Abstract
Responsiveness to EDRF-releasing substances and inhibitory nerve stimulation of canine isolated penile corpus cavernosum with and without saponin treatment were investigated.
Histological studies demonstrated that saponin did not detach endothelial cells from underlying tissues, but induced degenerative changes in the endothelial cells selectively.
In the cavernous strips contracted with phenylephrine, addition of acetylcholine, sodium nitroprusside, ATP and Ca2+ ionophore A23187 induced relaxations, but substance P and bradykinin did not change the muscle tone.
Acetylcholine-induced relaxation was significantly attenuated but not abolished by NG-nitro-L-arginine (L-NOARG). L-arginine restored the response inhibited by L-NOARG. The L-NOARG resistant relaxation was not influenced by 1H[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) but was suppressed in the strips contracted with K+. Treatment with saponin abolished the relaxation elicited by acetylcholine and A23187 but did not influence the response to nitroprusside and ATP. The ATP-induced relaxation was attenuated by aminophylline.
Transmural electrical stimulation at 2–20 Hz produced endothelium-independent relaxations which were abolished by tetrodotoxin and L-NOARG but unaffected by treatment with saponin. In saponin-treated cavernous strips, the neurogenic relaxation was not affected by acetylcholine, physostigmine, atropine and vasoactive intestinal peptide (VIP) but was abolished by ODQ.
It is concluded that acetylcholine-induced relaxations are endothelium-dependent and mediated partly by NO and also by other substances from the endothelium. The endothelium-independent relaxation to ATP is likely to be mediated by P1 purinoceptors. The function of nitrergic nerve does not seem to be prejunctionally modulated by acetylcholine and VIP.
Keywords: Corpus cavernosum, nitric oxide, acetylcholine, saponin, endothelium-dependent relaxation, nitrergic function, transmural electrical stimulation, ATP
Introduction
Although cholinergic and vasoactive intestinal peptide (VIP)-releasing nerves have been considered to be involved in provoking penile erection associated with an increase in intracavernous pressure (Willis et al., 1981; Andersson et al., 1984; Saenz de Tejada et al., 1988; Kim et al., 1995), accumulated data from recent in vitro and in vivo studies strongly suggest that nitric oxide (NO) synthesized from L-arginine mainly mediates the relaxation of penile corpus cavernosum muscle and the intracavernous pressor response to nerve stimulation in a variety of mammals (Burnett et al., 1992; Rajfer et al., 1992; Pickard et al., 1993; Hayashida et al., 1996; Ayajiki et al., 1997). NO is considered to be liberated from the endothelium in corpora cavernosa in response to EDRF-releasing substances, such as acetylcholine (Ignarro et al., 1990; Saenz de Tejada et al., 1988; Azadzoi et al., 1992), which induces relaxation of isolated cavernous strips. The presence of muscarinic receptors in human corpus cavernosum and in endothelial cells from this tissue has been demonstrated (Traish et al., 1990). However, little information is available concerning relaxing factors liberated from the endothelium in response to chemical stimuli.
Neurogenic relaxation of cavernous muscle strips is now recognized to be mediated by NO, and the involvement of endothelium-derived NO in the response has been excluded (Kim et al., 1991). Histochemical studies have demonstrated dense networks of neurons containing NO synthase (Burnett et al., 1993; Hayashida et al., 1996), cholinesterase (Shirai et al., 1972; Benson et al., 1980) and VIP (Gu et al., 1983; Hayashida et al., 1996) in the corpus cavernosum. Electrical stimulation of these neurons is expected to liberate NO, acetylcholine and VIP. Therefore, synthesis or/and release of neurotransmitter from nitrergic nerves that play a major role in increasing the intracavernous pressure may be modulated by neurogenic acetylcholine and VIP, as was seen in monkey and bovine cerebral arteries in response to endogenous acetylcholine (Toda & Ayajiki, 1990; Toda et al., 1997a).
The present study was undertaken to determine the actions and mechanisms of action of substances that liberate relaxing factors from the vascular endothelium, including acetylcholine, bradykinin, substance P, ATP and Ca2+ ionophore A23187 (Angus & Cocks, 1989), on strips of the canine corpus cavernosum and to clarify whether or not neurogenic relaxations of cavernous strips were influenced by histological impairment of endothelial cells and by endogenous and exogenous acetylcholine and VIP.
Methods
Preparation
The Animal Care and Use Committee at Shiga University of Medical Science approved the use of dog penises in this study. Male mongrel dogs, weighing 7–13 kg, were anaesthetized with intravenous injections of sodium thiopental (50 mg kg−1) and killed by bleeding from the carotid arteries. The penis was rapidly removed, and corpus cavernosum was isolated. The tunica albuginea was removed, and two or three strips (about 1×2×10 mm) were obtained. The specimens were fixed vertically between hooks in a muscle bath of 20 ml capacity containing the nutrient solution, which was aerated with a mixture of 95% O2 and 5% CO2 and maintained at 37±0.3°C. The hook anchoring the upper end of the strips was connected to the lever of a force-displacement transducer (Nihon-Kohden Kogyo Co., Tokyo, Japan). The resting tension was adjusted to 0.7 g, which is optimal for inducing the maximal contraction. Constituents of the solution were as follows (mM): NaCl 120, KCl 5.4, CaCl2 2.2, MgCl2 1.0, NaHCO3 25.0 and dextrose 5.6. The pH of the solution was 7.36–7.43. Before the start of experiments, all of the strips were allowed to equilibrate in the bathing media for 60–90 min, during which time the fluids were replaced every 10–15 min.
Some of the strips were placed between stimulating electrodes. The gaps between the strip and the electrodes were wide enough to allow undisturbed mechanical responses and yet sufficiently narrow to stimulate intramural nerve terminals effectively. A train of 0.2 ms square pulses of supramaximal intensity were transmurally applied at 2, 5 and 20 Hz for 100, 40 and 10 s, respectively. In order to evaluate the effects of tested drugs on the responses elicited by electrical stimulation, 5 Hz was selected, because submaximal and reproducible responses could be obtained. The stimulus pulses were delivered by an electronic stimulator (Nihon-Kohden Kogyo Co, Tokyo, Japan).
Functional study
Isometric contractions and relaxations were displayed on an ink-writing oscillograph. The contractile response to K+ was obtained at first by adding 30 mM K+ directly to the medium, and the strips were washed three times with fresh media and equilibrated for 30–40 min. The strips were partially contracted with phenylephrine (0.5–2 μM); the contraction (182±22 mg) was in a range between 30 and 40% of the contraction (495±55 mg) induced by 30 mM K+. Transmural electrical stimulation was applied repeatedly at intervals of 10 min until steady responses were obtained, and then blocking agents were applied. Acetylcholine, sodium nitroprusside and ATP were applied directly to the bathing media in cumulative concentrations, and the strips were repeatedly washed. At the end of each series of experiment, papaverine (0.1 mM) was applied to attain the maximal relaxation (100% relaxation), and relative values of the response to electrical and chemical stimulation were presented. Some of the strips were exposed for 45 min to saponin (1 mg ml−1) to induce endothelial impairment (Samata et al., 1986), and repeatedly rinsed with drug-free media (10 times, every 5 min).
Histological study
Corpus cavernosum strips treated with saponin (1 mg ml−1) or vehicle for 45 min were immersed for 2 h in a fixative containing 4% paraformaldehyde, 0.5% glutaraldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.4) at 4°C and then immersed for 24 h in a post-fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer at 4°C. Tissues were washed for 4 days with several changes of 0.1 M phosphate buffer containing 15% sucrose, embedded in 10% gelatin in 0.1 M phosphate buffer and then cut into 50 μm sections on a vibratome. The sections were osmificated for 1 h with 1% OsO4 in 0.1 M phosphate buffer at 4°C, dehydrated in graded ethanol, and embedded in epoxy resin. Ultrathin sections were cut and mounted on 200 mesh copper grids, stained with uranyl acetate and Reynold's solution and then observed under electron microscopy (H-7100, Hitachi, Japan).
Statistics and drugs used
The results shown in the text and figures are expressed as mean values ±s.e.mean. Statistical analyses were made using the Student's paired and unpaired t-test and the Tukey's test after one-way analysis of variance. Drugs used were NG-nitro-L-arginine (L-NOARG), vasoactive intestinal peptide (VIP), substance P, bradykinin (Peptide Institute, Minoh, Japan), L-arginine (Nacalai Tesque, Kyoto, Japan), acetylcholine chloride (Daiichi Co., Osaka, Japan), sodium nitroprusside (Merck, Darmstadt, Germany), ATP, aminophylline, indomethacin, physostigmine sulphate, 1-phenylephrine hydrochloride (Sigma Chemical, St. Louis, MO, U.S.A.), atropine sulphate, thiopental (Tanabe, Osaka), bretylium tosylate (Glaxo Wellcome, NC, U.S.A.), tetrodotoxin (Sankyo Co., Tokyo), Ca2+ ionophore A23187 (C. H. Boehringer Ingelheim, Elmsford, NY, U.S.A.) and papaverine hydrochloride (Dainippon Co., Osaka). ODQ (1H[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) was a generous gift from Dr S. Moncada. Responses to NO were obtained by adding the NaNO2 solution adjusted at pH 2 (Furchgott, 1988), and the concentrations of NaNO2 in the bathing media were expressed as those of NO.
Results
Effects of acetylcholine and other agonists
In corpus cavernosum strips partially contracted with phenylephrine, acetylcholine (10 nM–10 μM) produced a concentration-related relaxation. Dose-response curves of acetylcholine did not significantly differ in the first, second and 3rd trials; mean values of the relaxation at 100 nM were 25.0±3.0, 20.8±4.3 and 21.3±3.8% (n=6), respectively, and those at 1 μM were 65.2±5.9 and 66.5±6.5 and 66.0±6.1% (n=6), respectively. Therefore, the first dose-response curve was taken as a control, and blocking agents or saponin were applied before the 2nd curve was obtained.
Treatment with L-NOARG (10 μM) significantly attenuated the response, and the addition of L-arginine (1 mM) reversed the inhibition (Figure 1, left). Raising the concentration of L-NOARG to 100 μM produced the additional inhibition in the acetylcholine-induced relaxation (Figure 2), but did not abolish it. In order to test the possibility of the involvement of other factors such as EDHF in the acetylcholine-induced relaxation or for insufficient inhibition by 100 μM L-NOARG of the relaxation, the effect of ODQ (1 μM), a soluble guanylate cyclase inhibitor, was examined in the presence of L-NOARG (100 μM). This treatment did not produce further inhibition. However, as shown in Figure 2, the relaxation was markedly inhibited in the L-NOARG (100 μM)-treated strips when contracted with K+ (15–20 mM). Typical tracings of the response are illustrated in Figure 3. Under the same conditions, the sodium nitroprusside-induced relaxation was not significantly affected (n=4). Indomethacin (1 μM) did not alter the acetylcholine-induced relaxation (Figure 1, right), whereas atropine (1 μM) abolished the response at concentrations up to 10 μM (n=3).
Figure 1.
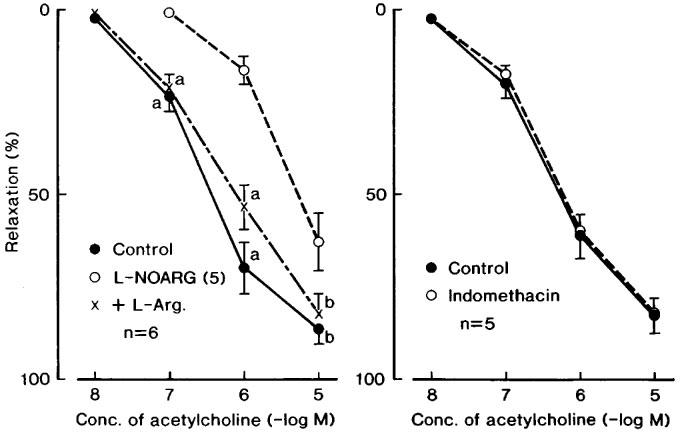
Modifications by L-NOARG (10 μM, 5 in the parenthesis) and L-NOARG plus L-arginine (L-Arg., 1 mM) (left figure) and by indomethacin (1 μM) (right) of the relaxant response to acetylcholine in corpus cavernosum strips contracted with phenylephrine. The ordinate represents relaxations relative to those induced by 0.1 mM papaverine. Significantly different from the value with L-NOARG, aP<0.01; bP<0.05 (Tukey's test). ‘n' denotes the number of strips from separate dogs. Vertical bars represent s.e.mean.
Figure 2.
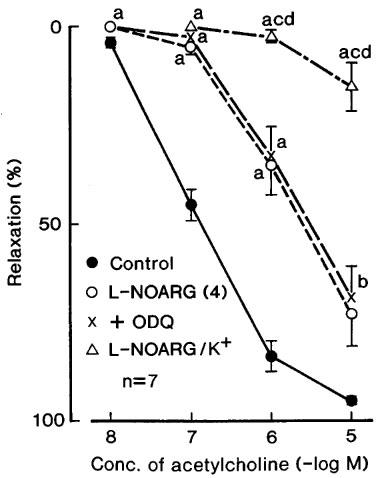
Modifications by L-NOARG (100 μM, 4 in the parenthesis), L-NOARG plus ODQ (1 μM) (+ODQ) and L-NOARG plus K+ (L-NOARG/K+) of the relaxant response to acetylcholine in corpus cavernosum strips. The strips were contracted with phenylephrine except for L-NOARG/K+ in which the preparations were contracted with K+ (15–20 mM), the concentration being adjusted to match the contraction induced by phenylephrine under control conditions. The ordinate represents relaxations relative to those elicited by 0.1 mM papaverine. Significantly different from control, aP<0.01, bP<0.05; significantly different from the value with ODQ, cP<0.01; significantly different from the value with 100 μM L-NOARG dP<0.01 (Tukey's test). ‘n' denotes the number of strips from separate dogs. Vertical bars represent s.e.mean.
Figure 3.
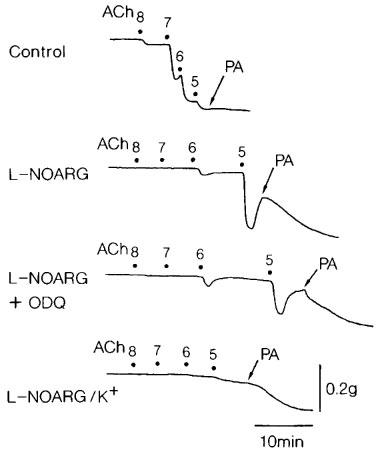
Tracings of the response to acetylcholine (ACh, 10 nM–10 μM) as affected by L-NOARG (100 μM), L-NOARG plus ODQ (1 μM) and L-NOARG plus ODQ (K+) in a corpus cavernosum strip. The preparation was contracted with phenylephrine in the upper three tracings but with K+ (15 mM) in the bottom tracing. PA represents 0.1 mM papaverine that produced the maximal relaxation. Numbers indicate the concentrations of the drugs in −log[M].
Substance P (10 nM) and bradykinin (1–100 nM) did not relax the strips (n=8), and one out of the eight strips responded to bradykinin with slight contraction.
ATP elicited a dose-related relaxation, which was attenuated by treatment with 20 μM aminophylline; mean values of the response to 0.1, 1 and 10 μM ATP were 4.3±1.7, 23.3±4.3 and 84.7±3.5% (n=6), respectively, in the control media and those were 0 (n=6, P<0.05 vs corresponding control; unpaired t-test), 5.7±1.9 (P<0.01) and 49.5±6.1% (P<0.001), respectively, in the aminophylline-containing media.
Modifications by treatment with saponin
Exposure for 45 min to bathing media containing saponin (1 mg ml−1) abolished or markedly attenuated the acetylcholine-induced relaxation but did not alter the dose-response curve of sodium nitroprusside (Figure 4). Relaxations to NO were not influenced by treatment with saponin; mean values of the response at 10 and 100 μM NO were 29.1±2.3 and 72.3±8.1% (n=5), respectively, in control media, and 31.6±10.5 and 75.4±8.9% (n=5), respectively, in the strips treated with saponin. Contractions induced by K+ and phenylephrine were also unaffected by treatment with saponin; mean values of the response to K+ (30 mM) before and after saponin treatment were 445±45 and 423±43 mg (n=4), respectively, and those to 10 μM phenylephrine before and after the treatment were 133.8±21.6 and 142.5±28.2% relative to those induced by 30 mM K+, respectively. Relaxations induced by ATP were not influenced by treatment with saponin (Figure 5, left). In contrast, the responses to A23187 (10 and 100 nM) were abolished by the same treatment (Figure 5, right).
Figure 4.
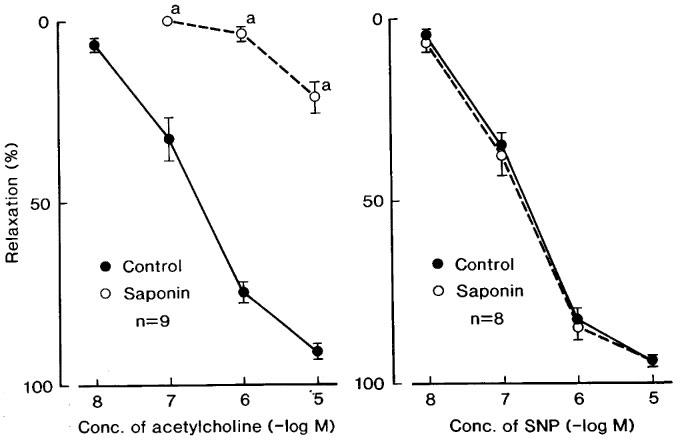
Concentration-response curves of acetylcholine (left figure) and sodium nitroprusside (SNP) (right) in corpus cavernosum strips before (control) and after treatment with saponin. The strips were contracted with phenylephrine. The ordinate represents relaxations relative to those induced by 0.1 mM papaverine. Significantly different from control, aP<0.001 (unpaired t-test). ‘n' denotes the number of strips from separate dogs. Vertical bars represent s.e.mean.
Figure 5.
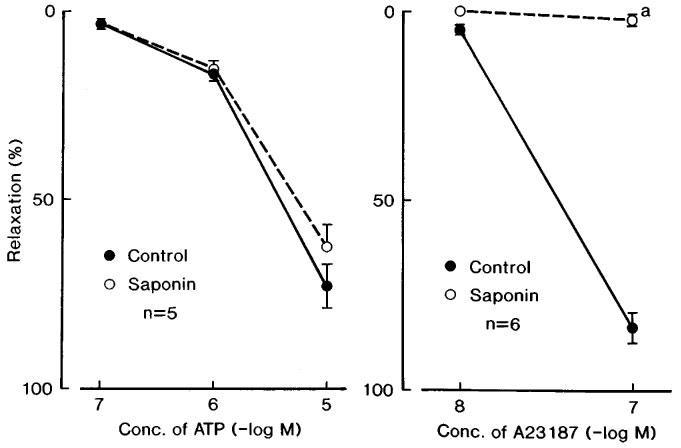
Concentration-response curves of ATP (left figure) and Ca2+ ionophore A23187 in corpus cavernosum strips before (control) and after treatment with saponin. The strips were contracted with phenylephrine. Significantly different from control, aP<0.001 (unpaired t-test). ‘n' denotes the number of strips from separate dogs. Vertical bars represent s.e.mean.
In order to determine whether NO liberated from the endothelium of copora cavernosa was involved in the relaxation induced by transmural electrical stimulation, the neurogenic response of the cavernous strips treated with bretylium (10 μM) and contracted with phenylephrine was compared before and after treatment with saponin. The frequency-response curve of nerve stimulation was not significantly influenced in the strips treated with saponin; mean values of the response relative to that induced by 0.1 mM papaverine in the control (n=7) and the saponin-treated strips (n=7) were 43.1±7.0 and 44.6±6.1% at 2 Hz, 59.6±6.1 and 60.6±5.0% at 5 Hz, and 61.3±4.8 and 65.1±3.7%, respectively. The stimulation-induced response was abolished by tetrodotoxin (0.3 μM) in all of the strips and by L-NOARG (10 μM) in three out of three strips tested.
Effects of acetylcholine and VIP on prejunctional receptors in saponin-treated strips
In cavernous strips previously treated with saponin and contracted with phenylephrine, the relaxation elicited by transmural electrical stimulation at 5 Hz was not influenced by acetylcholine (1 and 10 μM), atropine (0.1 μM) and physostigmine (0.1 μM). The mean value of the control response was 58.6±11.2% (n=5) relative to that induced by 0.1 mM papaverine, and changes caused by treatment with acetylcholine (1 and 10 μM), atropine (0.1 μM) and physostigmine (0.1 μM) were 1.3±2.7 (n=5), −3.8±3.9 (n=4), 5.2±3.8 (n=5) and 3.4±2.8% (n=5), respectively, the differences being statistically insignificant (P>0.05). These concentrations, which were sufficient to stimulate or inhibit prejunctional muscarinic receptors and to possibly inactivate cholinesterase in nitrergic nerve terminals (Toda et al., 1997a,1997b), did not alter the basal tone of cavernous strips by treatment with saponin. In eight strips without saponin treatment, no significant influence of physostigmine and atropine on the response to nerve stimulation was observed; mean values in control media and those containing these inhibitors were 50.9±8.6, 55.5±8.8 and 55.9±6.2%, respectively.
VIP (1 and 3 nM) relaxed the strips (14.9±2.5 and 21.0±3.8%, respectively, n=8) but did not significantly alter the response to nerve stimulation; mean values of the response to nerve stimulation before and after treatment with VIP in these two concentrations were 44.6±2.0, 46.1±2.6 and 51.7±3.8% (n=8), respectively. Additional treatment with 1 μM ODQ abolished the response (n=5).
Histological study
Ultrastructural changes in the endothelial cells in the sinus of corpus cavernosa induced by saponin treatment were examined. In the endothelial cells from strips without saponin treatment (control), such organelles as Golgi apparatus, rough endoplasmic reticulum, mitochondria and pinocytotic vesicles are clearly seen in the cytoplasm (Figure 6A). However, in the saponin-treated preparations, the endothelial cells were decreased in size and those organelles seen in controls were hardly visible under electron microscopy (Figure 6B and C). Most characteristically, the cytoplasmic processes were dramatically shrunken by the saponin treatment. They were folded (arrows in Figure 6B), and in severe cases these folds surrounded the perinuclear cytoplasm (arrows in Figure 6C). These results suggest that saponin treatment may induce degeneration of the endothelial cells in corpus cavernosa of penis. However, there was no ultrastructural change in the smooth muscle cells of saponium-treated strips.
Figure 6.
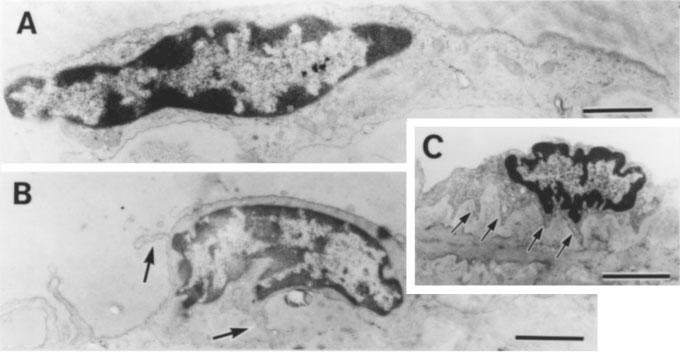
Electron photo micrographs of endothelial cells in the sinus of corpus cavernosa of penis in control (A) and saponin-treated (B and C) strips. Bars=2 μm. (A) Organelles such as Golgi apparatus, rough endoplasmic reticulum, mitochondria and pinocytotic vesicles are clearly seen in the cytoplasm. (B and C) Cell size is reduced compared to (A) and organelles seen in (A) are hardly seen in the cytoplasm. The cytoplasmic processes elongated laterally in (A) are dramatically shrunken and they are folded around the perinuclear cytoplasm (arrows).
Discussion
Canine cavernous strips contracted with phenylephrine responded to acetylcholine, nitroprusside, ATP and Ca2+ ionophore A23187 with relaxations but did not relax in response to peptides, such as substance P and bradykinin, which are reportedly strong vasodilators releasing NO from the endothelium in canine arteries (Furchgott, 1983). In contrast, these peptides relax cavernous strips from rabbits (Azadzoi et al., 1992). Treatment of the strips for 45 min with saponin (1 mg ml−1) abolished the relaxant response to acetylcholine and A23187 but failed to alter the response to nitroprusside, NO and ATP. Histological studies demonstrate that saponin did not detach the endothelial cells from underlying tissues, but induced degenerative changes in the endothelial cells, since such organelles as Golgi apparatus, rough endoplasmic reticulum, mitochondria and pinocytotic vesicles clearly seen in the cytoplasm of the strips without saponin treatment were hardly visible under electron microscopy in the saponin-treated strips. In contrast, such a degeneration is not observed in the smooth muscle cells. Therefore, treatment with saponin under the conditions used in this study is considered to selectively impair the endothelial function. Actually, contractions induced by K+ and phenylephrine were unaffected by treatment with saponin, as were nitroprusside-induced relaxations. Therefore, acetylcholine- and A23187-induced relaxations are considered to be endothelium-dependent as seen in those of a variety of blood vessels from dogs, whereas the response to ATP, an EDRF-releasing substance in canine arteries (Angus & Cocks, 1989), is independent of the endothelium in the corpus cavernosum. ATP-induced relaxations were attenuated by aminophylline, as those in canine cerebral arteries (Toda et al., 1982; Okamura et al., 1998), suggesting that the response is mediated by P1 purinoceptors and production of cyclic AMP (Williams, 1987).
Endothelium-dependent relaxations induced by acetylcholine were significantly attenuated by L-NOARG, and the inhibition was reversed by the addition of L-arginine, suggesting that NO from the endothelium is involved in the response. Similar findings have been reported in isolated rabbit and human cavernous strips (Kim et al., 1991). NO synthase binding sites and endothelial NO synthase are present on the endothelium of rat and human corpora cavernosa (Sullivan et al., 1996; Seftel et al., 1997). However, L-NOARG even in high concentrations and additional treatment with ODQ, an inhibitor of soluble guanylate cyclase (Garthwaite et al., 1995), did not abolish the relaxation. The remaining response was abolished in the strips exposed to high K+ solution. Under the same conditions, the sodium nitroprusside-induced relaxation was not significantly reduced. Therefore, acetylcholine may liberate a substance(s) that opens K+ channels, possibly resulting in hyperpolarization. The channel subtypes involved could not be analysed in the present study.
Histochemical studies have demonstrated that the corpus cavernosum is innervated by neurons containing NO synthase, cholinesterase and VIP. Release of acetylcholine from cholinergic nerves is detected by measurement of release of [3H]-acetylcholine in human corpus cavernosum strips (Blanco et al., 1988). Among the endogenous substances involved, NO is now recognized to play an important role in an elevation of intracavernous pressure and a penile erection (Burnett et al., 1992; Holmquist et al., 1991; Ayajiki et al., 1997). Although the possible involvement of acetylcholine and VIP as neurotransmitters in neurogenic relaxations is excluded (Hayashida et al., 1996), functional roles of these substances as neuromodulators have not been determined. The present study revealed that acetylcholine, physostigmine and atropine did not modify the relaxation mediated by NO produced from L-arginine in response to electrical nerve stimulation. Endogenous and exogenous acetylcholine does not seem to modulate functions of nitrergic nerve in canine corpus cavernosum. On the other hand, treatment with acetylcholine and physostigmine inhibited the response to nitrergic nerve stimulation in isolated monkey cerebral and porcine ciliary arteries, whereas atropine potentiated the response (Toda et al., 1997b; Toda et al., 1998). VIP, although it relaxed the strips, was also without effect on the neurogenic relaxation, suggesting that regulatory roles of endogenous VIP, even if released, are excluded.
Relaxations induced by electrical nerve stimulation and exogenous NO were not influenced by treatment with saponin. Despite the histological (Samata et al., 1986) and functional damage (present study) of endothelial cells by saponin, the vasodilator nerve and smooth muscle seem to be functionally unaffected. Further, the possibility that substances released by nerve stimulation elicit relaxation by a mediation of NO derived from the endothelium would be ruled out as reported in rabbit and human corpora cavernosa (Kim et al., 1991). The response to electrical stimulation was abolished by L-NOARG (present study; Pickard et al., 1993; Hayashida et al., 1996) and also by ODQ, despite the fact that acetylcholine-induced relaxations were partially inhibited by L-NOARG and the L-NOARG-resistant response was not susceptible to ODQ. These findings support the hypothesis that the neurogenic relaxation of cavernous smooth muscle is mediated solely by NO synthesized from L-arginine in nerve terminals in dogs. However, the fact that impairment of the endothelial function failed to reduce the response to nerve stimulation may indicate that acetylcholine in concentrations sufficient to liberate significant amounts of endothelium-derived NO is not released from cholinergic nerves.
In summary, acetylcholine liberates NO from the endothelium in canine copora cavernosa but does not act as a neurotransmitter or neuromodulator in inhibitory nerves. Acetylcholine-induced relaxations are likely to be mediated not only by NO but also other substance(s) from the endothelium. Despite the fact that an ability of the endothelium to release EDRF was proved by the use of acetylcholine and Ca2+ ionophore, there was no evidence for an endothelium-dependent response to ATP, substance P and bradykinin. Whether this is due to a lack of purinoceptors and substance P- and bradykinin-receptors in the endothelium of canine cavernous strips remains to be clarified.
Acknowledgments
This work was supported in part by the Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Culture and Sports, Japan.
Abbreviations
- ATP
adenosine triphosphate
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- L-NOARG
NG-nitro-L-arginine
- NO
nitric oxide
- ODQ
1H[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- VIP
vasoactive intestinal peptide
References
- ANDERSSON P.O., BLOOM S.R., MELLANDER S. Haemodynamics of pelvic nerve induced penile erection in the dog: possible mediation by vasoactive intestinal polypeptide. J. Physiol. (London) 1984;350:209–224. doi: 10.1113/jphysiol.1984.sp015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANGUS J.A., COCKS T.M. Endothelium-derived relaxing factor. Pharmac. Ther. 1989;41:303–351. doi: 10.1016/0163-7258(89)90112-5. [DOI] [PubMed] [Google Scholar]
- AYAJIKI K., HAYASHIDA H., OKAMURA T., TODA N. Pelvic nerve stimulation-induced pressor responses in corpus cavernosum of anesthetized dogs. Am. J. Physiol. 1997;273:H2141–H2145. doi: 10.1152/ajpheart.1997.273.5.H2141. [DOI] [PubMed] [Google Scholar]
- AZADZOI K.M., KIM N., BROWN M.L., GOLDSTEIN I., COHEN R.A., SAENZ DE TEJADA I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J. Urol. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- BENSON G.S., MCONNELL J., LIPSHULTZ L.J., CORRUIERE J.R., WOOD J. Neuromorphology and neuropharmacology of the human penis. J. Clin. Invest. 1980;65:506–513. doi: 10.1172/JCI109694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCO R., SAENZ DE TEJADA I., GOLDSTEIN I., KRANE R.J., WOTIZ H.H., COHEN R.A. Cholinergic neurotransmission in human corpus cavernosum. II. Acetylcholine synthesis. Am. J. Physiol. 1988;254:H468–H472. doi: 10.1152/ajpheart.1988.254.3.H468. [DOI] [PubMed] [Google Scholar]
- BURNETT A.L., LOWENSTEIN C.J., BREDT D.S., CHANG T.S.K., SNYDER S.H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- BURNETT A.L., TILLMAN S.L., CHANG T.S.K., EPSTEIN J.I., LOWENSTEIN C.J., BREDT D.S., SNYDER S.H., WALSH P.C. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J. Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F. Role of endothelium in responses of vascular smooth muscle. Circ. Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.Studies on relaxation of rabbit aorta by sodium nitrite: the basis for the proposal that the acid-activatable inhibitory factor from bovine retractor penis is inorganic nitrite and the endothelium-derived relaxing factor is nitric oxide Vasodilation: Vascular Smooth Muscle, Peptides, Autonomic Nerve, and Endothelium 1988New York: Raven Press; 401–414.ed. Vanhoutte, P.M. pp [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylate cyclase by 1H-[1,2,4]oxadiazolo[4,4-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GU J., POLAK J.M., PROBERT L., ISLAM K.N., MARANGO P.J., MINA S., ADRIAN T.E., MCGREGOR G.P., O'SHAAUGHNESSY D.J., BLOOM S.R. Peptidergic innervation of the human male genital tract. J. Urol. 1983;130:386–391. doi: 10.1016/s0022-5347(17)51174-x. [DOI] [PubMed] [Google Scholar]
- HAYASHIDA H., OKAMURA T., TOMOYOSHI T., TODA N. Neurogenic nitric oxide mediates relaxation of canine corpus cavernosum. J. Urol. 1996;155:1122–1127. [PubMed] [Google Scholar]
- HOLMQUIST F., STIEF C.G., JONAS U., ANDERSSON K.E. Effects of the nitric oxide synthase inhibitor NG-nitro-L-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol. Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUSH P.A., BUGA G.M., WOOD K.S., FUKUTO J.M., RAJFTER J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biopohys. Res. Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- KIM N., AZADZOI K.M., GOLDSTEIN I., SAENZ DE TEJADA I. A nitric oxide-like factor mediates nonadrenergic noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y.C., KIM J.H., DAVIES M.G., HAGEN P.O., CARSON C.C. Modulation of vasoactive intestinal polypeptide (VIP)-mediated relaxation by nitric oxide and prostanoids in the rabbit corpus cavernosum. J. Urol. 1995;153:807–810. [PubMed] [Google Scholar]
- OKAMURA T., AYAJIKI K., UCHIYAMA M., KAGAMI K., TODA N. Mechanisms underlying constrictor and dilator responses to perivascular nerve stimulation in canine lingual arteries. Eur. J. Pharmacol. 1998;354:43–50. doi: 10.1016/s0014-2999(98)00425-7. [DOI] [PubMed] [Google Scholar]
- PICKARD R.S., POWELL P.H., ZAR M.A. Evidence against vasoactive intestinal polypeptide as the relaxant neurotransmitter in human cavernosal smooth muscle. Br. J. Pharmacol. 1993;108:497–500. doi: 10.1111/j.1476-5381.1993.tb12831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJFER J., ARONSON W.J., BUSH P.A., DOREY F.J., IGNARRO L.J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. New Engl. J. Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- SAENZ DE TEJADA I., BLANCO R., GOLDSTEIN I., AZADZOI K., DE LAS MORENAS A., KRANE R.J., COHEN R.A. Cholinergic neurotransmission in human corpus cavernosum: I. Responses of isolated tissues. Am. J. Physiol. 1988;254:H459–H467. doi: 10.1152/ajpheart.1988.254.3.H459. [DOI] [PubMed] [Google Scholar]
- SAMATA K., KIMURA T., SATOH S., WATANABE H. Chemical removal of the endothelium by saponin in the isolated dog femoral artery. Eur. J. Pharmacol. 1986;128:85–91. doi: 10.1016/0014-2999(86)90561-3. [DOI] [PubMed] [Google Scholar]
- SEFTEL A.D., VAZIRI N.D., NI Z., RAZMJOUEI K., FOGARTY J., HAMPEL N., POLAK J., WANG R.Z., FERGUSON K., BLOCK C., HAAS C. Advanced glycation end products in human penis: elevation in diabetic tissue, site of deposition, and possible effect through iNOS or eNOS. Urology. 1997;50:1016–1026. doi: 10.1016/S0090-4295(97)00512-8. [DOI] [PubMed] [Google Scholar]
- SHIRAI M., SASAKI K., RIKIMARU A. Histochemical investigation on the distribution of adrenergic and cholinergic nerves in human penis. Tohoku J. Exp. Med. 1972;107:403–404. doi: 10.1620/tjem.107.403. [DOI] [PubMed] [Google Scholar]
- SULLIVAN M.E., BELL C.R., DASHWOOD M.R., MILLER M.A., THOMPSON C.S., MIKHAILIDIS D.P., MORGAN R.J. Autoradiographic localization of nitric oxide synthase binding sites in normal and diabetic rat corpus cavernosum. Eur. J. Urol. 1996;30:506–511. doi: 10.1159/000474225. [DOI] [PubMed] [Google Scholar]
- TODA M., OKAMURA T., AZUMA I., TODA N. Modification by neurogenic acetylcholine of nitroxidergic nerve function in porcine ciliary arteries. Invest. Ophthal. Vis. Sci. 1997b;38:2261–2269. [PubMed] [Google Scholar]
- TODA N., AYAJIKI K. Cholinergic prejunctional inhibition of vasodilator nerve function in bovine basilar arteries. Am. J. Physiol. 1990;258:H983–H986. doi: 10.1152/ajpheart.1990.258.4.H983. [DOI] [PubMed] [Google Scholar]
- TODA N., AYAJIKI K., OKAMURA T. Inhibition of nitroxidergic nerve function by neurogenic acetylcholine in monkey cerebral arteries. J. Physiol., Lond. 1997a;498:453–461. doi: 10.1113/jphysiol.1997.sp021871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODA N., OKUNISHI H., TANIYAMA K., MIYAZAKI M. Responses to adenine nucleotides and related compounds of isolated dog cerebral, coronary and mesenteric arteries. Blood Vessels. 1982;19:226–236. doi: 10.1159/000158389. [DOI] [PubMed] [Google Scholar]
- TODA N., TODA M., AYAJIKI K., OKAMURA T. Cholinergic nerve function in monkey ciliary arteries innervated by nitroxidergic nerve. Am. J. Physiol. 1998;274:H1582–H1589. doi: 10.1152/ajpheart.1998.274.5.H1582. [DOI] [PubMed] [Google Scholar]
- TRAISH A.M., CARSON M.P., KIM N., GOLDSTEIN I., SAENZ DE TEJADA I. Characterization of muscarinic acetylcholine receptors in human penile corpus cavernosum: studies on whole tissue and cultured endothelium. J. Urol. 1990;144:1036–1040. doi: 10.1016/s0022-5347(17)39653-2. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M. Purine receptors in mammalian tissues: pharmacology and functional significance. Ann. Rev. Pharmacol. Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- WILLIS E., OTTESEN B., WAGNER G., SUNDLER F., FAHRENKRUG J. Vasoactive intestinal polypeptide (VIP) as a possible neurotransmitter involved in penile erection. Acta Physiol. Scand. 1981;113:545–547. doi: 10.1111/j.1748-1716.1981.tb06936.x. [DOI] [PubMed] [Google Scholar]


