Abstract
A sustained tone was produced in rat isolated anococcygeus muscles with guanethidine and clonidine and relaxant responses were elicited by electrical stimulation of its nitrergic nerves and by the three redox forms of nitrogen monoxide.
The nitroxyl anion (NO−) was donated by dissociation of Angeli's salt; the free radical (NO•) was from an aqueous solution of nitric oxide gas; the nitrosonium cation (NO+) was donated by dissociation of nitrosonium tetrafluoroborate.
The concentrations producing approximately 50% relaxations of the anococcygeus muscle were 0.3 μM for Angeli's salt (nitroxyl), 0.5 μM for NO• and 100 μM for nitrosonium tetrafluoroborate. Nitrergic nerve stimulation at 1 Hz for 10 s produced equivalent relaxant responses.
The superoxide generator pyrogallol (100 μM) had no effect on responses to nitrergic nerve stimulation or Angeli's salt but significantly reduced responses to NO• and nitrosonium tetrafluoroborate.
The NO• scavenger carboxy-PTIO (100 μM) had no effect on responses to nitrergic nerve stimulation or Angeli's salt but significantly reduced responses to NO• and nitrosonium tetrafluoroborate.
Hydroxocobalamin (30 μM) had no significant effect on responses to the nitrergic transmitter, enhanced the response to Angeli's salt, and significantly reduced responses to NO• and nitrosonium tetrafluoroborate.
The findings suggest that the nitroxyl anion donated by Angeli's salt is a better candidate than NO• to serve as the nitrergic transmitter in the rat anococcygeus muscle, although it still does not behave exactly like the transmitter.
Keywords: Angeli's salt, carboxy-PTIO, hydroxocobalamin, nitrergic transmitter, nitric oxide, nitrosonium cation, nitrosonium tetrafluoroborate, nitroxyl anion, pyrogallol
Introduction
This paper is concerned with the pharmacological activity of the three redox forms of nitric oxide: the nitrosonium anion (NO+), the free radical (NO•), and the nitroxyl cation (NO−) (Stamler et al., 1992). We have preferred the more generic term nitrogen monoxide since the unqualified term nitric oxide has generally been taken to mean the free radical, in which form nitrogen monoxide exists in the gaseous state, albeit in solution in biological systems with the exception of that in the airways.
The functional integrity of neuronal nitric oxide synthase (nNOS) is essential for nitrergic transmission and its nitrogen monoxide product from L-arginine is generally assumed to be NO• (Moncada et al., 1991). However, NO• has pharmacological properties that do not conform to those of the nitrergic transmitter in a number of tissues, of which the rat anococcygeus muscle has been most studied (for reviews, see Rand & Li, 1995b,1995c). Thus, the relaxant action of exogenous NO• (in aqueous solution) is blocked or greatly attenuated by superoxide generators such as pyrogallol and the scavenging agents carboxy-PTIO and hydroxocobalamin in concentrations that do not reduce relaxant responses to the nitrergic transmitter. These findings led to the suggestion that the nitrergic transmitter, at least in the rat anococcygeus muscle, is not NO• and might be an NO-donating adduct (Rand & Li, 1995b,1995c; Rand et al., 1997), although alternative explanations for the discrepancies have been proposed in terms of protection of transmitter NO• from inactivation. The latter are highlighted by the findings that endogenous antioxidants such as superoxide dismutase (SOD) and ascorbate can interact with certain superoxide generators and NO scavengers (Martin et al., 1994; Gibson & Lilley, 1997) (see Discussion).
It occurred to us that the simplest possibility is that the transmitter might be nitrogen monoxide, but not in the NO• redox form. Therefore, we set out to compare the actions of the nitrergic transmitter in the rat anococcygeus muscle with those of donors of nitroxyl (NO−) and nitrosonium (NO+) ions as well as NO• from nitric oxide gas in aqueous solution, and compared the effects of pyrogallol, carboxy-PTIO and hydroxocobalamin on their actions. A preliminary account of this work was presented to the Australasian Society for Clinical and Experimental Pharmacologists and Toxicologists (Karagiannis et al., 1998).
Methods
Tissue preparation
Male Sprague-Dawley rats (200–400 g) were killed humanely by narcotizing with carbon dioxide and decapitation. The paired anococcygeus muscles were removed and each was mounted under a resting tension of 1 g in 4 ml organ baths containing physiological salt solution (PSS) bubbled with 95% O2 and 5% CO2 and maintained at 37°C as described elsewhere (Gillespie, 1972; Li & Rand, 1989). The tension of the muscle was measured with an isometric transducer (Grass FT 03) and recorded with a MacLab acquisition system. Before experimental interventions, the isolated muscles were equilibrated for 30 min; during this period, the PSS in the organ bath was replaced every 10 min.
Experimental protocols
After the equilibration period, guanethidine (10–20 μM) and clonidine (0.3 μM) were added to block noradrenergic responses and to produce a stable increase in muscle tone. Responses to electrical stimulation or relaxant agents were elicited after the tone had reached a steady level (30–45 min). The mean tone was 7.93±0.69 g (n=36).
Relaxations induced by nitrergic nerve stimulation were elicited by electrical field stimulation (EFS) with 1 ms pulses of supramaximal voltage at various frequencies for 10 s periods delivered from a MultiStim system-D330 stimulator through a pair of platinum wire electrodes placed on either side of the muscle. Frequency-response curves (0.5–5 Hz) were constructed for EFS in muscles that were used for no other purpose.
Non-cumulative concentration-response curves were constructed with each of the three redox forms of nitrogen monoxide, fresh PSS being replaced after each concentration was tested. The forms and their sources are as follows. The nitrosonium cation (NO+) was donated by dissociation of nitrosonium tetrafluoroborate (NT; NOF4B) (Mohr et al., 1994). Free radical nitric oxide (NO•) was a saturated solution of nitric oxide gas (2 mM) in deoxygenated water, prepared as described elsewhere (Ishii et al., 1991; Rajanayagam et al., 1993). The nitroxyl or nitrosyl anion (NO−) was donated by Angeli's salt (AS; Na2ONNO2), which dissociates to yield NO− and NO2− (Zamora et al., 1995). Only one of the redox forms of nitrogen monoxide was used in any one muscle preparation.
When studying the effects of various drugs on responses to EFS or the redox forms of nitrogen monoxide, responses to one of these were elicited at 5 min intervals until the responses became constant. Approximately equivalent relaxations amounting to about 50% of the pre-existing tone were obtained with EFS at 1 Hz for 10 s, 0.3 μM AS (NO−), 0.5 μM NO•, and 100 μM NT (NO+); the bath was washed with fresh PSS after each response. Then ODQ (1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one) (1 μM) or one of the transmitter/NO•-differentiating agents pyrogallol (100 μM), carboxy-PTIO (100 μM) and hydroxocobalamin (30 μM) was added to the organ bath and the responses were repeated. Separate anococcygeus muscles were used for each pair of differentiating agent and relaxant stimulus. In addition, time-control experiments were carried out with the relaxants.
Drugs and reagents
In most experiments, the PSS had the following composition (mM): NaCl, 118; KCl, 4.7; NaHCO3, 25; MgSO4, 0.45; KH2PO4, 1.03; CaCl2, 2.5; D-(+)-glucose, 11.1; disodium edetate, 0.067; ascorbic acid, 0.14. However, ascorbic acid was omitted from the PSS in experiments in which carboxy-PTIO was used; this was because ascorbic acid reduces it to N-hydroxy-carboxy-PTIO, which does not react with NO• (Tsunoda et al., 1994).
The following drugs were used: guanethidine monosulphate, hydroxocobalamin, and pyrogallol were obtained from Sigma Chemical Co. (Castle Hill, NSW, Australia); nitrosonium tetrafluoroborate was obtained from Aldrich Chemical Co. (Castle Hill, NSW, Australia); Angeli's salt (sodium trioxydinitrate; hyponitric acid, disodium salt; Na2(NONO2) and carboxy-PTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide] were obtained from Sapphire Bioscience (Alexandria, NSW, Australia); clonidine was obtained from Boehringer (Ingelheim, Germany); ODQ (1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one) was from Cayman Chemical company (Denver, Colorado, U.S.A.). Saturated solutions containing 2 mM of NO• were prepared from nitric oxide gas (compressed gas, CIG, Australia) as previously described by Ranajayagam et al. (1993).
All drugs were dissolved in distilled water except for AS which was dissolved in 0.01 M NaOH, and ODQ which was dissolved in DMSO. Stock solutions were subsequently diluted in PSS. The maximum final concentrations for 0.01 M NaOH and DMSO in the organ baths were 0.75 and 0.5%, respectively. In these concentrations there was no effect on the tone of the muscle or on relaxant responses. The stock solution of 40 mM AS in 0.01 M NaOH was made freshly every 2 days and was kept on ice. The stock solution of 40 mM NT in distilled water was made freshly for each experiment.
Statistical analysis
Relaxations were measured as a percentage of the tone induced by guanethidine and clonidine and were converted to a percentage of the initial control relaxation when comparison were made after addition of modifying agents. Data are expressed as mean±standard error of the mean, with the number of experiments denoted by n. Relaxations to EFS, AS (NO−), NO• and NT (NO+) in the presence of drugs were compared with those obtained in the corresponding time control experiments (unpaired Student's t-test). Statistical analysis was performed using the software program SigmaStat for windows (Version 1.0, Jandel Scientific) taking P<0.05 to indicate a significant difference.
Ethics
The RMIT animal ethics committee approved these experiments.
Results
Responses to EFS and the redox forms of nitrogen monoxide
As illustrated in Figure 1, the relaxant responses to all three redox forms of nitrogen monoxide were reproducible and provided reasonable mimicry of the response to the nitrergic transmitter. Other control responses are shown in Figures 3, 5, 7 and 8.
Figure 1.
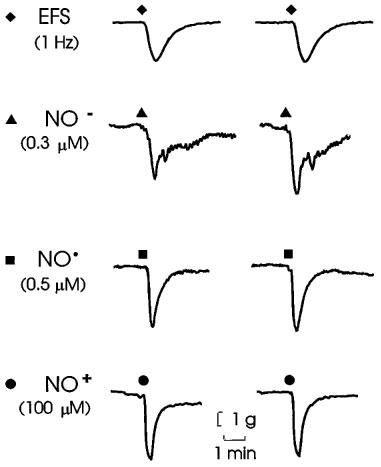
Representative relaxations of anococcygeus muscles produced by electrical field stimulation of nitrergic nerves (EFS; 1 Hz for 10 s), nitroxyl (NO−) donated from Angeli's salt, NO• from an aqueous solution of nitric oxide gas, and NO+ donated from nitrosonium tetrafluoroborate at the points indicated by the symbols in time-control experiments. The parameters of stimulation and concentrations used produced relaxations approximately equal to 50% of the pre-existing tone (see Figure 2).
Figure 3.
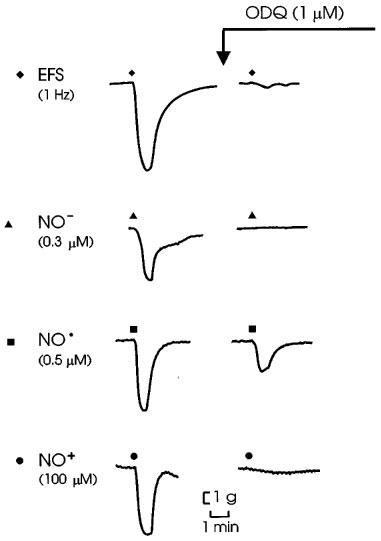
Representative tracings of effects of ODQ on responses to electrical field stimulation of nitrergic nerves (EFS; 1 Hz for 10 s), NO− donated from Angeli's salt, NO• and NO+ donated from nitrosonium tetrafluoroborate.
Figure 5.
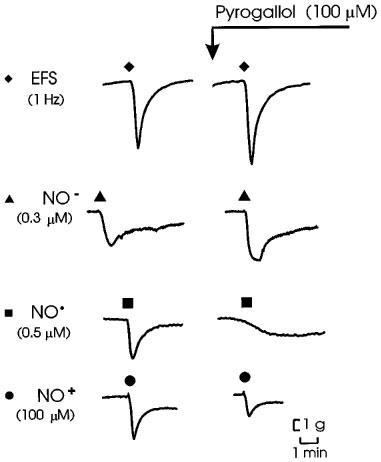
Representative tracings of effects of pyrogallol (100 μM) on responses to electrical field stimulation of nitrergic nerves (EFS; 1 Hz for 10 s). Angeli's salt (NO−; 0.3 μM), NO• (0.5 μM) and nitrosonium tetrafluoroborate (NO+; 100 μM).
Figure 7.
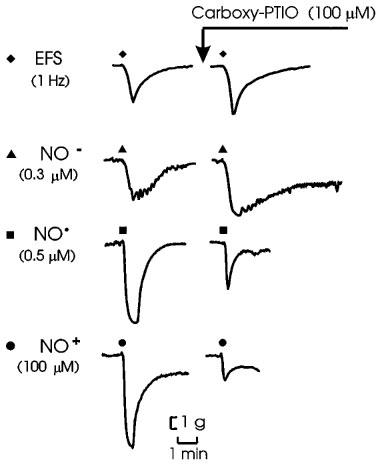
Representative tracings of effects of carboxy-PTIO (100 μM) on responses to electrical field stimulation of nitrergic nerves (EFS; 1 Hz for 10 s), Angeli's salt (NO−; 0.3 μM), NO• (0.5 μM) and nitrosonium tetrafluoroborate (NO+; 100 μM).
Figure 8.
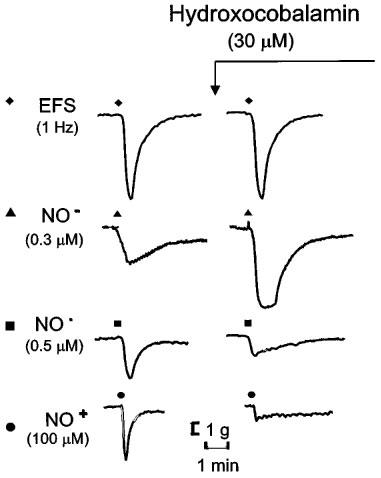
Representative tracings of effects of hydroxocobalamin (30 μM) on responses to electrical field stimulation of nitrergic nerves (EFS; 1 Hz for 10 s), Angeli's salt (NO−; 0.3 μM), NO• (0.5 μM) and nitrosonium tetrafluoroborate (NO+; 100 μM).
Concentration-response curves for the redox forms and a frequency-response curve for EFS are shown in Figure 2. AS (NO−) was slightly more potent than NO• and NT (NO+) was the least active, having about 0.5% of the potency of NO•. The concentrations producing relaxations of approximately 50% of the pre-existing tone were 0.3 μM AS (NO−), 0.5 μM NO•, and 100 μM NT (NO+). EFS at 1 Hz for 10 s produced an equivalent response.
Figure 2.
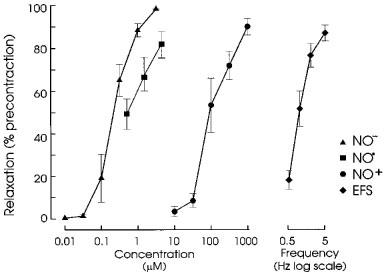
Concentration-response curves for the relaxant actions of nitroxyl (NO− donated from Angeli's salt), NO• in aqueous solution and nitrosonium (NO+ donated from nitrosonium tetrafluoroborate) and frequency-response curve for electrical field stimulation (EFS; for 10 s periods). I-bars indicate standard errors of means (n=3–6 for each point).
Effects of ODQ
ODQ (1 μM) produced an initial decrease in tone, but after 5–10 min the tone recovered to the initial level. Relaxant responses to EFS and all three redox forms of nitrogen monoxide were abolished or greatly reduced by ODQ. Representative tracings and grouped data are shown in Figures 3 and 4, respectively.
Figure 4.
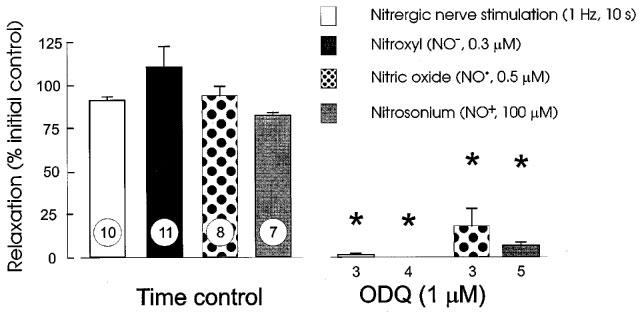
Blockade of responses to nitrergic nerve stimulation (EFS; 1 Hz for 10 s), Angeli's salt (NO−, 0.3 μM), NO• (0.5 μM) and nitrosonium tetrafluoroborate (NO+; 100 μM) by 1 μM ODQ. Column heights indicate means and I-bars are s.e.means with n indicated by the number at the foot of each column; * indicates significant difference from time control (unpaired t-test, P<0.05).
Pyrogallol
Pyrogallol (100 μM) had no effect on tone. Pyrogallol did not affect responses to EFS or AS NO−), but reduced response to NO• and NT (NO+) to about 50 and 45%, respectively, of their control levels. Representative records are shown in Figure 5 and group data are in Figure 6.
Figure 6.
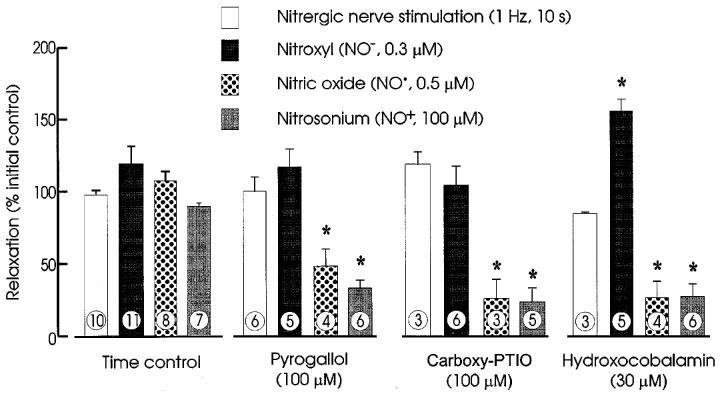
Effects of time controls, pyrogallol (100 μM), carboxy-PTIO (100 μM), and hydroxocobalamin (30 μM) on relaxations of rat anococcygeus muscles produced by nitrergic nerve stimulation (1 Hz for 10 s), Angeli's salt (NO−; 0.3 μM), NO• (0.5 μM) and nitrosonium tetrafluoroborate (NO+; 100 μM) expressed as percentages of relaxations before addition of a modifying agent (or elapse of time for controls). Column heights indicate means and I-bars are s.e.means. The number of muscles contributing to each mean is indicated at the foot of each column.
Carboxy-PTIO
In experiments with carboxy-PTIO, ascorbate was omitted from the PSS (see Methods). The responses to the redox forms of nitrogen monoxide and to EFS were not noticeably affected by its omission (compare control responses in Figure 7 with those in Figures 1, 3, 7 and 8). Carboxy-PTIO (100 μM) had no effect on tone and had no significant effects on responses to EFS or to AS (NO−) but reduced responses to NO• and NT (NO+) to less than 25% of control. Representative tracings and grouped data are shown in Figure 7 and grouped data are in Figure 6.
Hydroxocobalamin
Hydroxocobalamin (30 μM) had no effect on tone or on responses to EFS. Responses to AS (NO−) were enhanced to 140% of control, but responses to NO• and NT (NO+) were reduced to less than 10% of control. Representative tracings and grouped data are shown in Figures 8 and 6, respectively.
Discussion
Previous comparative studies of the smooth muscle relaxant activities of the redox forms of nitrogen monoxide have been largely confined to vascular tissues and related to the activity of endothelium derived relaxing factor (EDRF). We are not aware of other studies in which they were compared with the nitrergic transmitter except for a recent study by Goyal & He (1998), who compared the effects of NO• and NO+ in guinea-pig ileal circular muscle. Goyal & He (1998) used sodium nitroprusside as the NO+ donor and failed to test the effect of nitroxyl anions; therefore, their claim that the NO• redox form of NO is the nitrergic inhibitory neurotransmitter needs to be re-evaluated.
The validity of any conclusions arising from the findings presented in this paper depend on the assumptions made about Angeli's salt as a donor of nitroxyl anions and nitrosonium tetrafluoroborate as a donor of nitrosonium cations. It is apparent from their formulae that these ionic forms of nitrogen monoxide could arise by dissociation of the donor salts, although we have no direct evidence for the existence of the ionic forms, nor do we have any data about the dissociation constants of the salts. We have assumed complete dissociation: if it is incomplete, the potencies ascribed to the ionic forms would be higher. It has been stated that the rate constant for decomposition of AS is 5×10−3 s−1 and that 0.54 moles of NO• are released per mole of AS, as determined from the integrated chemiluminescence (Maragos et al., 1991), but this may not be a valid measure of NO− ions in solution, although it does suggest that part of the NO− released from AS is eventually oxidized to NO•.
Nitrosonium cation
It has been reported that NO+ lacked vasodilator activity (Feelisch et al., 1994). According to Pauling (1960), nitrosonium tetrafluoroborate contains the nitrosonium cation (NO+) [he terms it the nitrosyl cation]. Nitrosonium tetrafluoroborate had only about 0.5% of the relaxant potency of NO• on the rat isolated anococcygeus muscle and was not differentiated from NO• by any of the agents used (pyrogallol, carboxy-PTIO and hydroxocobalamin). Its low potency and its differentiation from the nigrergic transmitter in the rat anococcygeus muscle militate against its consideration as a candidate for the transmitter. On the available evidence, we could not discount the possibility that a portion of the nitrosonium ions donated by its dissociation was reduced to NO•. The lack of vasodilator activity of NO+ reported by Feelisch et al. (1994) may have been because too low a concentration had been tested, or possibly because a small portion was less readily converted to NO• in the aorta than in the anococcygeus muscle.
Nitroxyl anion
It has been found that donors of nitroxyl ions (NO−) have vasodilator activity (Fukuto et al., 1992a; Pino & Feelisch, 1994; Zamora et al., 1995). The EC50 values for relaxation of rabbit aortic rings by nitroxyl was 0.59 μM when it was donated from AS (Maragos et al., 1991) and 1.03 μM when it was donated from Piloty's salt (benzenesulphohydroxamic acid) (Nagasawa et al., 1995). Thus it appears that nitroxyl was considerably less potent than NO• in the rabbit aorta.
It has been suggested that conversion of nitroxyl (HNO) to NO• may account for the biological activity of NO− since it was accelerated by a variety of oxidants (Fukuto et al., 1993; Hobbs et al., 1994). On the other hand, Pino & Feelisch (1994) and Zamora et al. (1995) considered that the nitroxyl acted as such. Zamora et al. (1995) stated that AS dissociates to yield nitroxyl (NO−) and nitrite (NO2−) ions but the potency of NO− is sufficiently great that NO2− did not contribute to its pharmacological activity. The present findings support this view since the EC50 for the relaxant action of Angeli's salt was about 0.3 μM, which is at least three orders of magnitude less than that of a threshold concentration of NO2− (300 μM) on the rat anococcygeus muscle (Li et al., unpublished observation).
Pino & Feelisch (1994) showed that high concentrations of L-cysteine completely inhibited the relaxant response to NO− (from AS or sodium nitroxyl) but largely enhanced that to NO•, thereby distinguishing between these two redox forms of nitrogen monoxide. We also observed differences between NO− and NO• in our experiments, since pyrogallol, carboxy-PTIO and hydroxocobalamin reduced responses to NO•, but not those to AS. Furthermore, AS was slightly more potent than NO•. In addition, we also found in preliminary experiments that L-cysteine inhibited responses to both AS and nitrergic nerve stimulation but enhanced responses to NO• (Li et al., unpublished observations). These findings indicate that the pharmacologically active component derived from AS is distinct from NO• and is reasonably assigned to the nitroxyl anion (NO−), at least for the period between its addition to the organ bath and the triggering of the response.
Effects of ODQ
The relaxant responses to all three redox forms of nitrogen monoxide were abolished or markedly reduced by the inhibitor of soluble guanylate cyclase ODQ (Garthwaite et al., 1995). However, it has been reported that only the NO• form of nitrogen monoxide activates partially purified preparations of soluble guanylate cyclase (Dierks & Burstyn, 1996). This raises the possibility that the nitroxyl anions (NO−) donated by Angeli's salt were converted to NO•. Nevertheless, studies with agents that differentiate between NO• and the nitrergic transmitter revealed a number of differences between nitroxyl and NO•, suggesting that nitroxyl retained its putative identity at least until it had entered the smooth muscle effector cells containing soluble guanylate cyclase. Although there might then have been intracellular conversion of nitroxyl to NO• before activation of guanylate cyclase, it is difficult to reconcile this possibility with the greater relaxant potency of nitroxyl than of the free radical form of ntirogen monoxide on the anococcygeus muscle. This matter raises the question of the relevance of biochemical studies on more or less purified enzymes to their activity in an organized physiological milieu.
Effects of pyrogallol, carboxy-PTIO and hydroxocobalamin
The superoxide generator pyrogallol readily inactivates NO• and abolishes response to it and to EDRF by the formation of nitrate or peroxynitrite (Rubyani & Vanhoutte, 1986), however it does not affect the response to nitrergic nerve stimulation in the rat gastric fundus (de Man et al., 1998) and anococcygeus muscle (Liu et al., 1997; La & Rand, 1999). In the present experiments, pyrogallol (100 μM) reduced responses to NO• and NT (NO+) but did not produce any reduction of responses to AS (NO−) or nitrergic nerve stimulation. NO• reacts rapidly with superoxide (O2•−) to form peroxynitrite (ONOO−) at a rate that is described as near diffusion-limited (Huie & Padmaja, 1993). This rapid interaction is presumably because they are both free radicals. Nitroxyl (NO−) is not a free radical and its anionic nature might also impede its possible reaction with the superoxide anion.
Carboxy-PTIO is a free radical that reacts rapidly and specifically with NO•, forming nitrite free radical and the corresponding imidazolineoxyl (Akaike et al., 1993), which are pharmacologically inactive in the concentrations produced. Carboxy-PTIO in a concentration of 10 μM blocks responses to NO• in rabbit (Akaike et al., 1993) and rat (Rand & Li, 1995a) aortic preparations. Carboxy-PTIO has similar activity against NO• in rat anococcygeus muscles, but is completely devoid of any blocking effect against the nitrergic transmitter and concentrations of 300 μM or more enhance the response to the transmitter (Rand & Li, 1995a). In the present experiments, 100 μM carboxy-PTIO had no significant effects on responses of rat anococcygeus muscles to electrical field stimulation or AS (NO−) but reduced responses to NO• and NT (NO+) to about 25% of control. Although carboxy-PTIO does not reduce responses to nitrergic nerve stimulation in anococcygeus muscles from the rat (Rand & Li, 1995a), mouse (Lilley & Gibson, 1996) or pig (Li & Rand, 1999) or the porcine retractor penis (Li & Rand, 1999), it did block them in the bovine retractor penis (Paisley & Martin, 1996). This may indicate that the transmitter in the bovine retractor penis is in fact NO•, possibly because the oxidative conditions in this tissue favour its formation.
Hydroxocobalamin reacts with NO• (Rochelle et al., 1995; Kruszyna et al., 1998) and blocks relaxant responses to it and to EDRF in rat aortic preparations and to NO• in rat anococcygeus muscles in concentrations that have little or no effect on responses to nitrergic nerve stimulation (Rajanayagam et al., 1993; La et al., 1996). In the current series of experiments, 30 μM hydroxocobalamin reduced the responses to NO• and nitrosonium tetrafluoroborate to 25% of control, whereas the response to nitrergic nerve stimulation was not affected and the response to AS (NO−) was enhanced to 140% of control. One possible explanation for this effect is that hydroxocobalamin may accelerate the formation of NO• from NO− if there is an intracellular conversion of nitroxyl to NO• before activation of guanylate cyclase.
The findings with pyrogallol and carboxy-PTIO, which differentiate between NO• and the nitrergic transmitter, suggest that nitroxyl (NO−) has a closer correspondence to the nitrergic transmitter. The only discrepancy is enhancement of the response to nitroxyl but not of the nitrergic transmitter by hydroxocobalamin.
In cascade-type experiments, the material flowing from a nitrergically innervated donor tissue after nitrergic nerve stimulation behaves like NO• on a rabbit aortic preparation used as a sensitive detector tissue. This has been shown with donor tissues consisting of the dog ileocolonic junction (Bult et al., 1990; Boeckxstaens et al., 1991b), rat gastric fundus (Boeckxstaens et al., 1991a) and guinea-pig myenteric plexus (La, unpublished observations). However, within the donor tissue, the relaxant response to nitrergic nerve stimulation does not behave like NO•. A possible interpretation of these findings is that the transmitter is released and acts locally in a non-NO• form, possibly as NO−, and is converted into NO• by the time it reaches the rabbit aorta.
Possible relationships between nitroxyl anions and the nitrergic transmitter
It has been suggested that the primary product of nNOS is nitroxyl (Schmidt et al., 1996) and NO• is only formed when NO− is oxidized to NO• in a reaction in which Cu(II)-containing SOD is reduced to Cu(I)-containing SOD (Schmidt et al., 1996). Thus, in addition to its eponymous enzymatic activity, the Cu(II) and Cu(I) forms of SOD can be coupled to a reversible redox reaction with NO− and NO• (Murphy & Sies, 1991). The suggestion that NO− is the primary product of nNOS has been disputed with evidence that NO• is formed directly by nNOS in the absence of SOD (Xia & Zweier, 1997). However, studies with purified nNOS preparations do not necessarily indicate how the enzymatic reaction proceeds in an organized tissue.
The first step in the formation of a nitrogen monoxide from L-arginine by NOS is thought to be production of the intermediate compound Nω-hydroxy-L-arginine, and this can generate either NO• or NO−, depending on the oxidative conditions (Fukuto et al., 1992b). Our findings suggest that the oxidative conditions in the rat anococcygeus muscle favour the formation of NO−.
The postulate that NO− is the nitrergic transmitter raises a question about the mechanism of transmission. Since the pKa of HNO is 4.7–5.0, the ratio NO− : HNO is about 100 : 1 at physiological pH. Only the protonated form will diffuse freely across cell membranes, therefore there must be some mechanism for transporting NO− from its intraneuronal site of formation into the neuroeffector junction; the NO− may then be oxidized to NO• at the smooth muscle surface.
The status of explanations for NO• as the nitrergic transmitter
It has been argued that resistance of the nitrergic transmitter to agents that inactivate NO• is due to one or more protective agents (Gibson & Lilley, 1997). One such postulated protective agent is superoxide dismutase (SOD), which has been shown to be colocalized with nNOS in the rat anococcygeus muscle (Liu et al., 1997). The main SOD in the cytosol and extracellular fluid is Cu/Zn SOD, which can be irreversibly inhibited by the Cu-sequestering agent diethyldithiocarbamate (DETC). It has been shown that DETC treatment resulted in blockade of nitrergic transmission by superoxide generators in the bovine retractor penis muscle (Martin et al., 1994; Paisley & Martin, 1996). However there was no change in the differential blocking activity of certain superoxide generators such as pyrogallol in the rat anococcygeus muscle (Rand et al., 1997; La & Rand, 1999) or of the xanthine oxidase-derived superoxide in the rat gastric fundus (Lefebvre, 1996) or the mouse anococcygeus muscle (Lilley & Gibson, 1996), although DETC slightly increased the action of LY83583 in the rat gastric fundus (Lefebvre, 1996). Therefore, additional factors may also be involved.
Another postulated protective agent, ascorbate, is released along with the nitrergic transmitter from mouse anococcygeus muscles and it was postulated that the ascorbate acted as an antioxidant protecting NO• against scavenger molecules (Lilley & Gibson, 1997). However, the protective action of ascorbate against carboxy-PTIO is due to formation of a product that does not inactivate NO• (Tsunoda et al., 1994): for this reason we omitted ascorbate (usually 0.14 μM) from the PSS in experiments with carboxy-PTIO. It is highly unlikely that the amount of ascorbate released from the muscle would inactivate the 100 μM of carboxy-PTIO in a 4 ml organ bath. Recent study suggests that normal extracellular concentrations of ascorbic acid (30–150 μM) are not likely to prevent the interaction of NO• with superoxide under physiological conditions (Jackson et al., 1998). The amount of ascorbate released from nitrergically innervated mouse anococcygeus muscle is reported to be in the nM range (Lilley & Gibson, 1997). However, if the ascorbate was released selectively with the transmitter into the neuroeffector junction, there might be sufficient to overcome momentarily the effect of carboxy-PTIO in the junction at the moment of release. In our laboratory, no apparent differences have been observed in responses to nitrergic nerve stimulation or NO• in PSS with or without ascorbic acid in the present or in previous experiments (La & Rand, 1999). Furthermore, De Man et al. (1998) found that ascorbic acid in concentrations up to 3 μM had no effect on responses of rat gastric fundus strips to NO• or nitrergic nerve stimulation, even after inhibition of SOD with DETC.
If our hypothesis that the nitrergic transmitter is the nitroxyl anion (NO−) is correct, then the antioxidant mechanisms that have been postulated as protective of NO• are more likely to be concerned with the metabolism of NO−.
Conclusions
The nitroxyl anion (NO−) is a potent relaxant of the rat anococcygeus muscle and responses to it, like those to the nitrergic transmitter, are not sensitive to pyrogallol, carboxy-PTIO or hydroxocobalamin. On this basis, NO− appears to be a better candidate for the role of the nitrergic transmitter than NO•. However, the correspondence between the transmitter and NO− is not complete since hydroxocobalamin enhanced the response to NO− but not to the transmitter.
Acknowledgments
This work was supported by grants from the Smoking & Health Research Foundation of Australia and the National Health & Medical Research Council.
Abbreviations
- AS
Angeli's salt
- DETC
diethyldithiocarbamate
- DMSO
dimethylsulphoxide
- EDRF
endothelium derived relaxing factor
- EFS
electrical field stimulation
- nNOS
neuronal nitric oxide synthase
- NO•
nitric oxide free radical
- NO+
nitrosonium cation
- NO−
nitroxyl anion
- NT
nitrosonium tetrafluoroborate
- ODQ
1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one
- SOD
superoxide dismutase
References
- AKAIKE T., YOSHIDA M., MIYAMOTO Y., SATO K., KOHNO M., SASAMOTO K., MIYAZAKI K., MAEDA H. Antagonistic action of imidazolineoxy N-oxides against endoethlium-derived relaxing factor/.NO through a radical reaction. Biochem. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., BOGERS J.J., BULT H., DE MAN J.G., OOSTERBOSCH L., HERMAN A.G., VAN MAERCKE Y.M. Release of nitric oxide upon stimulation of non-adrenergic, non-cholinergic nerves in the rat gastric fundus. J. Pharmacol. Exp. Ther. 1991a;256:441–447. [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., RUYTJENS I.F., BULT H., DE MAN J.G., HERMAN A.G., VAN MAERCKE Y.M. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br. J. Pharmacol. 1991b;103:1085–1091. doi: 10.1111/j.1476-5381.1991.tb12304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULT H., BOECKXSTAENS G.E., PELCKMANS P.A., JORDAENS F.J., VAN MAERCKE Y.M., HERMAN A.G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- DE MAN J.G., DE WINTER B.Y., MOREELS T.G., HERMAN A.G., PELCKMANS P.A. S-Nitrosothiols and the nitrergic neurotransmitter in the rat gastric fundus: effects of antioxidants and metal chelation. Br. J. Pharmacol. 1998;123:1039–1046. doi: 10.1038/sj.bjp.0701692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIERKS E.A., BURSTYN J.N. Nitric oxide (NO•), the only nitrogen monoxide redox form capable of activating soluble guanylate cyclase. Biochem. Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., TE POEL M., ZAMORA R., DEUSSEN A., MONCADA S. Understanding the controversy over the identity of EDRF. Nature. 1994;368:62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- FUKUTO J.M., CHIANG K., HSZIEH R., WONG P., CHAUDHURI G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endoethlium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1992a;263:546–551. [PubMed] [Google Scholar]
- FUKUTO J.M., HOBBS A.J., IGNARRO L.J. Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems: the role of physiological oxidants and relevance to the biological activity of HNO. Biochem. Biophys. Res. Commun. 1993;196:707–713. doi: 10.1006/bbrc.1993.2307. [DOI] [PubMed] [Google Scholar]
- FUKUTO J.M., WALLACE G.C., HSZIEH R., CHAUDHURI G. Chemical oxidation of N-hydroxyguanidine compounds. Release of nitric oxide, nitroxyl and possible relationship to the mechanism of biological nitric oxide generation. Biochem. Pharmacol. 1992b;43:607–613. doi: 10.1016/0006-2952(92)90584-6. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GIBSON A., LILLEY E. Superoxide anions, free-radical scavengers, and nitrergic neurotransmission. Gen. Pharmacol. 1997;28:489–493. doi: 10.1016/s0306-3623(96)00281-9. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J.S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br. J. Pharmacol. 1972;45:404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOYAL R.K., HE X.D. Evidence for NO•redox form of nitric oxide as nitrergic inhibitory neurotransmitter in gut. Am. J. Physiol. 1998;275:G1185–1192. doi: 10.1152/ajpgi.1998.275.5.G1185. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J., FUKUTO J.M., IGNARRO L.J. Formation of free nitric oxide from l-arginine by nitric oxide synthase: direct enhancement of generation by superoxide dismutase. Proc. Nat. Acad. Sci. U.S.A. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- ISHII K., SHENG H., WARNER T.D., FÖRSTERMANN U., MURAD F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am. J. Physiol. 1991;261:H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- JACKSON T.S., XU A., VITA J.A., KEANYE J.F., JR Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- KARAGIANNIS J., LI C.G., RAND M.J. Comparison of the nitrergic transmitter with redox forms of nitrogen monoxide indicates that the transmitter may be the nitroxyl anion. Proc. Australas. Soc. Clin. Exp. Pharmacol. Toxicol. 1998;5:P2–104. [Google Scholar]
- KRUSZYNA H., MAGYAR J.S., ROCHELLE L.G., RUSSELL M.A., SMITH R.P., WILCOX D.E. Spectroscopic studies of nitric oxide (NO) interactions with cobalamins: reaction of NO with superoxocobalamin(III) likely accounts for cobalamins reversal of the biological effects of NO. J. Pharmacol. Exp. Ther. 1998;285:665–671. [PubMed] [Google Scholar]
- LA M., LI C.G., RAND M.J. Comparison of the effects of hydroxocobalamin and oxyhaemoglobin on responses to NO, EDRF and the nitrergic transmitter. Br. J. Pharmacol. 1996;117:805–810. doi: 10.1111/j.1476-5381.1996.tb15264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA M., RAND M.J. Effects of pyrogallol, hydroquinone and duroquinone on responses to nitrergic nerve stimulation and NO in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;126:342–348. doi: 10.1038/sj.bjp.0702277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Influence of superoxide dismutase inhibition on the discrimination between NO and the nitrergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 1996;118:2171–2177. doi: 10.1111/j.1476-5381.1996.tb15659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J.Effects of hydroxocobalamin and carboxy-PTIO on nitrergic transmission in porcine anococcygeus and retractor penis muscles Br. J. Pharmacol. 1999. in press [DOI] [PMC free article] [PubMed]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus muscle against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidants ascorbate and urate from nitrergically innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X., MILLER S.M., SZURSZEWSKI J.H. Protection of nitrergic neurotransmission by colocalization of neuronal nitric oxide synthase with copper zinc superoxide dismutase. J. Auton. Nerv. Syst. 1997;62:126–133. doi: 10.1016/s0165-1838(96)00113-0. [DOI] [PubMed] [Google Scholar]
- MARAGOS C.M., MORLEY D., WINK D.A., DUNAMS T.M., SAAVEDRA J.E., HOFFMAN A., BOVE A.A., ISAAC L., HRABIE J.A., KEEFER L.K. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.M.H., PAISLEY K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- MOHR S., STAMLER J.S., BRUNE B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Letters. 1994;18:223–227. doi: 10.1016/0014-5793(94)00596-6. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MURPHY M.E., SIES H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc. Natl. Ac. Sci. U.S.A. 1991;88:10860–10864. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGASAWA H.T., KAWLE S.P., ELBERLING E.G., DEMASTER, FUKUTO J.M. Prodrugs of nitroxyl as potential aldehyde dehydrogenase inhibitors vis-a-vis vascular smooth muscle relaxants. J. Med. Chem. 1995;38:1865–1871. doi: 10.1021/jm00011a005. [DOI] [PubMed] [Google Scholar]
- PAISLEY K., MARTIN W. Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br. J. Pharmacol. 1996;117:1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULING L. The Nature of the Chemical Bond 1960Ithaca, New York: Cornell University Press; 345Third Edition. p [Google Scholar]
- PINO R.Z., FEELISCH M. Bioassay discriminating between nitric oxide (NO•) and nitroxyl (NO−) using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- RAJANAYAGAM M.A., LI C.G., RAND M.J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br. J. Pharmacol. 1993;108:3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br. J. Pharmacol. 1995a;116:1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Ann. Rev. Physiol. 1995b;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G.Nitric oxide in the autonomic and enteric nervous systems Nitric Oxide in the Nervous System 1995cLondon: Academic Press; 228–279.ed. Vincent, S.R. pp [Google Scholar]
- RAND M.J., LI C.G., LA M., DE LUCA A., NAJBAR-KASZKIEL A.T., JIANG F., DI IULIO J.L., ELLIS A. Nitrergic transmisison: discovery and continuing studies. Asia Pacific J. Phamacol. 1997;12:137–148. [Google Scholar]
- ROCHELLE L.G., MORANA S.J., KRUSZYNA H., RUSSELL M.A., WILCOX D.E., SMITH R.P. Interactions between hydroxocobalamin and nitric oxide (NO): evidence for a redox reaction between NO and reduced cobalamin and reversible NO binding to oxidized cobalamin. J. Pharmacol. Exp. Ther. 1995;275:48–52. [PubMed] [Google Scholar]
- RUBYANI G.M., VANHOUTTE P.M. Superoxide and hyperoxia inactive endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., HOFMANN H., SCHINDLER U., SHUTENKO Z.S., CUNNINGHAM D.D., FEELISCH M. No .NO from NO synthase. Proc. Natl. Ac. Sci. U.S.A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S., SINGEL D.J., LOSCALZO J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- TSUNODA R., OKAMURA K., ISHIZAKA H., MATSUNAGA T., TABUDI T., YASHUE H., AKAIKE T., SATO K., MAEDA H. Vasodilator effect of carboxy-2-phenyl-4.4.5.5-tetramethylimidazoline-1-oxyl in the coronary circulation: in vivo and in vitro studies. Eur. J. Pharmacol. 1994;262:55–63. doi: 10.1016/0014-2999(94)90028-0. [DOI] [PubMed] [Google Scholar]
- XIA Y., ZWEIER J.L. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc. Natl. Ac. Sci. U.S.A. 1997;94:12705–12710. doi: 10.1073/pnas.94.23.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMORA R., GRZESIOK A., WEBER H., FEELISCH M. Oxidative release of nitric oxide accounts for guanylate cyclase stimulating, vasodilator and anti-platelet activity of Piloty's acid. Biochem. J. 1995;312:333–339. doi: 10.1042/bj3120333. [DOI] [PMC free article] [PubMed] [Google Scholar]


