Abstract
The effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of soluble guanylyl cyclase (sGC), were investigated in aortic rings and ventricular cardiomyocytes from rats. The production of cyclic GMP was stimulated by NO•-donors or carbachol. Additionally, the effects of ODQ were studied in cytosolic extracts from both tissues in which the cyclic GMP production was stimulated by S-nitroso-N-acetylpenicillamine (SNAP).
In endothelium-intact aortic rings, SNAP (100 μM), 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (DETA NONOate; 100 μM), or carbachol (10 μM) increased cyclic GMP levels about 4 fold. These effects were abolished by ODQ (50 μM).
In cardiomyocytes, SNAP (100 μM), DETA NONOate (100 μM), or carbachol (10 μM) increased cyclic GMP levels about 2 fold. These effects were not affected by ODQ (50 μM).
In cytosolic extracts from aortic rings and cardiomyocytes, SNAP (100 μM) induced about 50 fold increases in cyclic GMP levels. ODQ (50 μM) reduced these effects by about 50%.
In extracts from cardiomyocytes, increases by SNAP (100 μM) of cyclic GMP levels were attenuated by myoglobin dependent on concentration: at 300 μM myoglobin, SNAP (100 μM) increased cyclic GMP levels only 3 fold. Inhibitory effects of ODQ (50 μM) were abolished by 300 μM myoglobin.
It is suggested that both NO• and ODQ can bind to myoglobin which, at high concentrations, can diminish their effects on sGC. Such a scavenger function of myoglobin could explain why NO• and ODQ exert only minor effects in cardiomyocytes (with high myoglobin content), but strong effects in aortic tissue (virtually devoid of myoglobin).
Keywords: Cyclic GMP, heart muscle, aortic smooth muscle, ODQ, myoglobin
Introduction
Soluble guanylyl cyclase (sGC; EC 4.6.1.2.) is considered the key enzyme mediating a variety of physiological processes in response to nitric oxide (NO•), including smooth muscle relaxation, inhibition of platelet aggregation, and synaptic transmission (Moncada et al., 1991; Garthwaite, 1995). Binding of NO• to the ferrous haem iron of sGC induces a change in haem geometry leading to a stabilization of the enzyme in its activated form that catalyzes the formation of cyclic GMP from GTP, with the concomitant release of pyrophosphate (Arnold et al., 1977; Ohlstein et al., 1982; Traylor & Sharma, 1992). Increases in cyclic GMP levels are associated with an activation of cyclic GMP-dependent protein kinases and subsequent phosphorylation of numerous intracellular target proteins (Murad, 1986).
In heart muscle, both muscarinic agonists and NO•-donors have been found to increase cyclic GMP levels in multicellular (George et al., 1975; Diamond et al., 1977; Lindemann & Watanabe, 1988) and single cell preparations (Stein et al., 1993; MacDonell et al., 1995; Kojda et al., 1996). These effects are assumed to be due to activation of sGC (see Hare & Colucci, 1995). One support for this view comes from the finding that the effects of carbachol and NO•-donors on the heart are attenuated by methylene blue, often used as an inhibitor of sGC (Balligand et al., 1993; Levi et al., 1994). However, the latter substance has been shown to exhibit a number of non-specific effects besides sGC inhibition, including generation of superoxide anions and inhibition of NO•-synthase activity (Mayer et al., 1993). Recently, the introduction of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), a potent and selective inhibitor of sGC (Garthwaite et al., 1995), has helped to identify more precisely the sGC-mediated effects in various tissues (Brunner et al., 1996; Cellek et al., 1996; Hebeiss & Kilbinger, 1996; Moro et al., 1996). The link between activation of sGC and the increase in cyclic GMP in heart muscle can, therefore, be more firmly established by the use of ODQ.
In this study, we investigated the effects of NO•-donors and carbachol on cyclic GMP levels in aortic tissue and isolated ventricular cardiomyocytes from rats, using ODQ as a selective inhibitor of sGC. The effects of NO•-donors or carbachol on cyclic GMP levels were abolished by ODQ in aortic preparations. Surprisingly, in cardiomyocytes, these effects were not affected by ODQ. This suggested that either a cardiac isoform of sGC exists that is not inhibited by ODQ or that sGC cannot be reached by ODQ in intact cardiomyocytes. A preliminary account of this work has been presented (Wegener et al., 1998).
Methods
Preparations
Sprague-Dawley rats (200–300 g) of either sex were anaesthetized with ether and bled from the carotid arteries. The heart and the thoracic aorta were quickly removed and immersed in oxygenated Tyrode's solution containing (in mM): NaCl 137, KCl 5.4, CaCl2 1.8, MgCl2 1, NaHCO3 12, NaH2PO4 0.42, glucose 5.6; bubbled with 95% O2+5% CO2, pH 7.4; 37°C. Ventricular cardiomyocytes were isolated as described previously (Wegener & Nawrath, 1995). Briefly, the hearts were enzymatically digested by perfusion with a collagenase-containing buffer solution via the aorta using the Langendorff-setup. Single myocytes were obtained by mechanical dispersion of the ventricular tissue. The suspension of cardiomyocytes was passed through a 250 μm nylon gaze, centrifuged (25×g, 15 min), and resuspended in buffered salt solution (in mM): NaCl 137, KCl 5.4, MgCl2 0.5, CaCl2 1.8, glucose 5, HEPES 10; pH 7.4. This cell suspension was centrifuged through a gradient of 0.6 % Ficoll and washed three times (25×g, 15 min). Microscopically, the final cell pellet contained exclusively cardiomyocytes, identified by their shape and size. 50–80% of the cells displayed clear cross striations. The cell pellet prepared from one heart was divided among 18 microtubes for further experiments.
After the connective tissue had been removed, the isolated thoracic aorta was cut into rings of 1 mm in width which were transferred to 21 microtubes containing buffered salt solution. The endothelium remained intact during the procedure as shown in parallel experiments in which the rings, pre-contracted by phenylephrine (3 μM), showed a strong relaxation in response to carbachol (3 μM).
Determination of cyclic GMP levels in aortic rings and cardiomyocytes
Aortic rings and cardiomyocytes were pre-treated with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; 100 μM) for 15 min in microtubes to inhibit breakdown of the cyclic nucleotide. The preparations were then incubated with test drugs or vehicle for 10 min at room temperature in the presence of IBMX. After the incubation, the preparations were immediately frozen in liquid nitrogen and stored at −20°C. Cyclic GMP was extracted by addition of four volumes 1 N HClO4 followed by sonification and centrifugation (13,000×g, 15 min). The pellets were resuspended in 1 ml of 2 M NaOH overnight and the protein concentration was then determined by the method of Bradford (1976). The supernatants were neutralized with KOH (5 M), centrifuged again (3,000×g, 2 min), and their cyclic GMP content was determined by a radio-immuno assay using 125I-labelled cyclic GMP according to Steiner et al. (1972).
Determination of sGC activity in cytosolic extracts
The activity of sGC in cytosolic extracts was determined according to Arnold et al. (1977). For each set of experiments, the thoracic aorta and the cardiomyocytes from both ventricles were prepared from the same rat each. The preparations were transferred to hypo-osmotic solutions containing 50 mM TRIS (pH 7.4) and incubated for 4 h at 4°C. After sonification and centrifugation (13,000×g, 20 min), the supernatants were used as the source of cytosolic sGC activity and for the estimation of protein concentration according to Bradford (1976). 20 μl of the supernatants were added to microtubes containing the assay buffer giving a final volume of 100 μl. The assay buffer contained (in mM): TRIS 200 (pH 7.4), MgCl2 4 IBMX 0.5, creatine phosphate 15, dithiothreitol 1, 1 μg creatine kinase (150–300 units/mg protein), 20 mg bovine serum albumin, and vehicle or drugs at the concentrations indicated. The reaction was started by addition of GTP (1 mM) and terminated after 10 min at 37°C by addition of 1 ml sodium acetate buffer (50 mM; pH 4), followed by rapid freezing in liquid nitrogen. The samples were then centrifuged (13000×g, 15 min), and the content of cyclic GMP in the supernatants was measured by a radio-immunoassay using 125l-labelled cyclic GMP according to Steiner et al. (1972).
Chemicals
S-nitroso-N-acetylpenicillamine (SNAP), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), 2-(4-carboxyphenyl)-4,4, 5,5-tetramethylimidazoline-1-oxyl-3-oxide (Carboxy-PTIO), and 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (DETA NONOate) were obtained from Calbiochem (Bad Soden, Germany), 125l-cyclic GMP-TME (2.2 mCi mmol−1) from Biotrend (Köln, Germany). All other chemicals used were at least p.a. grade and purchased from Sigma (St Louis MO, U.S.A.). Stock solutions of IBMX and ODQ were prepared in dimethylsulphoxide (DMSO) and further diluted with buffered salt solution to achieve the concentrations indicated. The final amount of DMSO in test solutions did not exceed 0.5 % (v v−1) which did not significantly affect the cyclic GMP levels measured. Stock solutions of myoglobin (Sigma, St Louis MO, U.S.A.) were prepared according to Martin et al., 1985.
Evaluation of results
Data are presented as means±s.e.mean. Concentration-response curves were fitted by sigmoidal functions (correlation coefficient >0.99) using GraphPad Prism 2.0 (GraphPad Software Inc., San Diego, CA, U.S.A.). Statistical analysis was performed using unpaired Student's t-test. P-values <0.05, <0.01, and <0.001 were considered as significant and marked by one, two, and three asterisks, respectively. Absence of significance was marked by N.S.
Results
The levels of cyclic GMP were determined in endothelium-intact aortic rings and in ventricular cardiomyocytes in response to (1) the NO•-donors SNAP (Salas et al., 1994) and DETA NONOate (Seccia et al., 1996), (2) carbachol, either alone or in combination with ODQ, an inhibitor of sGC (Garthwaite et al., 1995), or carboxy-PTIO, an NO• scavenger (Rand & Li, 1995).
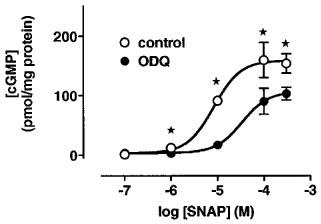
In cardiomyocytes, SNAP increased cyclic GMP levels in a concentration-dependent manner both under control conditions and in the presence of ODQ (50 μM; Figure 1); the EC50 were about 4 μM under both conditions.
Figure 1.
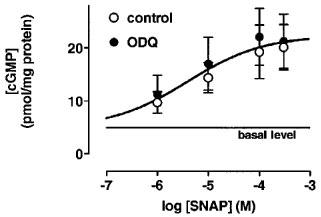
Concentration-dependent effects of SNAP on cyclic GMP levels in the absence and presence of ODQ in cardiomyocytes. The cyclic GMP levels (in pmol mg−1 protein) amounted to 4.9±0.4 under control conditions and to 9.7±2 at 1 μM SNAP, to 14.4±2.9 at 10 μM SNAP, to 19.3±5.1 at 100 μM SNAP, and to 20.1±4.3 at 300 μM SNAP (n=9 each). In the presence of ODQ (50 μM), the cyclic GMP levels (in pmol mg−1 protein) were 11.2±3.6 at 1 μM SNAP, 17±5 at 10 μM SNAP, 22.1±5.4 at 100 μM SNAP, and 21.3±5.1 at 300 μM SNAP (n=9 each). The data were statistically not different in the absence and presence of ODQ (50 μM) and were approximated by one sigmoid concentration-response curve with an EC50 of 4 μM. Data represent means±s.e.mean.
SNAP (100 μM) increased the cyclic GMP level to 384% and to 223% of control in aortic rings and cardiomyocytes, respectively (Figure 2a and b). ODQ (50 μM) abolished this response in aortic rings but not in cardiomyocytes. Carboxy-PTIO (500 μM) reduced the effect of SNAP by 69 % in aortic rings and by 64 % in cardiomyocytes. ODQ (50 μM) and carboxy-PTIO (500 μM) alone significantly reduced the control values in aortic rings but were ineffective in cardiomyocytes (Figure 2).
Figure 2.
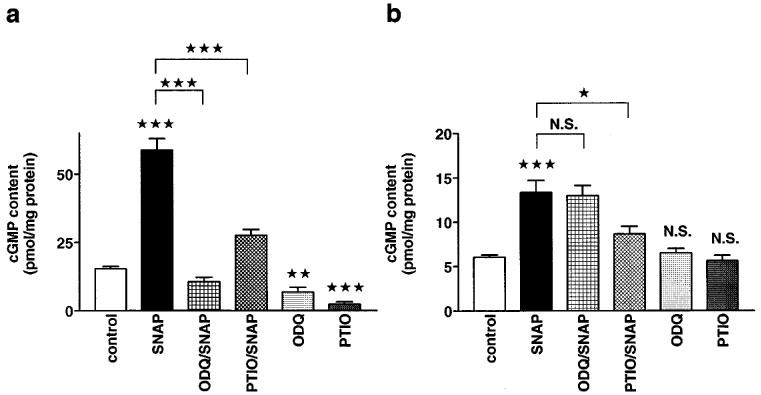
Effects of ODQ on SNAP-induced increases in cyclic GMP levels in aortic rings and cardiomyocytes. (a) Cyclic GMP levels in aortic rings with intact endothelium. The cyclic GMP levels (in pmol mg−1 protein) were 15.3±0.9 (n=25) under control conditions, 58.8±4.1 (n=12) in the presence of SNAP (100 μM), 10.5±1.7 (n=11) in the presence of both ODQ (50 μM) and SNAP (100 μM), 27.6±2 (n=10) in the presence of both carboxy-PTIO (500 μM) and SNAP (100 μM), 6.8±1.7 (n=10) in the presence of ODQ (50 μM) alone, and 2.3±0.9 (n=7) in the presence of carboxy-PTIO (500 μM) alone. (b) Cyclic GMP levels in isolated cardiomyocytes. The cyclic GMP levels (in pmol/mg protein) were 6±0.3 (n=45) under control conditions, 13.4±1.4 (n=15) in the presence of SNAP (100 μM), 13±1.1 (n=12) in the presence of both ODQ (50 μM) and SNAP (100 μM), 8.6±0.9 (n=9) in the presence of both carboxy-PTIO (500 μM) and SNAP (100 μM), 6.5±0.5 (n=12) in the presence of ODQ (50 μM) alone, and 5.6±0.9 (n=6) in the presence of carboxy-PTIO (500 μM) alone. Columns represent means±s.e.mean. Statistically significant differences (P<0.05) are marked by *.
The effects of DETA NONOate, which exhibits a longer lasting NO•-release than SNAP (Seccia et al., 1996), on increases in cyclic GMP content were also investigated. DETA NONOate (100 μM) increased the cyclic GMP content to 307% and to 198% of control in aortic rings and in cardiomyocytes, respectively (Figure 3a and b). ODQ (50 μM) abolished the effect of DETA NONOate in aortic rings but not in cardiomyocytes, as also seen in the first series of experiments using SNAP. Carboxy-PTIO (500 μM) reduced the effect of DETA NONOate from 307 to 21% of control in aortic rings and from 198 to 115% of control in cardiomyocytes.
Figure 3.
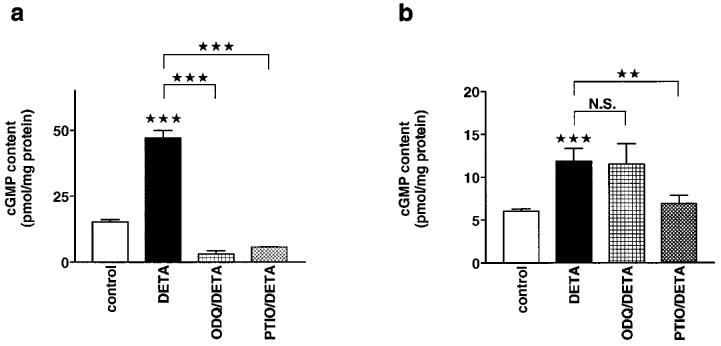
Effects of ODQ on DETA NONOate-induced increases in cyclic GMP levels in aortic rings and cardiomyocytes. (a) Cyclic GMP levels in aortic rings with intact endothelium. The cyclic GMP levels (in pmol mg−1 protein) were 15.3±0.9 (n=25) under control conditions, 47.1±2.7 (n=9) in the presence of DETA NONOate (100 μM), 3.2±1.2 (n=6) in the presence of ODQ (50 μM) and DETA NONOate (100 μM), and 5.8±0.1 (n=6) in the presence of carboxy-PTIO (500 μM) and DETA NONOate (100 μM). (b) Cyclic GMP levels in isolated cardiomyocytes. The cyclic GMP levels (in pmol mg−1 protein) reached 6±0.3 (n=45) under control conditions, 11.9±1.5 (n=9) in the presence of DETA NONOate (100 μM), 11.5±2.4 (n=9) in the presence of ODQ (50 μM) and DETA NONOate (100 μM), and 6.9±1 (n=9) in the presence of carboxy-PTIO (500 μM) and DETA NONOate (100 μM). Columns represent means±s.e.mean. Statistically significant differences (P<0.05) are marked by *.
Muscarinic agonists have been reported to increase cyclic GMP levels in both vascular smooth muscle (Moncada et al., 1991) and isolated cardiomyocytes (Stein et al., 1993). Therefore, the effects of carbachol on cyclic GMP levels were also studied in the presence of ODQ or carboxy-PTIO. Carbachol (10 μM) increased the cyclic GMP content to 251% and to 228% of control in aortic rings and in cardiomyocytes, respectively (Figure 4a and b). Both ODQ (50 μM) and carboxy-PTIO (500 μM) abolished this effect in aortic rings but not in cardiomyocytes.
Figure 4.
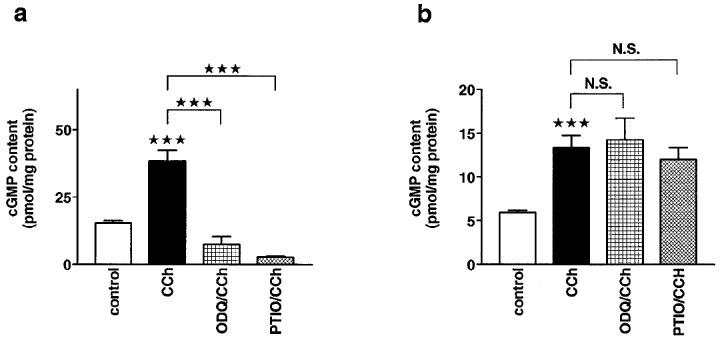
Effects of ODQ on carbachol-induced increases in cyclic GMP levels in aortic rings and cardiomyocytes. (a) Cyclic GMP levels in aortic rings with intact endothelium. The cyclic GMP levels (in pmol mg−1 protein) were 15.3±0.9 (n=25) under control conditions, 39.5±3.9 (n=12) in the presence of carbachol (10 μM), 7.4±2.8 (n=9) in the presence of ODQ (50 μM) and carbachol (10 μM), 2.7±0.4 (n=6) in the presence of carboxy-PTIO (500 μM) and carbachol (10 μM). (b) Cyclic GMP levels in isolated cardiomyocytes. The cyclic GMP levels (in pmol mg−1 protein) reached 6±0.3 (n=45) under control conditions, 13.7±1.5 (n=27) in the presence of carbachol (10 μM), 14.3±2.5 (n=20) in the presence of ODQ (50 μM) and carbachol (10 μM), 10.6±1.4 (n=3) in the presence of carboxy-PTIO (500 μM) and carbachol (10 μM). Columns represent means±s.e.mean. Statistically significant differences (P<0.05) are marked by *.
In the last series of experiments, the effects of SNAP on cyclic GMP levels were investigated under cell-free conditions. To this end, cytosolic extracts were obtained from aortic tissue and from cardiomyocytes. In cytosolic extracts from cardiomyocytes, SNAP increased cyclic GMP levels in a concentration-dependent manner; the EC50 was 8 μM (Figure 5). ODQ (50 μM) attenuated these responses; in the presence of this drug, the EC50 for SNAP was 33 μM (Figure 5). In both cytosolic extracts from aortic tissue and from cardiomyocytes, SNAP (100 μM) increased the cyclic GMP concentration more than 50 fold (Figure 6). ODQ (50 μM) reduced the effects of SNAP (100 μM) by about 50% in both extracts (Figure 6). ODQ alone (50 μM) did not influence the cyclic GMP concentration in either extract.
Figure 5.
Concentration-dependent effects of SNAP on cyclic GMP levels in the absence and presence of ODQ in cytosolic extracts from cardiomyocytes. The cyclic GMP levels (in pmol mg−1 protein) amounted to 3.4±0.8 under control conditions and to 2.1±1 at 0.1 μM SNAP, to 12.1±2.5 at 1 μM SNAP, to 91±5 at 10 μM SNAP, to 160±21 at 100 μM SNAP, and to 154±16 at 300 μM SNAP (n=4 each). In the presence of ODQ (50 μM), the cyclic GMP levels (in pmol mg−1 protein) were 3.4±1 at 0.1 μM SNAP, 3±0.9 at 1 μM SNAP, 17±1.6 at 10 μM SNAP, 90±22 at 100 μM SNAP, and 103±11 at 300 μM SNAP (n=4 each). Both data sets were fitted by sigmoidal concentration-response curves; the EC50 were 8 μM under control conditions and 33 μM in the presence of ODQ (50 μM). Data represent means±s.e.mean. Statistically significant differences (P<0.05) are marked by *.
Figure 6.
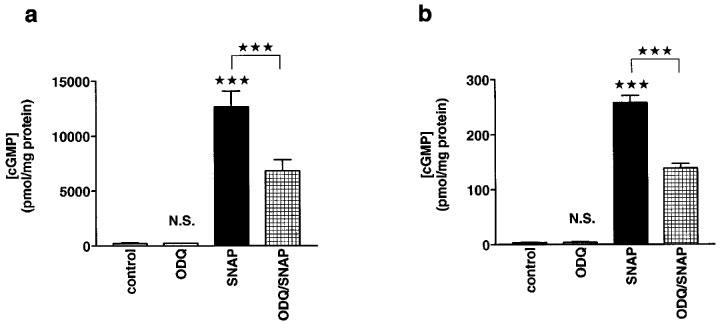
Effects of ODQ on SNAP-induced increases in cyclic GMP levels in cytosolic extracts from aortic rings and cardiomyocytes. (a) Cyclic GMP levels in extracts from aortic rings. The cyclic GMP levels (in pmol mg−1 protein) were 212±50 (n=9) under control conditions, 200±70 (n=6) in the presence of ODQ (50 μM), 12600±1500 (n=6) in the presence of SNAP (100 μM), and 6800±1000 (n=6) in the presence of both ODQ (50 μM) and SNAP (100 μM). (b) Cyclic GMP levels in extracts from cardiomyocytes. The cyclic GMP levels (in pmol mg−1 protein) were 3.4±0.3 (n=18) under control conditions, 4±1.2 (n=6) in the presence of ODQ (50 μM), 258±13 (n=12) in the presence of SNAP (100 μM), and 138±9 (n=12) in the presence of both ODQ (50 μM) and SNAP (100 μM). Columns represent means±s.e.mean. Statistically significant differences (P<0.05) are marked by *.
Since ODQ has been reported to act on the haem-group of soluble guanylyl cyclase (Schrammel et al., 1996), the haem-group of other proteins may also be a target for ODQ. One candidate for such a protein is myoglobin which is intracellularly present in cardiomyocytes at concentrations of about 200 μM (Doeller & Wittenberg, 1991). To test this hypothesis, the effects of ODQ on the SNAP-induced increase in cyclic GMP concentration were investigated in the presence of myoglobin under cell-free conditions to simulate the situation in intact cells. The effects of different myoglobin concentrations on SNAP-induced cyclic GMP increases in the cytosolic extract from cardiomyocytes are shown in Figure 7. Myoglobin attenuated the increase in cyclic GMP levels by SNAP in a concentration-dependent manner: in the presence of 30 μM myoglobin, SNAP (100 μM) increased the cyclic GMP level about 50 fold, whereas the increase was only 3 fold at 300 μM myoglobin. The presence of myoglobin attenuated also the inhibitory effects of ODQ (50 μM) on the SNAP-induced increase in cyclic GMP levels in a concentration-dependent manner (Figure 7). At myoglobin concentrations up to 30 μM, ODQ (50 μM) reduced the effect of SNAP (100 μM) on the cyclic GMP concentration by about 50%. At myoglobin concentrations of 100 and 300 μM, the inhibitory effect of ODQ on the SNAP-induced increase in cyclic GMP concentration was absent thereby resembling the results obtained in intact cells (Figures 2b and 7).
Figure 7.
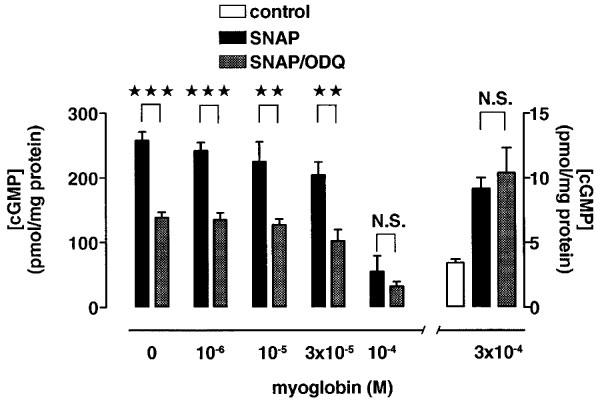
Effects of ODQ on SNAP-induced increases in cyclic GMP level in cytosolic extracts from cardiomyocytes at different concentrations of myoglobin. The cyclic GMP levels obtained under control conditions and in the presence of 300 μM myoglobin are shown at different scale (right). Under control conditions, the cyclic GMP levels (in pmol mg−1 protein) were 3.2±0.3 (n=6). In the presence of SNAP (100 μM), the cyclic GMP levels (in pmol mg−1 protein) increased to 257±13 (n=12) without myoglobin, to 242±13 (n=3) at 1 μM myoglobin, to 225±31 (n=5) at 10 μM myoglobin, to 205±19 (n=3) at 30 μM myoglobin, to 55±24 (n=6) at 100 μM myoglobin, and to 9.2±0.8 (n=6) at 300 μM myoglobin. In the presence of both ODQ (50 μM) and SNAP (100 μM), the cyclic GMP levels (in pmol mg−1 protein) were 138±9 (n=12) without myoglobin, 135±11 (n=3) at 1 μM myoglobin, 128±9 (n=5) at 10 μM myoglobin, 102±17 (n=3) at 30 μM myoglobin, 33±7 (n=6) at 100 μM myoglobin, and 10.4±0.9 (n=6; inset) at 300 μM myoglobin. Columns represent means±s.e.mean. Statistically significant differences (P<0.05) are marked by *.
Discussion
The present study demonstrates that ODQ, a selective inhibitor of sGC (Garthwaite et al., 1995), inhibits the effects of carbachol and NO•.-donors on cyclic GMP levels in rat aortic tissue but not in intact rat ventricular cardiomyocytes. In contrast, ODQ inhibits the NO•-donor-induced increases in cyclic GMP concentration in cytosolic extracts from both aortic tissue and cardiomyocytes. This indicates the presence of an intracellular factor in intact cardiomyocytes which counteracts the effect of ODQ. One candidate for this factor is myoglobin which is present in cardiomyocytes at about 200 μM (Doeller & Wittenberg, 1991). ODQ has been shown spectroscopically to interact with the haem group of sGC (Schrammel et al., 1996) and haemoglobin (Moro et al., 1996) and may also interact with the haem group of myoglobin. This view is supported by the finding that addition of myoglobin to the cytosolic extract from cardiomyocytes abolished the inhibitory action of ODQ on sGC activity. Therefore, a scavenger function of myoglobin seems likely in cardiomyocytes reducing the effects of ODQ, whereas in aortic tissue, which contains no detectable amounts of myoglobin (Ishibashi et al., 1993), the drug is more effective.
It is well accepted that the effects of NO• are inhibited by haemoglobin and myoglobin which act as scavengers of free NO• (Mittal et al., 1978; Martin et al., 1985; Ignarro, 1990). In the present study, activation of extracted sGC by NO•-donors was also diminished by myoglobin at concentrations reported in mammalian striated muscle cells (200–300 μM; Doeller & Wittenberg, 1991; Jurgens et al., 1994). Therefore, high concentrations of exogenous NO•. may be required to reach intracellular sGC in cardiomyocytes. Indeed, negative inotropic effects induced by gaseous NO•. were only observed at very high concentrations (about 100 μM) in rat cardiomyocytes (Wyeth et al., 1996). The role of NO• in mediating cardiac contractility seems, therefore, to be questionable under physiological conditions because these concentrations are not reached by endogenous NO• production which is in the range of 1–50 nM (Gross & Wolin, 1995). However, under pathophysiological circumstances, the situation may change because endogenous NO• production can reach concentrations up to 10 μM (Gross & Wolin, 1995). In addition, endogenous NO•, derived from NO• synthase, which is present in cardiomyocytes (Balligand et al., 1995), may affect cardiac contractility if the enzyme exists in close vicinity to target proteins like sGC in a subcellular compartment in which myoglobin is absent.
Ishibashi et al. (1993) proposed that the high level of myoglobin in the myocardium as compared to aortic tissue accounts for the small effects of NO• on cardiac contractility as compared to the marked effects of NO• on vascular smooth muscle. From this point of view, the discrepancy in the observed effects of NO• and related substances on cardiac contractility in mammals (i.e. positive inotropic effects (Strauer, 1973), negative inotropic effects (Smith et al., 1991; Flesch et al., 1997), and absence of effects (Weyrich et al., 1994; Nawrath et al., 1995) may be related to differences in the myoglobin concentration in the preparations and species used. For example, rat cardiac muscle contains 7 fold more myoglobin than guinea pig cardiac muscle (500 and 70 mg 100 g−1, respectively; O'Brien et al., 1992). In addition, rabbit ventricular cardiac muscle was found to contain 3 fold more myoglobin than rabbit atrial cardiac muscle (Ishibashi et al., 1993). The situation may be different in non-mammalian striated muscle preparations containing lower myoglobin levels than mammalian striated muscle (O'Brien et al., 1992): for example, frog skeletal muscle is normally devoid of myoglobin (Baylor & Pape, 1988). Therefore, it seems possible that the described effects of NO• and related substances in frog cardiomyocytes (Méry et al., 1993), which were antagonised by ODQ (Abi-Gerges et al., 1997), are related to a low level of myoglobin in these cells.
Muscarinic agonists increase cyclic GMP levels in heart muscle and decrease cardiac contractility especially when previously stimulated by β-agonists (Lindemann & Watanabe, 1988). Their effects have been ascribed partially to activation of NO•-synthase and NO•-mediated activation of sGC (Hare & Colucci, 1995). However, several studies have challenged this view by the demonstration that constitutive NO•-synthase is involved neither in carbachol-induced increases in cyclic GMP levels in isolated cardiomyocytes (Stein et al., 1993) nor in inotropic or chronotropic responses induced by muscarinic agonists in atrial preparations (Nawrath et al., 1995). In addition, it has been reported that cyclic GMP does not mediate muscarinic effects on cardiac contractility (MacDonell et al., 1995). Since the effects of carbachol on cyclic GMP levels in cardiomyocytes were not affected by ODQ (this study), or NO•-synthase inhibitors (Stein et al., 1993), further experiments are necessary to clarify the involvement of sGC in this reaction cascade.
Carboxy-PTIO reversed the cyclic GMP increases produced by carbachol in aortic rings but not in cardiomyocytes. This discrepancy may be ascribed to the fact that the cyclic GMP measurements were performed in intact preparations (aorta) and isolated cardiomyocytes (heart). Carboxy-PTIO acts as a scavenger for NO• molecules outside the cell if NO• acts as an inter-cellular messenger (as it is the case in the aorta), but not if NO• acts as an intra-cellular messenger (as it may be the case in cardiomyocytes).
We suggest that, in rat ventricular cardiomyocytes, the failure of ODQ to inhibit increases in cyclic GMP content induced by NO•-donors or carbachol is due to binding to myoglobin, which is present in high amounts in these cells. On the other hand, ODQ effectively inhibits the increases in cyclic GMP content induced by NO•-donors or carbachol in aortic tissue, in which myoglobin is not detectable (Ishibashi et al., 1993).
Acknowledgments
We thank Drs A. Mülsch (Frankfurt, Germany) and B. Mayer (Graz, Austria) for helpful comments on the current study. This work was supported by grants (to H. Nawrath) from the Deutsche Forschungsgemeinschaft (Germany) and the Umweltministerium of Rheinland-Pfalz (Germany).
Abbreviations
- Carboxy-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DETA NONOate
2,2′-(hydroxynitrosohydrazino)bis-ethanamine
- IBMX
3-isobutyl-1-methylxanthine
- NO•
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluble guanylyl cyclase
- SNAP
S-nitroso-N-acetylpenicillamine
References
- ABI-GERGES N., HOVE-MADSEN L., FISCHMEISTER R., MÉRY P.F. A comparative study of the effects of three guanylyl cyclase inhibitors on the L-type Ca2+ and muscarinic K+ currents in frog cardiac myocytes. Br. J. Pharmacol. 1997;121:1369–1377. doi: 10.1038/sj.bjp.0701249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNOLD W.P., MITTAL C.K., KATSUKI S., MURAD F. Nitric oxide activates guanylate cyclase and increases guanosine 3′: 5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLIGAND J.L., KELLY R.A., MARSDEN P.A., SMITH T.W., MICHEL T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc. Natl. Acad. Sci. U.S.A. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLIGAND J.L., KOBZIK L., HAN X., KAYE D.M., BELHASSEN L., O'HARA D.S., KELLY R.A., SMITH T.W., MICHEL T. Nitric oxide-dependent parasympathetic signalling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J. Biol. Chem. 1995;270:14582–14586. doi: 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- BAYLOR S.M., PAPE P.C. Measurement of myoglobin diffusivity in the myoplasm of frog skeletal muscle fibres. J. Physiol. (Lond.) 1988;406:247–275. doi: 10.1113/jphysiol.1988.sp017379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRUNNER F., SCHMIDT K., NIELSEN E.B., MAYER B. Novel guanylyl cyclase inhibitor potently inhibits cyclic GMP accumulation in endothelial cells and relaxation of bovine pulmonary artery. J. Pharmacol. Exp. Ther. 1996;277:48–53. [PubMed] [Google Scholar]
- CELLEK S., KASAKOV L., MONCADA S. Inhibition of nitrergic relaxations by a selective inhibitor of the soluble guanylate cyclase. Br. J. Pharmacol. 1996;118:137–140. doi: 10.1111/j.1476-5381.1996.tb15376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., TENEICK R.E., TRAPANI A.J. Are increases in cyclic GMP levels responsible for the negative inotropic effects of acetylcholine in the heart. Biochem. Biophys. Res. Commun. 1977;79:912–918. doi: 10.1016/0006-291x(77)91197-4. [DOI] [PubMed] [Google Scholar]
- DOELLER J.E., WITTENBERG B.A. Myoglobin function and energy metabolism of isolated cardiac myocytes: effect of sodium nitrite. Am. J. Physiol. 1991;261:H53–H62. doi: 10.1152/ajpheart.1991.261.1.H53. [DOI] [PubMed] [Google Scholar]
- FLESCH M., KILTER H., CREMERS B., LENZ O., SUDKAMP M., KUHN-REGNIER F., BOHM M. Acute effects of nitric oxide and cyclic GMP on human myocardial contractility. J. Pharmacol. Exp. Ther. 1997;281:1340–1349. [PubMed] [Google Scholar]
- GARTHWAITE J. Neural nitric oxide signalling. Trends Neurosci. 1995;18:51–2. [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSON E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GEORGE W.J., BUSUTTIL R.W., PADDOCK R.J., WHITE L.A., IGNARRO L.J. Opposing regulatory influences of cyclic guanosine monophosphate and cyclic adenosine monophosphate in the control of cardiac muscle contraction. Recent Adv. Stud. Cardiac Struct. Metab. 1975;8:243–250. [PubMed] [Google Scholar]
- GROSS S.S., WOLIN M.S. Nitric oxide: pathophysiological mechanisms. Annu. Rev. Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- HARE J.M., COLUCCI W.S. Role of nitric oxide in the regulation of myocardial function. Prog. Cardiovasc. Dis. 1995;38:155–166. doi: 10.1016/s0033-0620(05)80004-0. [DOI] [PubMed] [Google Scholar]
- HEBEISS K., KILBINGER H. Differential effects of nitric oxide donors on basal and electrically evoked release of acetylcholine from guinea-pig myenteric neurones. Br. J. Pharmacol. 1996;118:2073–2078. doi: 10.1111/j.1476-5381.1996.tb15646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGNARRO L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- ISHIBASHI T., HAMAGUCHI M., KATO K., KAWADA T., OHTA H., SASAGE H., IMAI S. Relationship between myoglobin contents and increases in cyclic GMP produced by glyceryl trinitrate and nitric oxide in rabbit aorta, right atrium and papillary muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:553–561. doi: 10.1007/BF00166750. [DOI] [PubMed] [Google Scholar]
- JURGENS K.D., PETERS T., GROS G. Diffusivity of myoglobin in intact skeletal muscle cells. Proc. Natl. Acad. Sci. USA. 1994;91:3829–3833. doi: 10.1073/pnas.91.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJDA G., KOTTENBERG K., NIX P., SCHLUTER K.D., PIPER H.M., NOACK E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ. Res. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- LEVI R.C., ALLOATTI G., PENNA C., GALLO M.P. Guanylate-cyclase-mediated inhibition of cardiac Ica by carbachol and sodium nitroprusside. Pflügers Arch. 1994;426:419–426. doi: 10.1007/BF00388305. [DOI] [PubMed] [Google Scholar]
- LINDEMANN J.P., WATANABE A.M.Mechanism of adrenergic and cholinergic regulation of myocardial contractility Physiology and Pathophysiology of the Heart 1988Boston: Kluwer Acad. Publishers; 423–452.Second edition. ed. Sperelakis, N. pp [Google Scholar]
- MACDONELL K.L., TIBBITS G.F., DIAMOND J. cGMP elevation does not mediate muscarinic agonist-induced negative inotropy in rat ventricular cardiomyocytes. Am. J. Physiol. 1995;269:H1905–H1912. doi: 10.1152/ajpheart.1995.269.6.H1905. [DOI] [PubMed] [Google Scholar]
- MARTIN W., VILLANI G.M., JOTHIANANDAN D., FURCHGOTT R.F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J. Pharmacol. Exp. Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- MAYER B., BRUNNER F. & K. , SCHMIDT K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- MÉRY P.F., PAVOINE C., BELHASSEN L., PECKER F., FISCHMEISTER R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J. Biol. Chem. 1993;268:26286–26295. [PubMed] [Google Scholar]
- MITTAL C.K., ARNOLD W.P., MURAD F. Characterization of protein inhibitors of guanylate cyclase activation from rat heart and bovine lung. J. Biol. Chem. 1978;253:1266–1271. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MORO M.A., RUSSEL R.J., CELLEK S., LIZASOAIN I., SU Y., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. Cyclic GMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAD F. Cyclic guanosine monophosphate as a mediator of vasodilation. J. Clin. Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAWRATH H., BÄUMNER D., RUPP J., OELERT H. The ineffectiveness of the NO-cyclic GMP signaling pathway in the atrial myocardium. Br. J. Pharmacol. 1995;116:3061–3067. doi: 10.1111/j.1476-5381.1995.tb15964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BRIEN P.J., SHEN H., MCCUTCHEON L.J., O'GRADY M., BYRNE P.J., FERGUSON H.W., MIRSALIMI M.S., JULIAN R.J., SARGEANT J.M., TREMBLAY R.R., BLACKWELL T.E. Rapid, simple and sensitive microassay for skeletal and cardiac muscle myoglobin and hemoglobin: use in various animals indicates functional role of myohemoproteins. Mol. Cell. Biochem. 1992;112:45–52. doi: 10.1007/BF00229642. [DOI] [PubMed] [Google Scholar]
- OHLSTEIN E.H., WOOD K.S., IGNARRO L.J. Purification and properties of heme-deficient hepatic soluble guanylate cyclase: effects of heme and other factors on enzyme activation by NO, NO-heme, and protoporphyrin IX. Arch. Biochem. Biophys. 1982;218:187–198. doi: 10.1016/0003-9861(82)90335-6. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br. J. Pharmacol. 1995;116:1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS E., MORO M.A., ASKEW S., HODSON H.F., BUTLER A.R., RADOMSKI M.W., MONCADA S. Comparative pharmacology of analogues of S-nitroso-N-acetyl-DL-penicillamine on human platelets. Br. J. Pharmacol. 1994;112:1071–1076. doi: 10.1111/j.1476-5381.1994.tb13192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMMEL A., BEHRENDS S., SCHMIDT K., KOESLING D., MAYER B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- SECCIA M., PERUGINI C., ALBANO E., BELLOMO G. Inhibition of Cu2+-induced LDL oxidation by nitric oxide: a study using donors with different half-time of NO release. Biochem. Biophys. Res. Commun. 1996;220:306–309. doi: 10.1006/bbrc.1996.0401. [DOI] [PubMed] [Google Scholar]
- SMITH J.A., SHAH A.M., LEWIS M.J. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J. Physiol. (Lond.) 1991;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN B., DRÖGEMÜLLER A., MÜLSCH A., SCHMITZ W., SCHOLZ H. Ca++-dependent constitutive nitric oxide synthase is not involved in the cyclic GMP-increasing effects of carbachol in ventricular cardiomyocytes. J. Pharmacol. Exp. Ther. 1993;266:919–925. [PubMed] [Google Scholar]
- STEINER A.L., PARKER C.W., KIPNIS D.M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J. Biol. Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- STRAUER B.E. Der Mechanismus der Nitroglyzerinwirkung vom Aspekt der Myokardkontraktilität. Z. Kardiol. 1973;62:97–113. [PubMed] [Google Scholar]
- TRAYLOR T.G., SHARMA V.S. Why NO. Biochemistry. 1992;31:2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]
- WEGENER J.W., CLOSS E.I., FÖRSTERMANN U., NAWRATH H. Failure of ODQ to inhibit activation of soluble guanylyl cyclase in ventricular myocytes from rat hearts. Naunyn Schmiedeberg's Arch. Pharmacol. 1998;357:R47. [Google Scholar]
- WEGENER J.W., NAWRATH H. Extracellular site of action of phenylalkylamines on L-type calcium current in rat ventricular myocytes. Naunyn Schmiedeberg's Arch Pharmacol. 1995;352:322–330. doi: 10.1007/BF00168564. [DOI] [PubMed] [Google Scholar]
- WEYRICH A.S., MA X.L., BUERKE M., MUROHARA T., ARMSTEAD V.E., LEFER A.M., NICOLAS J.M., THOMAS A.P., LEFER D.J., VINTEN-JOHANSEN J. Physiological concentrations of nitric oxide do not elicit an acute negative inotropic effect in unstimulated cardiac muscle. Circ. Res. 1994;75:692–700. doi: 10.1161/01.res.75.4.692. [DOI] [PubMed] [Google Scholar]
- WYETH R.P., TEMMA K., SEIFEN E., KENNEDY R.H. Negative inotropic actions of nitric oxide require high doses in rat cardiac muscle. Pflügers Arch. 1996;432:678–684. doi: 10.1007/s004240050185. [DOI] [PubMed] [Google Scholar]


