)00270-2. [DOI] [PubMed] [Google Scholar]
- OTTO B., STEUSLOFF A., JUST I., AKTORIES K., PFITZER G. Role of Rho proteins in carbacol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. J. Physiol. 1996;496:317–329. doi: 10.1113/jphysiol.1996.sp021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZI H., KWON S.-C., TAJIMI M., KARAKI H. Changes in cytosolic Ca2+ and contraction induced by various stimulants and relaxants in canine tracheal smooth muscle. Pflügers Arch. 1990;416:351–359. doi: 10.1007/BF00370740. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.A., RAEBURN D., RODGER I.W., THOMSON N.C. Comparison of in-vivo responsiveness and in-vitro smooth muscle sensitivity to methacholine. Thorax. 1984;39:837–843. doi: 10.1136/thx.39.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO K., OZAKI H., KARAKI H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator Fura 2. J. Pharmacol. Exp. Ther. 1988;246:294–300. [PubMed] [Google Scholar]
- SATOH S., KREUTZ R., WILM C., GANTEN D., PFITZER G. Augmented agonist-induced Ca2+-sensitization of coronary artery contraction in genetically hypertensive rats. Evidence for altered signal transduction in the coronary smooth muscle cells. J. Clin. Invest. 1994;94:1397–1403. doi: 10.1172/JCI117475. [DOI] [PMC free article] [PubMed] [Google Scholar]


