Abstract
Nociceptin, also known as orphanin FQ, is an endogenous ligand for the orphan opioid receptor-like receptor 1 (ORL1) and involves in various functions in the central nervous system (CNS). On the other hand, nocistatin is recently isolated from the same precursor as nociceptin and blocks nociceptin-induced allodynia and hyperalgesia.
Although ORL1 receptors which display a high degree of sequence homology with classical opioid receptors are abundant in the hippocampus, little is known regarding their role in learning and memory.
The present study was designed to investigate whether nociceptin/orphanin FQ and nocistatin could modulate impairment of learning and memory induced by scopolamine, a muscarinic cholinergic receptor antagonist, using spontaneous alternation of Y-maze and step-down type passive avoidance tasks in mice.
While nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) administered 30 min before spontaneous alternation performance or the training session of the passive avoidance task, had no effect on spontaneous alternation or passive avoidance behaviours, a lower per cent alternation and shorter median step-down latency in the retention test were obtained in nociceptin (1.5 and/or 5.0 nmol mouse−1, i.c.v.)-treated normal mice.
Administration of nocistatin (1.5 and/or 5.0 nmol mouse−1, i.c.v.) 30 min before spontaneous alternation performance or the training session of the passive avoidance task, attenuated the scopolamine-induced impairment of spontaneous alternation and passive avoidance behaviours.
These results indicated that nocistatin, a new biologically active peptide, ameliorates impairments of spontaneous alternation and passive avoidance induced by scopolamine, and suggested that these peptides play opposite roles in learning and memory.
Keywords: Nocistatin, nociceptin, orphanin FQ, κ-opioid receptor, dynorphin A, spontaneous alternation, passive avoidance, learning and memory, cholinergic neuronal system
Introduction
Nociceptin, also known as orphanin FQ, is an endogenous ligand for the orphan opioid receptor-like receptor 1 (ORL1) and has some structural homology with the endogenous opioid peptide dynorphin A (1-17) (Meunier et al., 1995, Meunier, 1997). When administered intracerebroventricularly (i.c.v.) to mice, nociceptin induces hyperalgesia and a decrease in motor activity (Reinscheid et al., 1995) and stimulates locomotor and exploratory behaviour (Florin et al., 1996). On the other hand, nocistatin, which was recently isolated from the same precursor as nociceptin, blocks nociceptin-induced allodynia and hyperalgesia, and attenuates pain evoked by prostaglandin E2 (Okuda-Ashitaka et al., 1998) and alleviates nociceptin-induced impairment of learning and memory (Hiramatsu & Inoue, 1999).
Opioid peptides acting on opioid receptors can modulate hippocampal synaptic functions (Wagner et al., 1993; Xie & Lewis, 1995). Although ORL1 receptors which display a high degree of sequence homology with classical opioid receptors are abundant in the hippocampus, little is known regarding their role in synaptic function. Recently, Sandin et al. (1997) showed that nociceptin microinjected into the hippocampus impaired spatial learning in rats. Yu et al. (1997) suggested that nociceptin could function as an inhibitory modulator regulating synaptic transmission and synaptic plasticity in the hippocampus. Further, Manabe et al. (1998) showed that mice lacking the nociceptin receptor have better learning ability and memory, and larger long-term potentiation in the hippocampal CA1 region than control mice. These findings suggest that activation of ORL1 receptors plays an important role in synaptic plasticity involved in learning and memory.
It is well known that cholinergic neuronal systems play an important role in the cognitive deficits associated with ageing and neurodegenerative diseases (Bartus et al., 1982; Newhouse, 1990). Although investigation of learning and memory has focused primarily on cholinergic neurotransmission, reports of increased κ-opioid receptor density in the brain of Alzheimer's patients (Hiller et al., 1987) and dynorphin A-(1-8)-like immunoreactivity in the hippocampus of aged rats (Jiang et al., 1989) suggest that disruption of opioidergic neurotransmission may also play a role in the cognitive deficits associated with Alzheimer's disease and ageing. Recent studies have indicated that neuropeptides modulate learning and memory processes in experimental animals (see Kovacs & De Wied, 1994). We reported previously that the κ-opioid receptor agonists dynorphin A (1-13) and U-50,488H improve impairments of learning and memory in mice and rats by κ-opioid receptor-mediated and/or non-opioid mechanisms (Hiramatsu et al., 1995; 1996a,1996b; 1997; 1998a,1998b,1998c; Itoh et al., 1993). However, the mechanisms underlying the improvement of memory by neuropeptides are still unknown. Here, we investigated whether the orphan neuropeptides nocistatin and nociceptin might be physiologically significant, i.e. endowed with central biological activity in vivo.
Methods
Animals
Seven-week-old male ddY mice (Japan SLC, Japan) were kept in a regulated environment (23±1°C, 50±5% humidity), with a 12 h light/dark cycle (light on 0800 h–2000 h) and given food and tap water ad libitum. Experimental protocols concerning the use of laboratory animals were approved by the committee of Meijo University and followed the guidelines of the Japanese Pharmacological Society (Folia Pharmacol. Japon., 1992, 99: 35A) and the interministerial decree of May 25th, 1987 (the Ministry of Education).
Drugs and treatments
Nociceptin and nocistatin (Peptide Inst. Inc., Osaka, Japan) were dissolved in 0.9 % saline. Both peptides were administered 30 min before the Y-maze session or the training session of the passive avoidance test into the lateral ventricle (i.c.v.) of the mouse brain according to the method of Haley & McCormick (1957) in a volume of 5 μl mouse−1 under brief ether anaesthesia. Scopolamine hydrobromide (scopolamine, Tokyo Chemical Industry, Co., Ltd., Japan) was dissolved in 0.9% saline and injected subcutaneously (s.c.) just before administration of these peptides. Control animals were injected with vehicle i.c.v. under brief ether anaesthesia.
Spontaneous alternation behaviour
Immediate working memory performance was assessed by recording spontaneous alternation behaviour in a single session in a Y-maze (Hiramatsu et al., 1997) made of black painted wood. Each arm was 40 cm long, 12 cm high, 3 cm wide at the bottom and 10 cm wide at the top, and converged in an equilateral triangular central area. The procedure was basically the same as that described previously (Sarter et al., 1988): each mouse, naive to the maze, was placed at the end of one arm and allowed to move freely through the maze during an 8-min session. The series of arm entries was recorded visually. Entry was considered to be completed when the hind paws of the mouse had been completely placed in the arm. Alternation was defined as successive entries into the three different arms (A, B or C), on overlapping triplet sets (ex. ACBABACBAB=5). Percentage alternation was calculated as the ratio of actual to possible alternation (defined as the total number of arm entries minus two), multiplied by 100 as shown in following equation; % alternation={ ( Number of alternations ) / ( Total arm entries−2) }×100.
Step-down type passive avoidance task
A step-down type of passive avoidance task was used, as described previously (Hiramatsu et al., 1995) with some modifications. The apparatus consisted of a transparent acrylic rectangular cage (30×30 ×40 cm high) with a grid floor with a wooden platform (4×4×4 cm) in the centre, set in a semi-soundproof wooden outer box (35×35×90 cm high). Illumination was provided by a 15-W illumination lamp above the apparatus. An electric current (1 Hz, 500 ms, 80 V DC) was delivered to the grid floor by an isolated stimulator (SEN-3201, Nihon Koden, Japan).
Each mouse was placed on the wooden platform. When the mouse stepped down from the platform onto the grid floor, an electric shock was delivered for 15 s. The retention test was carried out 24 h after the training session in a manner similar to the training except that no electric shock was delivered to the grid floor. Each mouse was placed on the platform and step-down latency was recorded. An upper cut-off time of 300 s was set.
Responses to electric shock
The responses to electric shock during the training session were recorded. The following scores were given based on the responses to electric shock: 3=jumping, 2=vocalization, 1=flinching, 0=no response. Shock sensitivity is shown as the total score, which was the sum of each score for 15 s.
Data analysis
The behavioural data are expressed in terms of median, interquartile, and/or 10th and 90th percentile ranges. The significance of differences was evaluated using the Mann-Whitney U-test for comparisons between two groups such as control and scopolamine alone, and Kruskal-Wallis non-parametric one-way analysis of variance followed by Bonferroni's test for multiple comparisons. The criterion for significance was P<0.05 in all statistical evaluations.
Results
Effects of nociceptin and nocistatin on spontaneous alternation performance in normal mice
Administration of nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) in the Y-maze dose-dependently decreased the per cent alternation (Figure 1A). The effects of nociceptin (1.5 and 5.0 nmol) were significant. Although nociceptin at 5.0 nmol mouse−1 induced loss of the righting reflex about 5–10 min after i.c.v. administration, no apparent behavioural disturbances and no changes in the number of total arm entries were observed during the Y-maze session (Figure 1B).
Figure 1.

Effects of nociceptin on spontaneous alternation (A) and total arm entries (B) in the Y-maze in normal mice. Mice were treated with nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) 30 min before the test. The data are expressed as median and interquartile ranges. Figures in parentheses show the numbers of mice used. *P<0.05, **P<0.01 vs control (Bonferroni's test).
On the other hand, nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) had no effect on the per cent alternation or the number of total arm entries in the Y-maze (Figure 2).
Figure 2.

Effects of nocistatin on spontaneous alternation (A) and total arm entries (B) in the Y-maze in normal mice. Mice were treated with nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) 30 min before the test. The data are expressed as median and interquartile ranges. Figures in parentheses show the numbers of mice used.
Effects of nociceptin and nocistatin on scopolamine-induced impairment of spontaneous alternation performance
In the Y-maze test, scopolamine (1.65 μmol kg−1, s.c.) significantly decreased the per cent alternation. Administration of nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) had no apparent effect on the scopolamine-induced impairment of spontaneous alternation (Figure 3A). Scopolamine markedly increased the total number of arm entries. Nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) dose-dependently reversed the scopolamine-induced increase of the total number of arm entries, and 5.0 nmol of nociceptin significantly decreased these entries induced by scopolamine (Figure 3B).
Figure 3.
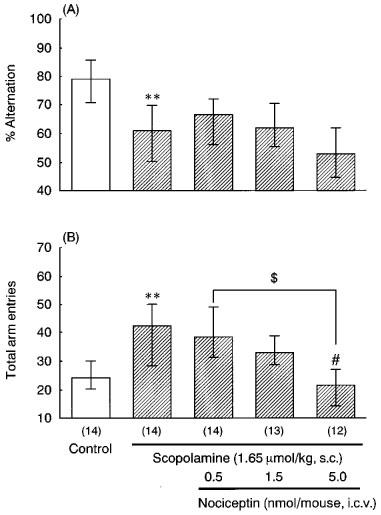
Effects of nociceptin on scopolamine-induced impairment of spontaneous alternation (A) and total arm entries (B) in the Y-maze. Mice were treated with scopolamine (1.65 μmol kg−1, s.c.) and nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) 30 min before the test. The data are expressed as median and interquartile ranges. Figures in parentheses show the numbers of mice used. **P<0.01 vs control for scopolamine alone (Mann-Whitney U-test), #P<0.05 vs scopolamine alone (Bonferroni's test).
On the other hand, administration of nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) dose-dependently reversed the scopolamine-induced impairment of spontaneous alternation (Figure 4A). Although 1.5 nmol mouse−1 of nocistatin partially reversed the scopolamine-induced increase in total number of arm entries, at 5.0 nmol nocistatin showed no such effect (Figure 4B).
Figure 4.
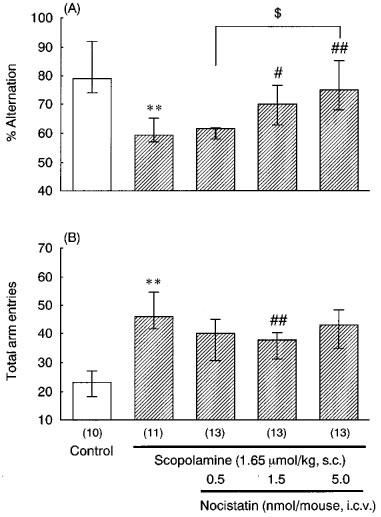
Effects of nocistatin on scopolamine-induced impairment of spontaneous alternation (A) and total arm entries (B) in the Y-maze. Mice were treated with scopolamine (1.65 μmol kg−1, s.c.) and nocistatin (0.5– 5.0 nmol mouse−1, i.c.v.) 30 min before the test. The data are expressed as median and interquartile ranges. Figures in parentheses show the numbers of mice used. **P<0.01 vs control for scopolamine alone (Mann-Whitney U-test), #P<0.05, ##P<0.01 vs scopolamine alone (Bonferroni's test).
Effects of nociceptin and nocistatin on acquisition of memory in the passive avoidance test in normal mice
Administration of nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) significantly shortened the step-down latencies in the retention test in a dose-dependent manner (Figure 5A). On the other hand, nocistatin had no significant effect on the step-down latencies in the retention test (Figure 5B).
Figure 5.

Effects of nociceptin (A) and nocistatin (B) on the passive avoidance response in normal mice. Mice were treated with nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) (A) or nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) (B) 30 min before the training session, and the retention test was carried out 24 h after training. Step-down latency values are shown as median (horizontal bar), first and third quartiles (vertical column) and 10th and 90th percentiles (vertical line). Figures in parentheses show the numbers of mice used. **P<0.01 vs control (Bonferroni's test).
Effects of nociceptin and nocistatin on scopolamine-induced impairment of learning and memory in the passive avoidance test
In the passive avoidance test, scopolamine (0.1 μmol kg−1, s.c.) significantly shortened the step-down latency. Administration of nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) had no apparent effect on the scopolamine-induced impairment of learning and memory (Figure 6A).
Figure 6.

Effects of nociceptin (A) and nocistatin (B) on scopolamine-induced impairment of the passive avoidance response. Mice were treated with scopolamine (0.1 μmol kg−1, s.c.) and nociceptin (0.5–5.0 nmol mouse−1, i.c.v.) (A) or nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) (B) 30 min before the training session, and the retention test was carried out 24 h after training. Step-down latency values are shown as median (horizontal bar), first and third quartiles (vertical column) and 10th and 90th percentiles (vertical line). Figures in parentheses show the numbers of mice used. **P<0.01 vs control for scopolamine alone (Mann-Whitney U-test), #P<0.05 vs scopolamine alone (Bonferroni's test).
On the other hand, administration of nocistatin (0.5–5.0 nmol mouse−1, i.c.v.) 30 min before the training session in the passive avoidance test dose-dependently reversed the scopolamine-induced impairment of learning and memory (Figure 6). Neither nociceptin nor nocistatin induced significant changes in the response to electric shocks after scopolamine administration at the same dose range as used in the passive avoidance test (data not shown).
Discussion
Since the isolation of nociceptin, an endogenous ligand for the ORL1 receptor (Meunier et al., 1995; Reinscheid et al., 1995), studies have been initiated to elucidate the relationship between nociceptin and opioid peptides. Nociceptin is structurally similar to dynorphin A but lacks the N-terminal tyrosine characteristic of opioid peptides and is derived from a novel precursor (Nothacker et al., 1996). Furthermore, although the anatomy of the nociceptin system has been described in the rat brain (Anton et al., 1996; Sim et al., 1996; Sim & Childers, 1997), it is important to compare the effects on learning and memory function of nociceptin and κ-opioid peptides to define the role of ORL1 receptors in the brain because the ORL1 receptor has been reported to bind dynorphin A (Zhang & Yu, 1995).
Although investigations of learning and memory have focused primarily on cholinergic neurotransmission, peptidergic neurotransmission also plays an important role in the cognitive deficits associated with Alzheimer's disease and ageing (Hiller et al., 1987; Jiang et al., 1989). Although structurally similar to endogenous opioid peptides, especially dynorphin A, nociceptin possesses different characteristics in its pharmacological profile in that it does not bind strongly to classical opioid receptors (Meunier, 1997; Reinscheid et al., 1995). In contrast with our results of dynorphin A (1-13) (Hiramatsu et al., 1995; 1996a; 1997; 1998a), the present study indicated that nociceptin impaired learning and/or memory since it decreased the per cent alternation in the Y-maze and shortened the step-down latency in passive avoidance tests. These results are in agreement with those of a previous study indicating that nociceptin injected into the hippocampus markedly impaired spatial learning in the rat (Sandin et al., 1997). Furthermore, Yu et al. (1997) suggested that since nociceptin, but not the inactive analogue des-Phe1-nociceptin, inhibited the induction of long-term potentiation, activation of ORL1 receptors may play an important role in learning and memory. These findings are also consistent with the dense localization of the ORL1 receptor in the hippocampus, cingulate and frontal cortex (Sim & Childers, 1997).
During preparation of this manuscript, Manabe et al. (1998) have reported that mice lacking the nociceptin receptor possessed greater learning ability and had better memory than control mice. Therefore, they concluded that the nociceptin system seemed to play negative roles in learning and memory at the whole-animal level. Our results also support their hypothesis because nociceptin at the dosage used here impaired spontaneous alternation and learning and/or memory in the passive avoidance tests. Further, nocistatin, which antagonizes nociceptin-induced hyperalgesia (Okuda-Ashitaka et al., 1998) and learning and memory (Hiramatsu & Inoue, 1999), alleviated scopolamine-induced learning and memory impairment.
The hippocampal formation is believed to play an important role in information processing in a variety of memory tasks (Morris et al., 1982). The CA3 region in the hippocampus in densely innervated by the mossy fibre pathway, containing opioid peptides such as dynorphins (McGinty et al., 1983). Previous studies have shown that microinjection of dynorphin into the CA3 region of the dorsal hippocampus at nanomolar concentrations impairs spatial learning through an action on κ-opioid receptors (Sandin et al., 1997). In contrast with these results, we previously suggested that κ-opioid receptor agonists improve learning and memory by acting on the impaired cholinergic system (Hiramatsu et al., 1998b). This raises the important question of whether nociceptin and/or nocistatin also interact with cholinergic systems. Therefore, we investigated the effects of these peptides after blockade of cholinergic receptors by scopolamine.
Although nociceptin and ORL1 receptors have some similarity with dynorphin A and κ-opioid receptors, respectively, nociceptin did not ameliorate the scopolamine-induced impairment of learning and memory. Interestingly, nocistatin blocked scopolamine-induced impairment of learning and memory without affecting the acquisition of memory in normal mice. This finding may be in agreement with the previous reports that nocistatin blocks nociceptin-induced allodynia and hyperalgesia (Okuda-Ashitaka et al., 1998) and memory impairment (Hiramatsu & Inoue, 1999). Since nocistatin does not bind to the ORL1 receptor (Okuda-Ashitaka et al., 1998), these two peptides may have opposite roles in the central nervous system (CNS). This is interesting because nocistatin precursor is the same as that of nociceptin and these peptides have opposing effects in the CNS.
Nociceptin is known to have many effects on the CNS including alterations of spontaneous activity, antinociception and aversive motivation (Florin et al., 1996; Meunier et al., 1995; Reinscheid et al., 1995). Therefore, pre-training administration of nociceptin and nocistatin may alter locomotor activity, pain sensitivity to electric shocks and/or motivation, and these effects may alter the behavioural test conditions in a non specific manner. Evaluation of the pain response (flinching and vocalization) to electric shocks showed that the drug tested in avoidance studies had no significant effect on pain sensitivity as compared with the control group. Thus, nociceptin-induced learning and memory impairments and improvement by nocistatin are not because of antinociceptive and motor effects.
In conclusion, the results of our study provide the first evidence that injection of nocistatin ameliorates impaired learning and memory processes. Although the functional significance and the precise nature of the interaction between these peptides and the cholinergic systems in the CNS is unknown, these endogenous peptides may play an important role in synaptic plasticity involved in learning and memory. Our data have implications for further clarifying the role of the neuropeptide systems in the hippocampus in memory function. However, considerable research is still necessary to fully understand the potential utility of nocistatin or nociceptin antagonists in the treatment of cognitive dysfunction.
Acknowledgments
This study was supported in part by grants from the Japan Smoking Research Foundation, the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan and INSERM/JSPS Joint Research Project, and by Grants-in-Aids for Scientific Research (No. 09672340 and High-Tech Research Center Project) from the Ministry of Education, Science, Sports and Culture, Japan.
Abbreviations
- CNS
central nervous system
- i.c.v.
intracerebroventricular
- κ
kappa
- ORL1
orphan opioid receptor-like receptor 1
- s.c.
subcutaneous
References
- ANTON B., FEIN J., TO T., LI X., SILBERSTEIN L., EVANS C.J. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J. Comp. Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- BARTUS R.T., DEAN R.L., BEER B., LIPPA A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- FLORIN S., SUAUDEAU C., MEUNIER J.C., COSTENTIN J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- HALEY T.J., MCCORMICK W.G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br. J. Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLER J.M., ITZHAK Y., SIMON E.J. Selective changes in mu, delta and kappa opioid receptor binding in certain limbic regions of the brain in Alzheimer's disease patients. Brain Res. 1987;406:17–23. doi: 10.1016/0006-8993(87)90764-5. [DOI] [PubMed] [Google Scholar]
- HIRAMATSU M., INOUE I. Effects of nocistatin on nociceptin-induced impairment of learning and memory in mice. Eur. J. Pharmacol. 1999;367:151–155. doi: 10.1016/s0014-2999(99)00003-5. [DOI] [PubMed] [Google Scholar]
- HIRAMATSU M., HYODO T., KAMEYAMA T. U-50488H, a selective κ-opioid receptor agonist, improves carbon monoxide-induced delayed amnesia in mice. Eur. J. Pharmacol. 1996b;315:119–125. doi: 10.1016/s0014-2999(96)00622-x. [DOI] [PubMed] [Google Scholar]
- HIRAMATSU M., MORI H., MURASAWA H., KAMEYAMA T. Dynorphin A (1-13) improves galanin-induced impairment of memory accompanied by blockage of reductions in acetylcholine release in rats. Br. J. Pharmacol. 1996a;118:255–260. doi: 10.1111/j.1476-5381.1996.tb15396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAMATSU M., MURASAWA H., MORI H., KAMEYAMA T. Reversion of muscarinic autoreceptor agonist-induced acetylcholine decrease and learning impairment by dynorphin A (1-13), an endogenous κ-opioid agonist. Br. J. Pharmacol. 1998a;123:920–926. doi: 10.1038/sj.bjp.0701671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAMATSU M. , MURASAWA H. , NABESHIMA T., KAMEYAMA T. Effects of U-50,488H on scopolamine-, mecamylamine- and dizocilpine-induced learning and memory impairment in rats. J. Pharmacol. Exp. Ther. 1998b;284:858–867. [PubMed] [Google Scholar]
- HIRAMATSU M., INOUE K., AMBO A., SASAKI Y., KAMEYAMA T. Des-tyrosine1-dynorphin analogs reverse impairment of learning and/or memory in non-opioid receptor mediated mechanism in mice. 28th Neurosci. Abstr. 1998c;24:684. [Google Scholar]
- HIRAMATSU M., SASAKI M., KAMEYAMA T. Effects of dynorphin A-(1-13) on carbon monoxide-induced delayed amnesia in mice studied in a step-down type passive avoidance task. Eur. J. Pharmacol. 1995;282:185–191. doi: 10.1016/0014-2999(95)00330-n. [DOI] [PubMed] [Google Scholar]
- HIRAMATSU M., SASAKI M., NABESHIMA T., KAMEYAMA T. Effects of dynorphin A (1-13) on carbon monoxide-induced delayed amnesia in mice. Pharmacol. Biochem. Behav. 1997;56:73–79. doi: 10.1016/S0091-3057(96)00159-1. [DOI] [PubMed] [Google Scholar]
- ITOH J., UKAI M., KAMEYAMA T. Dynorphin A-(1-13) markedly improves scopolamine-induced impairment of spontaneous alternation performance in mice. Eur. J. Pharmacol. 1993;236:341–345. doi: 10.1016/0014-2999(93)90469-x. [DOI] [PubMed] [Google Scholar]
- JIANG H.-K., OWYANG V., HONG J.-S., GALLAGHER M. Elevated dynorphin in the hippocampal formation of aged rats: Relation to cognitive impairment on a spatial learning task. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS G.L., DE WIED D. Peptidergic modulation of learning and memory processes. Pharmacol. Rev. 1994;46:269–291. [PubMed] [Google Scholar]
- MANABE T., NODA Y., MAMIYA T., KATAGIRI H., HOUTANI T., NISHI M., NODA T., TAKAHASHI T., SUGIMOTO T., NABESHIMA T., TAKESHIMA H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- MCGINTY J.F., HENRIKSEN S.J., GOLDSTEIN A., TERENIUS L., BLOOM F.E. Dynorphin is contained within hippocampal mossy fibers: immunochemical alterations after kainic acid administration and colchicine-induced neurotoxicity. Proc. Natl. Acad. Sci., U.S.A. 1983;80:589–593. doi: 10.1073/pnas.80.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER J.C. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur. J. Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G., GARRUD P., RAWLINS J.N., O'KEEFE J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- NEWHOUSE A. Cholinergic drug studies in dementia and depression. Adv. Exp. Med. Biol. 1990;282:65–76. doi: 10.1007/978-1-4613-0665-8_6. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.P., REINSCHEID R.K., MANSOUR A., HENNINGSEN R.A., ARDATI A., MONSMA F.J. , JR, WATSON S.J., CIVELLI O. Primary structure and tissue distribution of the orphanin FQ precursor. Proc. Natl. Acad. Sci., U.S.A. 1996;93:8677–8682. doi: 10.1073/pnas.93.16.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., MINAMI T., TACHIBANA S., YOSHIHARA Y., NISHIUCHI Y., KIMURA T., ITO S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J. , JR, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- SANDIN J., GEORGIEVA J., SCHÖTT P.A., ÖGREN S.O., TERENIUS L. Nociceptin/Orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- SARTER M., BODEWITZ G., STEPHENS D.N. Attenuation of scopolamine-induced impairment of spontaneous alternation behavior by antagonist but not inverse agonist and antagonist β-carboline. Psychopharmacology. 1988;94:491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- SIM L.J., CHILDERS S.R. Anatomical distribution of mu, delta, and kappa opioid- and nociceptin/orphanin FQ-stimulated [35S]guanylyl-5′-O-(gamma-thio)-triphosphate binding in guinea pig brain. J. Comp. Neurol. 1997;386:562–572. doi: 10.1002/(sici)1096-9861(19971006)386:4<562::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- SIM L.J., XIAO R., CHILDERS S.R. Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTPγS binding in rat brain. Neuroreport. 1996;7:729–733. doi: 10.1097/00001756-199602290-00012. [DOI] [PubMed] [Google Scholar]
- WAGNER J.J., TERMAN G.W., CHAVKIN C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE C.W., LEWIS D.V. Endogenous opioids regulate long-term potentiation of synaptic inhibition in the dentate gyrus of rat hippocampus. J. Neurosci. 1995;15:3788–3795. doi: 10.1523/JNEUROSCI.15-05-03788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU T.P., FEIN J., PHAN T., EVANS C.J., XIE C.W. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- ZHANG S., YU L. Identification of dynorphins as endogenous ligands for an opioid receptor-like orphan receptor. J. Biol. Chem. 1995;270:22772–22776. doi: 10.1074/jbc.270.39.22772. [DOI] [PubMed] [Google Scholar]


