Abstract
The effects of ethyl alcohol and wine (red and white) on haemostatic parameters and experimental thrombosis were studied in rats; NO was evaluated as a possible mediator of these effects.
We found that red wine (12% alcohol) supplementation (8.4±0.4 ml d−1 in drinking water, for 10 days) induced a marked prolongation of ‘template' bleeding time (BT) (258±13 vs 132±13 s in controls; P<0.001), a decrease in platelet adhesion to fibrillar collagen (11.6±1.0 vs 32.2±1.3%; P<0.01) and a reduction in thrombus weight (1.45±0.33 vs 3.27±0.39 mg; P<0.01).
Alcohol-free red wine showed an effect similar to red wine. In contrast, neither ethyl alcohol (12%) nor white wine (12% alcohol) affected these systems.
All these effects were also observed after red wine i.v. injection (1 ml kg−1 of 1 : 4 dilution) 15 min before the experiments.
The effects of red wine were prevented by the NO inhibitor, Nωnitro-L-arginine-methyl ester (L-NAME). L-arginine, not D-arginine, reversed the effect of L-NAME on red wine infusion.
Red wine injection induced a 3 fold increase in total radical-trapping antioxidant parameter values of rat plasma with respect to controls, while white wine and alcohol did not show any effect.
Our study provides evidence that red wine modulates primary haemostasis and prevents experimental thrombosis in rats, independently of its alcohol content, by a NO-mediated mechanism.
Keywords: Haemostasis, platelet adhesion, bleeding, venous thrombosis, nitric oxide, plasma antioxidant capacity
Introduction
The protective effect of a moderate daily consumption of alcoholic beverage on the risk of cardiovascular disease has been described by a number of epidemiological studies (Renaud and De Lorgeril 1992; Goldberg et al., 1995; Rimm et al., 1996; Hein et al., 1993; Gaziano et al., 1996). Several mechanisms have been proposed that mediate the protective effect of alcohol in ischaemic vascular disease. In particular, alcohol increases the prostacyclin/thromboxane ratio (Landolfi & Steiner, 1984) and decreases platelet aggregability (Renaud et al., 1992); moreover, it increases the release of plasminogen activator and lowers the levels of fibrinogen (Veenstra et al., 1990; Laug, 1983; Ridker et al., 1994).
The possibility that the type of alcoholic drink, beside alcohol itself, influences the risk of cardiovascular disease has been suggested. The so called ‘French paradox' proposed that low cardiovascular mortality rate in France was due, at least in part, to regular consumption of wine, more than of beer (Renaud and De Lorgeril 1992). The Copenaghen City Heart Study showed among wine but not beer or spirit drinkers a lower cardiovascular mortality, similar to that observed in moderate alcohol drinkers (Groenbaek et al., 1995). Lately, results of a large prospective study performed in Eastern France, appeared to confirm the association between the regular consumption of alcohol, mostly wine, and lower mortality for coronary artery disease (Renaud et al., 1998).
From epidemiological studies, whether the beneficial effects of alcohol consumption have to be ascribed to wine in a substantial way remains an unresolved issue. It is also unknown whether red or white wine equally contribute to the potential benefits of alcohol. The observation that red wine administration to healthy subjects increased plasma high-density lipoprotein cholesterol and apolipoprotein A-1 plasma concentrations while white wine did not (Lavy et al., 1994), suggested that other components in red wine rather than its alcoholic content might play a role in cardiovascular prevention.
Red wine contains many other compounds that might influence the process of thrombosis (Rice-Evans et al., 1997). Polyphenolic red wine components inhibited platelet aggregation and platelet-leukocyte interactions (Bertelli et al., 1995; Pellegrini et al., 1996; Pace-Asciak et al., 1995; Rotondo et al., 1996, 1998). Flavonoids and other red wine ingredients, which were recognized as strong antioxidants and oxygen free radical scavengers, enhanced in in vitro and in ex vivo experiments, the generation of nitric oxide (NO), a platelet inhibitor and vasodilator (Fitzpatrick et al., 1993; van Acker et al., 1995; Andriambeloson et al., 1997). Fitzpatrick et al. showed that wine and other grape products induced a NO-dependent relaxation of aortic rings (Fitzpatrick et al., 1993). Later, Andriambeloson et al. identified polyphenol compounds and leucocyanidol as responsible for this NO-dependent effect (Andriambeloson et al., 1997). However, no data are available on the relevance of such mechanisms in vivo.
The aims of this study were the following: (1) To establish whether ethyl alcohol and wines (red and white) would exert comparable effects on haemostatic parameters and on experimental thrombosis; (2) To evaluate whether removal of alcohol from wine would also remove its potential effects on haemostasis and thrombosis and (3) To determine the role of NO production as a possible mediator of the effects of alcoholic beverages on haemostasis and thrombosis.
We provide here evidence that red wine, but not white wine or ethyl alcohol administration to rats induces NO production in vivo and tentatively suggest a clinically-relevant mechanism to explain, in part, the protective effects of moderate red wine consumption on cardiovascular disease in man.
Methods
Animals
Male Sprague-Dawley rats, from Mario Negri Sud breeding facility, weighing 300–370 g were used for this study, housed under controlled conditions and fed a standard diet ad libitum. Procedures involving animals and their care were conducted in conformity with the Institution guidelines that are in compliance with national (D.L. n. 116, G.U. suppl. 40, February 18, 1992) and international laws and policies (EEC Council Directive 86/609, OJ L 358,I, Dec. 12, 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publication N. 85-23, 1985 and Guidelines for the Use of Animals in Biomedical Research., Thromb Haemost 58, 1078-1084, 1987).
Experimental design
Oral administration
Rats were supplemented with drinking water for 10 days, with red wine (12% alcohol by volume), white wine (12% alcohol by volume) or ethyl alcohol (12%). Three different drinking solutions were prepared by adding one part of red wine, white wine or ethyl alcohol to three or seven parts of drinking water. Since rats drank an average of 32±3 ml d−1 of these solutions, the daily amount of wines or ethyl alcohol was the following: 8.4±0.4 or 4.2±0.2 ml d−1 of red wine, 8.4±0.4 ml d−1 of white wine and 8.3±0.4 or 4.1±0.3 ml d−1 of ethyl alcohol.
In some experiments alcohol-free red wine was administered at a daily amount of 8.8±0.2 ml in drinking water for 10 days. Alcohol-free red wine was obtained by red wine lyophilization and resuspension in an equal volume of drinking water.
BT, ex vivo platelet studies, experimental thrombosis induction and laboratory analyses were performed after 10 days of supplementation. Groups of 15 animals were studied for each experiment.
To test the role of NO, a group of animals (ten rats) was supplemented for 10 days with red wine, containing 1.0 g l−1 of L-NAME, an inhibitor of NO formation, or with L-NAME alone diluted in drinking water.
Intravenous (i.v.) administration
One part of red wine, white wine, ethyl alcohol or alcohol-free red wine was diluted in three parts of saline solution (NaCl 0.9%). One ml kg−1 of this solution was injected i.v. in rats and experiments were performed after 15 min (this time interval was chosen on the basis of preliminary experiments, data not shown). Groups of 15 animals were used.
To investigate the role of NO on intravenous injection of red wine, groups of animals (ten rats) were pretreated with L-NAME, at the dose of 30 mg kg−1, i.v. Since the inhibitory effect of L-NAME was detected only when it was given before red wine administration and lasted no more than 20 min, we administered L-NAME 2 min before wine injection (preliminary experiments, data not shown). Fifteen minutes after the treatments, BT was measured or blood was drawn from the heart for ex vivo platelet studies and laboratory analyses.
When venous thrombosis was studied, L-NAME or saline solution were also infused in the femoral vein after inferior vena cava ligature for the whole experiment. The infusion led to a reduction in thrombus weight, due to continuous washing of the thrombus or to blood dilution at the site of thrombus formation.
L-arginine, the precursor of NO synthesis in vascular endothelium and its stereoisomer D-arginine, were used to reverse the effect of L-NAME on red wine-induced effects. Pretreatment with L-NAME (30 mg kg−1, i.v.) 2 min before wine injection was followed by the injection of L-arginine or D-arginine (300 mg kg−1) 5 min after (Remuzzi et al., 1990).
To rule out a role of prostacyclin on red wine effects, aspirin was used at a dose (5 mg kg−1, i.v.) effectively inhibiting vascular cyclo-oxygenase (Cerletti et al., 1986).
‘Template' bleeding time
BT was measured as described (Wollny et al., 1997). Briefly, rats were placed in a plastic cylinder with several openings from one of which the animal's tail was protruding. A standardized device was applied longitudinally on the dorsal part of the tail between 6 and 9 cm from the tip, taking care to avoid large veins. Immediately after injury, the tail was placed into a cylinder with isotonic saline solution at 37°C. BT was measured in seconds from the time when the tail was surgically cut until bleeding stopped completely (no rebleeding within 30 s).
Platelet preparation
After oral or i.v. treatment, blood was drawn from the heart into plastic syringes containing 3.13% sodium citrate (1 : 9 v v−1) from rats anaesthetized with sodium pentobarbital (40 mg kg−1), injected intraperitoneally. Suspensions of washed platelets were prepared as previously described (Rotondo et al., 1997). Platelet-rich plasma (PRP) was obtained by consecutive centrifugation at 200×g for 15 min and then at 800×g for 3 min at room temperature. Then PRP was removed and pooled, the residual blood sample was centrifuged at 2000×g for 10 min to obtain platelet-poor plasma (PPP). The PRP platelet number was adjusted to 3×105 platelets μl−1 with autologous PPP.
Platelet adhesion to fibrillar collagen
Ex vivo platelet adhesion was carried out as described (Radomski et al., 1987). Briefly, washed platelet samples (0.75×108 platelets in 0.25 ml) were incubated in duplicate in an aggregometer (Elvi Logos, Milano, Italy) at 37°C and stirred at 900 r.p.m. EDTA (5 mM) was added to prevent platelet aggregation. After 5 min, collagen (50 μg ml−1) was added and the samples were stirred for a further 15 min. Platelets were counted optically using a Burker chamber, after sample dilution by the Unopette system (Becton-Dickinson, N.J., U.S.A.), before and 15 min after collagen addition and a difference in platelet count was taken as an index of their adhesion to collagen.
Platelet aggregation
Platelet aggregation in PRP was induced by collagen and ADP as previously described (Remuzzi et al., 1990; Di Minno et al., 1979). The threshold-aggregating concentration was defined as the lowest concentration of aggregating agent, which induced irreversible platelet aggregation, starting within 3 min of addition of aggregating agents to PRP.
Experimental thrombosis
Experimental thrombosis was induced by ligature of the inferior vena cava (Reyers et al., 1989). Rats were anaesthetized with sodium pentobarbital (40 mg kg−1, bolus IP) and the rat abdomen was surgically opened on the median line. After a careful dissection, a tight ligature (with cotton thread) was placed around the inferior vena cava, just below the left renal vein. Two hours later, the abdomen was reopened under anaesthesia, the thrombus, if present, was removed, washed in distilled water, blotted on filter paper and placed in a desiccator; 24 h later, the dry weight of the thrombus was recorded.
Laboratory analysis
Fibrinogen was measured according to Clauss method; 200 μl of diluted plasma were incubated with 200 μl of thrombin (100 U ml−1, Ortho Diagnostics, N.J., U.S.A.) and clotting time was measured by an electromagnetic coagulometer. The fibrinogen levels were calculated using a standard curve.
Procoagulant activity was assessed by a one-stage clotting assay (Napoleone et al., 1997); 100 μl of plasma were placed at 37°C and after 30 s, 100 μl of 25 mmol l−1 CaCl2 at 37°C were added and the time of clot formation was recorded.
Euglobulin clot lysis time (ECLT) was assayed as previously described (Johnson et al., 1964).
Ethanol analysis in whole blood
Ethanol analysis in whole blood was performed by Headspace gas chromatography with capillary column (Correa & Custodio Pedroso, 1997) (Perkin Elmer GC-Autosystem XL chromatograph, fitted with CP-Wax 57 CB fused silica capillary column 50×0.25 mm I.D.; df=0.2 mm; Chrompack, The Netherlands). The column temperature was initially set at 40°C (0 min) and then programmed at 7°C min−1 to 100°C for 4 min. The injector and the flame ionization detection (FID) system were at 210°C. Helium (He) was used as carrier gas at 1.2 ml min−1. Air and He were set at 450 μl min−1, respectively. The split rate was 1/50. The integrator was used with attenuation setting of 2.
Ethanol aqueous working solutions were prepared from 4% (v v−1) ethanol standard solutions (Carlo Erba, Rodano, MI, Italy). The internal standard (analytical grade, 99.5% purity) n-propanol was purchased from Riedel-de Haën, (Seelze, Germany).
One μl of internal standard was added to 200 μl of whole blood in a 2 ml glass vial. The vial was rapidly sealed with silicone rubber septum cap and an aluminum crimp seal and incubated for 30 min at 70°C. The upper gas phase was homogenized three times by pulling and pushing the vapour phase using the injection syringe. After this, a homogenized 100 μl gas aliquot was withdrawn through the rubber cap with a 100 μl gas-tight Hamilton syringe (model 1705N, Supelco) and injected directly into the gas chromatograph. This syringe was equilibrated at 50°C, in order to prevent internal condensation on the walls (Tangerman, 1997).
Calibration curves were performed in whole blood spiked with ethanol standard to obtain concentrations ranging from 0.016 to 3.156 g l−1.
Whole blood was obtained from rats (n=6) ten days after red wine supplementation and 5 and 15 min after its intravenous injection.
Characterization of red and white wines
Red wine (1995 Montepulciano D'Abruzzo 12% alcohol by volume) and white wine (1996 Trebbiano D'Abruzzo 12% alcohol by volume) were kindly supplied by Cantina Miglianico, Miglianico, Italy.
Samples of red and white wine were analysed by HPLC (Varian 9010 Solvent delivery system, with Varian 9065 Polychrom diode array detector), following the method described by Goldberg et al. (1996), slightly modified as follows: 10 ml aliquots of freshly opened wine bottles were filtered through Minisart 0.45 mm (Sartorius) filter pad and stored at 4°C, protected against direct light. Analyses were completed within a week. Samples of 20 μl of filtered wine were directly injected into the column (ODS Hypersil 5 mm, purchased by Sigma-Aldrich) and eluted with the following gradient: solvent A: acetic acid, solvent B: methanol, solvent C: bidistilled water. Zero time conditions: 5% A, 15% B, 80% C; flow rate: 0.4 ml min−1 for 5 min, 5% A, 20% B, 75% C; flow rate 0.5 ml min−1 for 30 min; 5% A, 45% B, 50% C for 10 min.
Every run was followed by 10 min equilibrium period with the zero time solvent mixture prior to injection of the next sample.
Total phenols were analysed according to the Folin Ciocalteu method (Goldberg et al., 1996), using gallic acid as the standard, and the results are given as gallic acid equivalents (GAE). Total flavonoids, total anthocyanins, free anthocyanins, and the difference between total flavonoids and total anthocyanins, here indicated as non-anthocyanin-flavonoids, were estimated colorimetrically according to Di Stefano et al. (1989), calibrating against (+)-catechin and expressing results as (+)-catechin equivalents for total flavonoids and non-anthocyanin-flavonoids; cyanidin chloride was used as standard for anthocyanin determination.
Tannin content was determined following the method described by Serafini et al. (1997) and here slightly modified. Lyophilized wine samples were rehydrated with bidistilled water and the calibration curve was made using tannic acid ranging from 0.78 to 12.5 mg l−1.
Phenolic composition of red and white wine
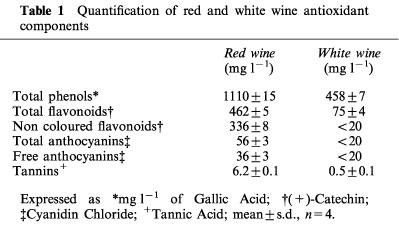
As shown in Figure 1, red wine contained a higher amount of phenolic compounds than white wine. The prominent peaks corresponded to protocatechin acid and gallic acid, identified with numbers 1 and 2 respectively, (+)-catechin and (−)-epicatechin, (peaks 3 and 4 respectively), Trans-Resveratrol (peak 5) and rutin (peak 6), were present only in red wine. This difference in polyphenolic content between red and white wine is more evident from the results reported in Table 1; the total phenol content in red wine exceeded more than two times that of white wine; total flavonoid content in red wine was more than six times and tannins were 12 times higher than in white wine. The content of other substances reported in Table 1 for white wine were below the detection limit and are indicated as <20 mg l−1.
Figure 1.
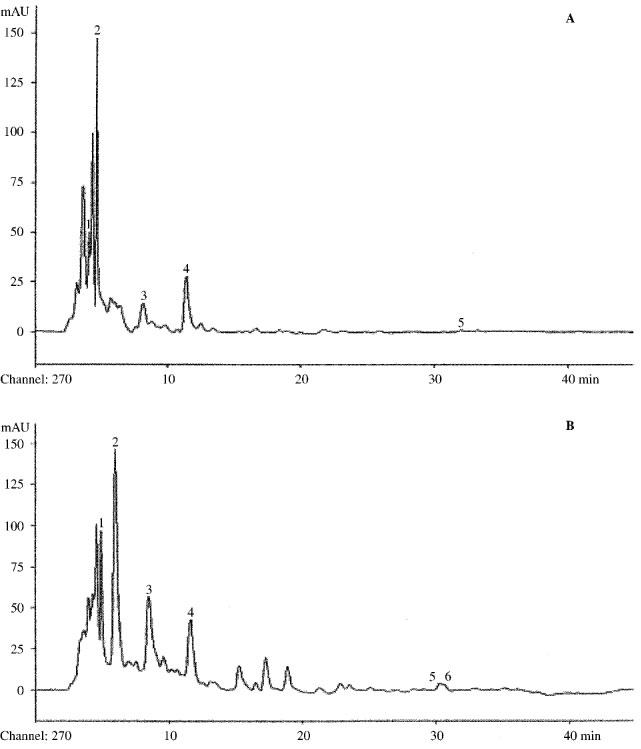
Comparison between HPLC chromatograms at 278 nm of white and red wines used. Chromatogram (A) refers to white wine (Trebbiano d'Abruzzo 1996); chromatogram (B) to red wine (Montepulciano d'Abruzzo 1995). Identified componds are: 1. Prothocatechuic acid; 2. Gallic acid; 3. (+)-Catechin; 4. (−)-Epicatechin; 5. trans-resveratrol; 6. Rutin.
Table 1.
Quantification of red and white wine antioxidant components
Total Radical-trapping Antioxidant Parameter (TRAP)
TRAP assay was performed by measuring in plasma the rate of peroxidation induced by 2,2′-diazobis(2-amidinopropane)dihydrochloride (ABAP) through the loss of fluorescence of R-Phycoerythrin (R-PE) (Ghiselli et al., 1995). Briefly, the reaction mixture consisted of 1.5×10−8 M R-PE in 75 mM phosphate buffer, pH 7.0. Eighty μl of freshly prepared and diluted plasma (1 : 6) or any other reagent were added to 2.0 ml final volume, and the resulting solution was maintained at 37°C for 5 min in fluorimeter cuvettes. The oxidation reaction was started by adding ABAP to a final concentration of 4.0 mM, and decay of R-PE fluorescence was monitored every 5 min on Perkin-Elmer LS-5 Luminescens Spectrometer equipped with thermostatically controlled cell holder; monochromators were operating at excitation wavelength 495 nm and emission wavelength 575 nm. When plasma was added to the reaction mixture, a period of complete protection of R-PE was observed. The length of this lag-phase (T) was directly related to total plasma antioxidant capacity. To quantify the TRAP, the T produced by plasma was compared to the T produced by a known amount of Trolox (a water-soluble analogue of vitamin E), according to the following proportion: Trolox concentration (μmol l−1) : T Trolox=X : T Plasma.
Drugs
L-NAME, L-arginine and D-arginine (Sigma Chemical Co. St. Louis, MO, U.S.A.), aspirin (Flectadol 1000, Maffioni Spa, Milan, Italy) and collagen (Semmelweis Milano, Italy) were dissolved in saline. 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) was purchased from Aldrich Chemical Co. (Milwaukee, MI, U.S.A.); 2,2′-azobis-(2-amidinopropane) dihydrochloride ABAP was a gift from Dr A. Ghiselli (National Institute of Nutrition, Rome, Italy) and R-Phycoerythrin (R-PE) from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Ethyl alcohol was from Carlo Erba, Milan, Italy.
Statistical analysis
All the results are presented as mean±s.e.mean. The analysis of Variance (ANOVA CR) was used for the comparison among groups, followed by Dunnett test for multiple comparison. Statistical significance was defined at P<0.05.
Results
Oral administration
Haemostatic parameters
Neither alcohol nor white wine administration, at the same doses and equivalent concentrations, showed any effect on BT. In contrast, red wine supplementation significantly prolonged BT in rats, in a dose-dependent manner. Alcohol-free red wine also showed a significant effect, even if slightly lower than the corresponding dose of red wine (Table 2).
Table 2.
Effect of 10 day supplementation with ethyl alcohol, white wine, red wine or alcohol-free red wine
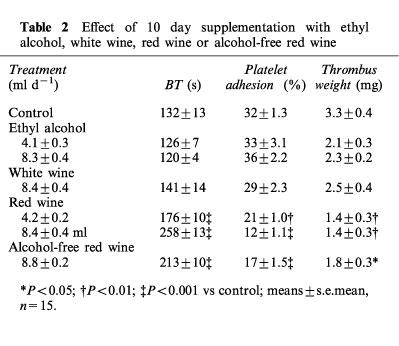
As shown in Table 2, ex vivo platelet adhesion to fibrillar collagen was significantly decreased in rats given red wine compared to controls. The effect was dose-dependent and was not observed in animals supplemented with equivalent doses of ethyl alcohol or white wine.
Ex vivo platelet aggregation responses induced either by collagen or ADP, were not changed following red wine administration (data not shown).
Fibrinogen levels, one-stage clotting assay and ECLT values were not altered in any treatment group (data not shown).
Experimental thrombosis
Ethyl alcohol and white wine administration induced a slight, however non significant reduction in thrombus weight (Table 2). Red wine, in contrast, showed a significant antithrombotic activity, by reducing thrombus weight up to 58% (Table 2). A similar effect was observed when alcohol-free red wine was supplemented.
Role of NO production
The NO synthase inhibitor, L-NAME, administered at the concentration of 1.0 g l−1, which did not show any effect per se, completely abolished the prolongation of BT, the decrease in platelet adhesion and the reduction in thrombus weight induced by red wine (Figure 2A, B and C). L-arginine administration (10 g l−1, in drinking water) for 10 days, significantly prolonged BT (126±8 vs 207±17 s, P<0.001); therefore, it could not be used to revert the effects of L-NAME.
Figure 2.
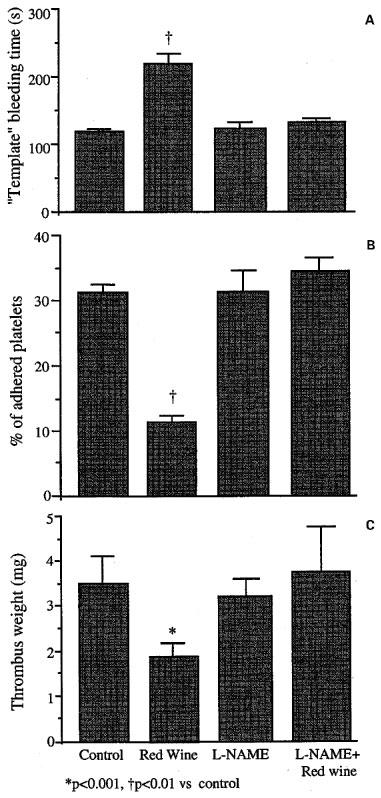
Effect of NO inhibition by L-NAME (1.0 g l−1 day−1 for 10 days) on (A) BT prolongation, (B) platelet adhesion to fibrillar collagen and (C) thrombus weight induced by red wine (8.8±0.2 ml day−1); 10 days supplementation.
Intravenous administration
Haemostatic parameters
When ethyl alcohol or white wine were administered at the dose of 1 ml kg−1 of 1 : 4 dilution i.v., no change in BT was observed (161±14 and 151±3 s, respectively; P=0.1).
Red wine, in contrast, significantly prolonged BT in rats if compared with controls (133±4 vs 284±10 s, P<0.001).
Intravenous injection of red wine also inhibited platelet adhesion to fibrillar collagen (13±3 vs 33±1%, P<0.01). Alcohol-free red wine showed similar effects (133±4 vs 260±11 s, P<0.001).
Fibrinogen levels, one-stage clotting assay and ECLT values were not altered in any treatment group (data not shown).
Experimental thrombosis
Thrombus weight after inferior vena cava ligature was not modified after acute injection of ethyl alcohol or white wine (3.4±0.3 vs 3.30±0.8 and 3.21±0.8 mg, respectively). In contrast, red wine and alcohol-free red wine reduced by about 50% thrombus weight (3.4±0.3 vs 1.6±0.3 and 1.8±0.4 mg, respectively, P<0.05).
Role of NO production
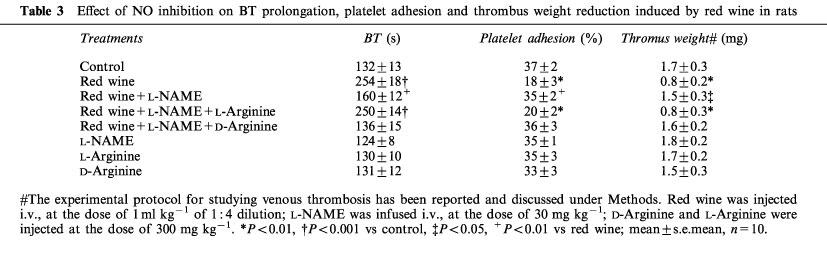
The effects of red wine on BT and platelet adhesion were completely abolished by L-NAME pretreatment. Administration of L-arginine substantially reversed the effect of L-NAME, while D-arginine did not. Neither L-NAME nor L-arginine or D-arginine per se modified the BT or platelet adhesion. L-NAME totally prevented also the antithrombotic activity of red wine, an effect prevented by L-arginine but not by D-arginine (Table 3).
Table 3.
Effect of NO inhibition on BT prolongation, platelet adhesion and thrombus weight reduction induced by red wine in rats
Evaluation of cyclo-oxygenase involvement
BT prolongation and thrombus weight reduction induced by the i.v. injection of red wine were unchanged by aspirin administration at the dose of 5 mg kg−1 (259±14 vs 284±10 s and 0.86±0.22 vs 0.84±0.24 mg, respectively, n=10). As previously shown, aspirin per se did not affect either BT (133±15 vs 133±4 s, n=10) or thrombus weight (2.8±0.5 vs 2.8±0.6 mg, n=10).
TRAP assay
Figure 3 shows plasma TRAP values measured in samples taken 15 min after treatments. TRAP did not change significantly after alcohol (163±43.5 vs 281±28 μM, n=10) or white wine (178±46 μM, n=10) administration. Red wine injected rats had TRAP values about three times higher than control animals (493±59 μM, n=10).
Figure 3.
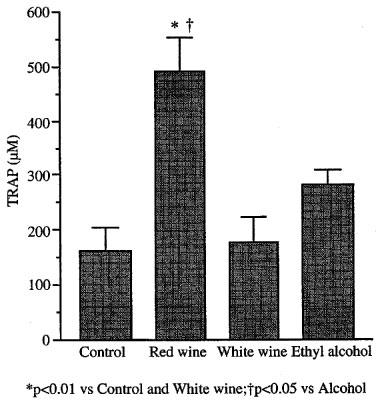
Total radical trapping antioxidant parameter (TRAP) of freshly prepared rat plasma 15 min after i.v. injection (1 ml kg−1 of 1 : 4 dilution) of red wine, white wine or ethyl alcohol.
Ethanol analysis in whole blood
Ethanol concentration in whole blood was 11.2±2 μg ml−1 after 10 day oral administration of 8.4±0.4 ml d−1 red wine in drinking water (mean±s.e.mean, n=4). Intravenous administration of 1 ml kg−1 of 1 : 4 diluted red wine resulted in blood ethanol concentrations of 32.5±3 and 7.9±1.4 μg ml−1, respectively 5 and 15 min after injection (mean±s.e.mean, n=3).
Discussion
There is epidemiological evidence that red wine can be beneficial in reducing the risk of cardiovascular disease above that expected from its alcohol content (Renaud and De Lorgeril, 1992; 1998; Gronbaek et al., 1995). An antithrombotic activity of red wine has also been demonstrated in experimental models of thrombosis (Demrow et al., 1995).
This study in the rat shows that red wine modified haemostatic parameters and prevented experimental thrombosis, independently of its alcohol content. White wine was ineffective while alcohol-free red wine was as effective as the original beverage, supporting the hypothesis that red wine components other than alcohol were responsible for the observed effects. The remarkable difference in phenolic components measured in red and white wine and the increased radical-trapping antioxidant activity in the plasma of animals given red wine strongly support the hypothesis that red wine antioxidant polyphenols may be implicated.
Ethyl alcohol, at the same doses and equivalent concentrations of red wine, did not affect haemostatic parameters, measured as BT and platelet adhesion to fibrillar collagen. In contrast, it tended to decrease thrombus weight after inferior vena cava ligature; however, the effect was observed only after 10 days oral ethyl alcohol administration but not after acute intravenous injection. These findings confirm that long term alcohol consumption has antithrombotic properties, although suggesting a different mechanism from that of non-alcoholic red wine components. Whether non-alcoholic red wine components and alcohol interact in inhibiting thrombus formation remains to be determined.
This study also provides evidence that red wine induces NO production in vivo and may prevent experimental thrombosis by modulating NO-dependent haemostatic mechanism(s) such as platelet-vessel wall (collagen) interaction.
Red wine supplementation, either after 10 day oral or acute intravenous administration in rats, markedly prolonged BT and inhibited platelet adhesion to fibrillar collagen as well as significantly reduced thrombus weight after inferior vena cava ligature. In all cases, L-NAME, an inhibitor of NO formation, prevented the effects of red wine, indicating the involvement of NO in this process. The possibility to revert the effect of L-NAME by L-arginine, the precursor of NO synthesis in vascular endothelium but not by its stereoisomer, D-arginine, clearly strengthens the role of NO in red wine-induced effects.
The red wine ability to inhibit experimental thrombosis has already been reported (Demrow et al., 1995), as well as the induction of NO-dependent effect in in vitro studies (Fitzpatrick et al., 1993; van Acker et al., 1995; Andriambeloson et al., 1997). The novelty of our observation is that red wine and its alcohol-free component prevent thrombosis and modulate primary haemostasis via NO in vivo. This is, to our knowledge, the first in vivo evidence that NO has antithrombotic properties.
BT has been extensively used to evaluate primary haemostasis and particularly the contribution of platelets and vascular tone to the arrest of bleeding in humans and in animals. We recently reported that this test is modulated by NO production in an experimental model of haemolysis in rats, the effect of NO on BT being mediated by inhibition of platelet adhesion (Wollny et al., 1997). Therefore, we evaluated the adhesion of platelets to fibrillar collagen after both chronic and acute wine administration. In both cases, after red wine consumption, platelet adhesion to fibrillar collagen was markedly decreased, while no change in platelet aggregation was observed. L-NAME administration also reverted the effect of red wine on platelets.
Adhesion of platelets to the subendothelial matrix, after vessel damage, is a triggering mechanism of thrombus formation, therefore, platelet inhibition by red wine could, at least partially, explain the prevention of thrombus growth.
No effect was observed on platelet aggregation after either acute or chronic red wine administration. These findings are in agreement with previous data, that NO-inhibited platelet adhesion, but not aggregation, was involved in BT prolongation in rats (Remuzzi et al., 1990; Wollny et al., 1997).
BT in rats can also be prolonged by prostacyclin increase as a consequence of its ability to induce vasodilation and to inhibit platelet aggregation (Villa & de Gaetano, 1979). On the other hand, alcohol has been described to increase prostacyclin production (Landolfi & Steiner, 1984). The administration of aspirin, at a concentration totally inhibiting prostacyclin synthesis by vascular walls in rats (Cerletti et al., 1986), did not revert the effect of red wine in prolonging BT, thus suggesting that prostacyclin is not involved in such a mechanism. Alcohol and wine have also been reported to affect the haemostatic process by increasing the release of plasminogen activator and lowering the levels of fibrinogen (Veenstra et al., 1990; Laug, 1983; Ridker et al., 1994). In our experimental system, no changes in fibrinogen levels, clotting activities or in the total fibrinolytic capacity of plasma, were observed, after either wine or alcohol administration.
Increase in NO levels by red wine components has been described in in vitro and ex vivo experiments (Gryglewski et al., 1987; Andriambeloson et al., 1997). Wine contains a large number of compounds with antioxidant properties, including phenolic flavonoids, tannins, anthocyanins and natural antifungal compounds, such as trans-resveratrol (Rice-Evans et al., 1997). In particular, tannic acid, but neither resveratrol nor malvidin produced an endothelium-dependent relaxation of intact rat aortic rings, that was L-NNA-inhibitable, while quercetin-induced relaxation was not reversed by NO synthase inhibition (Fitzpatrick et al., 1993).
Chromatographic resolution of the wines used in our experiments confirmed that red wine contained greater amounts of antioxidant substances than white wine. In particular, the tannin content was 12 times higher in red wine than in white wine. The greater content in antioxidant substances of red wine as compared to white wine induced a higher antioxidant potential in vivo as it has been shown by the evaluation of the antioxidant capacity of rat plasma after wine intake. Animals given red wine showed a 4 fold increase in the TRAP capacity as compared to controls or animals given white wine or ethyl alcohol. These results strongly support the concept that the effect of red wine in prolonging BT, through a NO-dependent mechanism, can be mediated by its high content in antioxidant substances and tannins. However, further studies are necessary to specifically identify the molecular compounds responsible for the in vivo effect described here.
The mechanism of the NO increase is unknown. It is possible that red wine components decrease degradation of basal levels of NO, preventing its destruction by superoxides (van Acker et al., 1995). However, NO increase might also result from a stimulation of its synthesis by endothelial cells, as reported by in vitro experiments (Andriambeloson et al., 1997; Gryglewski et al., 1987). We have no evidence to support either mechanism; however, it can be conceived that both are acting in in vivo conditions.
The mechanism of platelet inhibition by red wine is different from that of other platelet-inhibiting substances, such as aspirin. Therefore, the effect of its moderate consumption in the prevention of coronary artery disease might be additive to that of aspirin. According to this hypothesis, Rotondo et al. (1996) showed that trans-resveratrol, a phenolic compound present in several red wines, inhibited human platelet aggregation induced by thrombin and cathepsin G also in the presence of aspirin.
Similarly to drugs interfering with primary haemostasis (Eristland et al., 1995; Steering Commitee of the Physicians' Health Study, 1989), red wine may increase the tendency to bleed. Some studies have shown that moderate alcohol drinkers had an elevated risk of subarachnoid haemorrhage (Stampfer et al., 1988; Donahue et al., 1986). Although this effect has not been specifically described for red wine, the mechanisms that mediate the protective effect of wine against ischaemic vascular disease might also increase the risk of bleeding. This possibility can simply be the counterpart of red wine antithrombotic activity, and in conditions of low risk for thrombosis, or in hypertensive subjects it should be taken into careful consideration.
In conclusion, moderate red wine consumption induces, independently of its alcohol content, impairment of primary haemostasis and prevention of thrombosis in rats. These effects are mediated by a NO-dependent mechanism, possibly triggered by antioxidant substances present in red wine. Our study may offer experimental support and biological plausibility to the observed epidemiological protection from coronary artery disease associated with moderate red wine consumption.
Limitations of the study
Wine is a complex mixture of many different compounds, whose analysis is difficult and necessarily incomplete. We identified in the wines used for this study the major classes of components and showed differences in their concentrations between red and white wine; however, some substances, potentially responsible for the effect measured, could have been missed in our analysis. The effects of red wine described here should be interpreted as properties of a dietary component as a whole: extrapolation to pharmacological effects of some well-identified wine components is not allowed and needs further experiments.
‘Wine' composition depends indeed on a wide spectrum of different conditions, such as the type of grape, the region where it is grown, the methods of grape cultivation and wine production. Therefore the reported effects of Montepulciano D'Abruzzo on haemostasis and thrombosis cannot be necessarily generalized to all red wines, but only to wines with similar characteristics. Flesch et al. lately demonstrated that only Italian and French red wines produced ‘en barrique' (Bordeaux, Chateauneuf-du-Pape, Barolo) were able to induce a NO-dependent relaxation of rat aorta and human coronary arteries. In contrast red wines not produced ‘en barrique' (Valpolicella, Ahr Spatburgunder), or white wines (produced ‘en barrique' Rioja, Chardonnay, Mosel-Riesling) or ethanol did not show any effect (Flesch et al., 1998). Furthermore, Chateauneuf-du-Pape, but not white wine Chateau Villotte Bordeaux inhibited platelet activity and thrombosis in dogs (Demrow et al., 1995).
Finally, it should be noted that, following red wine lyophilization to remove alcohol, oxidation of certain constituents, such as volatile aromas, likely has occurred; therefore, it is conceivable that lyophilized versus non lyophilized red wine may differ more than by alcohol contents. However, such unidentified substances should not have major effects on the NO-dependent changes in haemostasis and thrombosis induced by red wine, since no statistically significant difference was observed in the effects of non-lyophilized and lyophilized red wine.
Acknowledgments
The Authors wish to thank the Animal Care Unit staff for their valuable assistance and Mr Stefano Manarini for platelet aggregation evaluation. This work was partially supported by the Regione Abruzzo (POM 1994/1996- Sottoprogramma 3, misura 3.1- Ricerca e Sperimentazione, A.R.S.S.A.). The methods for wine characterization were developed with the support of a grant from European Union (FAIR CT97 6321) T. Wollny was on leave of absence from Medical School, Bialystok, Poland.
Abbreviations
- BT
‘template' bleeding time
- NO
nitric oxide
- L-NAME
Nωnitro-L-arginine-methyl ester
- TRAP
total radical-trapping antioxidant parameter
References
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTELLI A.E.E., GIOVANNINI L., GIANESSI D., MIGLIORI M., BERNINI W., FREGONI M., BERTELLI A. Antiplatelets activity of synthetic and natural resveratrol in red wine. Int. J. Tiss. Reac. 1995;17:1–3. [PubMed] [Google Scholar]
- CERLETTI C., GAMBINO M.C., GARATTINI S., DE GAETANO G. Biochemical selectivity of oral versus intravenous aspirin in rats. Inhibition by oral aspirin of cyclooxygenase activity in platelets and presystemic but not systemic vessels. J. Clin. Invest. 1986;78:323–326. doi: 10.1172/JCI112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORREA C.L., CUSTODIO PEDROSO R. Headspace gas chromatography with capillary column for urine alcohol determination. J. Chromatography. 1997;704:365–368. doi: 10.1016/s0378-4347(97)00445-3. [DOI] [PubMed] [Google Scholar]
- DEMROW H.S., SLANE P.R., FOLTS J.D. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- DI MINNO G., SILVER M.J., DE GAETANO G. Prostaglandins as inhibitors of human platelet aggregation. Br. J. Haematol. 1979;43:637–647. doi: 10.1111/j.1365-2141.1979.tb03797.x. [DOI] [PubMed] [Google Scholar]
- DI STEFANO R., CRAVERO M.C., GENTILINI N. Metodi per lo studio dei polifenoli dei vini. L'Enotecnico, Maggio. 1989. pp. 83–89.
- DONAHUE R.P., ABBOTT R.D., REED D.M., YANO K. Alcohol and hemorrhagic stroke. The Honolulu Heart Program. JAMA. 1986;255:2311–2314. [PubMed] [Google Scholar]
- ERITSLAND J., ARNESEN H., SELJEFLOT I., KIERULF P. Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagul. Fibrinolysis. 1995;6:17–22. doi: 10.1097/00001721-199502000-00003. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK D.F., HIRSCHFIELD S.L., COFFEY R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- FLESCH M., SCHWARZ A., BOHM M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am. J. Physiol. 1998;275:H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- GAZIANO J.M., GODFRIED S.L., HENNEKENS C.H. Alcohol and coronary heart disease. Trends Cardiovasc. Med. 1996;6:175–178. doi: 10.1016/S1050-1738(96)00067-9. [DOI] [PubMed] [Google Scholar]
- GHISELLI A., SERAFINI M., MAIANI G., AZZINI E., FERRO-LUZZI A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995;18:29–36. doi: 10.1016/0891-5849(94)00102-p. [DOI] [PubMed] [Google Scholar]
- GOLDBERG D.M., HAHN S.E., PARKES J.G. Beyond alcohol: beverage consumption and cardiovascular mortality. Clin. Chim. Acta. 1995;237:155–187. doi: 10.1016/0009-8981(95)06069-p. [DOI] [PubMed] [Google Scholar]
- GOLDBERG D.M., TSANG E., KARUMANCHIRI A., DIAMANDIS E.P., SOLEAS G., NG E. Method to assay the concentrations of phenolic constituents of biological interest in wines. Anal. Chem. 1996;68:1688–1694. doi: 10.1021/ac951083i. [DOI] [PubMed] [Google Scholar]
- GRONBAEK M., DEIS A., SORENSON T.I., BECKER U., SCHNOHR P., JENSEN G. Mortality associated with moderate intakes of wine, beer, or spirits. B.M.J. 1995;6:1165–1169. doi: 10.1136/bmj.310.6988.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., KORBUT R., ROBAK J., SWIES J. On the mechanism of antithrombotic action of flavonoids. Biochem. Pharmacol. 1987;36:317–322. doi: 10.1016/0006-2952(87)90288-7. [DOI] [PubMed] [Google Scholar]
- HEIN H.O., SORENSEN H., SAUDICANI P., GYNTELBERG F. Alcohol consumption, Lewis phenotypes, and risk of ischaemic heart disease. Lancet. 1993;341:392–396. doi: 10.1016/0140-6736(93)92987-5. [DOI] [PubMed] [Google Scholar]
- JOHNSON A.J., SEMAR M., NEWMAN J. Blood Coagulation: hemorrhage and thrombosis. New York: Grune and Stratton; 1964. Estimation of fibrinolytic activity by whole blood euglobulin clot lysis. [Google Scholar]
- LANDOLFI R., STEINER M. Ethanol raises prostacyclin in vivo and in vitro. Blood. 1984;64:679–682. [PubMed] [Google Scholar]
- LAUG W.E. Ethyl alcohol enhances plasminogen activator secretion by endothelial cells. JAMA. 1983;250:772–776. [PubMed] [Google Scholar]
- LAVY A., FURHMAN B., DANKNER G., BEN-AMOTZ A., PRESSER D., AVIRAM M. Effect of dietary supplementation of red or white wine on human blood chemistry, hematology and coagulation: Favorable effect of red wine on plasma high-density lipoprotein. Ann. Nutr. Metab. 1994;38:287–294. doi: 10.1159/000177823. [DOI] [PubMed] [Google Scholar]
- NAPOLEONE E., DI SANTO A.M., LORENZET R. Monocytes upregulate endothelial cell expression of tissue factor: a role for cell-cell interaction and cross-talk. Blood. 1997;89:541–549. [PubMed] [Google Scholar]
- PACE-ASCIAK C.R., HAHN S., DIAMANDIS E.P., SOLEAS G., GOLDBERG D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin. Chim. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- PELLEGRINI N., PARETI F.I., STABILE F., BRUSAMOLINO A., SIMONETTI P. Effects of moderate consumption of red wine on platelet aggregation and haemostatic variables in healthy volunteers. Eur. J. Clin. Nutr. 1996;50:209–213. [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- REMUZZI G., PERICO N., ZOJA C., CORNA D., MACCONI D., VIGANÒ G. Role of endothelium-derived nitric oxide in the bleeding tendency of uremia. J. Clin. Invest. 1990;86:1768–1771. doi: 10.1172/JCI114904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENAUD S.C., BESWICK A.D., FEHILY A.M., SHARP D.S., ELWOOD P.C. Alcohol and platelet aggregation: the Caerphilly prospective heart disease study. Am. J. Clin. Nutr. 1992;55:1012–1017. doi: 10.1093/ajcn/55.5.1012. [DOI] [PubMed] [Google Scholar]
- RENAUD S.C., DE LORGERIL M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- RENAUD S.C., GUÉGUEN R., SCHENKER J., D'HOUTAUD A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology. 1998;9:184–188. [PubMed] [Google Scholar]
- REYERS I., DE GAETANO G., DONATI M.B. Venostasis-induced thrombosis in rats is not influenced by circulating platelet or leukocyte number. Agents Actions. 1989;28:137–141. doi: 10.1007/BF02022994. [DOI] [PubMed] [Google Scholar]
- RICE-EVANS C.A., MILLER N.J., PAGANGA G. Antioxidant properties of phenolic compounds. Trends Plant. Sc. 1997;2:152–159. [Google Scholar]
- RIDKER P.M., VAUGHAN D.E., STAMPFER M.J., GLYNN R.J., HENNEKENS C.H. Association of moderate alcohol consumption and plasma concentrations of endogenous tissue-type plasminogen activator. JAMA. 1994;272:929–933. doi: 10.1001/jama.1994.03520120039028. [DOI] [PubMed] [Google Scholar]
- RIMM E.B., KLATSKY A., GROBBEE D., STAMPFER M.J. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirit. B.M.J. 1996;312:731–736. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTONDO S., EVANGELISTA V., MANARINI S., DEGAETANO G, CERLETTI C. Different requirement of intracellular calcium and protein kinase C for arachidonic acid release and serotonin secretion in cathepsin G-activated platelets. Throm. Haemost. 1997;78:919–925. [PubMed] [Google Scholar]
- ROTONDO S., RAJTAR G., MANARINI S., CELARDO A., ROTILIO D., DE GAETANO G., EVANGELISTA V., CERLETTI C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br. J. Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTONDO S., ROTILIO D., CERLETTI C., DE GAETANO G. Red wine, aspirin and platelet function. Thromb. Haemost. 1996;76:818–819. [PubMed] [Google Scholar]
- SERAFINI M., MAIANI G., FERRO-LUZZI A. Effect of ethanol on red wine tannin-protein (BSA) interactions. J. Agric. Food. Chem. 1997;45:3148–3151. [Google Scholar]
- STAMPFER M.J., COLDITZ G.A., WILLETI D.C., SPEIZER F.E., HENNEKENS C.H. A prospective study of moderate alcohol consumption and the risk of coronary heart disease and stroke in women. N. Engl. J. Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- STEERING COMMITTEE OF THE PHYSICIANS' HEALTH STUDY Final report on the aspirin component of the ongoing Physicians' Health Study. N. Engl. J. Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- TANGERMAN A. Highly sensitive gas chromatographic analysis of ethanol in whole blood, serum, urine and fecal supernatants by the direct injection method. Clinical Chemistry. 1997;43:1003–1009. [PubMed] [Google Scholar]
- VAN ACKER S.A., TROMP M.N., HAENEN G.R., VAN DER VIJGH W.J., BAST A. Flavonoids as scavengers of nitric oxide radicals. Biochem. Biophys. Res. Commun. 1995;214:755–759. doi: 10.1006/bbrc.1995.2350. [DOI] [PubMed] [Google Scholar]
- VEENSTRA J., KLUFT C., OCKHUIZEN T.H., VD POL H., WEDEL M., SCHAAFSMA G. Effects of moderate alcohol consumption on platelet function, tissue-type plasminogen activator and plasminogen activator inhibitor. Thromb. Haemost. 1990;63:345–348. [PubMed] [Google Scholar]
- VILLA S., DE GAETANO G. Bleeding time in laboratory animals. IV. Effects of prostacyclin, pyrimido-pyrimidine compounds and aspirin in rats. Thromb. Res. 1979;15:727–732. doi: 10.1016/0049-3848(79)90182-8. [DOI] [PubMed] [Google Scholar]
- WOLLNY T., IACOVIELLO L., BUCZKO W., DE GAETANO G., DONATI M.B. Prolongation of bleeding time by acute hemolysis in rats: a role for nitric oxide. Am. J. Physiol. 1997;272:H2875–H2884. doi: 10.1152/ajpheart.1997.272.6.H2875. [DOI] [PubMed] [Google Scholar]


