Abstract
Adenosine-5′-triphosphate (ATP) is a potent coronary vasodilator. Because of the efficient hydrolysis of ATP, adenosine-5′-diphosphate (ADP) and adenosine-5′-monophosphate (AMP) by ectonucleotidases located in the coronary endothelium ATP-induced vasodilation may be mediated via both P1 (AMP and adenosine) and P2Y (ATP and ADP) receptors. We have used the change in total coronary resistance (TCR) induced by intravascular ATP in the isolated working rat heart to determine both the component of the vasodilation mediated via P2Y receptors and the identity of the subclass of receptor involved.
The dose response for ATP revealed a half maximal effect at an apparent ATP concentration of 0.08±0.009 μM. The response was saturated at apparent ATP concentrations greater than 0.23 μM. Contrary to much of the current literature, the perfusion of a 0.25 μM concentration of adenosine resulted in the identical response to an equimolar concentration of ATP suggesting a significant role for adenosine in coronary vasodilation.
The non-selective P1 receptor antagonist 8-(p-Sulfophenyl)theophylline (8-SPT) was used to show that the response to ATP was mediated via both P1 and P2Y receptors. Whilst 8-SPT abolished the effect of adenosine it reduced the effect of ATP by only 50%. Thus, at a saturating concentration of ATP, P1 and P2Y receptors were shown to contribute equally to the observed vasodilation.
Uridine-5′-triphosphate (UTP), ADP and adenosine-5′-O-thiotriphosphate (ATPγS) were used to characterize the component of coronary vasodilation that was mediated via P2Y receptors. UTP at 0.25 μM was ineffective and did not induce vasodilation. Perfusion with 0.25 μM ADP resulted in a vasodilation that was identical to 0.25 μM ATP. In the absence of 8-SPT the perfusion of 0.25 μM ATPγS produced a vasodilation that was significantly (P<0.05) less than ATP. However, the vasodilation due to ATPγS, like that of adenosine, but unlike that of both ATP and ADP, was abolished in the presence of 8-SPT. The ability of ADP to induce vasodilation combined with both the lack of response to UTP and the ability of 8-SPT to abolish the vasodilation induced by ATPγS suggested very strongly that the component of ATP-induced coronary vasodilation in the isolated working rat heart that was mediated via P2Y receptors was achieved by the action of ADP (and not ATP) at P2Y1 receptors.
These results suggest that the vasodilatory action of intravascular ATP in the coronary circulation should be attributed to the dual and equal activities of adenosine and ADP acting at P1 and P2Y1 receptors respectively.
Keywords: Working rat heart; vasodilation; ATP; adenosine; P1 receptor; P2Y receptor; 8-(p-Sulfophenyl)theophylline, suramin
Introduction
ATP is released in the heart under both physiological and pathophysiological conditions (Forrester & Williams, 1977; Vials et al., 1987). Intravascular ATP may induce a significant coronary vasodilation in the isolated rat heart (Hopwood & Burnstock, 1987). Upon entry into the coronary circulation ATP is immediately hydrolyzed to ADP, AMP and adenosine via a cascade of extracellular ecto-nucleotidases (Gordon, 1986). The nucleotides ATP, ADP and AMP and the nucleoside adenosine have each been shown to induce coronary vasodilation (Bunger et al., 1975), adenosine and AMP via P1 and ATP and ADP via P2Y receptors (Burnstock, 1978). For this reason the respective roles of P1 and P2Y receptors in mediating the vasodilatory response induced by intravascular ATP are often in question. We have used an isolated working heart model to characterize the vasodilation induced by intravascular ATP in the coronary circulation of the rat. The heart model we have used is a close approximation of a working heart in vivo and has been developed to take into account inotropic and chronotropic influences upon coronary vasodilation (Wright et al., 1999). We have defined the vasodilatory response to a continuous perfusion of ATP in the terms of its sensitivity to the apparent ATP concentration and, at the minimum apparent concentration of ATP required to produce a maximum coronary vasodilation, we have determined the relative contributions to this response of ligands for both P2Y and P1 receptors. The coronary vasodilation was extremely sensitive to the apparent concentration of ATP and, at saturation, was mediated to an approximately equal extent by P1 and P2Y receptors. The latter involving only the P2Y1 receptor.
Methods
Heart perfusion
The investigation was performed in accordance with the Home Office Guidance on the Operation of The Animals (Scientific Procedures) Act 1986, published by Her Majesty's Stationery Office, London.
We have used an adaptation (Snaith et al., 1992; Wright et al., 1995; Korchazhkina et al., 1998) of an isolated working heart preparation first described by Westerhof et al. (1971). Male Wistar rats, 230–280 g in weight, were lightly anaesthetized with 4% ether in oxygen, heparinized by intravenous injection (500 I.U. kg−1) and the heart excised and transferred to ice-cold heparinized saline. After cannulation of the aorta, the coronary arteries of the spontaneously beating heart were perfused at 10–12 ml min−1 using a peristaltic pump. When the left atrium was cannulated, the preparation was converted to the working mode with an atrial filling pressure of 15 mmHg. The heart then performed external work against a 3-component lumped parameter model of the rat aortic input impedance (Korchazhkina et al., 1998) with the component values adjusted to correspond to values that we measured in vivo (Wright, 1991). In this preparation the coronary flow is determined by the left ventricular power output and the balance of the model and the coronary impedances. The heart was allowed to stabilize for 30 min. Thereafter it was perfused according to one of the protocols described below. The presence of a second left atrial perfusion line allowed us to switch from one buffer to another without delay.
The normal perfusion buffer contained (mM): HEPES 25.0, NaCl 118.5, KCl 4.8, MgSO4 1.2, CaCl2 1.4 and glucose 11.0 at pH 7.42 and 37°C. The perfusion buffer was oxygenated (pO2>85 kPa) and its temperature controlled using a hollow-fibre miniature membrane oxygenator (Microsafe, Polystan A/S, Denmark). The coronary effluent was not recirculated.
Physiological measurements
The following techniques were used to monitor cardiac performance. Pressure in the model ‘aorta' was recorded using a Gaeltec 16CT luer-lock catheter-tip straingauge transducer coupled to a Biodata Microlink AN1DS interface module (Biodata Limited, Manchester, U.K). Model ‘aortic' flow was monitored using a 3 mm i.d. cannulating transducer of a Skalar MDL 1401 electromagnetic flowmeter (Skalar Limited, Delft, The Netherlands). Data were acquired over a 30 s period. Both pressure and flow signals were digitized by a Microlink 12-bit A-D converter at 500 Hz and the digital records were stored on optical discs for subsequent computation of functional parameters using C programmes developed by one of the authors. All computations were performed on a single waveform derived as an average of eight consecutive waveforms. Total coronary resistance (TCR) was calculated as mean aortic pressure divided by mean coronary flow. The latter was measured by timed collection of the total coronary effluent.
Experimental protocols
Dose-response experiments
A 15 min period of perfusion using normal buffer was followed by a 5 min perfusion of the same buffer but containing ATP in the concentration range of 0.05–5.00 μM. The perfusion with ATP was followed by a 20 min period of wash-out with normal buffer and this served to demonstrate the reversibility of the response. Each replicate of each concentration of ATP was performed on a new heart. Control hearts were perfused with normal buffer for the 40 min experimental period.
Pharmacological determinations of the vasodilatory response
A 15 min period of perfusion using normal buffer was followed by a 5 min perfusion with the same buffer but including one of a number of different additives (ATP, ADP, adenosine, UTP, ATPγS) at a concentration of 0.25 μM (Figure 1). This was the lowest concentration of ATP which produced the maximum change in the TCR (see Results). After treatment with the additive the heart was perfused for a further 15 min with normal buffer (wash-out) to demonstrate the reversibility of the response. Experiments with UTP were terminated after this period of wash-out. In experiments using ATP, ADP, ATPγS and adenosine the heart was then treated for a subsequent 15 min period with either 10 μM of the P1 receptor antagonist 8-SPT or 5 μM of the P2 receptor antagonist suramin plus 10 μM 8-SPT (ATP treatment only). Hearts which had been pretreated with antagonists were then perfused for a further 5 min with buffer containing the corresponding antagonist and either ATP, ADP, ATPγS or adenosine. Physiological measurements relevant to the determination of TCR were made during the first and fifth minutes of each of the 5 min periods of continuous perfusion with either the agonist alone or the agonist in combination with 8-SPT (see, for example, Figure 4).
Figure 1.
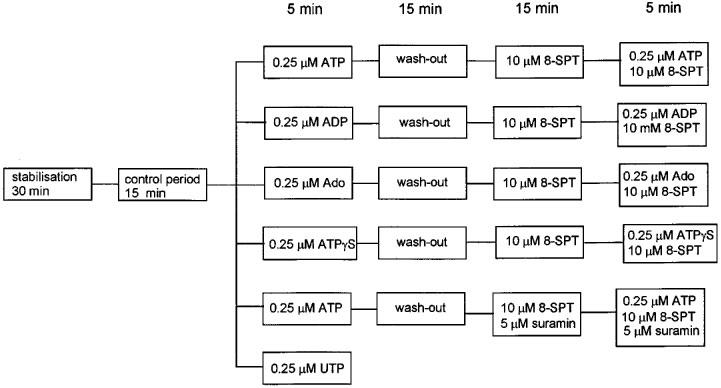
Summary of experimental steps used in the pharmacological analyses. The perfusion period for each step is given in minutes.
Figure 4.
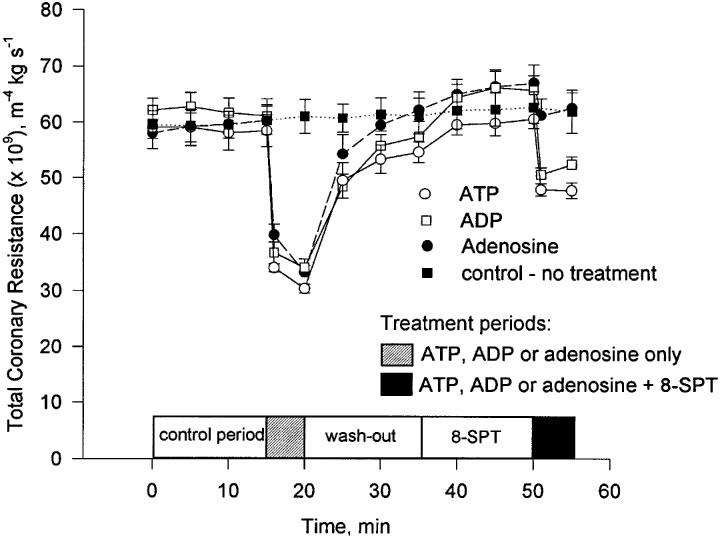
The change in TCR during the experimental period following treatment with ATP, ADP and adenosine (nucleotide concentration=0.25 μM). Mean and s.e.mean are plotted, n=5.
Control hearts were perfused with normal buffer using the identical experimental protocol.
Measurement of ATP and its hydrolysis products by HPLC
We have used an adaptation of a method of measurement of ATP by HPLC (Stocchi et al., 1985). A Waters (Waters Corporation, MA, U.S.A.) HPLC system which included a 2690 Separations Module and a 996 Photodiode Array Detector was used to measure ATP and the products of its hydrolysis in the coronary effluent. Separation of the nucleotides/nucleosides was achieved using a Waters Nova-Pak C18 column (3.9×300 mm). A guard column, Nova-Pak C18 (3.9×20 mm), was placed before the main column to eliminate contaminating moeities. Samples of coronary effluent were collected and prior to analysis were filtered through nylon 0.2 μm filters and maintained at 4°C during measurement. The column temperature was maintained at 30°C and a mobile phase flow rate of 0.5 ml min−1 was used. A reverse phase two step gradient elution was used. The first step of the elution comprised 30 mM KH2PO4 buffer at pH 6.0 over 7 min for the separation of uric acid, ATP, ATPγS and ADP. In the second step AMP, inosine and adenosine were eluted using a linear gradient of acetonitrile (0–15%) over a further 18 min period. After every run the column was equilibrated by pumping 5 column volumes of 100% 30 mM KH2PO4. The range of wavelengths scanned was 190–300 nm, and the absorption maxima for ATP, ATPγS, ADP, AMP and adenosine were 260 nm, inosine 247 nm and uric acid 290 nm. Standards which were prepared in the normal perfusion buffer, were injected after every five samples throughout the measurements to ensure the stability of the resolution and retention times. The detection limit was determined to be between 0.6 and 1.0 pmol of nucleotide.
Reagents
AnalaR grade NaCl, KCl, MgSO4, CaCl2 and glucose, ARISTAR grade KOH, NaOH and HCl, HiperSolv HPLC-grade acetonitrile were all purchased from BDH (Poole, Dorset, U.K.). HEPES, UTP and inosine were obtained from Sigma (Poole, Dorset, U.K.). Adenosine, ATP, ADP, AMP, ATPγS were supplied by Boehringer Mannheim (East Sussex, U.K.). Suramin hexasodium, 8-(p-Sulfophenyl)theophylline were purchased from Research Biochemicals International (Sigma-Aldrich Poole, Dorset, U.K.). HPLC-grade KH2PO4 was obtained from Fisher Scientific U.K. Ltd. All solutions including buffers were prepared using HPLC-grade water (conductivity less than 0.05 μS cm−1).
Statistical analyses
Five hearts were used in each treatment group. Results are presented as the mean±s.e. mean. The variables were compared by one-way ANOVA and values were considered to be significantly different if P<0.05.
Results
Effect of intravascular ATP on the components of the total coronary resistance
Intravascular ATP increased the coronary hydraulic power (3.47±0.29 to 5.75±0.31 mW, n=5, P<0.05, for an apparent ATP concentration of 0.25 μM) with an increase in the proportion of coronary flow that occurred during systole (for example, 28.03±1.66 before and 32.7±0.63% during perfusion with ATP, n=5, P<0.05). The same effect was observed for ADP and adenosine when added alone. Intravascular ATP, ADP and adenosine at the concentrations used in these experiments had no affect upon heart rate.
Effect of intravascular ATP on total coronary resistance–dose-response curve
The initial value of TCR for the preparation was 61.7±1.08×109 m−4 kg s−1 (n=53). ATP caused a dose-dependent reduction in TCR. A significant reduction in TCR was observed at apparent concentrations of ATP as low as 0.05 μM. This effect was saturated (TCR=28.8± 0.61×109 m−4 kg s−1, n=38) at apparent concentrations of ATP>0.23 μM (Figure 2a). The half maximal response, [ATP]50%, was calculated to be 0.08±0.009 μM (Figure 2b).
Figure 2.
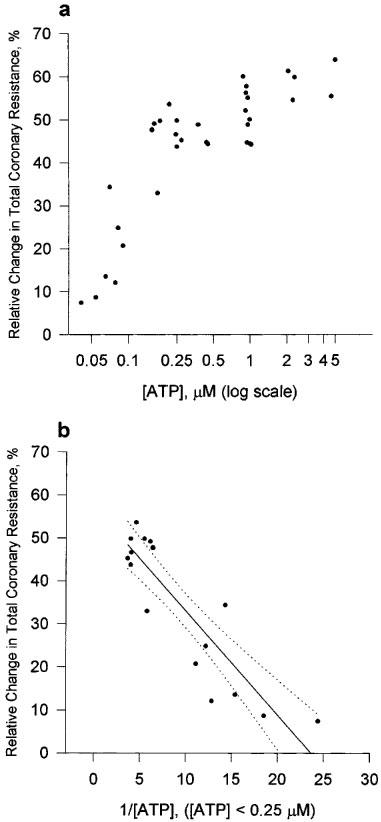
Dose-response curve for ATP on the isolated working rat heart preparation. (a) The relative change in TCR is plotted against [ATP]. TCR was calculated as (TCRpretreatment−TCRposttreatment)/TCRpretreatment×100%. TCRpretreatment refers to the 15th minute of the control period. TCRposttreatment refers to the 5th minute of the treatment period. (b) Transformed data used to determine the apparent ATP concentration that resulted in a 50% change in TCR. Linear regression analysis gave a straight line with r2=0.7893; b(0)=57.238; b(1)=−2.4175. Dashed lines represent 95% confidence interval.
Hydrolysis of ATP
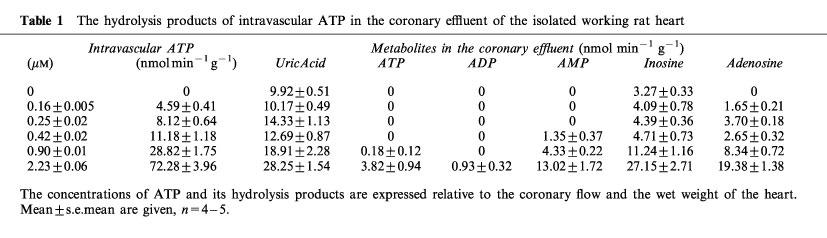
The hydrolysis of intravascular ATP was extremely efficient. For example, at an intravascular concentration of ATP of 2.23±0.06 μM less than 6% of this ATP was not hydrolyzed during its passage through the coronary vessels (Table 1). ADP was rarely if ever found in the coronary effluent. The main products of ATP hydrolysis measured in the coronary effluent were AMP, adenosine, inosine and uric acid. These results demonstrated the high activity in our preparations of the ecto-nucleotidases known to be located in the coronary endothelium (Fleetwood et al., 1989).
Table 1.
The hydrolysis products of intravascular ATP in the coronary effluent of the isolated working rat heart
Pharmacological assessment of reduction in total coronary resistance
The effect of intravascular adenosine
The influence of an equimolar concentration (0.25 μM) of adenosine and ATP on TCR was determined. Adenosine caused a reduction in TCR that was not significantly different to that of ATP (Figure 3). The P1 receptor antagonist, 8-SPT, abolished this effect of adenosine whilst only reducing the effect due to ATP by approximately 50% (Figures 3 and 4).
Figure 3.
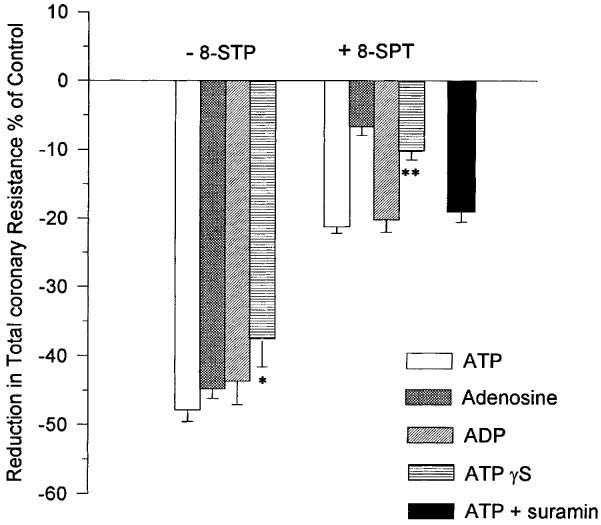
The influence of ATP, ADP, ATPγS, adenosine and ATP+suramin (nucleotide concentration=0.25 μM) in the presence and absence of the P1 receptor antagonist 8-SPT on TCR. Mean and s.e. mean are plotted, n=5. *Indicates a significant difference (P<0.05) with ATP; **Indicates a significant difference (P<0.05) with ATP and ADP but not with adenosine.
The effect of intravascular UTP on the total coronary resistance
The continuous perfusion of the heart with 0.25 μM UTP for 5 min did not result in a significant change in TCR (Figure 5).
Figure 5.
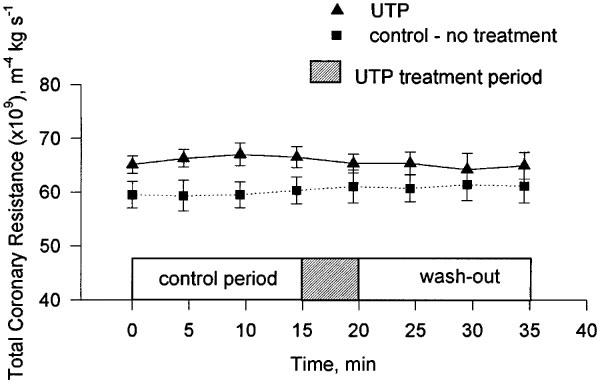
The change in TCR during the experimental period following treatment with 0.25 μM UTP. Mean and s.e.mean are plotted, n=5.
The effect of intravascular ADP on the total coronary resistance
The influence on TCR of an equimolar concentration (0.25 μM) of ADP and ATP was compared. ADP caused a reduction in TCR that was not significantly different to that of ATP (Figure 3). The P1 receptor antagonist, 8-SPT, reduced this effect of ADP by approximately 50%. A result which was not significantly different to that of ATP (Figures 3 and 4).
The effect of intravascular ATPγS on the total coronary resistance
Perfusion with ATPγS produced a significant reduction in the TCR. The reduction was less than that measured for equimolar concentrations of either adenosine, ATP or ADP. However, the difference was only significant (P<0.05) for ATP (Figure 3). 8-SPT reduced the response to ATPγS to the extent that the change in TCR was significantly lower than that for ATP (P<0.05) but not significantly different than that for adenosine. Analysis by HPLC of coronary effluents showed that only 25.6±2.96% of ATPγS remained intact. The concomitant increases in the amounts of inosine, adenosine and uric acid in the coronary effluent strongly suggested that ATPγS was hydrolyzed during passage through the coronary vessels.
The influence of suramin on ATP-induced changes in total coronary resistance
The non-selective P2 receptor antagonist suramin was found to have a significant influence on TCR. In the absence of added ATP, suramin at a concentration of 300 μM produced a reduction in TCR which was equivalent to that resulting from ATP concentrations greater than 0.25 μM. A 10 fold reduction in suramin concentration to 30 μM also influenced TCR causing a reduction from 61.5±4.18 to 42.1±2.30×109 m−4 kg s−1. A concentration of 5 μM suramin had no effect on TCR either when administered alone or in combination with 10 μM 8-SPT. When this concentration of suramin was used as an antagonist of the P2 receptor in the presence of 8-SPT it was found to have no influence on the reduction in TCR attributable to ATP (Figure 3).
Evidence for the desensitization of P2Y receptors
Continuous perfusion for 5 min of each of the agonists ATP, ATPγS and ADP did not result in any increase in TCR between the first and fifth minutes (Figure 4). The only changes in TCR, if any, were further reductions during this period. These results do not support a desensitization of P2Y receptors during the period of perfusion (Wilkinson et al., 1994).
Discussion
Intravascular ATP is a potent vasodilation
The response of the isolated working rat heart to intravascular ATP was a dose-dependent reduction in total coronary resistance (TCR). The response was saturated at apparent concentrations of ATP greater than 0.23 μM (Figure 2a). TCR is influenced by both the vasoactive state of the coronary vessels and their compression during the systolic phase of myocardial contraction (Spaan 1995). Coronary resistance could be decreased by a reduction in cardiac contractile force during systole. However, in both this study and a previous one (Korchazhkina et al., 1998), perfusion of coronary vessels with ATP resulted in an increase in coronary hydraulic power suggesting that ATP affects TCR by relaxing vessels and not by a reduction in myocardial compression.
The application of HPLC to the measurement of ATP and its hydrolysis products in the coronary effluent demonstrated that the breakdown of intravascular ATP was almost instantaneous and resulted in the appearance of a number of metabolites (Table 1). Prominent amongst these was the known vasodilator adenosine. The respective roles of ATP and adenosine in vasodilation are controversial, thus it is possible that both ATP and adenosine contributed to the ATP-induced vasodilation observed in our experiments.
Adenosine is at least as effective as ATP in inducing coronary vasodilation
It has been known for some time that both adenosine (Berne, 1963; 1980) and ATP (Paddle & Burnstock, 1974) are potent coronary vasodilators and that their actions are mediated via specific receptors (Burnstock, 1978). These receptors have recently been assigned the designations P1 (adenosine) and P2Y (ATP) (Fredholm et al., 1997; Burnstock, 1997). To discriminate the respective roles of these receptors in the vasodilatory response we have used the non-selective P1 receptor antagonist 8-SPT (Cave et al., 1993). To validate the use of this antagonist in our model we perfused hearts with adenosine (0.25 μM) in the presence and absence of 8-STP. Adenosine alone caused a reduction in TCR which was equivalent to that of an equimolar concentration of ATP. Adenosine appeared to be at least as effective as ATP in inducing coronary vasodilation in our model of the isolated working rat heart. The response to adenosine was all but abolished in the presence of 8-SPT (Figure 3).
ATP-induced coronary vasodilation is mediated to an equal extent via both P1 and P2Y receptors
When hearts were perfused with 0.25 μM ATP, a concentration of ATP that we have shown to result in the maximum reduction in TCR (see Figure 2a), and in the presence of 8-SPT the response to ATP was reduced by as much as 50% (Figure 3). This result showed that approximately half of the vasodilation induced by ATP was due to AMP and adenosine acting at P1 receptors. The remainder of the vasodilation was likely to be mediated through a number of possible P2Y receptors.
ATP acts via ADP at P2Y1 receptors to induce coronary vasodilation
One method of discriminating between P2Y receptors is to measure their response to the pyrimidine nucleotide UTP (Yang et al., 1996; Nicholas et al., 1996). We were unable to show any change in TCR upon perfusion of hearts with 0.25 μM UTP (Figure 5). This result implicated P2Y1 as the principal P2Y receptor mediating the non-P1 specific response to intravascular ATP since this is the only P2Y receptor that is not known to be activated by UTP (Nicholas et al., 1996). ADP is an agonist for the UTP-inactive P2Y1 receptor (Nicholas et al., 1996). In our experiments ADP was at least as effective a vasodilator as ATP (Figure 3) and the response to ADP in the presence of 8-SPT was identical to that of ATP again lending further weight to the involvement of the P2Y1 receptor. That this receptor was an important mediator of vasodilation was also the conclusion of both Houston et al. (1987) and Matsumoto et al. (1997) working with canine blood vessels. The nucleotide specificity of the P2Y1 receptor has recently been questioned (Leon et al., 1997) and it has been suggested that it is specific for ADP and antagonised by ATP. We have used ATPγS, a non-hydrolyzable analogue of ATP (Cusack et al., 1983), to investigate the possibility that the P2Y-specific vasoactivity observed in our experiments was mediated via ADP and not ATP. We found that the reduction in TCR caused by ATPγS was all but abolished by the P1 receptor antagonist 8-SPT (Figure 3). A small reduction in TCR was observed, however, this was not significantly different to the affect of an equimolar concentration of adenosine in the presence of 8-SPT. In contrast with previous research (Cusack et al., 1983; Pearson et al., 1985) we found that a high proportion of ATPγS was hydrolyzed during single passage through the coronary vessels. It is unlikely that the hydrolysis proceeded via ADP. It is more likely that AMP was the first hydrolysis product. As such the small reduction in TCR observed when ATPγS was perfused in the presence of 8-SPT might simply reflect differences in the potency of the P1 receptor antagonist 8-SPT for AMP and adenosine. The abolition of the vasodilatory action of ATPγS by 8-SPT strongly suggested that the P2Y1 receptor is specific for ADP. To provide categorical evidence for the involvement of P2Y receptors in the ATP-induced vasodilation, we have attempted to block the response by perfusing hearts with ATP in the presence of both the non-selective P2 antagonist suramin (Hoyle et al., 1990) and 8-SPT. Unfortunately, suramin on its own proved to be a strong vasodilator. Only when the suramin concentration was reduced to 5 μM could it be used without any influence upon the vasodilatory status of the heart. At this concentration it had no effect upon the P2Y-mediated vasodilatory response to ATP. This may confirm previous misgivings about the application of suramin as an antagonist of P2Y receptors in isolated heart preparations (Hoyle et al., 1990).
The expression of receptors for nucleotides and nucleosides may be mediated by the vasoactive state of vessels
The isolated working rat heart was extremely sensitive to the perfusion of ATP. However, whilst the vasodilatory response was saturated at an apparent ATP concentration of 0.23 μM, we have demonstrated that the response at this concentration was mediated, to an approximately equal extent, via both P1 and P2Y receptors. Thus 50% of the vasodilation induced by the perfusion of 0.23 μM ATP must be attributed to the actions of adenosine and AMP. This observation, which is contrary to some of the published literature (Godecke et al, 1996; Hopwood & Burnstock, 1987; Fleetwood & Gordon, 1987), that the perfusion of equimolar concentrations of adenosine or ATP resulted in identical vasodilatory responses suggested a significant role for adenosine in ATP-induced vasodilation. The component of the vasodilation that was mediated via P2Y receptors was shown using UTP and ADP to involve only the P2Y1 receptor. We were unable to show any effect of UTP on vasodilation. Receptors for UTP have been demonstrated in primary microvascular endothelial cells from the rat heart (Godecke et al., 1996) and UTP has been shown to induce vasodilation in isolated rat aorta (Garcia-Velasco, et al, 1995), isolated guinea-pig hearts (Vials & Burnstock, 1993) and isolated rat hearts (Godecke et al., 1996). That UTP did not induce coronary vasodilation in our isolated rat heart model may be accounted for by differences in methods of tissue or organ preparation. For example, to our knowledge UTP-induced vasodilation has only been observed in constricted or pre-constricted tissue. In the isolated working heart preparation used in our research it is not necessary to alter the vascular state of the heart in order to study either vasodilation or vasoconstriction (Korchazhkina et al., 1998). We believe that this model is a close approximation of the normal physiological state of the heart in vivo. As such our results compel us to suggest that ADP, and not ATP, is the nucleotide responsible for triggering vasodilation via P2Y1 receptors in the coronary endothelium. Differences in the preparations of tissues and organs used to study vasodilation may also help to explain the wide range of potencies observed for other vasoactive agents. For example, Godecke et al. (1996) working with Langendorff-perfused rat hearts reported half maximal responses for ATP and adenosine of 0.6 and 4.0 μM respectively. These values are an order of magnitude higher than those observed in the present study. How the physiological state of a tissue might influence its pharmacological receptivity remains to be understood. Certainly, there are precedents for the physiological status of a membrane altering the expression of an ATP receptor (Sromek & Harden, 1998). Whether the vasoactive state of a vessel is a determinant of the expression or availability for binding of different receptors will have to be established.
Acknowledgments
This research was supported by The Wellcome Trust, The Royal Society, the W.E. Dunn Trust and the North Staffordshire Heart Committee.
Abbreviations
- 8-SPT
8-(p-Sulfophenyl)theophylline
- ADP
Adenosine-5′-diphosphate
- AMP
Adenosine-5′-monophosphate
- ATP
Adenosine-5′-triphosphate
- ATPγS
Adenosine-5′-O-thiotriphosphate
- TCR
Total coronary resistance
- UTP
Uridine-5′-triphosphate
References
- BERNE R.M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am. J. Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- BERNE R.M. The role of adenosine in the regulation of coronary blood flow. Circ. Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- BUNGER R., HADDY F.J., GERLACH E. Coronary responces to dilating substances and competitive inhibition by theophylline in the isolated perfused guinea pig heart. Pflugers. Arch. 1975;358:213–224. doi: 10.1007/BF00587218. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.In cell Membrane Receptors for Drugs and Hormones: A Multidiciplinary Approach 1978Raven Press. New York, NY; 107–118.L. Bolis and R.W. Straub, Eds [Google Scholar]
- BURNSTOCK G. Review. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- CAVE A.C., COLLIS C.S., DOWNEY J.M., HEARSE D.J. Improved functional recovery by ischemic preconditioning is not mediated by adenosine in the globally ischemic isolated rat heart. Cardiovasc. Res. 1993;27:663–668. doi: 10.1093/cvr/27.4.663. [DOI] [PubMed] [Google Scholar]
- CUSACK N.J., PEARSON J.D., GORDON J.L. Stereoselectivity of ectonucleotidases on vascular endothelial cells. Biochem. J. 1983;214:975–981. doi: 10.1042/bj2140975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEETWOOD G., COADE S.B., GORDON J.L., PEARSON J.D. Kinetics of adenine nucleotide catabolism in coronary circulation of rats. Am. J. Physiol. 1989;256:H1565–H1572. doi: 10.1152/ajpheart.1989.256.6.H1565. [DOI] [PubMed] [Google Scholar]
- FLEETWOOD G., GORDON J.L. Purinoceptors in the rat heart. Br. J. Pharmacol. 1987;90:219–227. doi: 10.1111/j.1476-5381.1987.tb16843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORRESTER T., WILLIAMS C.A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J. Physiol. 1977;268:371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSON K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature for P1 and P2 receptors. TiPS. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-VELASCO G., SANCHEZ M., HIDALGO A., GARCIA DE BOTO M.J. Pharmacological dissociation of UTP- and ATP-elicited contractions and relaxations in isolated rat aorta. Eur. J. Pharmacol. 1995;294:521–529. doi: 10.1016/0014-2999(95)00576-5. [DOI] [PubMed] [Google Scholar]
- GODECKE S., DECKING U.K.M., GODECKE A., SCHRADER J. Cloning of the rat P2U receptor and its potential role in coronary vasodilation. Am. J. Physiol. 1996;270:C570–C577. doi: 10.1152/ajpcell.1996.270.2.C570. [DOI] [PubMed] [Google Scholar]
- GORDON J.L. Extracellular ATP: effects, sources and fate. Biochem. J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD A.M., BURNSTOCK G. ATP mediates coronary vasoconstriction via P2X-purinoceptors and coronary vasodilation via P2Y-purinoceptors in the isolated perfused rat heart. (1987) Eur. J. Pharmacol. 1987;136:49–54. doi: 10.1016/0014-2999(87)90777-1. [DOI] [PubMed] [Google Scholar]
- HOUSTON D.A., BURNSTOCK G., VANHOUTTE P.M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol. Exp. Ther. 1987;241:501–506. [PubMed] [Google Scholar]
- HOYLE C.H.V., KNIGHT G.E., BURNSTOCK G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br. J. Pharmacol. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORCHAZHKINA O., WRIGHT G., EXLEY C. Action of Al-ATP on the isolated working rat heart. J. Inorg. Biochem. 1998;69:153–158. doi: 10.1016/s0162-0134(97)10012-5. [DOI] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.-P., GACHET C. Yhe P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Letters. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO T., NAKANE T., CHIBA S. Pharmacological analysis of responses to ATP in the isolated and perfused canine coronary artery. Eur. J. Pharmacol. 1997;334:173–180. doi: 10.1016/s0014-2999(97)01167-9. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., LAZAROWSKI E.R., WATT W.C., LI Q., BOYER J., HARDEN T.K. Pharmacological and second messenger signalling selectivities of cloned P2Y receptors. J. Autonomic Pharmacol. 1996;16:319–323. doi: 10.1111/j.1474-8673.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- PADDLE B.M., BURNSTOCK G. Release of ATP from perfused heart during coronary vasodilation. Blood vessels. 1974;11:110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- PEARSON J.D., COADE S.B., CUSACK N.J. Characterisation of ectonucleotidases on vascular smooth-muscle cells. Biochem. J. 1985;230:503–507. doi: 10.1042/bj2300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNAITH C.D., WRIGHT G., LOFKIN M. The effects of aspartate and 20oxoglutarate upon glycolytic energy metabolites and mechanical recovery following global ischaemia in isolated rat hearts. J. Mol. Cell. Cardiol. 1992;24:305–315. doi: 10.1016/0022-2828(92)93167-i. [DOI] [PubMed] [Google Scholar]
- SPAAN J.A.E. Mechanical determinats of myocardial perfusion. Basic Res. Cardiol. 1995;90:89–102. doi: 10.1007/BF00789439. [DOI] [PubMed] [Google Scholar]
- SROMEK S.M., HARDEN T.K. Agonist-induced internalization of the P2Y2 receptor. Mol. Pharmacol. 1998;54:485–494. doi: 10.1124/mol.54.3.485. [DOI] [PubMed] [Google Scholar]
- STOCCHI V., CUCCHIARINI L., MAGNANI M., CHIARANTINI L., PALMA P., CRESCENTINI G. Simultaneous extraction and reverse-phase high performance liquid chromatographic determination of adenine and pyridine nucleotides in human red blood cells. Analytical Biochemistry. 1985;146:118–124. doi: 10.1016/0003-2697(85)90405-1. [DOI] [PubMed] [Google Scholar]
- VIAL C., OWEN P., OPIE L.H., POSEL D. Significance of release of adenosine triphosphate and adenosine induced by hypoxia or adrenaline in perfused rat heart. J. Mol. Cell. Cardiol. 1987;19:187–197. doi: 10.1016/s0022-2828(87)80561-8. [DOI] [PubMed] [Google Scholar]
- VIALS A.J., BURNSTOCK G. Effect of pyrimidines on the guinea-pig coronary vasculature. Br. J. Pharmacol. 1993;110:1091–1097. doi: 10.1111/j.1476-5381.1993.tb13926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTERHOF N., ELZINGA G., SIPKEMA P. An artificial arterial system for pumping hearts. J. Appl. Physiol. 1971;31:776–781. doi: 10.1152/jappl.1971.31.5.776. [DOI] [PubMed] [Google Scholar]
- WILKINSON G.F., PURKISS J.R., BOARDER M.R. Differential heterologous and homologous desensitization of two receptors for ATP (P2Y purinoceptors and nucleotide receptors) coexisting on endothelial cells. Mol. Phamacol. 1994;45:731–736. [PubMed] [Google Scholar]
- WRIGHT G. Effects of vasoactive drugs upon haemodynamic power and input impedance in normal rats. Cardiovasc. Res. 1991;25:923–929. doi: 10.1093/cvr/25.11.923. [DOI] [PubMed] [Google Scholar]
- WRIGHT G., KINGSTON M.A., ROSS I.S. The role of metabolic acidosis upon cardiac mechanical performance during severe acute hypoxia and reoxygenation is small and transient. Cardiovasc. Res. 1995;29:611–615. [PubMed] [Google Scholar]
- WRIGHT G., KORCHAZHKINA O.V., ZHANG S. Evaluation of a lumped parameter model for isolated working rat hearts. Med. Biol. Eng. Comput. 1999;37:1–6. doi: 10.1007/BF02513286. [DOI] [PubMed] [Google Scholar]
- YANG S., BUXTON I.L.O., PROBERT C.B., TALBOT J.N., BRADLEY M.E. Evidence for a discrete UTP receptor in cardiac endothelial cells. Br. J. Pharmacol. 1996;117:1572–1578. doi: 10.1111/j.1476-5381.1996.tb15323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


