Abstract
Effects of two tachykinin NK1 receptor selective agonists (septide and [Sar9, Met(O2)11]SP) were compared on the increases in mean arterial pressure (MAP), heart rate (HR) and motor behaviour following intracerebroventricular (i.c.v.) administration in unanaesthetized rat, and on the vascular permeability increases to intradermal (i.d.) injection in the anaesthetized rat. Moreover, five tachykinin NK1 receptor selective antagonists (LY303870, LY306740, LY303241, SR140333 and RP67580) were tested against the two agonists to compare their pharmacological profile.
[Sar9, Met(O2)11]SP and septide (10–100 pmol per rat, i.c.v.) were equipotent in increasing MAP and HR, yet they had dissimilar time-course. Both agonists increased dose-dependently face washing and sniffing while [Sar9, Met(O2)11]SP was the sole to produce grooming. septide was more potent than [Sar9, Met(O2)11]SP (6.5–650 pmol) in increasing vascular permeability.
For most centrally mediated responses, LY303870 and RP67580 were significantly more potent in inhibiting septide than [Sar9, Met(O2)11]SP. In some parameters, greater blockade was achieved when antagonists (particularly LY306740) were given 1 h instead of 10 min prior to i.c.v. septide.
All antagonists except LY303241 blocked dose-dependently the increases in vascular permeability to equipotent doses of [Sar9, Met(O2)11]SP and septide. LY303870 and LY306740 were more potent against septide.
The antagonism afforded by LY303870, LY306740 and LY303241 was stereoselective and only SR140333 was found to cause central and peripheral non specific effects.
The data confirm a distinct pharmacological profile for septide in vivo. RP67580 and LY306740 are currently the most valuable tachykinin NK1 receptor antagonists for in vivo studies in rat.
Keywords: Substance P, septide, tachykinin NK1 receptor, tachykinin antagonists, blood pressure, behaviour, vascular permeability
Introduction
The tachykinin NK1 receptor selective agonist septide ([pGlu6, Pro9]SP(6–11)) was reported to display a pharmacological profile distinct from that of classical tachykinin NK1 receptor agonists in various bioassays. In those functional studies, the affinity of septide for the NK1 receptor was either similar or greater than that of the classical agonists, and the tachykinin NK1 receptor antagonists were more potent against the responses induced by septide. Surprisingly, septide showed no appreciable affinity for the tachykinin NK1 receptor in competition binding experiments against radiolabelled substance P (SP) (Chassaing et al., 1992; Petitet et al., 1992; Maggi et al., 1993; Palma et al., 1994; Maggi, 1995; Torrens et al., 1995). That was claimed to be due either to the activation of a tachykinin NK1 receptor subtype (Petitet et al., 1992; Glowinski, 1995), to the differential activation of second messenger pathways (Sagan et al., 1996), to the existence of two distinct binding sites on the same receptor or to the presence of two putative distinct conformers of the tachykinin NK1 receptor (Maggi & Schwartz, 1997). However, the action of septide on the release of dopamine from striatal slices of rat brain raised two discrepancies with previous studies (Gauchy et al., 1996). First, the atypical tachykinin NK1 receptor agonist has a stimulatory effect on the dopamine release evoked by N-methyl-D-aspartate (NMDA) while the classical tachykinin NK1 receptor agonist [Pro9]SP has an inhibitory effect. Secondly, the tachykinin NK1 receptor selective antagonist RP 67580 is less active against septide than [Pro9]SP on in vitro dopamine release. Thus, the existence of a specific functional site for septide remains unsettled and requires further pharmacological characterization, especially in vivo.
Over the last decade, tremendous effort has been deployed on the development of potent and selective tachykinin NK1 receptor antagonists (Otsuka & Yoshioka, 1993; Vassout et al., 1994; Regoli et al., 1994; Maggi, 1995; Beattie et al., 1995). Following the discovery of the first non-peptide tachykinin NK1 receptor selective antagonist CP96345 (Snider et al., 1991) which exerts non-specific effects on verapamil-sensitive calcium channels and neurotransmission (Schmidt et al., 1992; Guard et al., 1993; Wang et al., 1994), several other compounds were disclosed: RP67580 (Garret et al., 1991), SR140333 (Emonds-Alt et al., 1993), CP99994 (MacLean et al., 1993) and L733060 (Harrison et al., 1994). These non-peptide antagonists are more potent, selective and stable than the previous peptide tachykinin NK1 receptor antagonists (Maggi et al., 1993; Regoli et al., 1994; Maggi, 1995).
Recently, a non-peptide antagonist, LY303870, was reported to bind with high affinity and selectivity to peripheral (Ki=0.15 nM) and central (Ki=0.10 nM) human tachykinin NK1 receptors (Table 1). It was found to be active in various in vitro and in vivo bioassays such as the blockade of interleukin-6 secretion from U-373 MG human astrocytoma cells evoked by SP, SP-induced rabbit vena cava contractions and [Sar9,Met (O2)11]SP-induced guinea-pig bronchoconstriction and pulmonary microvascular leakage (Gitter et al., 1995; Hipskind et al., 1995). It also binds potently to the rat and mouse tachykinin NK1 receptors, although with lower affinity. In vivo assays demonstrate that LY303870 potently inhibits nociceptive behavioural responses in mouse and rat (Iyengar et al., 1997). Binding affinities of two analogues of this series, namely LY306740 and LY303241 (Table 1), were lower than that of LY303870 at human tachykinin NK1 receptors but greater at rodent tachykinin NK1 receptors (Hipskind et al., 1996). We reported that the three compounds are potent, specific and selective antagonists when tested against the hypotensive and salivary responses to intravenous SP in anaesthetized rat (Cellier et al., 1996).
Table 1.
Structure of the NK1 receptor selective non-peptide antagonists tested in this study

The present study was undertaken to compare: (a) the cardiovascular and behavioural responses induced by intracerebroventricularly (i.c.v.) administered [Sar9,Met(O2)11]SP and septide in unanaesthetized rat, and (b) the increases in plasma protein extravasation to both agonists injected intradermally (i.d.) to anaesthetized rat. Five tachykinin NK1 receptor antagonists (LY303870, LY306740, LY303241, SR140333 and RP67580) were used to further characterize the central and peripheral effects of the two agonists. Part of this work has been presented elsewhere (Cellier et al., 1997).
Methods
Animal source and care
Male Wistar rats (275–300 g) were purchased 3–5 days prior to experiments from Charles River, St-Constant, Québec, Canada and housed four to five per cage under a 12 h light-dark cycle in a room with controlled temperature (20°C), humidity (53%) with food (Charles River Rodent) and tap water available ad libitum. The care of animals and research protocols conformed to the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and approved by the Animal Care Committee of the Université de Montréal.
I.c.v. effects on cardiovascular system and motor behaviour
Animal preparation
Male Wistar rats (n=337) were anaesthetized with an intraperitoneal (i.p.) injection of 65 mg kg−1 sodium pentobarbitone (Somnotol; M.T.C. Pharmaceuticals, Cambridge, Ontario, Canada) and a polyethylene catheter (PE-20; Intramedics, Clay Adams, NJ, U.S.A.) was inserted with a guide cannula into the right lateral cerebral ventricle (i.c.v.) (coordinates: 0.6 mm caudal to the bregma, 1.3 mm lateral to the midline, 5 mm vertical from the skull surface) with the use of a stereotaxic apparatus (David Kopf Instrumentation, Tujunga, CA, U.S.A.), as described earlier (Picard et al., 1994). Thereafter, the rats were allowed to recover in individual plastic cages (40 cm×23 cm×20 cm) and housed in the same controlled conditions. Placement of the cannula was verified post mortem with Evans Blue dye.
Two days later, rats were re-anaesthetized with sodium pentobarbitone (65 mg kg−1) and an intravascular siliconized (Sigmacote, Sigma, St-Louis, MO, U.S.A.) PE-50 catheter, filled with physiological saline containing 50 i.u. ml−1 heparin sodium salt (Sigma, St-Louis, MO, U.S.A.), was inserted into the abdominal aorta through the femoral artery for direct blood pressure recording and exteriorized at the back of the neck. Recovery from anaesthesia was monitored closely under a warming lamp to maintain the body temperature of animals. Rats with apparent abnormal behaviour were immediately humanely killed with an overdose of pentobarbital. Thereafter, rats were housed individually in polyethylene cage with a top grid and returned to their resident room. Experimental protocols were initiated 24 h later, in conscious and unrestrained rats.
Measurement of cardiovascular and behavioural parameters
Blood pressure and heart rate were measured respectively with a Statham pressure transducer (P23ID) and a cardiac tachometer (model 7P4) (triggered by the arterial blood pressure pulse) coupled to a Grass polygraph (model 79; Grass Instruments Co., Quincy, MA, U.S.A.). The behavioural activity was measured according to a previous study (Picard et al., 1994). Briefly, during every consecutive period of 15 s, a score of 1 or 0 was given systematically depending on whether the animal showed the specific type of behaviour or not, whatever its frequency, intensity or duration during that period. Summation of scores for the first 30 min period following the i.c.v. injection gave the behavioural scores for face washing, grooming and sniffing in each experiment. The maximal theoretical score was 120 (15 s intervals×30 min). Wet dog shakes behaviour was measured according to the number of episodes during the first 30 min period, whatever the intensity. Both cardiovascular and behavioural responses were measured 1 h after the rats were transported to the testing room. They remained in their resident cage but the top grid was removed and had no more access to the food and water.
Experimental protocols
Dose-response curve to i.c.v. [Sar9, Met(O2)11]SP and septide
Rats initially received an i.c.v. injection of artificial cerebrospinal fluid (aCSF; 1 μl) followed 60 min later by a single dose of either [Sar9, Met(O2)11]SP (10 pmol (n=9), 25 pmol (n=9), 65 pmol (n=8) or 100 pmol (n=8)) or septide (10 pmol (n=12), 25 pmol (n=9), 65 pmol (n=6) or 100 pmol (n=6)) to construct a complete dose-response curve. Each rat was selected randomly and injected with only one of the two agonists for the remainder of the protocol. Increasing doses of [Sar9, Met(O2)11]SP or septide were given at 24 h intervals on day 1 (10 pmol), day 2 (25 pmol), day 3 (65 pmol) and day 4 (100 pmol). Control rats (n=18) received only the vehicle (aCSF) each day of experiment. Peptides were administered in a volume of 1 μl of vehicle followed by 5 μl flush volume of aCSF which corresponds to the void volume of the catheter. Each dose was calculated per rat in 1 μl solution.
One dose of 25 pmol [Sar9, Met(O2)11]SP was tested on mean arterial blood pressure (MAP), heart rate (HR), face washing, grooming and sniffing behaviours of seven naive rats on four consecutive days to assess its reproducible i.c.v. effects. Likewise, the i.c.v. effects of 25 pmol septide on MAP, HR, face washing and sniffing were assessed on four consecutive days in a second group of seven naive rats.
I.c.v. effects of tachykinin NK1 receptor antagonists
Rats that had 24 h previously received 25 pmol of either [Sar9, Met(O2)11]SP or septide (the lowest dose which produced significant behavioural responses to both agonists) were given i.c.v. either LY303870 (against [Sar9, Met(O2)11]SP: n=5; against septide: n=5–6), LY306740 (against [Sar9, Met (O2)11]SP: n=9–6); against septide: n=7–5, LY303241 (against [Sar9, Met(O2)11]SP: n=6–4; against septide: n=4), SR140333 (against [Sar9, Met(O2)11]SP: n=7; against septide: n=5) or RP67580 (against [Sar9, Met(O2)11]SP: n=7–5; against septide: n=8) and then 10 min later were given 25 pmol i.c.v. of the same agonist. The doses of antagonists were chosen on the basis of a previous study which reported a potent inhibition of the i.c.v. effects of 25 pmol SP with 6.5 nmol of RP67580 (Picard et al., 1994). All antagonists were injected at increasing doses of 0.065, 0.65, 6.5 and 65 nmol with the exception of SR140333 which was not injected at 65 nmol because 6.5 nmol SR140333 caused deleterious motor effects and increases of mean arterial pressure and heart rate. Each rat received only one agonist and one antagonist in a random fashion.
Stereoselectivity of LY303870, LY306740 and LY303241
To verify whether the antagonists are enantiomer selective, the opposite (S) enantiomers of LY303870 (LY306155) (against [Sar9, Met(O2)11]SP: n=8; against septide: n=4), LY306740 (LY307679) (against [Sar9, Met(O2)11]SP: n=15; against septide: n=8) and LY303241 (LY303374) (against septide: n=4) were tested i.c.v. in five separate groups of rats 10 min prior to [Sar9, Met(O2)11]SP or septide, according to the same protocol as described above. Only one agonist and one enantiomer were injected to each rat.
Time-dependent effects of the antagonists
Experiments performed on isolated organs showed that the antagonist activity of SR140333 increases in parallel with its time of preincubation before the addition of the agonist (Emonds-Alt et al., 1993). Therefore, we examined the time-dependent action of the five antagonists against the i.c.v. effects of septide. Thus, 24 h after a first injection of 25 pmol septide, rats were injected i.c.v. 1 h before the injection of 25 pmol septide with either LY303870 (n=9), LY306740 (n=6), LY303241 (n=11), SR140333 (n=7) or RP67580 (n=10) at the previously calculated ID50 dose against septide-induced MAP response. Septide was also reinjected alone 24 h afterward to evaluate the reversibility of any inhibition. Each rat received only one antagonist.
I.d. effects on vascular permeability
Animal preparation and experimental procedures
These experiments were conducted in sodium pentobarbitone (65 mg kg−1, i.p) anaesthetized male Wistar rats (n=262) as vascular permeability was markedly reduced in urethane anaesthetized rats (Couture & Kérouac, 1987). A polyethylene tubing (PE-50; Intramedics, Clay Adams, NJ, U.S.A.) filled with physiological saline (0.9 % NaCl) was inserted into the left jugular vein and used for drug injections.
Vascular permeability was measured by the leakage of plasma protein-bound Evans Blue dye into the rat dorsal skin as described previously (Couture & Kérouac, 1987). Briefly, Evans Blue dye (Sigma; St. Louis, MO, U.S.A.) was injected i.v. (35 mg kg−1) in a volume of 0.1 ml saline (0.9% NaCl) per 100 g of body weight and then flushed with 0.2 ml of saline. Five minutes later, agonists and/or antagonists were injected i.d. in 100 μl volume in the shaved rat dorsal skin. Rats were killed by exsanguination 30 min after i.d. injections. The dorsal skin was removed and the blue injection sites were punched out (15 mm diameter). Tissues were weighted and placed in 4 ml of formamide 99% (American Chemicals; Montréal, Canada). After incubation at 50°C for 24 h, the Evans Blue content of each sample was measured with a spectrophotometer (Shimadzu, UV 160U) at 620 nm. The effects of agonists on vascular permeability were assessed by measuring the amount of Evans Blue dye in the treated skin minus that found in the control skin injected with Krebs vehicle. Results were expressed in ng Evans Blue per mg of fresh tissue.
Experimental protocols
Dose-response curve to i.d. [Sar9, Met(O2)11]SP and septide
In a first series of experiments, dose-response curves to [Sar9, Met(O2)11]SP and septide were measured on vascular permeability. Each rat received only one agonist either at the single dose of 650 pmol or at three doses (0.65, 6.5 and 65 pmol). Each dose was injected i.d. in duplicate in eight rats. Krebs was also injected in two additional sites as control.
I.d. effects of tachykinin NK1 receptor antagonists
The inhibitory effect of several doses of tachykinin NK1 receptor antagonists on the increases of vascular permeability induced by equipotent doses of [Sar9, Met(O2)11]SP (650 pmol) and septide (65 pmol) was compared. Each animal received only one agonist and one antagonist administered separately and together in duplicate (total of six sites). Krebs was injected in two additional sites as control. The doses (pmol) of antagonists tested against [Sar9, Met(O2)11]SP were: LY303870 (650, n=5; 6500, n=6; 65,000, n=4), LY306740 (650, n=4; 6500, n=5), LY303241 (6500, n=4), SR140333 (65, n=4; 650, n=7; 6500, n=4) and RP67580 (65, n=4; 650, n=5; 6500, n=4). In order to verify the stereoselectivity of the action of Eli Lilly antagonists, 6500 pmol of the (S) enantiomers of LY303870 (LY306155; n=8) and LY306740 (LY307679; n=4) were also tested against [Sar9, Met (O2)11]SP. The antagonists and (S) enantiomers were also tested against septide at the following doses (pmol): LY303870 (65, n=8; 650, n=4; 6500, n=7), LY306740 (65, 650 and 6500, n=4 for each dose), LY303241 (6500, n=4), SR140333 (65, n=9; 650, n=5; 6500, n=4) and RP67580 (65, n=7; 650, n=6; 6500, n=6), LY306155 (6500, n=4) and LY307679 (6500, n=8).
In another series of experiments, the specificity of active antagonists was further examined by determining their ability to antagonize the vascular permeability effect induced by bradykinin (10 nmol) and histamine (10 nmol). The agonists and each antagonist (6500 pmol) were injected alone or together in eight additional groups of rats (for bradykinin : LY303870 (n=5), LY306740 (n=6), SR140333 (n=8) RP67580 (n=4) and for histamine: LY303870, LY306740 and SR140333 (n=6 for each compound) and RP67580 (n=5)) according to the same protocol as described for the tachykinin NK1 selective agonists.
Drugs and solutions
The composition of aCSF was (in mM): NaCl 128.6, KCl 2.6, MgCl2, 2.0 and CaCl2, 1.4; pH adjusted to 7.2 while that of the Krebs' solution was (in mM): NaCl 118.1, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, dextrose 5.5 and NaHCO3 25.0; pH adjusted to 7.2. The latter solution was filtered just before use. Septide (pGlu-Phe-Phe-Pro-Leu-Met-NH2) (MW: 763.4) and bradykinin (MW: 1060.3) were purchased from Peninsula Laboratories (Belmont, CA, U.S.A.) whereas [Sar9, Met(O2)11]SP (MW: 1392.9) was from Bachem Bioscience Inc. (King of Prussia, PA, U.S.A.). Histamine (MW: 184.1) and heparin sodium salt (porcine, grade 1-A) were purchased from Sigma Company (St-Louis, Missouri, U.S.A.). The non-peptide antagonists LY303870 (MW: 559.8), LY306740 (MW: 559.8) and LY303241 (MW: 553.7) and the opposite (S) enantiomers LY306155 (MW: 559.8), LY307679 (MW: 559.8) and LY303374 (MW: 553.7) were synthesized by Eli Lilly (Indianapolis, IN, U.S.A.) (Hipskind et al., 1995; 1996). SR140333 (MW: 656.1) was obtained from Dr J.C. Brelière of Sanofi Research (Montpellier, France) while RP67580 (MW: 438.6) was provided by Dr C. Garret (Rhone Poulenc, Paris, France). Structure of all antagonists are shown in Table 1. Septide and antagonists were solubilized in dimethyl sulphoxide (DMSO; Fisher Scientific, Montréal, Québec, Canada) and then the solution was completed with pure distilled water in the case of LY303870, LY303740, LY303241 and their (S) enantiomers or with an aqueous solution of 10% v w−1 of hydroxypropyl-γ-cyclodextrine (MW 1655.09; Research Biochemicals International, Natick, MA, U.S.A.) in the case of septide, RP67580 and SR140333. [Sar9, Met(O2)11]SP was dissolved directly in aCSF. The stock solutions (10 mg ml−1) were stored in aliquots of 100 μl at −20°C until use. Agonists and antagonists were injected in aCSF (i.c.v.) or Krebs (i.d.) containing less than 5% DMSO except for the i.c.v. doses of 6.5 nmol (50% DMSO) of Eli Lilly antagonists. In all experiments, vehicle was tested as control and no significant changes were seen on any parameters when compared to aCSF (i.c.v.) or Krebs (i.d.) values.
Statistical analysis of data
Results are expressed as means±s.e.mean. The potency of the antagonists was evaluated by the ID50 values which represent the dose of the compound that blocks by 50% the effect of the agonist on either cardiovascular system and motor behaviour (i.c.v.) or vascular permeability (i.d.). Pairwise comparisons were made with a Student's t-test for paired samples. For parametric values, statistical differences were evaluated with a one-way or two-way (time- or dose-dependent effects) analysis of variance (ANOVA) followed by a Dunnett's test (multiple comparisons), by a Student's t-test (simple comparison) or by a Tukey's test (multiple comparisons for vascular permeability). Statistical differences on non-parametric episodes of behaviours were evaluated with a Kruskal-Wallis test and a post hoc Wilcoxon Mann-Whitney. The uniformity of the variances between groups was verified a priori with the Bartlett test. Only probability values (P) less than 0.05 were considered to be statistically significant.
Results
I.c.v. effects on cardiovascular system and motor behaviour
Cardiovascular effects of [Sar9, Met(O2)11]SP and septide
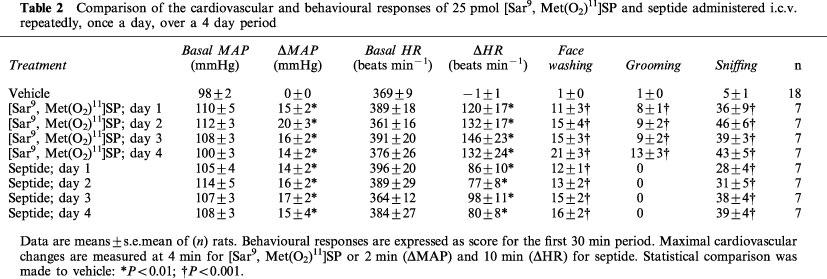
The cardiovascular effects of i.c.v. injected [Sar9, Met (O2)11]SP and septide are shown in Figure 1. [Sar9, Met(O2)11]SP (10–100 pmol; n=8–9) evoked dose- and time-dependent increases in MAP (ranging from 6±3 to 25±3 mmHg) and in HR (from 63±24 to 133±18 beats min−1). The MAP and HR responses to [Sar9, Met(O2)11]SP peaked at 4 min except at the highest dose of 100 pmol (5–10 min). Septide (10–100 pmol; n=12–6) also evoked dose- and time-dependent increases in MAP (from 10±1 to 21±5 mmHg) while its effect on HR reached a maximum at 25 pmol (from 44±10 to 91±12 beats min−1). The maximal MAP responses to 10 and 25 pmol septide were reached at 2 min compared with 10 and 25 min for the highest doses of 65 and 100 pmol, respectively. The HR response to septide peaked at 2 min (10 pmol), 8–10 min (25 and 65 pmol) and 20 min (100 pmol). The maximal increases in MAP and HR elicited by both agonists were not significantly different at any doses. As shown in Table 2, the MAP and HR to 25 pmol of either [Sar9, Met(O2)11]SP or septide were not significantly different when injected i.c.v. repeatedly, once a day, over a 4 day period.
Figure 1.

Time-course effects on changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) of [Sar9, Met(O2)11)]SP injected i.c.v. at 10 (n=9), 25 (n=9), 65 (n=8) and 100 (n=8) pmol per animal and septide injected i.c.v. at 10 (n=12), 25 (n=9), 65 (n=6) and 100 (n=6) pmol per animal for a period of 30 min post-injection in conscious rats. Basal values were: MAP: 107±3 mmHg: HR 395±10 beats min−1 for [Sar9, Met(O2)11]SP and MAP: 107±3 mmHg; HR: 400±8 beats min−1 for septide. Statistical significance of differences between vehicle (n=18) and [Sar9, Met(O2)11]SP or septide values were calculated with a two-way ANOVA followed by a Dunnett's test and are indicated by: *P<0.05; **P<0.01.
Table 2.
Comparison of the cardiovascular and behavioural responses of 25 pmol [Sar9, Met(O2)11]SP and septide administered i.c.v. repeatedly, once a day, over a 4 day period
Behavioural effects of [Sar9, Met(O2)11]SP and septide
The i.c.v. administration of [Sar9, Met(O2)11]SP (25–100 pmol) increased dose-dependently behavioural activity during the first 30 min post-injection (sniffing>grooming=face washing>wet dog shake) (Figure 2). The dose of 10 pmol failed to affect behavioural activity when compared to vehicle values. In contrast, septide increased sniffing and face washing at the lowest dose of 10 pmol while higher doses produced effects somewhat similar in intensity to those evoked by [Sar9, Met(O2)11]SP. However, septide failed to induce increases in grooming at any doses and wet dog shakes were only slightly increased by 25, 65 and 100 pmol septide (Figure 2). As shown in Table 2, the increases in face washing, grooming and sniffing induced by 25 pmol [Sar9, Met(O2)11]SP were reproducible when the agonist was injected i.c.v. repeatedly, once a day, over a 4 day period. Similarly, face washing and sniffing responses to 25 pmol septide were stable over the same period.
Figure 2.
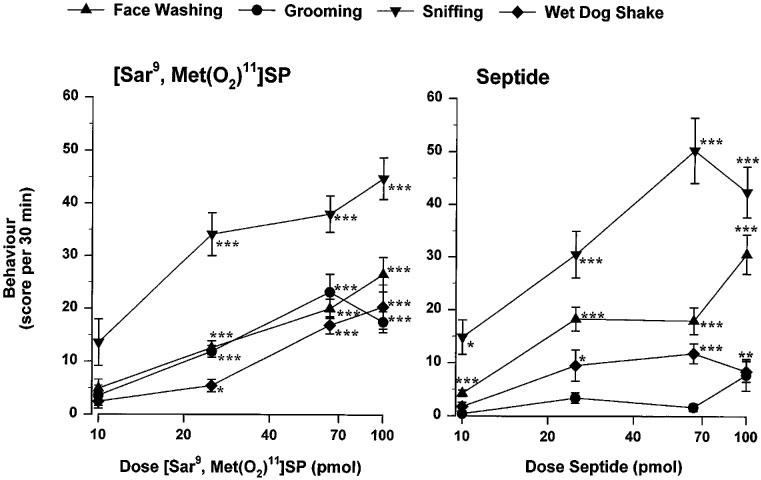
Dose-response curves elicited by i.c.v. injection of [Sar9, Met(O2)11]SP and septide(10–100 pmol per animal; same rats than Figure 1) on face washing, grooming, sniffing and wet dog shakes during the first 30 min post-injection period in conscious rats. The vehicle score for each behaviour was as follows: 1±0 (face washing); 1±0 (grooming); 5±1 (sniffing); 2±1 (wet dog shake). Statistical significance of differences between NK1 agonists and vehicle (n=18) values were calculated with a Kruskal-Wallis test and a post hoc Wilcoxon Mann-Whitney test and are indicated by: *P<0.05; **P<0.01; ***P<0.001.
Action of the tachykinin NK1 receptor selective antagonists against [Sar9, Met(O2)11]SP and septide
The effects of the five tachykinin NK1 receptor antagonists against the MAP and HR responses to an equipotent dose (25 pmol) of [Sar9, Met(O2)11]SP and septide injected i.c.v. are shown in Table 3. The MAP effect of [Sar9, Met(O2)11]SP was antagonized by SR140333, RP67580 and LY303870 while LY306740 and LY303241 were inactive. The HR response to [Sar9, Met(O2)11]SP was inhibited only by RP67580 and LY303870. Conversely, the MAP response to septide was antagonized by SR140333, RP67580, LY303870, LY303241 and LY306740 whereas its HR effect was only inhibited by RP67580, LY303870 and LY303241. LY303870 and RP67580 were significantly more potent in inhibiting septide than [Sar9, Met(O2)11]SP effect on both parameters. Basal MAP and HR values were not significantly different among the different experimental groups on day 1 (Table 3) and over the 4 days of experiments (data not shown).
Table 3.
Potency of NK1 antagonists and their (S) enantiomers (in parenthesis) in inhibiting the cardiovascular responses to equipotent doses of NK1 selective agonists (25 pmol)
The effects of the NK1 antagonists against the behaviour induced by an equipotent dose of 25 pmol of [Sar9, Met(O2)11]SP and septide injected i.c.v. are described in Table 4. The [Sar9, Met(O2)11]SP-induced face washing was inhibited by RP67580, LY303870 and LY306740 whereas the sniffing episodes was only blocked by RP67580. The grooming elicited by [Sar9, Met(O2)11]SP was inhibited by SR140333, RP67580, LY306740 and LY303870. The septide-induced face washing was inhibited by RP67580, LY303870, SR140333 and LY306740 while the sniffing episodes elicited by septide was inhibited by RP67580, LY303870 and LY306740. RP67580 and LY303870 but not LY306740 were about 10 fold more potent in blocking septide than [Sar9, Met(O2)11]SP-induced face washing.
Table 4.
Potency of NK1 antagonists and their (S) enantiomers (in parenthesis) in inhibiting the behavioural responses to equipotent doses of NK1 selective agonists (25 pmol)
Stereoselectivity of LY303870, LY306740 and LY303241
The actions of the (S) enantiomers of Eli Lilly antagonists versus the MAP and HR responses to 25 pmol of either [Sar9, Met(O2)11]SP or septide are described in Table 3. The (S) enantiomers of LY303870 (LY306155) and LY306740 (LY307679) were inactive against both the MAP and HR responses to [Sar9, Met(O2)11]SP and septide. The (S) enantiomer of LY303241 (LY303374) was also found inactive against the MAP and HR responses to septide. LY303374 was not tested against [Sar9, Met(O2)11]SP because LY303241 was found inactive against this agonist.
The (S) enantiomers were tested against the NK1 agonists-induced behaviours (Table 4). LY306155 had no effect on the face washing and sniffing elicited by [Sar9, Met(O2)11]SP but inhibited to some extent the grooming episodes; it was significantly less potent than LY303870. LY307679 was also less potent than LY306740 at inhibiting face washing or grooming elicited by [Sar9, Met(O2)11]SP. LY306155 antagonized the septide-induced face washing and sniffing but with less efficacy than LY303870. LY307679 antagonized the septide-induced face washing behaviour with a potency that did not differ significantly from that of LY306740 whereas it was unable to modify the sniffing induced by septide. In contrast to LY303241, the (S) compound LY303374 inhibited at high doses the sniffing episodes elicited by septide.
Time-dependent effects of the antagonists
The i.c.v. effects of the antagonists against the cardiovascular and behavioural responses to septide (25 pmol i.c.v., 10 min or 1 h later) are shown in Tables 5 and 6. When injected 1 h earlier, LY303870 inhibited the MAP and sniffing responses to septide whereas it was inactive against the HR and face washing responses. The inhibitory action of LY303870 against septide-induced increases in MAP was greater when administered 1 h rather than 10 min before the agonist (48±11 vs 97±16% inhibition; P<0.05) whereas the inhibition of sniffing was not significantly different (41±9 vs 54±9). All the cardiovascular and behavioural responses to i.c.v. septide were inhibited with a greater potency by LY306740 when the antagonist was injected 60 min beforehand instead of 10 min (P<0.01). Conversely, the inhibition of the septide-induced hypertension exerted by LY303241 was over at 60 min after the injection of this antagonist (2±17 vs 49±8%; P<0.05). The inhibitory effect of SR140333 against the septide-induced rise in MAP was still present 1 h post-injection (5±1 vs 18±2 mmHg; P<0.01) and was not different from that observed when SR140333 was injected 10 min beforehand (51±15 vs 52±3% inhibition). Whereas SR140333 injected 1 h before septide inhibited the agonist's effects on HR, face washing and sniffing (90±14, 86±7 and 64±4%, respectively), it was inactive on those responses when injected 10 min prior to septide (11±13, 5±16 and 29±11%, respectively). The inhibitory effect of RP 67580 on septide-induced increases in MAP was greater at 1 h than at 10 min (69±5 vs 47±3%; P<0.01) but was similar on HR, face washing and sniffing responses. None of the three tachykinin NK1 receptor antagonists inhibited the cardiovascular and behavioural responses to i.c.v. septide when the agonist was re-injected 24 h later (data not shown).
Table 5.
Central responses to 25 pmol septide and their inhibition by NK1 antagonists injected 60 min beforehand
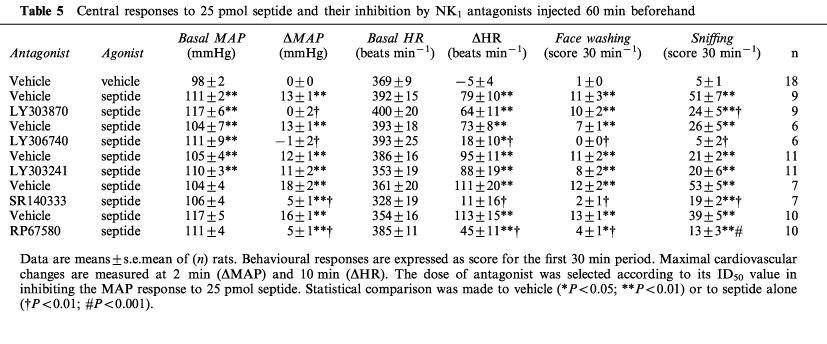
Table 6.
Level of inhibition of the central responses to 25 pmol septide by NK1 antagonists injected i.c.v. either 10 or 60 min beforehand

Direct i.c.v. effects of tachykinin NK1 receptor antagonists
LY306740, LY303870, LY303241 and RP67580 had no direct cardiovascular or motor effects when injected i.c.v. at 6.5 nmol (data not shown). The (S) enantiomers of LY303870 (LY306155) and LY306740 (LY307679) were also devoid of any direct effects. In contrast, the Sanofi antagonist SR140333 caused at 6.5 nmol barrel rotation (clockwise) that was accompanied by increases in MAP and HR in all the 17 tested rats (data not shown).
I.d. effects on vascular permeability
Effects of [Sar9, Met(O2)11]SP and septide on vascular permeability
As shown in Figure 3, i.d. administration of [Sar9, Met(O2)11]SP and septide evoked dose-dependent increases of vascular permeability. Values (in ng mg−1 of fresh tissue) were ranging from 1.8±0.6 to 9.6±1.6 for [Sar9, Met(O2)11]SP (0.65–650 pmol; n=8) and from 0.9±0.5 to 20.9±2.7 for septide (0.65–650 pmol; n=8). The increases of vascular permeability were significantly different from Krebs values from the doses of 6.5 pmol [Sar9, Met(O2)11]SP (P<0.05) and septide (P<0.001).
Figure 3.

Dose-response curves to i.d. administration of [Sar9, Met(O2)11]SP (0.65–650 pmol, n=8) and septide (0.65–650 pmol, n=8) on vascular permeability. Krebs values (8.6–12.1 ng mg−1 of fresh tissue) were subtracted from agonist values. Statistical significance of differences to Krebs were calculated with a one-way ANOVA followed by the Dunnett's test and are indicated by: *P<0.05; ***P<0.001.
Action of the tachykinin NK1 receptor selective antagonists against [Sar9, Met(O2)11]SP and septide
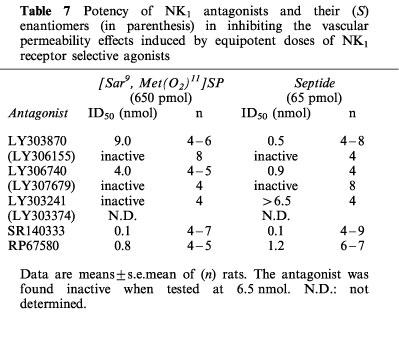
The inhibitory effects of tachykinin NK1 receptor antagonists (65 pmol–65 nmol) on the vascular permeability increases induced by 650 pmol of [Sar9, Met(O2)11]SP or 65 pmol of septide are shown in Figure 4a and b, respectively. The ID50 values determined from the curves of inhibition are presented in Table 7. The vascular permeability effect of [Sar9, Met(O2)11]SP was dose-dependently inhibited by SR140333, RP67580, LY306740 and LY303870, while LY303241 was ineffective. Conversely, the vascular permeability effect elicited by septide was dose-dependently antagonized by SR140333, LY303870, LY306740, RP67580 and LY303241.
Figure 4.
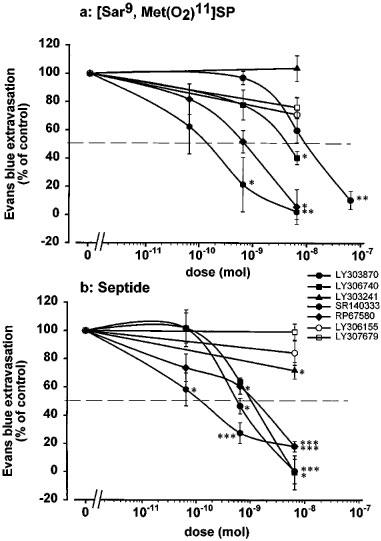
Inhibitory effects of antagonists (65 pmol–65 nmol) and their (S) enantiomers on vascular permeability increases induced by (a) [Sar9, Met(O2)11]SP (650 pmol) and (b) septide (65 pmol). Data are expressed as per cent of inhibition of vascular permeability induced by the agonist (n=4–9). Each rat received i.d. only one agonist and one dose of antagonist. Statistical significance of differences between the effect of the agonist without and with antagonist were calculated with a one-way ANOVA followed by the Tukey's test and are indicated by: *P<0.05; **P<0.01; ***P<0.001.
Table 7.
Potency of NK1 antagonists and their (S) enantiomers (in parenthesis) in inhibiting the vascular permeability effects induced by equipotent doses of NK1 receptor selective agonists
Stereoselectivity of LY303870 and LY306740
The effects of the (S) enantiomers of active Eli Lilly antagonists on vascular permeability increases induced by 650 pmol of [Sar9, Met (O2)11]SP or 65 pmol of septide are shown respectively on Figure 4a and b. The (S) enantiomers of LY303870 (LY306155) and LY306740 (LY307679) did not significantly affect the vascular permeability response elicited by both agonists. Since LY303241 was found inactive against [Sar9, Met(O2)11]SP and active only at very high doses against septide, its (S) enantiomer (LY303374) was not tested in this paradigm.
Effects of the tachykinin NK1 receptor antagonists against bradykinin and histamine
Bradykinin (10 nmol) and histamine (10 nmol) were used to further verify the specificity of the antagonists against the effects of tachykinin NK1 receptor agonists on vascular permeability (Figure 5). At this dose, bradykinin increased vascular permeability from 13.6±2.3 to 19.1±2.7 ng mg−1 of fresh tissue. When co-injected at 6.5 nmol, LY303870 (n=5), LY306740 (n=6) and RP67580 (n=4) did not significantly affect the response to bradykinin, while the same dose of SR140333 (n=8) reduced it significantly (P<0.001). At the doses assayed, none of the tachykinin NK1 receptor antagonists displayed a direct effect on vascular permeability when compared to Krebs values (LY303870, 0.0±0.7; LY306740, −1.1±0.2; SR140333, −0.7±0.5; RP67580, −1.8±1.2 ng mg−1 fresh tissue). At the dose of 10 nmol, histamine increased vascular permeability from 5.1±0.6 to 7.4±1.4 ng mg−1 of fresh tissue. When coinjected at 6.5 nmol, LY306740 (n=6) and RP67580 (n=5) did not significantly affect the response to histamine, while at the same dose LY303870 (n=6) and SR140333 (n=6) reduced it significantly (P<0.05). None of the antagonists displayed a direct effect when compared to Krebs values (LY303870, 0.0±1.1; LY306740, −1.1±0.6; SR140333, 0.7±0.9; RP67580, −2.7±0.5 ng mg−1 fresh tissue).
Figure 5.
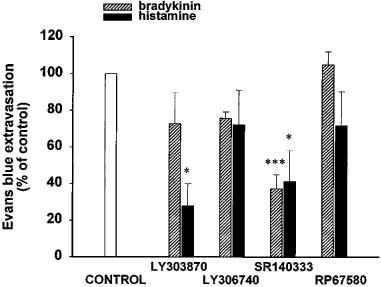
Effect of i.d. antagonists (6.5 nmol per 100 μl) on vascular permeability increases induced by bradykinin or histamine (10 nmol per 100 μl). Data are expressed as per cent of inhibition of vascular permeability induced by bradykinin (n=4–8) and histamine (n=5–6). Each rat received only one agonist and one antagonist. Statistical significance of differences between the effect of agonists without and with antagonist were calculated with a one-way ANOVA followed by the Tukey's test and are indicated by: *P<0.05, ***P<0.001.
Discussion
I.c.v. action of tachykinin NK1 receptor selective agonists
The classical tachykinin NK1 receptor agonist [Sar9, Met (O2)11]SP induces rapid increases of MAP and HR that are accompanied by intensive episodes of face washing, grooming and sniffing. These effects are consistent with earlier findings with SP (Itoi et al., 1992; Picard et al., 1994). The wet dog shakes behaviour was ascribed to the activation of tachykinin NK3 receptor on the basis of experiments using natural and selective agonists and antagonists (Picard et al., 1994). The i.c.v. effects of SP are inhibited by NK1 selective antagonists and are not affected by the injection of selective antagonists for both tachykinin NK2 and NK3 receptors (Tschöpe et al., 1992; Picard et al., 1994). However, in the presence of a NK1 antagonist, SP exerts a residual response that is abolished by the co-administration of a NK2 receptor antagonist (Picard et al., 1994). Since SP is not a highly selective agonist for the brain NK1 receptor, [Sar9, Met(O2)11]SP was used for comparison with septide.
The atypical tachykinin NK1 receptor agonist septide is as potent as [Sar9, Met(O2)11]SP in increasing MAP, HR and episodes of face washing, sniffing and wet dog shakes. Nevertheless, the same doses of [Sar9, Met(O2)11]SP and septide display dissimilar time-course effects on MAP and HR. Also, septide does not stimulate grooming, contrary to [Sar9, Met(O2)11]SP. These differences between the two agonists could be related to their differential intrastriatal modulation of dopamine release, as underscored by Gauchy et al. (1996). However, the substantia nigra was suggested to be the site of action of NK1 agonists on grooming in the rat (Stoessl et al., 1991). Hence, the reason which could explain the differential behavioural responses to septide remains unknown and could be attributed to differential pharmacokinetic features, receptor isoforms (long and truncated receptor) or to the activation of different signal transduction mechanisms (see below).
I.d. action of tachykinin NK1 receptor selective agonists, bradykinin and histamine
Septide and [Sar9, Met(O2)11]SP dose-dependently increase vascular permeability in agreement with previous studies (Devor et al., 1989; Ahluwalia et al., 1995). In our study, septide is at least ten times more potent than [Sar9, Met(O2)11]SP on vascular permeability although septide and [Sar9, Met(O2)11]SP were found equipotent on oedema formation (Ahluwalia et al., 1995). The reason for this difference in the order of potency of agonists between these two studies is presently unknown. However, in the latter study, more than one agonist was injected to a rat at doses which caused systemic effects.
The effect of i.d. administration of bradykinin on vascular permeability was ascribed to the activation of B2 receptor in the rat skin (Whalley, 1987). In the present study the dose of 10 nmol of bradykinin was selected to increase vascular permeability to the same magnitude than that evoked by septide and [Sar9, Met(O2)11]SP. This dose of bradykinin is about ten times greater than its ED10 (1.3 nmol) value (Whalley, 1987). The effect of histamine on vascular permeability at the dose of 10 nmol is consistent with earlier studies in the rat skin (Saria et al., 1983).
Effects of tachykinin NK1 receptor antagonists in the rat central nervous system
Both MAP and HR responses to [Sar9, Met(O2)11]SP and septide are dose-dependently inhibited by LY303870 and RP67580 whereas SR140333 (injected 10 min before agonist) inhibits only the increase in MAP induced by both tachykinin NK1 agonists. LY306740 inhibits only the MAP response induced by septide when injected 10 min earlier. This finding suggests a dissociation between the MAP and HR responses which may indicate that each antagonist does not reach with the same ease the brain areas subserving the effects of the agonists on HR and MAP. This is suggested by the abolition of the HR response to septide by SR140333 and LY306740 injected 1 h before the agonist. Also, the effect of agonist on behavioural responses may derive from different sites of action which are not easily accessible by all antagonists. This may afford a reasonable explanation for the abolition of [Sar9, Met(O2)11]SP-induced sniffing by RP67580 only while the face washing and grooming are inhibited by LY303870, LY306740 and RP67580. The inhibition of the septide-induced increases in HR and face washing behaviour by LY 303870 is over 1 h post-injection while its efficacy in reducing the increases in MAP and sniffing is either greater or unchanged. Again, this suggests different sites of action for septide in the production of these various centrally mediated responses. The greater inhibition of the centrally mediated effects of septide provided by a longer period of treatment (1 h vs 10 min prior to the agonist) with SR 140333 is in agreement with in vitro studies on isolated organs (Emonds-Alt et al., 1993). In the present study, such a pharmacodynamic feature was also observed with LY306740 and to some extent with RP67580 and LY303870 on the MAP response. Thus, the antagonistic activity of some non-peptide antagonists can be underestimated if the period of contact with the tissue is not sufficient. For instance, LY306740 becomes the most potent of the five NK1 antagonists acting centrally in the present study if administered 1 h instead of 10 min before septide.
The inhibition of cardiovascular and behavioural responses elicited by i.c.v. [Sar9, Met(O2)11]SP and septide by LY303870, LY306740 and LY303241 is stereoselective since their (S) enantiomers were inactive or significantly less active. Only SR140333 elicits direct cardiovascular effects and barrel rotation when injected i.c.v. at 6.5 nmol. Those effects are unlikely due to the vehicle DMSO since the other antagonists and their enantiomers were dissolved in the same concentration of DMSO and were devoid of such direct effects.
Effects of tachykinin NK1 receptor antagonists in the periphery
The action of LY303870 and LY306740 on vascular permeability is stereoselective since their (S) enantiomers are not significantly active against the i.d. action of [Sar9, Met(O2)11]SP and septide. The antagonists LY306740 and RP67580 are also specific since they are inactive against the i.d. action of bradykinin and histamine. In contrast, SR140333 appears less specific since it exerts an inhibitory effect against bradykinin and histamine. LY303870 was also found to block histamine. The inhibition of bradykinin and/or histamine effects by SR140333 and LY303870 is unlikely related to the release of SP following the activation of sensory nerves by histamine and bradykinin because the other tachykinin NK1 receptor antagonists did not alter the increases in vascular permeability induced by these pro-inflammatory mediators. This is in agreement with a previous study with the NK1 receptor antagonist CP-96,345 which selectively inhibited tachykinin-induced vascular permeability without affecting that induced by histamine or bradykinin in the guinea-pig skin (Nagahisa et al., 1992). Thus, this evidence does not support earlier findings suggesting that bradykinin and histamine require intact primary sensory afferents to enhance cutaneous vascular permeability (Jancsó et al., 1980; Khalil & Helme, 1992; Geppetti, 1993). Nevertheless, the ability of SR140333 to prevent the effects of SP, bradykinin and histamine on vascular permeability may be an advantage for a drug to be used in the treatment of inflammation. Finally, the high specificity of RP67580 in this paradigm is congruent with the observation that this antagonist is also ineffective against bradykinin-induced vascular permeability in the rat knee joint (Cambridge & Brain, 1995).
Presence of a distinct pharmacological profile for septide
Because the rank order of potencies of the five tachykinin NK1 receptor antagonists in inhibiting the central and peripheral effects of septide and [Sar9, Met(O2)11]SP are strikingly differents, it is confirmed in vivo that septide displays a pharmacological profile distinct from that of the classical NK1 receptor agonist (Table 8). Moreover, the greater efficacy of several tachykinin NK1 receptor antagonists in inhibiting septide rather than [Sar9, Met(O2)11]SP is in agreement with earlier studies using other tachykinin NK1 receptor antagonists (Chassaing et al., 1992; Petitet et al., 1992; Maggi et al., 1993; Palma et al., 1994; Torrens et al., 1995). The distinct pharmacological profile of septide may be related to its association with a distinct isoform of the tachykinin NK1 receptor. For instance, evidence suggests the existence of truncated tachykinin NK1 receptor isoforms resulting from differential mRNA splicing (Fong et al., 1992) or proteolytic maturation in the rat salivary gland (Boyd et al., 1991; Kage et al., 1993; Mantyh et al., 1996). Recently, Maggi & Schwartz (1997) presented two models to explain how SP and septide might bind to the same receptor, but compete poorly in radioligand-binding assays. The model suggesting the existence of two receptor conformations with a single site on the tachykinin NK1 receptor appears the most attractive explanation and takes into account the activation of distinct second messenger pathways by SP and septide (Sagan et al., 1996).
Table 8.
Synopsis of the rank order of potency of tachykinin NK1 antagonists in inhibiting the biological action of [Sar9, Met(O2)11]SP and septide in the rat
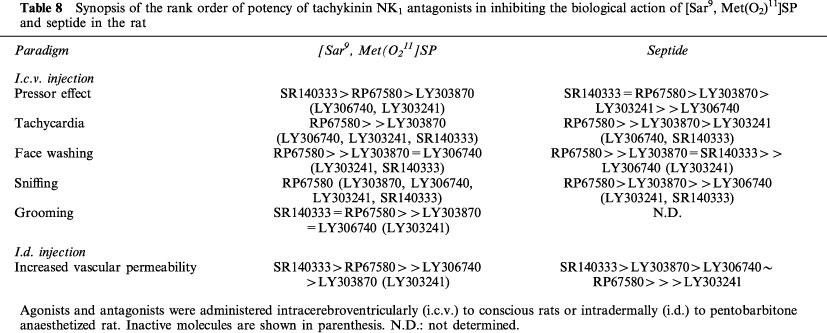
What are the best NK1 antagonists to be used in the rat?
In most central and peripheral effects induced by either tachykinin NK1 receptor agonist, RP67580 was generally found more potent than the other non-peptide antagonists. This might be due to its high affinity for the rat tachykinin NK1 receptor (Beaujouan et al., 1993). However, LY306740 appears more potent than RP67580 if pre-administered 1 h prior to the agonist (Tables 5 and 6), suggesting that the time of contact with the tissue is highly relevant in the case of LY306740. The latter exhibits high affinity for the rat NK1 receptor while LY303870 has a greater affinity for the human NK1 receptor (Hipskind et al., 1996). LY303870 and LY306740 readily cross the blood-brain barrier to interact with central NK1 receptors after systemic administration (parenteral and/or oral routes) (Cellier et al., 1995; Iyengar et al., 1997), yet RP67580 has poor brain penetration in rat (Holzer-Petsche & Rordorf-Nikolic, 1995). Thus, LY306740 seems a better pharmacological tool to block central NK1 receptors in rats. On the other hand, RP67580 would be more appropriate for in vivo studies aim at blocking exclusively the peripheral tachykinin NK1 receptor.
LY303241 was found a weak antagonist (even after a long period of pre-injection) which is congruent with its ineffectiveness to antagonize the hyperalgesic effect of intrathecally administered [Sar9,Met(O2)11]SP in the rat tail-flick test (Couture, R. & Boucher, S., unpublished observation). Nevertheless, LY303241 was more potent than LY306740 and LY303870 in inhibiting the hypotensive response to i.v. SP in rat (Cellier et al., 1996). These in vivo differences in the efficacy of LY303241 are unknown at the present time.
Conclusion
The higher potency of NK1 antagonists to prevent central and peripheral responses mediated by septide and the differential rank order of potencies of NK1 antagonists to block septide and [Sar9,Met(O2)11]SP may be explained by the existence of receptor isoforms or by the activation of different signalling pathways following the stimulation of the same receptor protein by the two agonists. However, the latter possibility can be best explained if there are two active conformations of a single tachykinin NK1 receptor. Nevertheless, the present study highlights striking differences in the pharmacodynamic features of the agonists and antagonists which may have consequences in the interpretation of in vivo studies. Finally, RP67580 and LY306740 represent the most valuable antagonists currently available for in vivo studies in rat and may serve to discriminate between the central and peripheral effects mediated by tachykinin NK1 receptor on the basis of their brain penetration index.
Acknowledgments
E. Cellier and L. Barbot should both be considered as first author as they contributed equally to this work. Authors acknowledge Dr C. Garret (Rhone Poulenc, France) and Dr J.C. Brelière (Sanofi Recherche, France) for the donation of RP67580 and SR140333, respectively. This work was supported by the Medical Research Council of Canada (MRCC) and the Kidney Foundation of Canada. E. Cellier was a recipient of a research traineeship from the Heart and Stroke Foundation of Canada.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- i.d.
intradermal
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- DMSO
dimethyl sulphoxide
- HR
heart rate
- MAP
mean arterial pressure
- MW
molecular weight
- SP
substance P
- NMDA
N-methyl-D-aspartate
References
- AHLUWALIA A., GIULIANI S., MAGGI C.A. Demonstration of a ‘septide-sensitive' inflammatory response in rat skin. Br. J. Pharmacol. 1995;116:2170–2174. doi: 10.1111/j.1476-5381.1995.tb15050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEATTIE D.T., CONNOR H.E., HAGAN R.M. Recent developments in tachykinin NK1 receptor antagonists: prospects for the treatment of migraine headache. Can. J. Physiol. Pharmacol. 1995;73:871–877. doi: 10.1139/y95-120. [DOI] [PubMed] [Google Scholar]
- BEAUJOUAN J.C., HEUILLET E., PETITET F., SAFFROY M., TORRENS Y., GLOWINSKI J. Higher potency of RP67580, in the mouse and the rat compared with other nonpeptide and peptide tachykinin NK1 antagonists. Br. J. Pharmacol. 1993;108:793–800. doi: 10.1111/j.1476-5381.1993.tb12880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD N.D., MACDONALD S.G., KAGE R., LUBER-NAROD J., LEEMAN S.E. Substance P receptor. Biochemical characterization and interactions with G proteins. Ann. N. Y. Acad. Sci. 1991;632:79–93. doi: 10.1111/j.1749-6632.1991.tb33096.x. [DOI] [PubMed] [Google Scholar]
- CAMBRIDGE H., BRAIN S.D. Mechanism of bradykinin-induced plasma extravasation in the rat knee joint. Br. J. Pharmacol. 1995;115:641–647. doi: 10.1111/j.1476-5381.1995.tb14980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELLIER E., BARBOT L., HIPSKIND P.A., IYENGAR S., COUTURE R.Central and peripheral effects of three novel non-peptide NK-1 receptor antagonists against septide in rat International Tachykinin Conference: Tachykinins in Health and Disease 1997. 7–11 September 1997, Cairns, Great Barrier Reef, Australia. Program and abstracts book, 26
- CELLIER E., FAYOLLE C., HIPSKIND P.A., IYENGAR S., COUTURE R. Peripheral effects of three novel non-peptide tachykinin NK1 receptor antagonists in the anaesthetized rat. Eur. J. Pharmacol. 1996;318:377–385. doi: 10.1016/s0014-2999(96)00808-4. [DOI] [PubMed] [Google Scholar]
- CELLIER E., PICARD P., FAYOLLE C., HIPSKIND P.A., IYENGAR S., COUTURE R.Peripheral and central effects of three novel non peptide NK-1 receptor antagonists in the rat International Symposium: TACHYKININS'95 - From Basic Science to Clinical Applications 1995. Florence (Italy), October 16–18, Abstract Book p. 92. Fondazione Internazionale Menarini
- CHASSAING G., LAVIELLE S., BRUNISSEN A., CARUETTE A., GARRET C., PETITET F., SAFFROY M., BEAUJOUAN J.C., TORRENS Y., GLOWINSKI J. [Pro9]SP and [pGlu6, Pro9]SP(6–11) interact with two different receptors in the guinea-pig ileum as demonstrated with new SP antagonists. Neuropeptides. 1992;23:73–79. doi: 10.1016/0143-4179(92)90081-7. [DOI] [PubMed] [Google Scholar]
- COUTURE R., KÉROUAC R. Plasma protein extravasation to mammalian tachykinins in rat skin: influence of anaesthetic agents and acetylcholine antagonist. Br. J. Pharmacol. 1987;91:265–273. doi: 10.1111/j.1476-5381.1987.tb10281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVOR M., PAPIR-KRICHELI D., NACHMIAS E., ROSENTHAL F., GILON C., CHOREV M., SELINGER Z. Substance P-induced cutaneous plasma extravasation in rats is mediated by NK-1 tachykinin receptors. Neurosci. Letts. 1989;103:203–208. doi: 10.1016/0304-3940(89)90576-4. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO V., VAN BROECK D., SOUBRIE P., LE FUR G., BRELIÈRE J.C. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- FONG T.M., ANDERSON S.A., YU H., HUANG R.R., STRADER C.D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- GARRET C., CARRUETTE A., FARDIN V., MOUSSAOUI S., PEYRONEL J.F., BLANCHARD J.C., LADURON P.M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUCHY C., DESBAN M., GLOWINSKI J., KEMEL M.L. Distinct regulations by septide and the neurokinin-1 tachykinin receptor agonist [Pro9]substance P of the N-methyl-D-aspartate-evoked release of dopamine in striosome- and matrix-enriched areas of the rat striatum. Neuroscience. 1996;73:929–939. doi: 10.1016/0306-4522(96)00099-1. [DOI] [PubMed] [Google Scholar]
- GEPPETTI P. Sensory neuropeptide release by bradykinin : mechanisms and pathophysiological implications. Regul. Pept. 1993;47:1–23. doi: 10.1016/0167-0115(93)90268-d. [DOI] [PubMed] [Google Scholar]
- GITTER B.D., BRUNS R.F., HOWBERT J.J., WATERS D.C., THRELKELD P.G., COX L.M., NIXON J.A., LOBB K.L., MASON N.R., STENGEL P.W., COCKERHAM S.L., SILBAUGH S.A., GEHLERT D.R., SCHOBER D.A., IYENGAR S., CALLIGARO D.O., REGOLI D., HIPSKIND P.A. Pharmacological characterization of LY303870: a novel, potent and selective nonpeptide substance P (neurokinin-1) receptor antagonist. J. Pharmacol. Exp. Ther. 1995;275:737–744. [PubMed] [Google Scholar]
- GLOWINSKI J. The ‘septide-sensitive' tachykinin receptor: still an enigma. Trends Pharmacol. Sci. 1995;16:365–367. doi: 10.1016/s0165-6147(00)89076-8. [DOI] [PubMed] [Google Scholar]
- GUARD S., BOYLE S.J., TANG K.W., WATLING K.J. , MCKNIGHT A.T., WOODRUFF G.N. The interaction of the NK1 receptor antagonist CP-96,345 with L-type calcium channels and its functional consequences. Br. J. Pharmacol. 1993;110:385–391. doi: 10.1111/j.1476-5381.1993.tb13821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON T., WILLIAMS B.J., SWAIN C.J., BALL R.G. Piperidine-ether based hNK1 antagonists 1: determination of the relative and absolute stereochemical requirements. Bioorg. Med. Chem. Lett. 1994;4:2545–2550. [Google Scholar]
- HIPSKIND P.A., HOWBERT J.J., BRUNS R.F., CHO S.S.Y., CROWELL T.A., FOREMAN M.M., GEHLERT D.R., IYENGAR S., JOHNSON K.W., KRUSHINSKI J.H., LI D.L., LOBB K.L., MASON N.R., MUEHL B.S., NIXON J.A., PHEBUS L.A., REGOLI D., SIMMONS R.M., THRELKELD P.G., WATERS D.C., GITTER B.D. 3-aryl- 1,2-diacetamidopropane derivatives as novel and potent NK-1 receptor antagonists. J. Med. Chem. 1996;39:736–748. doi: 10.1021/jm950616c. [DOI] [PubMed] [Google Scholar]
- HIPSKIND P.A., HOWBERT J.J., CHO S., CRONIN J.S., FORT S.L., GINAH F.O., HANSEN G.J., HUFF B.E., LOBB K.L., MARTINELLI M.J., MURRAY A.R., NIXON J.A., STASZACK M.A., COPP J.D. Practical and enantiospecific synthesis of LY303870, a novel NK-1 antagonist. J. Org. Chem. 1995;60:7033. [Google Scholar]
- HOLZER-PETSCHE U., RORDORF-NIKOLIC T. Central versus peripheral site of action of the tachykinin NK1 antagonist RP 67580 in inhibiting chemonociception. Br. J. Pharmacol. 1995;115:486–490. doi: 10.1111/j.1476-5381.1995.tb16359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOI K., TSCHÖPE C., JOST N., CULMAN J., LEBRUN C., STAUSS B., UNGER T. Identification of the central tachykinin receptor subclass involved in substance P-induced cardiovascular and behavioral responses in conscious rats. Eur. J. Pharmacol. 1992;219:435–444. doi: 10.1016/0014-2999(92)90485-m. [DOI] [PubMed] [Google Scholar]
- IYENGAR S., HIPSKIND P.A., GEHLERT D.R., SCHOBER D., GITTER B.D., MCMILLIAN C., COUTURE R., LI D., SIMMONS R.M.A. LY303870: a centrally active NK-1 antagonist with a long duration of action. J. Pharmacol. Exp. Ther. 1997;280:774–785. [PubMed] [Google Scholar]
- JANCSÓ G., KIRÁLY E., JANCSÓ-GÁBOR A. Chemosensitive pain fibres and inflammation. Int. J. Tiss. Reac. 1980;2:57–66. [Google Scholar]
- KAGE R., LEEMAN S.E., BOYD N.D. Biochemical characterization of two different forms of the substance P receptor in rat submaxillary gland. J. Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- KHALIL Z., HELME R.D. The quantitative contribution of nitric oxide and sensory nerves to bradykinin-induced inflammation in rat skin microvasculature. Brain. Res. 1992;589:102–108. doi: 10.1016/0006-8993(92)91167-d. [DOI] [PubMed] [Google Scholar]
- MACLEAN S., GANONG A., SEYMOUR P.A., SNIDER R.M., DESAI M.C., ROSEN T., BRYCE D.K., LONGO K.P., REYNOLDS L.S., ROBINSON G., SCHMIDT A.W., SIOK C., HEYM J. Pharmacology of CP-99,994, a nonpeptide antagonist of the tachykinin NK1 receptor. J. Pharmacol. Exp. Ther. 1993;267:472–479. [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., SCHWARTZ T.W. The dual nature of the tachykinin NK1 receptor. Trends Pharmacol. Sci. 1997;18:351–355. doi: 10.1016/s0165-6147(97)01107-3. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., ROVERO P., GIACHETTI A. Tachykinin receptors and tachykinin receptor antagonists. J. Auton. Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- MANTYH P.W., ROGERS S.D., GHIRLARDI J.R., MAGGIO J.E., MANTYH C.R., VIGNA S.R. Differential expression of two isoforms of the neurokinin-1 (substance P) receptor in vivo. Brain Res. 1996;719:8–13. doi: 10.1016/0006-8993(96)00050-9. [DOI] [PubMed] [Google Scholar]
- NAGAHISA A., KANAI Y., SUGA O., TANIGUCHI K., TSUCHIYA M., LOWE J.A., III, HESS H.-J. Antiinflammatory and analgesic activity of a non-peptide substance P receptor antagonist. Eur. J. Pharmacol. 1992;217:191–195. doi: 10.1016/0014-2999(92)90847-w. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- PALMA C., GOSO C., MANZINI S. Different susceptibility to neurokinin 1 receptor antagonists of substance P and septide-induced interleukin-6 release from U373 MG human astrocytoma cell line. Neurosci. Letts. 1994;171:221–224. doi: 10.1016/0304-3940(94)90644-0. [DOI] [PubMed] [Google Scholar]
- PETITET F., SAFFROY M., TORRENS Y., LAVIELLE S., CHASSAING G., LOEUILLET D., GLOWINSKI J., BEAUJOUAN J.C. Possible existence of a new tachykinin receptor subtype in the guinea pig ileum. Peptides. 1992;13:383–388. doi: 10.1016/0196-9781(92)90125-m. [DOI] [PubMed] [Google Scholar]
- PICARD P., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of centrally administered tachykinins in the rat: characterization of receptors with selective antagonists. Br. J. Pharmacol. 1994;112:240–249. doi: 10.1111/j.1476-5381.1994.tb13058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGOLI D., BOUDON A., FAUCHÈRE J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- SAGAN S., CHASSAING G., PRADIER L., LAVIELLE S. Tachykinin peptides affect differently the second messenger pathways after binding to CHO-expressed human NK-1 receptors. J. Pharmacol. Exp. Ther. 1996;276:1039–1048. [PubMed] [Google Scholar]
- SARIA A., LUNDBERG J.M., SKOFITSCH G., LEMBECK F. Vascular protein leakage in various tissues induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn-Schmied. Arch. Pharmacol. 1983;324:212–218. doi: 10.1007/BF00503897. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A.W., MCLEAN S., HEYM J. The substance P receptor antagonist CP-96,345 interacts with Ca2+ channels. Eur. J. Pharmacol. 1992;19:351–352. doi: 10.1016/0014-2999(92)90498-s. [DOI] [PubMed] [Google Scholar]
- SNIDER R.M., CONSTANTINE J.W., LOWE J.A., III, LONGO K.P., LEBEL W.S., WOODY H.A., DROZDA S.E., DESAI M.A., VINICK F.J., SPENCER R.W., HESS H.J. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991;251:435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- STOESSL A.J., SZCZUTKOWSKI E., GLENN B., WATSON I. Behavioural effects of selective tachykinin agonists in midbrain dopamine regions. Brain Res. 1991;565:254–262. doi: 10.1016/0006-8993(91)91657-m. [DOI] [PubMed] [Google Scholar]
- TORRENS Y., BEAUJOUAN J.C., SAFFROY M., GLOWINSKI J. Involvement of septide-sensitive tachykinin receptors in inositol phospholipid hydrolysis in the rat urinary bladder. Peptides. 1995;16:587–594. doi: 10.1016/0196-9781(95)00016-d. [DOI] [PubMed] [Google Scholar]
- TSCHÖPE C., PICARD P., CULMANN J., PRAT A., ITOI K., REGOLI D., UNGER T., COUTURE R. Use of selective antagonists to dissociate the central cardiovascular and behavioural effects of tachykinins on NK1 and NK2 receptors in the rat. Br. J. Pharmacol. 1992;107:750–755. doi: 10.1111/j.1476-5381.1992.tb14518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASSOUT A., SHAUB M., GENTSCH C., OFNER S., SCHILLING W., VEENSTRA S. CGP49823, a novel NK-1 receptor antagonist: Behavioural effects. Neuropeptides. 1994;26:38. [Google Scholar]
- WANG Z.Y., TUNG S.R., STRICHARTZ G.R., HÅKANSON R. Non-specific actions of the non-peptide tachykinin receptor antagonists, CP-96,345, RP67580 and SR48968, on neurotransmission. Br. J. Pharmacol. 1994;111:179–184. doi: 10.1111/j.1476-5381.1994.tb14041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHALLEY E.T. Receptor mediating the increase in vascular permeability to kinins: comparative studies in rat, guinea-pig and rabbit. Naunyn-Schmied. Arch. pharmacol. 1987;336:99–104. doi: 10.1007/BF00177758. [DOI] [PubMed] [Google Scholar]


