Abstract
The in vitro hemisected spinal cord from young rat was used to investigate the mechanism of serotoninergic modulation of primary afferent-mediated synaptic transmission in the dorsal horn through activation of the 5-HT3 receptor.
Dorsal root-evoked excitatory post-synaptic potentials (DR-EPSPs) were recorded intracellularly from dorsal horn neurones. Extracellular recordings of the population primary afferent depolarization (PAD) and the dorsal rootevoked dorsal root reflex (DR-DRR) were made from segmental dorsal roots.
5-Hydroxytryptamine (5-HT) and the selective 5-HT3 receptor agonist 1-(m-chloro-phenyl)-biguanide hydrochloride (m-ChPB) (10 and 50 μM) induced statistically significant reductions of the DR-EPSP amplitude (P<0.001) and duration (P<0.001) in the majority of dorsal horn neurones. The 5-HT3 receptor selective antagonists 3-Tropanyl-indole-3-carboxylate hydrochloride (Tropisetron, 10 μM) and N-(1-Azabicyclo[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide (Y-25130, 10 μM) abolished m-ChPB-induced DR-EPSP attenuation and partially blocked the 5-HT effect.
m-ChPB (50 μM)-induced DR-EPSP amplitude and duration attenuation was retained in the presence of the GABAA receptor antagonist bicuculline (30 μM), the GABAB receptor antagonist saclofen (50 μM) and the opioid receptor antagonist naloxone (50 μM).
Both 5-HT and m-ChPB (10 and 50 μM) induced a PAD but the mean peak amplitude of 5-HT-induced PAD was significantly greater than PAD to m-ChPB (98.6±12 μV compared to 51.8±10 μV for 50 μM of agonist, respectively). Tropisetron partially reduced 5-HT-induced PAD and abolished m-ChPB-induced PAD. 5-HT, but not m-ChPB, significantly (P<0.001) reduced the peak amplitude of the DR-DRR and this action of 5-HT was unaffected by Tropisetron or Y-25130.
These data provide experimental evidence for a putative cellular mechanism at the level of the dorsal horn for anti-nociceptive effects of 5-HT3 receptor activation. This 5-HT3-mediated modulation of sensory afferent transmission was evidently independent of inhibitory GABA- or opioid-dependent interneuronal pathways. The extent to which the 5-HT3 receptor could be involved in the operation of endogenous analgesia and sensory modulation by descending monoamine bulbo-spinal pathways is discussed.
Keywords: Serotonin receptor, 5-HT3 receptor, sensory afferent, nociception, spinal cord, electrophysiology
Introduction
The pharmacology of serotonin (5-HT)-induced physiological actions in the mammalian central nervous system is complex; presently seven classes of receptor (5-HT1–7) are recognized on the basis of radioligand binding assays, cloning and primary sequence analysis (Jenkinson et al., 1995). Within these receptor classes, the 5-HT3 receptor is unique in its interaction with a ligand-gated ion channel that conducts monovalent cations (Na+ and K+) and generates excitation (Derkach et al., 1989). All other 5-HT receptor subtypes are linked to adenyl cyclase or phospholipase C and belong to a superfamily of G-protein coupled receptors (Hoyer et al., 1994; Jenkinson et al., 1995). The distribution of the 5-HT3 receptor is widespread, it has been localized in higher brain areas such as cortex and hippocampus as well as nuclei of the lower brainstem e.g., trigeminal nucleus and dorsal vagal complex (Hoyer et al., 1994). Within the spinal cord, it is localized most densely within the substantia gelatinosa (Kia et al., 1995; Laporte et al., 1996), a superficial dorsal horn area associated principally with the processing of nociceptive sensory afferent inputs. The significant drop in specific binding of high affinity 5-HT3 receptor radioligands such as [3H]-zacopride after capsaicin-induced elimination of small diameter unmyelinated afferents is interpreted as indicating a particular association with nociceptive afferent terminals (Hamon et al., 1989; Kidd et al., 1993). However, since a proportion of the [3H]-zacopride specific binding sites remained after the neurotoxin pre-treatment, this strongly suggests the presence of 5-HT3 receptors either on a population of larger diameter capsaicin-insensitive afferents or on post-synaptic targets in the dorsal horn e.g., spinal interneurones. Localization of this receptor on spinal intrinsic neurones is further supported by immunohistochemical and in situ hybridization analysis of 5-HT3 receptor distribution before and after unilateral dorsal rhizotomy (Kia et al., 1995).
In the context of nociception, 5-HT is a proposed mediator of endogenous analgesic mechanisms operating via descending pathways from brainstem nuclei to dorsal horn. One major component of this anti-nociceptive system originates in the periaqueductal gray (PAG) and via the nucleus raphe magnus it inhibits responses to innocuous and noxious stimuli of second order relay neurones, including spinothalamic tract neurones in vivo (Roberts, 1984). 5-HT immuno-positive fibres that originate from raphe nuclei somata are extensively distributed across dorsal and ventral laminae at all segmental levels of the spinal cord (Dahlstrom & Fuxe, 1964; Steinbusch, 1981). Evidence for a 5-HT involvement in anti-nociceptive processes is that 5-HT is released in the spinal cord as a consequence of nociceptive, sciatic nerve, nucleus raphe magnus or dorsolateral funiculus stimulation (Tyce & Yaksh, 1981; Sorkin et al., 1993) and exogenous 5-HT inhibits nociceptive units and raises behavioural nociceptive thresholds in vivo (Hamon et al., 1990).
A major issue still to be resolved in the field of 5-HT-induced analgesia and anti-nociception is the receptor subtype with which 5-HT interacts to achieve its physiological effects. Behavioural and electrophysiological studies utilizing 5-HT3 receptor-selective agonists and antagonists implicate an involvement of this subtype. In rodent behavioural studies, the 5-HT3 receptor agonist, 2-methyl-5-HT mimicked anti-nociceptive effects of 5-HT in tail flick and hot plate tests and intrathecal application of a selective 5-HT3 receptor antagonist blocked 5-HT-induced anti-nociception (Glaum et al., 1988; 1990). Dorsal horn units activated by mechanical stimuli were inhibited by PAG stimulation and by the potent and selective 5-HT3 receptor agonist 1-(m-chloro-phenyl)-biguanide hydrochloride (m-ChPB) administered via a microdialysis fibre (Peng et al., 1996). Furthermore, these PAG-induced inhibitions were offset by the 5-HT3 receptor antagonist Ondansetron. However, there is not a complete consensus of opinion in the literature regarding the anti-nociceptive consequences of 5-HT3 receptor activation. One behavioural study reported a 5-HT1 but not 5-HT3 receptor-induced anti-nociception (Xu et al., 1994b) and in another in vivo study, intrathecal m-ChPB enhanced the responsiveness of rat dorsal horn neurones to noxious thermal stimuli although these electrophysiological effects were not replicated in a test of tail flick latency using equivalent drug doses (Ali et al., 1996). One source of conflict may be the spinal versus supra-spinal site of action of various 5-HT analogues. This issue can be resolved by analysing the actions of these 5-HT compounds on an isolated rat spinal cord in which supra-spinal mechanisms are eliminated but primary afferent-mediated synaptic inputs onto dorsal horn neurones are preserved (Lopez-Garcia & King, 1996a).
Previously, we have described mainly inhibitory but also excitatory effects of exogenous 5-HT on primary afferent-evoked neurotransmission in young rat dorsal horn neurones in vitro (Lopez-Garcia & King, 1996b). There is no equivalent intracellular data on the effects of 5-HT3 receptor activation on dorsal horn neurones. In this in vitro study, an intracellular strategy and selective pharmacological tools were used to assess a putative 5-HT3 receptor-mediated modulation of dorsal root-evoked synaptic transmission. The mechanism of 5-HT3 receptor sensory neurotransmission modulation and antinociception is unknown but excitation of spinal inhibitory interneurones is one possibility that should be considered (Alhaider et al., 1991; Peng et al., 1996). To this end, the GABAA receptor antagonist bicuculline, the GABAB receptor antagonist saclofen and the opioid receptor antagonist naloxone were tested against the inhibitory actions of a selective 5-HT3 agonist. Polarization of sensory afferent terminals is proposed as a pre-synaptic mechanism of 5-HT spinal action (Holohean et al., 1990; Lopez-Garcia & King, 1996a,1996b; Khasabov et al., 1998) and 5-HT3 receptors are localized partly to nociceptive sensory afferent terminals (Hamon et al., 1989; Kidd et al., 1993; Kia et al., 1995). For this reason, we evaluated (a) 5-HT3-induced population primary afferent depolarisation (PAD) recorded extracellularly from a dorsal root and (b) the involvement of the 5-HT3 receptor in the dorsal root-evoked dorsal root reflex (DR-DRR).
Methods
The isolated rat spinal cord preparation
Under intraperitoneal urethane anaesthesia (2 g kg−1), the thoracic, lumbar and sacral spinal cord segments with attached segmental roots were isolated from 10–14 day-old Wistar rats and immediately placed into ice-cooled gas-saturated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) for hemisection. A hemisected spinal cord was placed securely into a Perspex custom-made experimental chamber that received ACSF at a constant perfusion rate of no less than 10 ml s−1. The composition of the ACSF was (in mM): NaCl 128, KCl 1.9, KH2PO4 1.2, MgSO4 1.3, CaCl2 2.4, NaHCO3 26, glucose 10, (pH 7.4; 21–24°C). A detailed description of this isolated preparation has been published previously (Lopez-Garcia & King, 1996a,1996b).
Electrophysiology
For intracellular recordings, spinal dorsal horn neurones were impaled with 3 M potassium acetate-filled sharp microelectrodes (90–140 MΩ) fabricated from borosilicate filamented glass capillaries (1.0 mm outer diameter, 0.58 mm inner diameter; purchased from Clark Electromedical Instruments, Pangbourne, U.K.). Neurones were selected for study on the basis of a stable minimum resting membrane potential (Vm) of −60 mV and action potentials overshooting 0 mV. A total population of 47 neurones with a mean resting membrane potential (Vm) of 75±1 mV and an input resistance (IR) of 81±11 MΩ was studied.
To elicit dorsal root-evoked excitatory post-synaptic potentials (DR-EPSPs), one dorsal root (L4–L6) was pulled into a borosilicate glass miniature suction electrode. Parameters of electrical stimulation were 250–300 μs/250–300 μA delivered at intervals of 30 s or 60 s (using ‘Neurolog System' modules, Digitimer Research Instrumentation, Welwyn Garden City, U.K.) which adequately recruited all main afferent classes (Thompson et al., 1990) without fatigue. In dorsal horn neurones, two DR-EPSP parameters, the mean±s.e.mean peak amplitude (mV) and the total duration(s), were measured. For each cell, a minimum of five captured waveforms in control and test conditions were measured and the data values averaged. The peak DR-EPSP amplitude and total duration were measured using visually placed horizontal and vertical cursors. Extracellular recordings of the population PAD (generated by exogenously applied 5-HT agonists) and DR-DRR (produced by electrical stimulation at 250 μs/250 μA of an adjacent ipsilateral dorsal root) were made from L4–L6 dorsal roots placed within fabricated miniature glass suction electrodes.
Extracellular and intracellular electrophysiological records were amplified with an Axoclamp-2B (Axon Instruments, Foster City, U.S.A.) and captured for on- or off-line computer-assisted analysis using ‘Spike 2' (Cambridge Electronic Design Ltd., Cambridge, U.K.). For comparison of drug effects, all data are expressed as a mean±s.e.mean percentage of the control value. For statistical comparison, a paired or unpaired Student's t-test was used with significance denoted by P<0.05.
Drug solutions and application
All drugs were dissolved in normal ACSF and superfused from separate gravity-fed reservoirs at known concentrations (as indicated below) for a fixed time period over the spinal cord preparation. The non-selective 5-HT receptor agonist Serotonin creatinine sulphate (5-Hydroxytryptamine, 5-HT) (Sigma-Aldrich, Poole, U.K.) and the selective 5-HT3 receptor agonist–1-(m-chloro-phenyl)-biguanide hydrochloride (m-ChPB) (Tocris Cookson, Bristol, U.K.) were superfused for 60 or 120 s at concentrations of 10 and 50 μM in the experimental analysis of DR-EPSPs, PAD and the DR-DRR. m-ChPB did not alter Vm while 5-HT produced small membrane depolarizations in a proportion of neurones tested; 10 and 50 μM 5-HT-induced depolarisations of 2.5±0.2 mV (n=7; 27%) and 4.8±1.4 mV (n=5; 36%) respectively. Neither agonist produced significant alterations in IR estimated from the slope of current-voltage plots before and after drug application. The mean IR after application of m-ChPB (10 μM) or 5-HT (10 μM) was 83±10 MΩ and 76±14 MΩ respectively, these values are not significantly different from the control value of 81±11 MΩ (P>0.4). The selective 5-HT3 receptor antagonists 3-Tropanyl-indole-3-carboxylate hydrochloride (Tropisetron) (Sigma-Aldrich, Poole, U.K.) and N-(1-Azabicyclo[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide hydrochloride (Y-25130) (Tocris Cookson, Bristol, U.K.) were applied at a concentration of 10 μM for 10 min prior to re-testing the 5-HT receptor agonists. Tropisetron and Y-25130 at this concentration did not significantly change IR or Vm. The GABAA receptor antagonist, (−)-bicuculline methochloride (30 μM), the GABAB receptor antagonist, saclofen hydrochloride (50 μM) and the opioid receptor antagonist, naloxone hydrochloride (50 μM) (all purchased from Tocris Cookson, Bristol, U.K.) were superfused for 10 min before application of the agonist, m-ChPB.
Results
5-HT3 receptor-induced modulation of primary afferent-induced synaptic transmission
Stimulation of a dorsal root elicited in dorsal horn neurones a polysynaptic DR-EPSP with a mean peak amplitude of 16.2±1.2 mV and duration of 15.4±1.0 s in the sampled cells (n=47). The predominant effect of 5-HT and the selective 5-HT3 receptor agonist m-ChPB superfused at concentrations of 10 and 50 μM was a reversible DR-EPSP attenuation (Figure 1 and Table 1). However, with both 5-HT and m-ChPB, a small percentage of dorsal horn neurones responded with either an increased DR-EPSP amplitude and duration or were unaffected (see Table 1). For example, after application of 10 μM m-ChPB, in 21.4% (6/28) of neurones the DR-EPSP amplitude increased from 15.6±0.9 to 17.9±1.0 mV (representing a 14.7% increase, P<0.05) and in 10.7% (3/28) there was no effect. After 10 μM 5-HT, the DR-EPSP was enhanced in one neurone and unaltered in another (Table 1).
Figure 1.
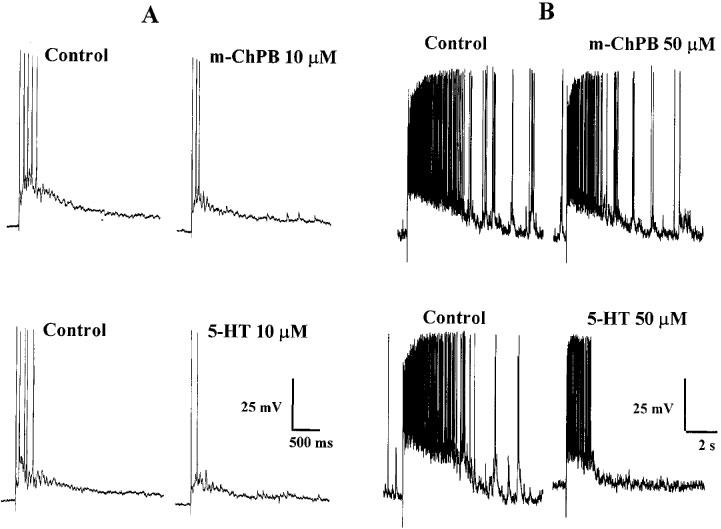
Inhibition of the DR-EPSP by 5-HT and m-ChPB. (A) Intracellular records from a single dorsal horn neurone superfused with 10 μM m-ChPB (upper traces) and 10 μM 5-HT (lower traces). (B) Records from another dorsal horn neurone superfused with 50 μM m-ChPB (upper traces) and 50 μM 5-HT (lower traces).
Table 1.
Effect of 10 and 50 μM 5-HT or m-ChPB on the amplitude and duration of the DR-EPSP in a population of dorsal horn neurones.

Considering the population as a whole, with the lower agonist concentration of 10 μM, the DR-EPSP amplitude was attenuated in 92.8% of neurones tested with 5-HT (n=28) and in 67.9% of neurones tested with m-ChPB (n=28). With the higher agonist concentration of 50 μM, 93.3% of neurones tested with 5-HT (n=15) and 70.6% of neurones tested with m-ChPB (n=17) responded with an attenuated DR-EPSP amplitude (Table 1). The percentage of neurones with a decreased DR-EPSP duration following application of 10 and 50 μM 5-HT or equivalent concentrations of m-ChPB is summarized in Table 1, it is apparent from these data that in the majority of neurones the duration was reduced. The predominant response to these two serotoninergic agonists was therefore a neuronal inhibition and reduced synaptic transmission. Following 10 μM m-ChPB, the DR-EPSP amplitude reduced from 14.3±1.4 to 10.9±1.1 mV (n=19) and the duration from 15.9±2.1 to 11.4±1.7 s (n=20) (P<0.05). With 50 μM m-ChPB the control DR-EPSP values were 17.2±1.1 mV (n=12) and 16.6±1.5 s (n=16) compared to 15.4±1.3 mV and 12.6±1.4 s (P<0.05) after the agonist. Following 10 μM 5-HT, the DR-EPSP amplitude reduced from 16.2±1.6 mV (n=26) to 11.4±1.7 mV and the duration from 13.9±1.5 s (n=25) to 8.6±1.2 s (P<0.05). With 50 μM 5-HT, the control DR-EPSP values were 16.3±1.9 mV (n=14) and 15.1±2.1 s (n=15) compared to 11.3±1.5 mV and 7.8±1.4 s (P<0.05) after the agonist. In order to compare the effects of the two agonists, the normalized data showing the DR-EPSP amplitude and duration as a mean±s.e.mean percentage from control values are represented in the histogram of Figure 2. The attenuation of DR-EPSP amplitude and duration by 10 or 50 μM 5-HT was greater than that induced by the equivalent concentration of the selective 5-HT3 agonist and this difference was statistically valid (P<0.05). For example, comparing data for 10 μM 5-HT and m-ChPB, the DR-EPSP amplitude was reduced to 77.5±2.9% (n=19) of the control value by m-ChPB and to 68.8±3.3% (n=26) of control by 5-HT.
Figure 2.
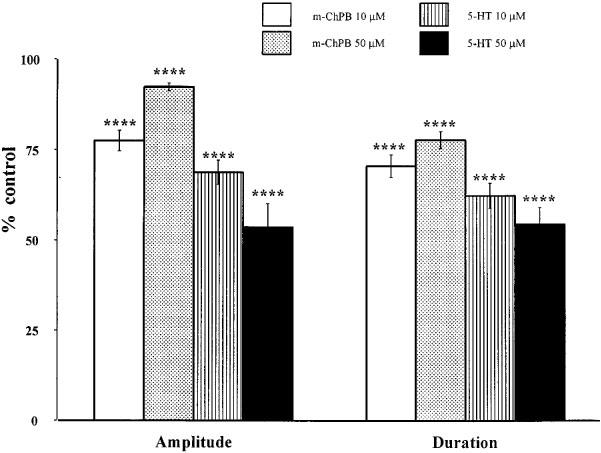
Quantified data for the effect of m-ChPB tested at 10 μM (white columns) and 50 μM (stippled columns) and 5-HT at 10 μM (lined columns) and 50 μM (black columns) on the DR-EPSP amplitude and duration. (Data presented as percentage of control values; ****P<0.001).
Two selective 5-HT3 receptor antagonists, Tropisetron and Y-25130 (10 μM), were tested against 10 μM 5-HT- and 10 μM m-ChPB-induced DR-EPSP attenuation to indicate involvement of this receptor in the modulation of the synaptic potential. The antagonists alone had no significant effect on DR-EPSP amplitude or duration. In a sample of seven neurones, the DR-EPSP amplitude and duration values were 15.9±1.1 mV and 14.7±1.1 s in control ACSF compared to 16.1±0.9 mV and 14.6±0.9 s in Tropisetron-containing ACSF. For Y-25130-containing ACSF, the corresponding amplitude and duration values were 16.1±1.1 mV and 14.8±0.7 s versus 16.3±1.1 mV and 15.1±0.4 s in control ACSF. Tropisetron (10 μM) or Y-25130 (10 μM) completely abolished the attenuation of amplitude and duration of DR-EPSPs induced by 10 μM m-ChPB but only partially antagonized the 5-HT-induced attenuation (Figures 3A and 4). The quantified data in the histogram of Figure 4 shows that while m-ChPB alone significantly reduced the DR-EPSP amplitude to 85.1±1.4% (n=10; P<0.001) of the control value, after Tropisetron or Y-25130 it had no significant effect on the DR-EPSP amplitude (values were 102.3±2 and 99.7±1.5% of the controls respectively, n=6). Similarly, the DR-EPSP duration was significantly reduced by m-ChPB to 76.5±4% of control (n=8, P<0.005) in normal ACSF but not after the antagonist pre-treatment (values of 98.2±2.1 and 100.6±2.6% after Tropisetron and Y-25130 respectively, n=6). When tested against 10 μM 5-HT, Tropisetron or Y-25130 (both at a concentration of 10 μM) partially reversed the effects on DR-EPSP amplitude and duration (Figures 3B and 4). 5-HT in control ACSF reduced the DR-EPSP amplitude and duration to 75.8±4.9% (P<0.005, n=9) and 62.6±8.2% (P<0.005, n=9) of control, respectively. After application of Tropisetron, the DR-EPSP amplitude and duration was 89.0±3.8 and 89.0±3.1% of control (n=7, P<0.01) respectively. Similarly, after Y-25130, the 5-HT-induced DR-EPSP reductions were smaller but remained statistically significant (n=7, P<0.01, see histogram of Figure 4).
Figure 3.
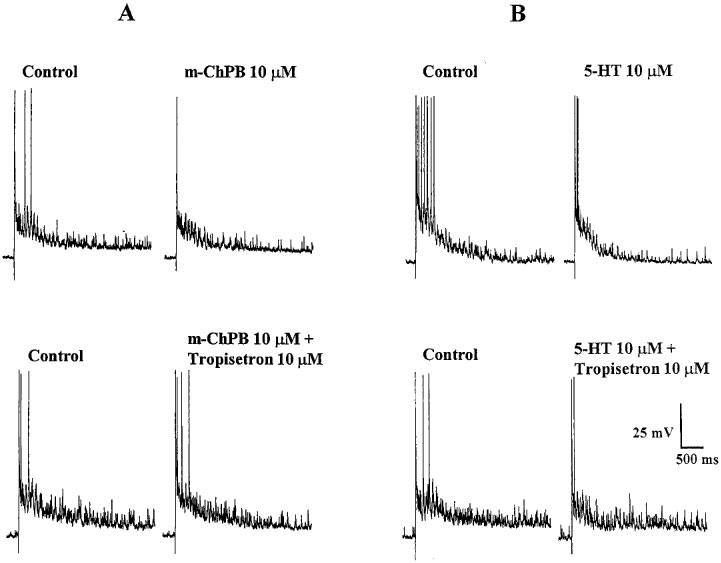
Antagonism of the effects of 5-HT and m-ChPB on the DR-EPSP by Tropisetron (10 μM). (A) 10 μM m-ChPB attenuated the DR-EPSP (upper traces) and this effect was abolished by Tropisetron (lower traces). (B) 10 μM 5-HT-induced attenuation of the DR-EPSP (upper traces) in a DH neurone is partially reversed by Tropisetron (lower traces). Records are from a single DH neurone.
Figure 4.

Quantified data expressed as percentage of control for the effects of 10 μM Tropisetron or 10 μM Y-25130 on 10 μM 5-HT or m-ChPB inhibition of the DR-EPSP amplitude (upper chart) and duration (lower chart) (**P<0.01; ***P<0.005; ****P<0.001). Note that the antagonists abolished m-ChPB-induced DR-EPSP inhibition and partially reduced the 5-HT effects.
To assess whether the 5-HT3-induced attenuation of the DR-EPSP was mediated through GABAA, GABAB or opioid receptor activation, m-ChPB was re-tested after superfusion of three selective antagonists, bicuculline, saclofen and naloxone. The quantitative data presented in Figure 5 indicates that a significant reduction in the amplitude and duration of the DR-EPSP was produced by m-ChPB (50 μM) in the presence of each of the antagonists. For example, with the GABAA receptor antagonist bicuculline (30 μM), the DR-EPSP amplitude was reduced to 73.6% of the control value (P<0.005, n=6) and the duration to 66.8% of control (P<0.01, n=6) by 50 μM m-ChPB. Similar DR-EPSP amplitude and duration attenuations were elicited by m-ChPB after prior superfusion with either saclofen (50 μM, n=4), a GABAB receptor antagonist, or naloxone (50 μM, n=4), an opioid receptor antagonist.
Figure 5.
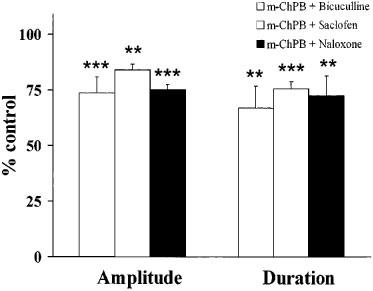
Quantified data (expressed as percentage of control) for inhibition of the DR-EPSP amplitude and duration by m-ChPB (50 μM) after pre-treatment with bicuculline (30 μM), saclofen (50 μM) or naloxone (50 μM) (**P<0.01; ***P<0.005).
Role of 5-HT3 receptors in primary afferent depolarizations (PAD) and the dorsal root-dorsal root reflex (DR-DRR)
Superfusion of 5-HT, tested at concentrations of 10 and 50 μM, generated a slow extracellularly recorded population PAD that peaked between 1–3 min and decayed slowly (Figure 6A). The peak amplitude of this agonist-induced PAD was 136.1±8.3 μV (n=32) for 10 μM 5-HT and 98.6±12.0 μV (n=18) for 50 μM 5-HT. Application of m-ChPB at equivalent concentrations also produced a slowly developing PAD that peaked at between 4–6 min (Figure 6A). For 10 and 50 μM m-ChPB, mean peak PADs of 53.1±3.2 μV (n=23) and 51.8±10 μV (n=11) respectively were generated. Comparing the two agonists, 5-HT-induced PAD was significantly greater in amplitude than m-ChPB-induced PAD (10 and 50 μM, P<0.005). To assess the involvement of 5-HT3 receptors in the generation of PAD, the antagonist Tropisetron (10 μM) was tested against 5-HT- and m-ChPB-induced PAD (Figure 6). Tropisetron abolished the m-ChPB-induced PAD and significantly reduced 5-HT-induced PAD (Figure 6A). The control value for PAD generated in this sample by 10 μM 5-HT was 95.8±21.8 μV compared to 44±10.1 μV after the antagonist (n=4; P<0.05) (Figure 6B).
Figure 6.
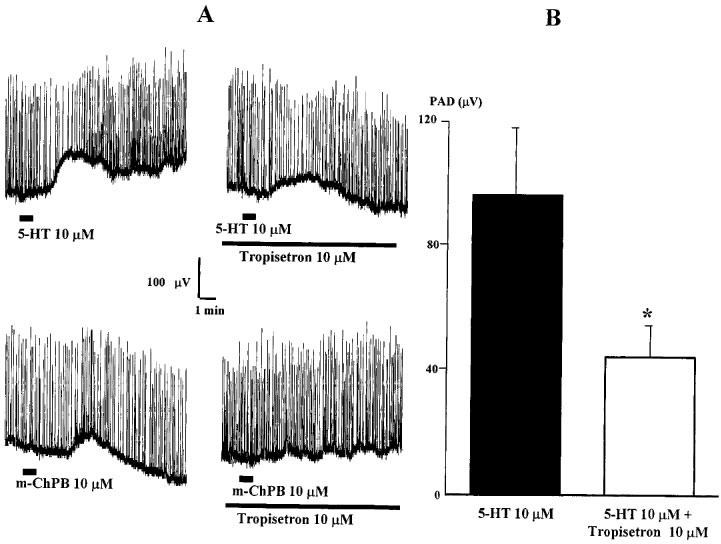
Population PAD generated by 10 μM 5-HT or m-ChPB in the absence or presence of Tropisetron. (A) 10 μM 5-HT (upper traces) and 10 μM m-ChPB (lower traces) produced PAD that was antagonised by Tropisetron (10 μM). (B) Histogram of data for PAD after superfusion of 5-HT in control ACSF (black column) or in 10 μM Tropisetron-containing ACSF (white column) (*P<0.05).
The modulation of DR-DRR by m-ChPB and 5-HT (10 and 50 μM) and the influence of the 5-HT3 antagonists Tropisetron and Y-25130 (10 μM) was investigated. The DR-DRR consisted of a short latency synchronized potential (latency <3 ms) that had a mean peak amplitude of 433±38 μV and was followed by a variable, long latency asynchronous potential (mean peak amplitude of 118±53 μM) with a total duration of 11.8±1 s (Figure 7A). Superfusion of 5-HT significantly decreased both components of the DR-DRR but this was not mimicked by m-ChPB (Figure 7A). In order to quantify the effects of 5-HT, the mean fast peak amplitude was determined (Figure 7b) after 10 and 50 μM 5-HT the values were reduced to 74.2±2.3% (n=22; P<0.001) and 59.1±4.9% (n=6, P<0.001) of control respectively. In contrast, m-ChPB at equivalent concentrations (10 μM, n=16; 50 μM, n=10) had no significant effect on the short latency DR-DRR amplitude (Figure 7A and B). Furthermore, Tropisetron (10 μM) itself did not modulate the DR-DRR and failed to reverse or antagonize 5-HT-induced DR-DRR amplitude attenuation. The quantified data in the histogram of Figure 7b shows that after Tropisetron pre-treatment, 5-HT significantly reduced the DR-DRR amplitude (P<0.005, n=4). Similar data was obtained for the effects of Y-25130 (10 μM, n=5) tested against 5-HT and the DR-DRR (Figure 7B), significant reduction of DR-DRR amplitude (P<0.001) was measured after 5-HT even in the presence of the antagonist (Figure 7B).
Figure 7.

The effects of 5-HT and m-ChPB on DR-DRR. (A) 5-HT (10 μM) reduced the amplitude of the DR-DRR whereas m-ChPB at a higher concentration (50 μM) had little effect on this potential. (B) The histogram of quantified effects of 5-HT and m-ChPB (10 and 50 μM) on the fast peak of DR-DRR in control ACSF and in presence of the antagonists Tropisetron and Y-25130 (both at 10 μM). Data shown as percentage of control values (***P<0.005; ****P<0.001).
Discussion
In the majority of spinal dorsal horn neurones in vitro, the dominant effect of 5-HT or the selective 5-HT3 receptor agonist m-ChPB was an attenuation of primary afferent-evoked synaptic transmission. Both the amplitude and the duration of DR-EPSPs were significantly reduced by these serotoninergic agonists in a high percentage of neurones. This inhibition of dorsal horn synaptic transmission by 5-HT confirms the results of previous electrophysiological studies in vivo (Roberts, 1984; El-Yassir et al., 1988; Hamon et al., 1990; Zemlan et al., 1994) and in vitro (Lopez-Garcia & King, 1996a,1996b). The fact that m-ChPB mimicked the effects of 5-HT on the DR-EPSP suggests that a portion of 5-HT's spinal action could be mediated through this receptor subtype. This view is reinforced by the finding that the selective 5-HT3 receptor antagonists, Tropisetron and Y-25130, fully abolished m-ChPB-induced DR-EPSP attenuation and partially reversed 5-HT-induced inhibitions. Thus, endogenous descending serotoninergic inhibition of spinal cord sensory afferent processing may operate partly via the 5-HT3 receptor type. Behavioural and electrophysiological studies have concluded that the 5-HT3 receptor plays an undeniable role in serotoninergic anti-nociception (Glaum et al., 1988; 1990; Danzebrink & Gebhart, 1991; Giordano, 1991; Rodgers & Shepherd, 1992; Jenkinson et al., 1995; Peng et al., 1996; Bardin et al., 1997). The fact that intrathecal 2-methyl-5-HT, a selective 5-HT3 receptor agonist, elicits antinociception in rats 10 days postnatal (Giordano, 1997) suggests an early emergence of monoamine-dependent endogenous analgesia. Since inhibition produced via the 5-HT3 receptor was less than that produced by 5-HT and the selective 5-HT3 receptor antagonists did not fully block 5-HT-induced DR-EPSP inhibition, it is unlikely that this receptor subtype can fully account for anti-nociceptive actions of 5-HT. Undoubtedly, an additional unidentified 5-HT receptor subtype is involved. A similar conclusion was reached by Peng et al. (1996) who reported that the selective 5-HT3 receptor antagonist ondansetron partially blocked periaqueductal gray-induced inhibition of dorsal horn nociceptive units in vivo. A candidate for this could be the 5-HT1 receptor that is associated with inhibition of dorsal horn neurotransmission (Zemlan et al., 1994; Lopez-Garcia & King, 1996a) and for which antinociceptive actions have been characterized (El-Yassir et al., 1988; Alhaider & Wilcox, 1993; Ali et al., 1994).
Whilst most studies have emphasized 5-HT inhibition of dorsal horn nociceptive inputs, 5-HT can have pro-nociceptive actions (Hamon et al., 1990; Wilcox & Alhaider, 1990) and 5-HT-induced excitation of dorsal horn neurones has been reported (Todd & Millar, 1983; Roberts, 1984; El-Yassir et al., 1988; Lopez-Garcia & King, 1996a; Hori et al., 1996). This seemingly contradictory data has been attributed to the non-selective action of 5-HT on heterogeneous receptors but the receptor subtype responsible for serotoninergic facilatory modulation of sensory neurotransmission in the dorsal horn is unknown. Pro-nociception has been ascribed to the 5-HT2 receptor (Hori et al., 1996) and the 5-HT1A receptor (Alhaider & Wilcox, 1993; Ali et al., 1994). In the case of 5-HT3, there are conflicting views in the literature. Some studies have failed to demonstrate 5-HT3 receptor-mediated control of nociception (Crisp et al., 1991; Xu et al., 1994a,1994b) and pro-nociceptive actions have been described (Ali et al., 1996; Oyama et al., 1996). In these in vitro studies where spinal actions of serotoninergic agonists were isolated from supraspinal effects, the response of dorsal horn neurones to 5-HT was heterogeneous and, in a small population of neurones, synaptic transmission was enhanced. Since a similar DR-EPSP augmentation was generated by m-ChPB, sensory amplification could, in part, be due to activation of 5-HT3 receptors and such an effect could account for reports of 5-HT3-mediated pro-nociception. It is proposed that the 5-HT3 receptor is coupled to a non-selective monovalent cation channel which when activated induces a large rapidly developing depolarizing inward current that desensitizes (Derkach et al., 1989; Yakushiji & Akaike, 1992). Isolated rat dorsal root ganglion neurones are depolarized by 5-HT and m-ChPB (Robertson & Bevan, 1991). However, since m-ChPB did not significantly depolarize or alter the input resistance of this population of dorsal horn neurones no proposal can be made concerning the mechanism of this synaptic facilitation action and further studies will be required.
In the context of anti-nociception and analgesia, what is the mechanism of 5-HT3-induced inhibition of primary afferent-mediated synaptic transmission and antinoception? Rhizotomy and capsaicin pre-treatment reduced but did not eliminate autoradiographic labelling of 5-HT3 receptors (Hamon et al., 1989; Laporte et al., 1995) and in situ hybridization studies have confirmed the presence of 5-HT3 receptors on intrinsic dorsal horn neurones (Kia et al., 1995). These data, taken together with morphological studies demonstrating axo-dendritic and axo-somatic synapses of 5-HT immuno-reactive fibres onto dorsal horn neurones (Ruda et al., 1982; Jankowska et al., 1995) give validity to a putative post-synaptic 5-HT action via 5-HT3 receptors. 5-HT-induced dorsal horn excitations have been associated with activation of GABA- or glycinergic inhibitory interneurones which, in turn, may inhibit sensory afferent transmission (Todd & Millar, 1983; Lopez-Garcia & King, 1996b; Peng et al., 1996). In vivo, the anti-nociceptive effects of a selective 5-HT3 receptor agonist are blocked by selective GABAA and GABAB antagonists (Alhaider et al., 1991). In contrast, DR-EPSP inhibition by m-ChPB in the isolated rat spinal cord was not abolished by bicuculline, saclofen or naloxone suggesting that, under these experimental conditions, modulation of dorsal horn neurotransmission was not solely dependent on GABA- or opioid-mediated interneurones. However, this study has not eliminated a possible involvement of other inhibitory interneuronal pathways e.g., glycinergic interneurones (Todd, 1990) and a more comprehensive study using a range of specific and selective antagonists would be informative. An alternative post-synaptic mechanisms could involve interactions with putative nociceptive afferent transmitters such as glutamate or substance P. In rat dorsal horn neurones in vitro, 5-HT depresses N-methyl-D-aspartate (NMDA) excitations (Lopez-Garcia, 1998). In spinal motoneurones, 2-methyl-5-HT reversibly reduced NMDA-induced excitations and this action was not offset by bicuculline, saclofen or naloxone (Holohean et al., 1995). 5-HT reduces substance P responses of dorsal horn neurones in vivo (Davies & Roberts, 1981) and the 5-HT3 receptor agonist, 2-methyl-5-HT attenuates substance P-induced pain-related behaviours (Wilcox, 1988).
Considering a putative pre-synaptic action of 5-HT acting via 5-HT3 receptors, two indicators of serotoninergic effect on primary afferent terminals, agonist-induced population PAD and stimulus-evoked DR-DRR, were examined. The 5-HT-induced PAD and inhibition of the DR-DRR confirms previously reported data of a similar nature (Preston & Wallis, 1980; Lopez-Garcia & King, 1996a,1996b). PAD is most likely due to a direct action of 5-HT on a population of fine calibre afferents including C-afferents (Lopez-Garcia & King, 1996b; Khasabov et al., 1998). This 5-HT-induced PAD was mimicked by m-ChPB (although the mean peak PAD was significantly smaller for this agonist) and was antagonized by Tropisetron which also abolished m-ChPB-induced PAD. These data are consistent with the anatomical localization of 5-HT3 receptors on primary afferent terminals particularly those of small diameter sensory capsaicin-sensitive afferents (Hamon et al., 1989; Kidd et al., 1993; Laporte et al., 1995) and suggest that this receptor subtype could be involved in a putative direct presynaptic inhibition of sensory afferent transmission generated by PAD. Whilst 5-HT significantly attenuated the DR-DRR, m-ChPB did not modulate this potential and Tropisetron had no effect on 5-HT-induced DR-DRR. These data suggest that the 5-HT3 receptor is not involved in the serotoninergic modulation of DR-DRR and the identity of this receptor is unknown. A number of studies have investigated the origin and neuropharmacology of this afferent potential and non-synaptic and synaptic components operating principally through excitatory amino acid receptors and the GABAA receptor have been demonstrated (Shimizu et al., 1995). The physiological significance of dorsal root potentials especially in vivo remains controversial and the classical view of a decreased presynaptic action potential and transmitter release following PAD such as has been proposed for muscle afferents (Eccles et al., 1961) require validation. Nonetheless, there is a view that presynaptic interactions between classes of sensory afferents and the dorsal root reflex could trigger allodynia (Cervero & Laird, 1996) and maintain or potentiate peripheral inflammation (Rees et al., 1994). In theory, a 5-HT-mediated attenuation of the dorsal root reflex could ameliorate or prevent the emergence of these pathological pain states.
In conclusion, these data provide a neuronal basis for the reported anti-nociceptive actions of ligand-gated 5-HT3 receptors localized on primary afferents and intrinsic neurones within the rat dorsal horn. Further anatomical and electrophysiological studies will be needed to establish the mechanism of 5-HT3-induced sensory inhibition following activation of descending bulbo-spinal pathways but these data suggest that it is likely to be reliant on both pre- and postsynaptic elements.
Acknowledgments
This study was supported by The Wellcome Trust. Technical assistance was given by Mrs J. Daniel.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- DR-EPSP
dorsal root-excitatory post-synaptic potential
- DR-DRR
dorsal root-dorsal root reflex
- IR
input resistance (MΩ)
- PAD
primary afferent depolarization
- Vm
membrane potential (mV)
- 5-HT
5-hydroxytryptamine, serotonin
References
- ALHAIDER A.A., LEI S.Z., WILCOX G.L. Spinal 5-HT3 receptor-mediated antinociception: possible release of GABA. J. Neurosci. 1991;11:1881–1888. doi: 10.1523/JNEUROSCI.11-07-01881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALHAIDER A.A., WILCOX G.L. Differential roles of 5-hydroxytryptaminelA and 5-hydroxytryptaminelB receptor subtypes in modulating spinal nociceptive transmission in mice. J. Pharmacol. Exp. Ther. 1993;265:378–385. [PubMed] [Google Scholar]
- ALI Z., WU G., KOZLOV A., BARASI S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Brain Res. 1994;661:83–90. doi: 10.1016/0006-8993(94)91184-3. [DOI] [PubMed] [Google Scholar]
- ALI Z., WU G., KOZLOV A., BARASI S. The role of 5HT3 in nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Neurosci. Lett. 1996;208:203–207. doi: 10.1016/0304-3940(95)12600-7. [DOI] [PubMed] [Google Scholar]
- BARDIN L., JOURDAN D., ALLOUI A., LAVARENNE J., ESCHALIER A. Differential influence of two serotonin 5-HT3 receptor antagonists on spinal serotonin-induced analgesia in rats. Brain Res. 1997;765:267–272. doi: 10.1016/s0006-8993(97)00566-0. [DOI] [PubMed] [Google Scholar]
- CERVERO F., LAIRD J.M. Mechanisms of allodynia: interactions between sensitive mechanoreceptors and nociceptors. NeuroReport. 1996;7:526–528. [PubMed] [Google Scholar]
- CRISP T., STAFINSKY J.L., SPANOS L.J., URAM M., PERNI V.C., DONEPUDI H.B. Analgesic effects of serotonin and receptor-selective serotonin agonists in the rat spinal cord. Gen. Pharmacol. 1991;22:247–251. doi: 10.1016/0306-3623(91)90441-8. [DOI] [PubMed] [Google Scholar]
- DAHLSTROM A., FUXE K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in cell bodies of brainstem neurons. Acta Physiol. Scand. 1964;232:1–55. [PubMed] [Google Scholar]
- DANZEBRINK R.M., GEBHART G.F. Evidence that spinal 5-HT1, 5-HT2 and 5-HT3 receptor subtypes modulate responses to noxious colorectal distension in the rat. Brain Res. 1991;538:64–75. doi: 10.1016/0006-8993(91)90377-8. [DOI] [PubMed] [Google Scholar]
- DAVIES J.E., ROBERTS M.H. 5-Hydroxytryptamine reduces substance P responses on dorsal horn interneurones: a possible interaction of neurotransmitters. Brain Res. 1981;217:399–404. doi: 10.1016/0006-8993(81)90018-4. [DOI] [PubMed] [Google Scholar]
- DERKACH V., SURPRENANT A., NORTH R.A. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- ECCLES J.R., ECCLES R.M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J. Physiol. (Lond.) 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-YASSIR N., FLEETWOOD-WALKER S.M., MITCHELL R. Heterogeneous effects of serotonin in the dorsal horn of rat: the involvement of 5-HT1 receptor subtypes. Brain Res. 1988;456:147–158. doi: 10.1016/0006-8993(88)90356-3. [DOI] [PubMed] [Google Scholar]
- GIORDANO J. Analgesic profile of centrally administered 2-methylserotonin against acute pain in rats. Eur. J. Pharmacol. 1991;199:233–236. doi: 10.1016/0014-2999(91)90462-y. [DOI] [PubMed] [Google Scholar]
- GIORDANO J. Antinociceptive effects of intrathecally administered 2-methylserotonin in developing rats. Dev. Brain Res. 1997;98:142–144. doi: 10.1016/s0165-3806(96)00186-1. [DOI] [PubMed] [Google Scholar]
- GLAUM S.R., PROUDFIT H.K., ANDERSON E.G. Reversal of the antinociceptive effects of intrathecally administered serotonin in the rat by a selective 5-HT3 receptor antagonist. Neurosci. Lett. 1988;95:313–317. doi: 10.1016/0304-3940(88)90677-5. [DOI] [PubMed] [Google Scholar]
- GLAUM S.R., PROUDFIT H.K., ANDERSON E.G. 5-HT3 receptors modulate spinal nociceptive reflexes. Brain Res. 1990;510:12–16. doi: 10.1016/0006-8993(90)90721-m. [DOI] [PubMed] [Google Scholar]
- HAMON M., COLLIN E., CHANTREL D., DAVAL G., VERGE D., BOURGOIN S.Serotonin receptors and the modulation of pain Serotonin and Pain 1990Elsevier Science Publisher B.V. (Biomedical Division)53–72.ed. Besson, J.M. pp [Google Scholar]
- HAMON M., GALLISSOT M.C., MENARD F., GOZLAN H., BOURGOIN S., VERGE D. 5-HT3 receptor binding sites are on capsaicin-sensitive fibres in the rat spinal cord. Eur. J. Pharmacol. 1989;164:315–322. doi: 10.1016/0014-2999(89)90472-x. [DOI] [PubMed] [Google Scholar]
- HOLOHEAN A.M., HACKMAN J.C., DAVIDOFF R.A. An in vitro study of the effects of serotonin on frog primary afferent terminals. Neurosci. Lett. 1990;113:175–180. doi: 10.1016/0304-3940(90)90299-o. [DOI] [PubMed] [Google Scholar]
- HOLOHEAN A.M., HACKMAN J.C., DAVIDOFF R.A. Modulation of frog spinal cord interneuronal activity by activation of 5-HT3 receptors. Brain Res. 1995;704:184–190. doi: 10.1016/0006-8993(95)01112-9. [DOI] [PubMed] [Google Scholar]
- HORI Y., ENDO K., TAKAHASHI T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J. Physiol. (Lond.) 1996;492:867–876. doi: 10.1113/jphysiol.1996.sp021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- JANKOWSKA E., MAXWELL D.J., DOLK S., KRUTKI P., BELICHENKO P.V., DAHLSTROM A. Contacts between serotoninergic fibres and dorsal horn spinocerebellar tract neurons in the cat and rat: a confocal microscopic study. Neuroscience. 1995;67:477–487. doi: 10.1016/0306-4522(95)00059-r. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H., BARNARD E.A., HOYER D., HUMPHREY P.P., LEFF P., SHANKLEY N.P. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. IX. Recommendations on terms and symbols in quantitative pharmacology. Pharmacol Rev. 1995;47:255–266. [PubMed] [Google Scholar]
- KHASABOV S.G., LOPEZ-GARCIA J.A., KING A.E. Serotonin-induced population primary afferent depolarisation in vitro: the effects of neonatal capsaicin treatment. Brain Res. 1998;789:339–342. doi: 10.1016/s0006-8993(98)00136-x. [DOI] [PubMed] [Google Scholar]
- KIA H.K., MIQUEL M.C., MCKERNAN R.M., LAPORTE A.M., LOMBARD M.C., BOURGOIN S., HAMON M., VERGE D. Localization of 5-HT3 receptors in the rat spinal cord: immunohistochemistry and in situ hybridization. NeuroReport. 1995;6:257–261. doi: 10.1097/00001756-199501000-00008. [DOI] [PubMed] [Google Scholar]
- KIDD E.J., LAPORTE A.M., LANGLOIS X., FATTACCINI C.M., DOYEN C., LOMBARD M.C., GOZLAN H., HAMON M. 5-HT3 receptors in the rat central nervous system are mainly located on nerve fibres and terminals. Brain Res. 1993;612:289–298. doi: 10.1016/0006-8993(93)91674-h. [DOI] [PubMed] [Google Scholar]
- LAPORTE A.M., DOYEN C., NEVO I.T., CHAUVEAU J., HAUW J.J., HAMON M. Autoradiographic mapping of serotonin 5-HT1A, 5-HT1D, 5-HT2A and 5-HT3 receptors in the aged human spinal cord. J. Chem. Neuroanat. 1996;11:67–75. doi: 10.1016/0891-0618(96)00130-5. [DOI] [PubMed] [Google Scholar]
- LAPORTE A.M., FATTACCINI C.M., LOMBARD M.C., CHAUVEAU J., HAMON M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B, and 5-HT3 receptors in the rat spinal cord. J. Neural Transm. 1995;100:207–223. doi: 10.1007/BF01276459. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A. Serotonergic modulation of the responses to excitatory amino acids of rat dorsal horn neurons in vitro: implications for somatosensory transmission. Eur. J. Neurosci. 1998;10:1341–1349. doi: 10.1046/j.1460-9568.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A., KING A.E. A novel methodology for simultaneous assessment of the effects of 5-hydroxytryptamine on primary afferent polarisation and synaptic transmission in rat dorsal horn neurones in vitro. J. Neurosci. Meth. 1996a;68:1–6. [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A., KING A.E. Pre- and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: implications for somatosensory transmission. Eur. J. Neurosci. 1996b;8:2188–2197. doi: 10.1111/j.1460-9568.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- OYAMA T., UEDA M., KURAISHI Y., AKAIKE A., SATOH M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci. Res. 1996;25:129–135. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- PENG Y.B., LIN Q., WILLIS W.D. The role of 5-HT3 receptors in periaqueductal gray-induced inhibition of nociceptive dorsal horn neurons in rats. J. Pharmacol. Exp. Ther. 1996;276:116–124. [PubMed] [Google Scholar]
- PRESTON P.R., WALLIS D.I. The pharmacology of dorsal root potentials recorded from the isolated cord of the rat. Br. J. Pharmacol. 1980;11:527–534. doi: 10.1016/0306-3623(80)90085-3. [DOI] [PubMed] [Google Scholar]
- REES H., SLUKA K.A., WESTLUND K.N., WILLIS W.D. Do dorsal root reflexes augment peripheral inflammation. Neuro-Report. 1994;5:821–824. doi: 10.1097/00001756-199403000-00021. [DOI] [PubMed] [Google Scholar]
- ROBERTS M.H.T. 5-Hydroxytryptamine and antinociception. Neuropharmacology. 1984;23:1529–1536. doi: 10.1016/0028-3908(84)90097-2. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B., BEVAN S. Properties of 5-hydroxytryptamine3 receptor-gated currents in adult rat dorsal root ganglion neurones. Br. J. Pharmacol. 1991;102:272–276. doi: 10.1111/j.1476-5381.1991.tb12165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODGERS R.J., SHEPHERD J.K. Attenuation of defensive analgesia in male mice by 5-HT3 receptor antagonists, ICS 205-930, MDL 72222, MDL 73147EF and MDL 72699. Neuropharmacology. 1992;31:553–560. doi: 10.1016/0028-3908(92)90187-t. [DOI] [PubMed] [Google Scholar]
- RUDA M.A., COFFIELD J., STEINBUSCH H.W. Immunocytochemical analysis of serotonergic axons in laminae I and II of the lumbar spinal cord of the cat. J. Neurosci. 1982;2:1660–1671. doi: 10.1523/JNEUROSCI.02-11-01660.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU T., YOSHIMURA M., BABA H., SHIMOJI K., HIGASHI H. Role of A delta afferent fibers in modulation of primary afferent input to the adult rat spinal cord. Brain Res. 1995;691:92–98. doi: 10.1016/0006-8993(95)00619-2. [DOI] [PubMed] [Google Scholar]
- SORKIN L.S., MCADOO D.J., WILLIS W.D. Raphe magnus stimulation-induced antinociception in the cat is associated with release of amino acids as well as serotonin in the lumbar dorsal horn. Brain Res. 1993;618:95–108. doi: 10.1016/0006-8993(93)90433-n. [DOI] [PubMed] [Google Scholar]
- STEINBUSCH H.W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- THOMPSON S.W.N., KING A.E., WOOLF C.J. Activity-dependent changes in rat ventral horn neurones in vitro; summation of prolonged afferent evoked postsynaptic depolarizations produce a d-APV sensitive windup. Eur. J. Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- TODD A.J. An electron microscope study of glycine-like immunoreactivity in laminae I-III of the spinal dorsal horn of the rat. Neuroscience. 1990;39:387–394. doi: 10.1016/0306-4522(90)90275-9. [DOI] [PubMed] [Google Scholar]
- TODD A.J., MILLAR J. Receptive fields and responses to iontophoretically applied noradrenaline and 5-hydroxytryptamine of units of laminae I-III of cat dorsal horn. Brain Res. 1983;288:159–167. doi: 10.1016/0006-8993(83)90090-2. [DOI] [PubMed] [Google Scholar]
- TYCE G.M., YAKSH T.L. Monoamine release from cat spinal cord by somatic stimuli: an intrinsic modulatory system. J. Physiol. (Lond.) 1981;314:513–529. doi: 10.1113/jphysiol.1981.sp013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX G.L. Pharmacological studies of grooming and scratching behavior elicited by spinal substance P and excitatory amino acids. Ann. N.Y. Acad. Sci. 1988;525:228–236. doi: 10.1111/j.1749-6632.1988.tb38608.x. [DOI] [PubMed] [Google Scholar]
- WILCOX G.L., ALHAIDER A.A.Nociceptive and anti-nociceptive action of serotonin agonists administered intrathecally 5-HT3Receptors and Pain 1990Amsterdam: Exerpta Medica; 205–206.ed. Besson, J.M. pp [Google Scholar]
- XU W., CUI X.C., HAN J.S. Spinal serotonin IA and IC/2 receptors mediate supraspinal mu opioid-induced analgesia. NeuroReport. 1994a;5:2665–2668. doi: 10.1097/00001756-199412000-00065. [DOI] [PubMed] [Google Scholar]
- XU W., QIU X.C., HAN J.S. Serotonin receptor subtypes in spinal antinociception in the rat. J. Pharmacol. Exp. Ther. 1994b;269:1182–1189. [PubMed] [Google Scholar]
- YAKUSHIJI T., AKAIKE N. Blockade of 5-HT3 receptor-mediated currents, in dissociated frog sensory neurones by benzoxazine derivative, Y-25130. Br. J. Pharmacol. 1992;107:853–857. doi: 10.1111/j.1476-5381.1992.tb14536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEMLAN F.P., MURPHY A.Z., BEHBEHANI M.M. 5-HT1A receptors mediate the effect of the bulbospinal serotonin system on spinal dorsal horn nociceptive neurons. Pharmacology. 1994;48:1–10. doi: 10.1159/000139156. [DOI] [PubMed] [Google Scholar]


