Abstract
The relative contributions of superoxide anion (O2−) and peroxynitrite (PN) were evaluated in the pathogenesis of intestinal microvascular damage caused by the intravenous injection of E. coli lipopolysaccharide (LPS) in rats. The superoxide dismutase mimetic (SODm) SC-55858 and the active peroxynitrite decomposition catalysts 5,10,15,20-tetrakis(2,4,6-trimethyl-3,5-disulphonatophenyl)-porphyrinato iron (III) and 5,10,15,20-tetrakis(N-methyl-4′-pyridyl)-porphyrinato iron (III) (FeTMPS, FeTMPyP respectively) were used to assess the roles of O2− and PN respectively.
The intravenous injection of LPS elicited an inflammatory response that was characterized by a time-dependent infiltration of neutrophils, lipid peroxidation, microvascular leakage (indicative of microvascular damage), and epithelial cell injury in both the duodenum and jejunum.
Administration of the SODm SC-55858, FeTMPS or FeTMPyP at 3 h post LPS reduced the subsequent increase in microvascular leakage, lipid peroxidation and epithelial cell injury. Inactive peroxynitrite decomposition catalysts exhibited no protective effects. Only, SC-55858 inhibited neutrophil infiltration.
Our results suggest that O2− and peroxynitrite play a significant role in the pathogenesis of duodenal and intestinal injury during endotoxaemia and that their removal by SODm and peroxynitrite decomposition catalysts offers a novel approach to the treatment of septic shock or clinical conditions of gastrointestinal inflammation. Furthermore, the remarkable protection of the intestinal epithelium by these agents suggests their use during chemo- and radiation therapy, cancer treatments characterized by gastrointestinal damage. Potential mechanisms through which these radicals evoke damage are discussed.
Keywords: Superoxide anions, peroxynitrite, intestinal injury
Introduction
Peroxynitrite (PN) is a highly reactive oxidant produced by the combination of nitric oxide (NO) with superoxide anion (O2−) at rates approaching the diffusion limit (Beckman et al., 1990; Ischiropoulos et al., 1992; Beckman & Crow, 1993; Huie & Padmaja, 1993). Under physiological conditions the removal of O2− occurs via endogenous superoxide dismutases (SOD). Under pathophysiological conditions such as endotoxic shock, the increase in the amount of both NO and O2− is such that the reaction between these two free radicals is favoured over that between O2− and SOD (Ischiropoulos et al., 1992; Beckman, 1994; Taylor et al., 1995). The overproduction of NO by the inducible nitric oxide synthase enzyme (iNOS) may play a role in the damage that is observed in the microvasculature and mucosa of the gut following the administration of LPS to rats (Salter et al., 1991; Boughton-Smith et al., 1993). Recent indirect evidence, however, points to PN as the culprit in such damage and raises the interesting possibility that the formation of peroxynitrite from NO and O2−, rather than NO itself, is the major cause of gastric injury observed in the gut during sepsis (Lamarque & Whittle, 1995a, 1995b). To date, the data that has been generated to support the role of peroxynitrite in vivo is indirect and has relied mainly on pharmacological (presumed inhibition of PN through reduction of NO by NOS inhibitors) and biochemical (measurement of nitrotyrosine, a marker for peroxynitrite mediated reactions) approaches. In order to explore directly the role(s) of peroxynitrite in disease, we have developed a class of porphyrin-containing compounds which catalytically decompose peroxynitrite to nitrate (Stern et al., 1996). These catalysts do not directly react with either O2− or NO (Stern et al., 1996) and therefore can be utilized to assess the direct contributions of peroxynitrite (Stern et al., 1996; Misko et al., 1998; Salvemini et al., 1998). Two of these catalysts, FeTMPS and FeTMPyP, were found to be effective both in vitro and in vivo at removing peroxynitrite and preventing its cytotoxic effects (Salvemini et al., 1998; Misko et al., 1998). More importantly, pharmacological use of these catalysts allowed us to demonstrate for the first time that, indeed, peroxynitrite plays a key role in vivo in the development of the inflammatory response since its removal resulted in potent anti-inflammatory effects (Salvemini et al., 1998).
In addition to its role in the formation of PN, little is known about the participation of O2− in the sequence of events underlying endotoxin-induced intestinal injury. This was examined in the present study by using a superoxide dismutase mimetic (SODm) which catalytically removes O2− without interfering with NO, ONOO− or other radicals such as the hydroxyl radical or hydrogen peroxide (Riley et al., 1996). These are low molecular weight, manganese containing, non-peptide molecules possessing the function and catalytic rates of native SOD enzymes, but having the advantage of being much smaller molecules (MW 400-500 vs MW 30,000 for the mimetic and native enzyme, respectively). In the present study we have used, SC-55858 a water soluble pentaaza macrocyclic Mn(II) complex containing the 2R, 3R, 8R, 9R-all-trans-fused cyclohexano substituents on the carbon atoms of the macrocycle (Figure 1) (Riley et al., 1997). SC-55858 is an excellent SOD catalyst (Mn(C18,H37N5)Cl2; Mo. Wt.=449.4) with a second-order catalytic rate constant at pH=7.4 of 1.2×10+8 M−1 s−1 rivaling that of the native Mn SOD enzyme (Riley et al., 1997). These mimetics potentially differ from other so-called metal based SODm such as the manganese complex with beta-octabromo-meso-tetrakis-(N-methylpyridinium-4-yl) porphyrin or manganese [III] tetrakis 4-benzoic acid porphyrin (Batinic-Haberle et al., 1997) or the manganese [III] tetrakis(benzoic acid) porphyrin (Day et al., 1995; Gardner et al., 1996) in that they are more chemically stable and they possess much faster rates for the dismutation of O2−. Additionally, the porphyrin complexes should not necessarily be regarded as SOD mimetic since they have not been shown to perform an actual catalytic dismutation (Weiss et al., 1993), but rather have been demonstrated to perform the stoichiometric dismutation of superoxide to hydrogen peroxide.
Figure 1.
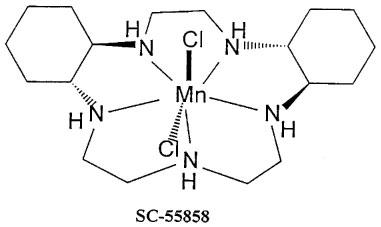
Structure of the superoxide dismutase mimetic SC55858.
SC-55858, FeTMPS and FeTMPyP were used to dissect pharmacologically the contributions of O2− and peroxynitrite to the intestinal injury resulting from systemic exposure to endotoxin.
Methods
Surgical procedure
Male Sprague Dawley rats (200–230 g) were anaesthetized with inactin (100 mg kg−1 intraperitoneally). The trachea was cannulated to facilitate respiration and body temperature maintained at 37°C by means of a heating pad. The left femoral vein was cannulated for the administration of drugs. Lipopolysaccharide from E. coli (LPS; 3 mg kg−1, serotype O111 : B4) was administered as a bolus intravenous (i.v) injection at a volume of 0.3 ml. Control animals received isotonic saline at the same volume and by the same route.
Drug administration
Drugs were dissolved in isotonic saline. All drugs were given by i.v. injection at a volume of 0.3 ml at 3 h (therapeutic protocol) after the administration of saline or LPS (a time point when damage was seen) and the animals sacrificed 2 h later (in order to determine effects of drugs on further damage). Control animals received isotonic saline at the same volume (0.3 ml) and by the same route (i.v.). In some experiments, animals received a single dose of colchicine (1.5 mg kg−1, Salvemini et al., 1995) 15 min before the injection of LPS. Animals were sacrificed at 3 and 5 h after LPS. Experiments were also performed with SODm and PN decomposition catalysts in the colchicine treated rats.
Myeloperoxidase activity
Myeloperoxidase (MPO), a haemoprotein located in azurophil granules of neutrophils, has been used as a biochemical marker for neutrophil infiltration into tissues (Bradley et al., 1982). In the present study, MPO was measured spectrophotometrically by a method similar to that described previously (Laight et al., 1994). At the specified times following the injection of LPS, segments of duodenums and jejunums were removed and homogenized in ice-cold phosphate buffer (50 mM, pH 6) containing 0.5% hexadecyltrimethylammonium bromide (HTAB), freeze-thawed three times and centrifuged (10,000×g, 15 min, 4°C). Following centrifugation, 10 μl aliquots of each of the supernatants were mixed with 200 μl of assay buffer (50 mM phosphate buffer, pH 6, containing 0.5% HTAB, 0.167 mg ml−1 O-dianisidine hydrochloride and 0.0005% hydrogen peroxide). Changes in absorbance at 460 nm were measured spectrophotometrically over 5 min and MPO activities were expressed as mU g−1 wet tissue).
Lipid peroxidation
Malondialdehyde (MDA) has been used as a biochemical marker for lipid peroxidation and was measured by a method similar to that described previously (Okhawa et al., 1979). Levels of MDA were measured at 0, 3 and 5 h post-injection of LPS. Segments of duodenum and jejunum were removed and immediately frozen in liquid nitrogen, and homogenized in potassium chloride (1.15%). 2 ml of chloroform was added to each homogenate and the samples were then spun for 30 min. The organic layer of the sample was removed and dried under nitrogen gas and reconstituted with 100 μl of saline. MDA production was estimated by measuring thiobarbituric acid (TBA)-reacting compounds. Briefly, to each sample was added the following reaction mixture: 20 μl of 8.1% sodium dodecyl sulphate (SDS), 150 μl of 20% acetic acid solution (pH 3.5), 150 μl of 0.8% TBA and 400 μl of distilled water. The mixture was heated at 95°C for 15 min and allowed to cool. To extract the chromogenic product, 100 μl distilled water and 500 μl of n-butanol and pyridine (15 : 1, v v−1, containing 0.05% butylated hydroxytoluene) was added to the samples which were centrifuged (4000 r.p.m., 10 min) to facilitate phase separation. The organic layer was removed and MDA measured by reading the absorbance at 532 nm. Results are expressed as nmol MDA g−1 tissue.
Intravascular volume
Changes in intravascular volume in the intestinal tissue were determined in an additional group of rats by administering 125I-labelled bovine serum albumin ([125I]-BSA) intravenously (0.5 ml; 0.5 μCi) 2 min before surgical removal of the jejunum. The tissue and plasma content of radiolabel was determined and intravascular volume expressed as μl g−1 tissue. This value was subtracted from that obtained in the plasma leakage studies to obtain a measure of the intestinal plasma albumin leakage.
Plasma leakage
Intestinal vascular permeability was determined as the leakage into the jejunal tissue of [125I] -BSA administered intravenously (0.5 ml; 0.5 μCi) together with either LPS (3 mg kg−1) or isotonic saline. At various times (1–6 h) after LPS or saline administration, segments (3–5 cm) of duodenal or jejunal tissues were ligated and removed. The intestinal tissues were rapidly washed, blotted dry and weighed. Blood (0.5 ml) was collected into tubes containing tri-sodium citrate (0.318% final concentration) and plasma prepared by centrifugation (10,000×g for 10 min). The [125I]-BSA content in segments of whole tissue and in aliquots of plasma (100 μl) was determined in a gamma counter. The total content of plasma in the intestinal tissues was expressed as μl g−1 tissue.
Intestinal epithelial cell isolation
Intestinal epithelial cells were isolated from segments of the intestine as described previously (Lentze et al., 1985). A segment of proximal small intestine was obtained and slowly flushed with 50 ml of a solution containing 0.15 M NaCl dithiothreitol (DTT). The segment was then filled with 5 ml of a solution containing (in mM): KCl 1.5, NaCl 96, sodium citrate 27, KH2PO4 8 and Na2HPO4 5.6 (pH 7.3); the proximal and the distal ends were ligated. The segments were then immersed for a period of 15 min in phosphate-buffered saline (PBS) kept at 37°C which was bubbled with 95% O2-5% CO2. The solution from inside the segments was removed and another containing 1.5 mM EDTA and 0.5 mM DTT was added for 5 min, after which epithelial cells were collected (Tepperman et al., 1993). The preparation was subsequently washed twice with PBS (pH 7.4), centrifuged for 5 min (800×g) and resuspended in a buffer containing N-2-hydroxyethylpiperazipine-N-2-ethane-sulphonic acid (HEPES, 10 mM), sucrose (320 mM), DTT (1 mM), soybean trypsin inhibitor (10 μg ml−1), leupeptin (10 μg ml−1) and aprotinin (2 μg ml−1). This technique yielded a preparation of epithelial cells at 99% purity (Tepperman et al., 1993), which was confirmed by formaldehyde fixation and staining with hematoxylin-eosin-safran (HES). In all experiments an aliquot of cells isolated from control, and from the experimental groups under investigation was examined for viability as determined by trypan blue dye exclusion (0.5% trypan blue in PBS). This technique has been shown to be a reliable index of epithelial cell injury (Tepperman et al., 1993). Results are expressed as per cent of damaged cells.
Materials
[125I]-bovine bovine serum albumin was obtained from Amersham International (U.S.A.). Human polymorphonuclear leukocyte myeloperoxidase was obtained from Calbiochem (La Jolla, CA, U.S.A.). All other chemicals and reagents were obtained from Sigma (St. Louis, MO, U.S.A.). SC-55858 and the PN decomposition catalysts were synthesized as described previously (Stern et al., 1996; Riley et al., 1996).
Statistical analysis
Results are expressed as mean±s.e.mean for (n) rats. The results were analysed by Student's unpaired t-test to determine the significant differences between means, or by a two-way ANOVA followed by a least significant procedure to determine the nature of this response. A P value of <0.05 was considered to be statistically significant.
Results
Selectivity of SODm and PN-decomposition catalysts
In a previous series of papers (Riley et al., 1996; 1997), we have shown that the pentaaza macrocyclic ligand complexes of Mn(II) can be not only highly active catalysts for the dismutation of O2− but that they are also highly selective. The complex SC-55858 has, for example, been found to catalytically dismute O2− at a rate exceeding 10+8 molecules of O2− per molecule of complex per second at pH=7.4 and 21°C, at a rate comparable to the native Mn SOD enzyme. Remarkably, this complex does not react with hydrogen peroxide under the same conditions (Riley et al., 1996; 1997), nor does it react with other biologically relevant oxidants such as PN or nitric oxide. Thus, in our assays to monitor catalytic catalase activity using oxygen electrodes (Marshall & Worsfold, 1978; Pasternack & Pysnik, 1983), in which total oxygen concentration evolved from the reaction of hydrogen peroxide with catalase (or any putative catalase mimic) is quantitatively monitored, no catalytic activity is observed between SC-55858 and H2O2, further no stoichiometric reaction is observed to occur between SC-55858 and H2O2 as monitored via spectrophotometric or electrochemical (cyclic voltammetric) techniques. The stopped flow assay developed for monitoring peroxynitrite catalytic activity (Stern et al., 1996) was utilized to assess the PN activity of SC-55858. No catalytic PN activity is observed with SC-55858, nor is there any stoichiometric reactivity with PN observed with SC-55858, as monitored spectrophotometrically. The PN catalysts utilized in this study were shown to possess no catalase nor any direct catalytic SOD activity (Stern et al., 1996). These substrate specificities allow us to probe directly the physiological roles that the small molecules, O2− and PN, play by studying the effects that such selective catalysts exhibit in vivo.
SODm and PN-decomposition catalysts reduce LPS-induced plasma leakage
In control rats or in rats that received an intravenous injection of LPS, the intravascular blood volume in the duodenum or jejunum as determined by the tissue level [125I]-BSA injected 2 min before the removal of these tissues did not change over the entire period of the experiment (6 h). LPS elicited a pronounced increase in plasma leakage in the jejunum and duodenum by 3 h (Figure 2A). The therapeutic injection of the SODm, SC-55858 (0.03–1 mg kg−1, n=5) (Figure 2B) or the PN decomposition catalysts, FeTMPS and FeTMPyP (1–30 mg kg−1, n=5) at 3 h post LPS (time point when significant damage was already seen) caused a dose-dependent inhibition of the leakage observed at the 5 h time point (Figure 3A and B). On the other hand, the inactive PN decomposition catalysts, H2TMPS or ZnTMPyP (30 mg kg−1, n=5) had no effect (Figure 3A and B).
Figure 2.
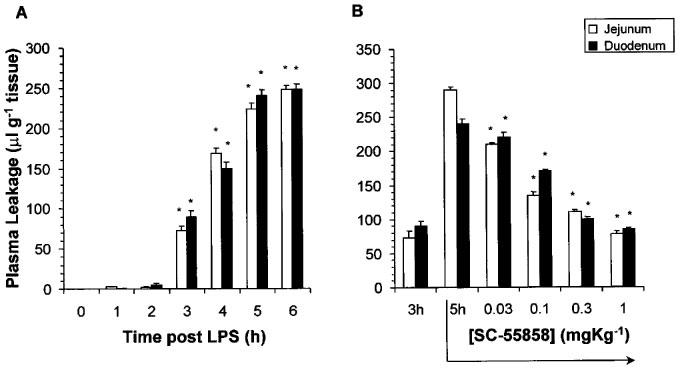
(A) Time-dependent induction of plasma leakage in the jejunum and duodenum following LPS (3 mg kg−1). (B) SC-55858 (0.03–1 mg kg−1) administered at 3 h post LPS, inhibited in a dose-dependent manner, the further increase in plasma leakage observed in jejunum and duodenum at the 5 h time-point. Each bar represents the mean±s.e.mean of five experiments. *P<0.05.
Figure 3.
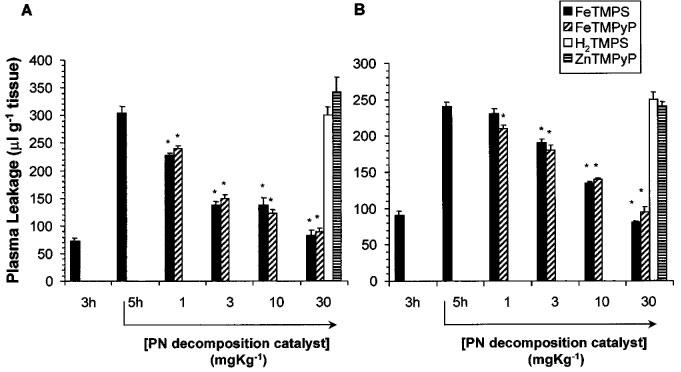
Effects of peroxynitrite decomposition catalysts on plasma leakage. FeTMPS (1–30 mg kg−1) or FeTMPyP (3–30 mg kg−1), administered at 3 h post LPS, inhibited in a dose-dependent manner, the increase in plasma leakage observed in jejunum (A) and duodenum (B) at the 5 h time-point. H2TMPS or ZnTMPyP (both at 30 mg kg−1) had no effect (A and B). Each bar represents the mean±s.e.mean of five experiments. *P<0.05.
Differential effects of SODm and PN-decomposition catalysts on LPS-induced neutrophil infiltration
The intravenous injection of LPS elicited a time-dependent neutrophil infiltration into the duodenum and jejunum as evidenced by the presence of MPO (an index of neutrophil influx) (Figure 4A). At the highest doses tested, the therapeutic injection (3 h post LPS) of the SODm, SC-55858 (1 mg kg−1, i.v, n=5), Figure 4B) prevented the subsequent increase in neutrophil infiltration observed at the 5 h time point. In contrast, FeTMPS and FeTMPyP (30 mg kg−1, i.v, n=5) had no effect (Figure 4B).
Figure 4.
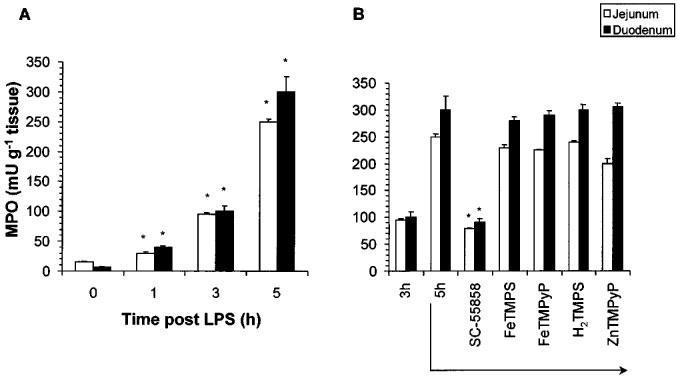
LPS caused a time-dependent infiltration of neutrophils into the jejunum and duodenum (A). The SODm, SC-55858 (1 mg kg−1) when given at 3 h post LPS attenuated further neutrophil accumulation observed at 5 h (B). No effect was seen with active (FeTMPS, FeTMPyP) or inactive (H2TMPS, ZnTMPyP) PN decomposition catalysts (30 mg kg−1) (B). Each bar represents the mean±s.e.mean of five experiments. *P<0.05.
SODm and PN-decomposition catalysts reduce LPS-induced lipid peroxidation
Lipids of biological membranes are often important targets for modification by O2− and peroxynitrite and MDA is a product that is formed as a consequence of free radical mediated lipid peroxidation. Consistent with the increase in vascular permeability, the intravenous injection of LPS elicited lipid peroxidation of the intestinal membranes as evidenced by the time-dependent increase in measurable MDA in both duodenal and jejunal preparations (Figure 5A). No lipid peroxidation products were observed at time 0 or 1 h after injection but significant peroxidation was observed at the 3 and 5 h time points (Figure 5A). At the highest doses tested, the therapeutic injection (3 h post LPS) of the SODm, SC-55858 (1 mg kg−1, i.v, n=5, Figure 5B) or FeTMPS and FeTMPyP (30 mg kg−1, i.v, n=5, Figure 5B) prevented the subsequent increase in intestinal epithelial cell damage observed at the 5 h time point. As expected, H2TMPS or ZnTMPyP (30 mg kg−1, i.v, n=5) had no effect (Figure 5B).
Figure 5.
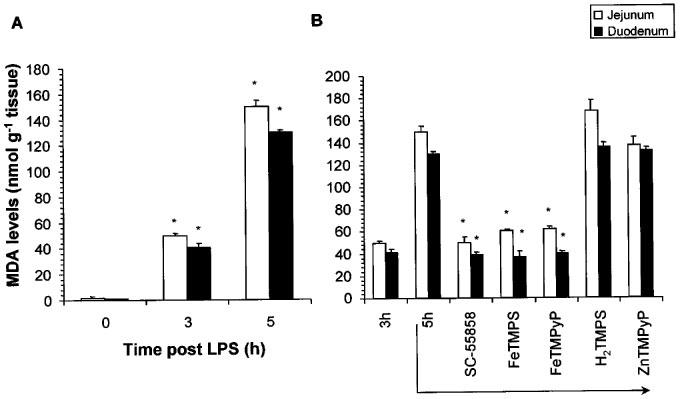
LPS caused a time-dependent increase in lipid peroxidation of jejunal and duodenal segments (A). SC-55858 (1 mg kg−1), FeTMPS or FeTMPyP (30 mg kg−1) when given at 3 h post LPS attenuated the lipid peroxidation observed at 5 h (B). The inactive PN decomposition catalysts (H2TMPS, ZnTMPyP; 30 mg kg−1) had no effect (B). Each bar represents the mean±s.e.mean of five experiments. *P<0.05.
LPS-induced epithelial cell damage and effects of SODm and PN-decomposition catalysts
At 3 and 5 h after the intravenous injection of LPS, the percentage of damaged intestinal epithelial cells was 26±2 and 74±3%, respectively (Figure 6A). At the highest doses tested, the therapeutic injection of the SODm, SC-55858 (1 mg kg−1, n=5, Figure 6B) or FeTMPS and FeTMPyP (30 mg kg−1, n=5, Figure 6B) at 3 h post LPS prevented the subsequent increase in intestinal epithelial cell damage observed at the 5 h time point (Figure 6B. H2TMPS or ZnTMPyP (30 mg kg−1, n=5) were ineffective (Figure 6B).
Figure 6.
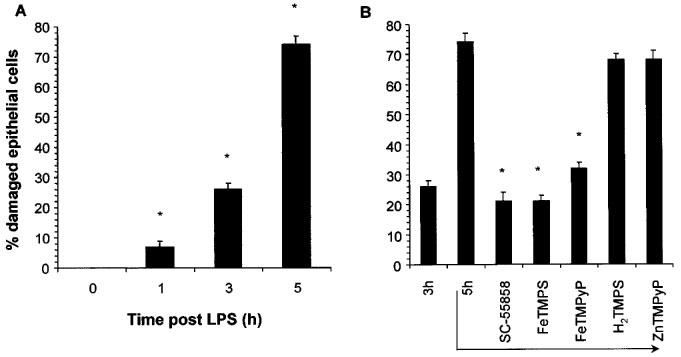
LPS caused a time-dependent damage of intestinal epithelial cells (A). SC-55858 (1 mg kg−1), FeTMPS or FeTMPyP (30 mg kg−1) when given at 3 h post LPS attenuated further epithelial cell damage observed at 5 h (B). The inactive peroxynitrite decomposition catalysts H2TMPS or ZnTMPyP had no protective effect (B). Each bar represents the mean±s.e.mean of five experiments. *P<0.05.
Neutrophil contribution to LPS-mediated intestinal damage: effects of colchicine treatment
To determine the relative contribution of neutrophils to endotoxin-induced intestinal damage, animals were made neutropenic by pre-treatment with colchicine as described in the Methods section: all parameters of inflammation were subsequently measured at 3 and 5 h after LPS. Colchicine inhibited neutrophil infiltration (from 95±3 and 250±5 to 15±7 and 20±3 mU MPO g−1 tissue at 3 and 5 h for LPS and LPS+colchicine respectively, n=5), lipid peroxidation (from 50±3 and 150±3 to 10±3 and 100±3 nmol MDA g−1 tissue at 3 and 5 h for LPS and LPS+colchicine respectively, n=5), plasma leakage (from 73±5 and 233±8 to 25±2 and 156±5 μl g−1 tissue at 3 and 5 h for LPS and LPS+colchicine respectively, n=5) and epithelial cell damage (from 26±2 and 74±3% to 3±2 and 45±5% damaged cells at 3 and 5 h for LPS and LPS+colchicine respectively, n=5). Interestingly, although colchicine was as effective in inhibiting neutrophil infiltration at 3 and 5 h, the degree of protection towards lipid peroxidation, plasma leakage and epithelial cell damage was greater at the 3 h time point. We next assessed the effects of the SODm and PN decomposition catalysts in colchicine-treated rats. For this purpose, SC-55858 (1 mg kg−1), FeTMPS or FeTMPyP (30 mg kg−1) were given 3 h after endotoxin. All drugs prevented damage seen at the 5 h time point. Levels of plasma leakage went from: 25±2 and 156±5 μl g−1 tissue for colchicine alone at 3 and 5 h to 20±3, 25±7 and 25±4 μl g−1 tissue for colchine in the presence of SC-55858, FeTMPS and FeTMPyP respectively at 5 h, n=5, levels of MDA went from: 10±3 and 100±3 nmol MDA g−1 tissue for colchicine alone at 3 and 5 h to 13±2, 12±1 and 10±2 nmol MDA g−1 tissue for colchine in the presence of SC-55858, FeTMPS and FeTMPyP respectively at 5 h, n=5, and percentage of damaged epithelial cells went from: 3±2 and 45±5% damaged cells for colchicine alone at 3 and 5 h to 2±0.3, 3±1 and 2±1% damaged cells for colchicine in the presence of SC-55858, FeTMPS and FeTMPyP respectively at 5 h, n=5.
Discussion
Some of the characteristics of endotoxin shock include hypotension, intravascular coagulation, increases in microvascular permeability and multi-organ damage with the lung and gastro-intestinal tract being prime targets (Schlag et al., 1991). Peroxynitrite is a powerful oxidant which is highly reactive towards biological molecules including protein and non-protein sulfhydryls, DNA, and membrane phospholipids (Beckman et al., 1990; Radi et al., 1991a, 1991b; Graham et al., 1993). Peroxynitrite is also stable enough to cross several cell diameters to reach targets before becoming protonated and decomposing (Crow & Beckman, 1995). It is, therefore, not surprising that evidence is increasing for a major role for peroxynitrite in the development of tissue damage during inflammation (Lamarque & Whittle, 1995a, 1995b; Dawson, 1995; Shigenawa et al., 1997) as well as in human subjects during sepsis (Fukuyama et al., 1997). Nevertheless, as indicated earlier, implications for the participation of peroxynitrite in diseases has relied on indirect evidence, mainly through the measurement of nitrotyrosine. The presence of nitrotyrosine at the site of injury does not however prove that peroxynitrite caused the damage, simply that it was formed. By using pharmacological agents specific for peroxynitrite decomposition (Salvemini et al., 1998; Misko et al., 1998), our results clearly indicate that peroxynitrite is the major cause of the intestinal damage following endotoxin administration. Thus, FeTMPS and FeTMPyP, which we have demonstrated to be active peroxynitrite decomposition catalyst (Stern et al., 1996; Misko et al., 1998; Salvemini et al., 1998) protected against microvascular injury, lipid peroxidation and epithelial cell injury.
The fact that the SOD mimetic, SC-55858, was also protective indicates that superoxide anions also play an important role in the damage evoked by endotoxin. Numerous cell types release superoxide anions during the inflammatory response and these include endothelial cells, epithelial cells, macrophages and neutrophils (Broner et al., 1988; Henson & Johnston 1988). As seen here, neutrophils do infiltrate into the intestine and their activation at the site of injury may contribute to O2− production and subsequent damage. Evidence does exist to support a damaging role of superoxide in the gastro-intestinal tract. For instance, the native enzyme SOD inhibits microvascular injury following ischaemia-reperfusion of the intestine and stomach (Droy-Lefaix et al., 1991; Haglind et al., 1994; Xia et al., 1995) and direct generation of superoxide anions from xanthine-xanthine oxidase infused intra-arterially provokes gastric mucosal injury (Espluges & Whittle, 1989; Lamarque & Whittle, 1995a, 1995b). Although neutrophils are probably the main source for the release of superoxide, and presumably playing a major role in the generation of peroxynitrite, a neutrophil involvement is not an obligatory requirement for the damage observed after endotoxin. Indeed, the protective effects of the peroxynitrite-decomposition catalysts were obtained despite the lack of effect by the catalysts on neutrophil infiltration. In contrast to peroxynitrite decomposition catalysts, SODm did inhibit neutrophil infiltration. Our findings with the SOD mimetic SC-55858 are consistent with a role for O2− in mediating neutrophil adhesion and infiltration (Scraufstatter et al., 1987; Warren et al., 1990) and with numerous reports indicating an inhibition of neutrophil infiltration at the site of inflammation by the native SOD enzyme (Hirschelmann & Bekemeier 1981; Boughton-Smith et al., 1993; Salvemini et al., 1996). Thus, besides inhibiting the formation of peroxynitrite, part of the protective effect of the SODm could be attributable to inhibition of neutrophil recruitment. Evidence does exist to suggest that the damaging effect of superoxide anions in the gastro-intestinal tract is neutrophil-independent since depletion of circulating neutrophils did not affect xanthine-xanthine oxidase induced mucosal damage (Deitch et al., 1990). Because neutrophils are only a part of the inflammatory cell infiltrate, we investigated the response of animals made neutropenic by colchicine treatment. Neutrophils appear to contribute to the damage observed at the earlier (3 h) but not later (5 h) time point since in neutropenic rats, plasma leakage, lipid peroxidation and epithelial cell injury were markedly attenuated at the 3 h time point, and only slightly attenuated at the later time point. This suggests that cells other than neutrophils are responsible for the damage during the later stages of the inflammatory response. SC-55858, FeTMPS and FeTMPyP did not lose their inhibitory activity in the colchicine treated animals suggesting that both O2− and peroxynitrite are still involved in the damage. Macrophages and epithelial cells are a likely source. These cells are able to generate large amounts of O2−, are induced by endotoxin to express iNOS and have as a consequence, the potential to generate peroxynitrite (Schlag et al., 1991; Gow et al., 1998).
There is no doubt that large amounts of NO are released from iNOS in animal models of endotoxaemia as well as in humans with sepsis. However, the use of selective inhibitors of iNOS for sepsis is problematic. This assertion stems from the fact that although iNOS inhibitors prevent the hyporeactivity and the hypotension following endotoxin administration it is very difficult to improve survival. It is therefore tempting to speculate that in the presence of a failing eNOS system (Forstermann & Leinert, 1995) optimal organ-perfusion and function may have to rely on some of the NO formed from iNOS. Thus, when the iNOS inhibitors prevent the release of NO from iNOS and eliminate part of its damaging effects (presumably through the formation of peroxynitrite) these same inhibitors will also remove a critical portion of NO required to maintain normal physiology. It is therefore possible that since neither SODm or peroxynitrite decomposition catalysts interact with NO (Misko et al., 1998), they are able to remove the harmful effects due to the overproduction of NO without compromising its ability to help maintain vital organ perfusion. This hypothesis is under current investigation. The pharmacological separation of the positive effects of NO from its negative effects in shock and other types of inflammatory disorders has, until now, been almost impossible.
In conclusion we have demonstrated that superoxide and peroxynitrite are associated with the intestinal damage evoked by endotoxin. The PN decomposition catalysts and SODm catalysts may offer a novel approach for manipulating the sequence of events that are associated with shock. The further use of these catalysts as pharmacological tools in animal models of human disease may lead us to a better understanding of when and where superoxide and peroxynitrite play key roles in the development of inflammatory diseases such as sepsis. This in turn should provide us with more effective treatment strategies for diseases in the clinic. In addition to inflammation, the future clinical utility for agents such as these may be as adjunct therapy for the gastro-intestinal damage associated with chemo- and radiation therapy for cancer.
In conclusion, SODm and peroxynitrite decomposition catalysts are not only useful tools for the pharmacological dissection of free radical-mediated pathology, but also offer promise as disease-modifying therapeutic agents capable of preserving the positive aspect of the double-edge sword of nitric oxide action.
Abbreviations
- FeTMPS
5,10,15,20-tetrakis(2,4,6-trimethyl-3,5 disulphonatophenyl)-porphyrinato iron (III)
- FeTMPyP
5,10,15,20-tetrakis(N-methyl-4′-pyridyl)-porphyrinato iron (III)
- H2TMPS
5,10,15,20-tetrakis(2,4,6-trimethyl-3,5-disulphonatophenyl)-porphyrin
- LPS
E. coli Lipopolysaccharide
- MDA
malondialdehyde
- MPO
myeloperoxidase
- O2−
superoxide anions
- PN
peroxynitrite
- SODm
superoxide dismutase mimic
- ZnTMPyP
5,10,15,20-tetrakis(N-methyl-4′-pyridyl)-porphyrinato zinc
References
- BATINIC-HABERLE I., LIOCHEV S.I., SPASOJEVIC I., FRIDOVICH I. A potent superoxide dismutase mimic: manganese beta-octabromo-meso-tetrakis-(N-methylpyridinium-4-yl)porphyrin. Arch. Biochem. Biophys. 1997;343:225–233. doi: 10.1006/abbi.1997.0157. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Peroxynitrite versus hydroxyl radical: The role of nitric oxide in superoxide-dependent cerebral injury. Ann. NY Acad. Sci. 1994;738:69–75. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.M., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., CROW J.P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., LASZLO F., WHITTLE B.J., MONCADA S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br. J. Pharmacol. 1993;110:1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation. Estimation of neutrophil content with an enzyme marker. J. Clin. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- BRONER C.W., SHENEP J.L., STIDHAM G.L., STOKES D.C., HILDNER B.S. Effects of scavengers of oxygen-derived free radicals on mortality in endotoxin-challenged mice. Crit. Care Med. 1988;16:848–851. doi: 10.1097/00003246-198809000-00006. [DOI] [PubMed] [Google Scholar]
- CROW J.P., BECKMAN J.S. The role of peroxynitrite in nitric oxide mediated toxicity. Current Topics in Microbiology and Immunology. 1995;196:51–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L. Nitric oxide: role in neurotoxicity. Clin. Exp. Pharm. Physiol. 1995;22:305–308. doi: 10.1111/j.1440-1681.1995.tb02005.x. [DOI] [PubMed] [Google Scholar]
- DAY B.J., SHAWEN S., LIOCHEV S.I., CRAPO J.D. A metalloprotin superoxide dismutase mimic protects against paraquat-induced endothelial cell injury, in vitro. J. Pharmacol. Exp. Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- DEITCH E.A., BRIDGES W., BERG R., SPECIAN R.D., GRANGER N. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J. Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- DROY-LEFAIX M.T., DROUET Y., GERAUD G., HOSFOD D., BRAQUET P. Superoxide dismutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Free Rad. Res. Commun. 1991;1213:725–735. doi: 10.3109/10715769109145852. [DOI] [PubMed] [Google Scholar]
- ESPLUGUES J.V., WHITTLE B.J. Gastric damage following local intra-arterial administration of reactive oxygen metabolites in the rat. Br. J. Pharmacol. 1989;97:1085–1092. doi: 10.1111/j.1476-5381.1989.tb12565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FÖRSTERMANN U., LEINERT H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn-Schmiedebergs Arch. Pharmacol. 1995;352:351–364. doi: 10.1007/BF00172772. [DOI] [PubMed] [Google Scholar]
- FUKUYAMA N., TAKEBAYASHI Y., HIDA M., ISHIDA H., ICHIMORI K., NAKAZAWA H. Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radical Biol. Med. 1997;22:771–774. doi: 10.1016/s0891-5849(96)00401-7. [DOI] [PubMed] [Google Scholar]
- GARDNER P.R., NGUYEN D.D.-H., WHITE C.W. Superoxide scavenging by Mn (II/III) tetrakis (1-methyl-4-pyridyl) porphyrin in mammalian cells. Arch. Biochem. Biophys. 1996;325:20–28. doi: 10.1006/abbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- GOW A.J., THOM S.R., ISCHIROPOULUS H. Nitric oxide and peroxynitrite mediated pulmonary cell death. Am. J. Physiol. 1998;274:L112–L118. doi: 10.1152/ajplung.1998.274.1.L112. [DOI] [PubMed] [Google Scholar]
- GRAHAM A., HOGG N., KALYANARAMAN B., O'LEARY V., DARLEY-USMAR V., MONCADA S. Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor. FEBS Lett. 1993;330:181–185. doi: 10.1016/0014-5793(93)80269-z. [DOI] [PubMed] [Google Scholar]
- HAGLIND E., XIA G., RYLANDER R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ. Shock. 1994;42:83–91. [PubMed] [Google Scholar]
- HENSON P.M., JOHNSTON R.B. Tissue injury and inflammation. Oxidants, proteinases and cationic proteins. J. Clin. Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHELMANN R., BEKEMEIER H. Effects of catalase, peroxidase, superoxide dismutase and 10 scavengers of oxygen radicals in carrageenan edema and in adjuvant arthritis of rats. Experientia. 1981;37:1313–1314. doi: 10.1007/BF01948381. [DOI] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Rad. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., BECKMAN J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- LAIGHT D.W., LAD N., WOODWARD B., WATERFALL J.F. Assessment of myeloperoxidase activity in renal tissue after ischemia/reperfusion. Eur. J. Pharmacol. 1994;292:81–88. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- LAMARQUE D., WHITTLE B.J.R. Involvement of superoxide and xanthine oxidase in neutrophil-independent rat gastric damage induced by NO donors. Br. J. Pharmacol. 1995a;116:1843–1848. doi: 10.1111/j.1476-5381.1995.tb16672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMARQUE D., WHITTLE B.J.R. Role of oxygen-derived metabolites in the rat gastric mucosal injury induced by nitric oxide donors. Eur. J. Pharmacol. 1995b;277:187–194. doi: 10.1016/0014-2999(95)00075-v. [DOI] [PubMed] [Google Scholar]
- LENTZE M.J., COLONY P.C., TRIER J.S. Glucocorticoid receptors in isolated intestinal epithelial cells in rats. Am. J. Physiol. 1985;249:G58–G65. doi: 10.1152/ajpgi.1985.249.1.G58. [DOI] [PubMed] [Google Scholar]
- MARSHALL M.J., WORSFOLD W. Superoxide dismutase: a direct, continuous assay using the oxygen electrode. Anal. Biochem. 1978;86:561–567. doi: 10.1016/0003-2697(78)90783-2. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., HIGHKIN M.K., VEENHUIZEN A.W., MANNING P.T., STERN M.K., CURRIE M.G, SALVEMINI D. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 1998;273:15646–15653. doi: 10.1074/jbc.273.25.15646. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI H., YAGI K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- PASTERNACK R.F., PYSNIK D.Catalysis of the Dispropostionation of Superoxide by Metalloporphyrins Oxy Radicals and Their Scavenger Systems 1983The Netherlands: Elsevier; eds. Cohen G & Greenwald R. p. 151 [Google Scholar]
- RADI R., BECKMAN J.S., BUSH K.M., FREEMAN B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991a;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- RADI R., BECKMAN J.S., BUSH K.M., FREEMAN B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991b;266:4244–4250. [PubMed] [Google Scholar]
- RILEY D.P., HENKE S.L., LENNON P.J., WEISS R.H., NEUMANN W.L., RIVERS W.J., ASTON K.W., SAMPLE K.R., RAHMAN H., LING C.-S., SHIEH J.-J., BUSCH D.H., SZULBINSKI W. Synthesis, characterization and stability of manganese (II) C-substituted 1,4,7,10,13-pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 1996;35:5213–5231. [Google Scholar]
- RILEY D.P., LENNON P.J., NEUMANN W.L., WEISS R.H. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese(II) complexes. J. Am. Chem. Soc. 1997;119:6522. [Google Scholar]
- SALTER M., KNOWLES R.G., MONCADA S. Widespread tissue distribution, species distribution and changes in activity of Ca2+-dependent and Ca2+-independent nitric oxide synthases. FEBS Lett. 1991;291:145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MANNING P.T., ZWEIFEL B.S., SEIBERT K., CONNOR J., CURRIE M.G., NEEDLEMAN P., MASFERRER J.L. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J. Clin. Invest. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.-Q., STERN M.K., CURRIE M.G., MISKO T.P. Peroxynitrite decomposition catalysts: novel therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P.S., BOURDON D.M., MARINO M.H., MANNING P.Y., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLAG G., REDL H., HALLSTRÖM S. The cell in shock: the origin of multiple organ failure. Resuscitation. 1991;21:137–180. doi: 10.1016/0300-9572(91)90044-y. [DOI] [PubMed] [Google Scholar]
- SCRAUFSTATTER I.U., HYSLOP P.A., JACKSON J., COCHRANE C.C. Oxidant injury of cells. Int. J. Tissue. React. 1987;9:317–324. [PubMed] [Google Scholar]
- SHIGENAGA M.K., LEE H.H., BLOUNT B.C., CHRISTEN S., SHIGENO E.T., YIP H., AMES B.N. Inflammation and NO(X)-induced nitration: assay for 3-nitrotyrosine by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3211–3216. doi: 10.1073/pnas.94.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN M.K., JENSEN M.P., KRAMER K. Peroxynitrite Decomposition Catalysts. J. Am. Chem. Soc. 1996;118:8735–8736. [Google Scholar]
- TAYLOR D.E., GHIO A.J., PIANTADOSI C.A. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch. Biochem. Biophys. 1995;316:70–76. doi: 10.1006/abbi.1995.1011. [DOI] [PubMed] [Google Scholar]
- TEPPERMAN B.L., BROWN J.F., WHITTLE B.J.R. Nitric oxide synthase induction and intestinal epithelial cell viability in rats. Am. J. Physiol. 1993;265:G214–G218. doi: 10.1152/ajpgi.1993.265.2.G214. [DOI] [PubMed] [Google Scholar]
- WARREN J.S., YABROFF K.R., MANDEL D.M., JOHNSON K.J., WARD P.A. Role of O2− in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free. Rad. Biol. Med. 1990;8:163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- WEISS R.H., FLICKINGER A.G., RIVERS W.J., HARDY M.D., ASTON K.W., RYAN U.S., RILEY D.P. Evaluation of putative superoxide dismutase mimics. J. Biol. Chem. 1993;268:23049. [PubMed] [Google Scholar]
- XIA Z.F., HOLLYOAK M., BARROW R.E., HE F., MULLER M.J., HERNDON D.N. Superoxide dismutase and leupeptin prevent delayed reperfusion injury in the rat small intestine during burn shock. J. Burn Care Rehab. 1995;16:111–117. doi: 10.1097/00004630-199503000-00004. [DOI] [PubMed] [Google Scholar]


