Abstract
Previous studies with indolyl derivatives as monoamine oxidase (MAO) inhibitors have shown the relevance of the indole structure for recognition by the active site of this enzyme. We now report a new series of molecules with structural features which determine the selectivity of MAO inhibition.
A benzyloxy group attached at position 5 of the indole ring is critical for this selective behaviour. Amongst all of these benzyloxy-indolyl methylamines, N-(2-propynyl)-2-(5-benzyloxyindol)methylamine FA-73 was the most potent MAO-B ‘suicide' inhibitor studied.
The Ki values for MAO-A and MAO-B were 800±60 and 0.75±0.15 nM, respectively. These data represent a selectivity value of 1066 for MAO-B, being 48 times more selective than L-deprenyl (Ki values of 376±0.032 and 16.8±0.1 nM for MAO A and MAO-B, respectively). The IC50 values for dopamine uptake in striatal synaptosomal fractions from rats were 150±8 μM for FA-73 and 68±10 μM for L-deprenyl whereas in human caudate tissue the IC50 values were 0.36±0.015 μM for FA-73 and 0.10±0.007 μM for L-deprenyl. Moreover, mouse brain MAO-B activity was 90% ex vivo inhibited by both compounds 1 h after 4 mg kg−1 adminstration, MAO-A activity was not affected.
These novel molecules should provide a better understanding of the active site of monoamine oxidase and could be the starting point for the design of further selective, non-amphetamine-like MAO-B inhibitors with therapeutic potential for the treatment of neurological disorders.
Keywords: MAO-B inhibitors, benzyloxy-tryptamine derivatives
Introduction
Monoamine oxidase (MAO; amine:oxygen oxidoreductase deaminating, flavin-containing; EC 1.4.3.4) is an enzyme located on the mitochondrial outer membrane which catalyses the oxidative deamination of biogenic amines such as the monoamine neurotransmitters and neuromodulators, as well as some exogenous bioactive monoamines (Youdim et al., 1988). Two MAO isoforms have been identified, MAO-A and MAO-B, which differ with respect to sensitivity to inhibitors and substrate specificity. MAO-A is sensitive to inhibition by low concentrations of the acetylenic inhibitor clorgyline, whereas MAO-B is sensitive to inhibition by L-deprenyl (Johnston, 1968). MAO-A selectively metabolizes serotonin (5-HT), while MAO-B has a greater affinity towards phenylethylamine (PEA) as substrate (Youdim & Finberg, 1983). Dopamine (DA), adrenaline and noradrenaline are substrates for both isoforms (Singer & Ramsay, 1993).
MAO-B inhibitors, including L-deprenyl, have been shown to prevent MPTP-induced Parkinson-like neurotoxicity in animals since MAO-B is responsible for oxidation of the pro-toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), to its toxic metabolite 1-methyl-pyridine ion (MPP+) (Heikkila et al., 1984). Inhibition of MAO-B has also been reported to protect against 3,4-methylenedioxy-methamphetamine (MDMA)-induced neurotoxicity (Sprague & Nichols, 1995), and some MAO-B inhibitors have been shown to have neuroprotective effects against the toxin N-2-(chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4) (Yu et al., 1994). L-deprenyl (selegiline) has been used in the initial treatment of Parkinson's disease (Tetrud & Langston, 1989), reducing the levodopa dose requirement (Myllylä et al., 1995). Because L-deprenyl is metabolized in vivo to D-methamphetamine and D-amphetamine (Heinonen et al., 1989), MAO-B inhibitors without amphetamine-like properties may be of interest as possible therapeutic agents in the treatment of neurodegenerative diseases.
Despite the large number of MAO inhibitors described (Balsa et al., 1991; 1994; Yu et al., 1992; Tipton et al., 1993; Avila et al., 1993; Kneubüler et al., 1995; Kalgutkar & Castagnoli, 1995; Pérez et al., 1996; Harfenist et al., 1996) and the fact that the primary structures of these enzymes have been established from the corresponding gene sequences (Back et al., 1988), the structural features responsible for selective MAO-A or MAO-B inhibition are still far from clear (Dostert et al., 1989).
Tryptamine is a non-selective MAO substrate, but when the hydrogen atom in position 5 of the indole ring is substituted by a hydroxyl group the MAO-A-specific substrate serotonin is formed. The synthesis of some 2-, and 3-substituted acetylenic and allenic derivatives of tryptamine has been previously reported, and it has been shown that these derivatives are selective inhibitors of MAO-A (Cruces et al., 1988; 1990; 1991). These compounds include 5-OH, 5-CH3O, and 5-H derivatives of these indoleamines, (Balsa et al., 1990; 1991; 1994; Avila et al., 1993; Pérez et al., 1996).
The present work was undertaken to study the in vitro effect of the benzyloxy group attached at the 5-position of a series of acetylenic and allenic derivatives of 2-(5-benzyloxyindolyl)methylamines on the selective inhibition of MAO-B. In addition, the effects of one of these compounds, the 5-benzyloxyindol derivative, FA-73, on the activity of monoamine oxidase-B ex vivo and its in vitro interaction with the dopamine uptake system has been studied.
Our results show that the presence of the benzyloxy group attached at the 5-position of the indole ring is critical for explaining the selectivity and potency of MAO-B inhibition. Furthermore, the benzyloxy-derivative FA-73 may be a compound of potential interest as a possible drug for treatment of neurodegenerative disorders.
Methods
Assays
Sprague-Dawley male rats weighing 250–300 g were used for all in vitro assays, while C57B1/6 male mice weighing 20–25 g were used for the ex vivo assays. The animals were purchased from Charles-River (France).
Preparation of rat-liver mitochondrial MAO
Liver mitochondrial fractions were prepared by differential centrifugation as previously described (Gómez et al., 1988). In order to study MAO-B or MAO-A activity, the mitochondrial fraction was incubated at 37°C for 60 min with selective inhibitors L-deprenyl (1.5×10−6 M) or clorgyline (1.5×10−6 M), followed by centrifugation at 20,000×g for 15 min. The pellet fraction was then resuspended in 50 mM potassium phosphate buffer, pH 7.2. Protein concentration was adjusted to 10 mg ml−1 in the same buffer.
MAO assays
MAO activity was determined radiochemically using 14C-labelled substrates as previously described (Fowler & Tipton, 1981). In brief, the enzyme preparations were incubated at 37°C for 10 min with the MAO-A-specific substrate 100 μM [14C]-5-HT (0.5 mCi mmol−1) or for 2 min with the MAO-B-specific substrate 20 μM [14C]-PEA (2.5 mCi mmol−1). The reactions were stopped by the addition of 100 μl of 2 M citric acid. Radioactive aldehyde products were extracted into toluene/ethyl acetate (1 : 1) containing 0.6% 2.5-diphenyloxazole (w v−1) before liquid scintillation counting.
IC50 assays
The mitochondrial fraction was pre-incubated for 30 min with different inhibitor concentrations (10−3–10−10 M) before MAO-A and MAO-B activity determination. The incubation-inhibitor time used in these experiments was previously determined as optimal to observe enzyme inhibition (Balsa et al., 1990).
Time-dependent inhibition
Mitochondrial fractions were incubated with each benzyloxy derivative, at a concentration similar to their IC50 values, for different periods of time prior to MAO determination.
Reversibility of the inhibition process
In order to test whether reversible or irreversible inhibition was produced by these indolealkylamine derivatives, 200 μl of enzyme preparation (8.5 mg ml−1) was incubated for 30 min at 37°C with 100 μl of different MAO inhibitors at a concentration near its IC50 value, in a final volume of 1 ml of 50 mM potassium phosphate buffer, pH 7.2, and after this the remaining activities were determined. The mixture was centrifuged three times for 10 min at 12,000×g. Regaining MAO-A, or MAO-B activities were determined after each washing. Controls in which the inhibitor was replaced by identical volume of buffer were run through the same procedure (Waldemeier et al., 1983).
Spectrophotometric assays: kinetic parameters of inhibition
After selective inhibition of MAO-A or MAO-B in the mitochondria as described above, the kinetic parameters were determined by a direct spectrophotometric analysis of reaction progress curves, using kynuramine (40 μM) or benzylamine (333 μM) as MAO-A and MAO-B substrates, respectively (Avila et al., 1993). In order to determine the apparent first-order rate constant (Kapp), inhibition progress curves were fitted to a first-order rate equation with the aid of the non-linear, regression-analysis computer program ENZFITTER (Elsevier-Biosoft). Kinetic parameters Ki, which define the affinity of the reversible step, and Kcat, which defines the velocity constant corresponding to the covalent step of the total inhibitory process, were determined by non-linear regression analysis of the Kapp versus inhibitor concentration.
Synaptosomal fraction
Male Sprague-Dawley rats were sacrificed by stunning and decapitation and their striata dissected at 4°C. Striatal tissues were homogenised (1 : 10 w v−1) in 0.32 M sucrose. A synaptosomal fraction was obtained by differential centrifugation through a sucrose gradient (Dodd et al., 1981).
Male human caudate tissues were obtained from the autopsy of three subjects (mean age±s.e.mean=50±5 years, mean post-mortem interval±s.e.mean=18±3 h) with no history of neurological disorder. Tissues were obtained from the Institut Anatòmic Forense de l'Hospital Clinic de Barcelona. They were freshly dissected and the synaptosomal fraction was rapidly prepared by the method described above.
Dopamine uptake assays
The synaptosomal preparation (100–125 μg protein) was preincubated for 5 min with Krebs buffer containing (in mM): NaCl 125, KCl 3, MgSO4.7H2O 1.2, CaCl2.2H2O 1.2, NaHCO3 22, NaH2PO4.H2O 1, glucose 10, equilibrated at pH 7.4 with 95% O2/5% CO2, containing 0.8 mM pargyline, 0.64 mM ascorbic acid and 100 nM [3H]-DA (specific activity 8000 d.p.m. pmol−1). Inhibition of dopamine uptake was assayed by adding the MAO inhibitors at different concentrations (10−3–10−8 M) and incubating the mixture for 4 min at 37°C. The reaction was stopped by the addition of 15 ml cold Krebs buffer. Samples were collected by vacuum filtration on to GF/C Watman filters (previously soaked in 0.1% polyethylenimine for 4 min) using a Brandel Cell Harvester. The filters were dissolved in Beckman scintillation mixture and the radioactivity was quantified by scintillation counting. Nonspecific uptake was obtained including a dopamine uptake blocker, i.e., 1 μM 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-[3-phenylpropyl]piperazine dihydrochloride (GBR-12909), in the appropriate tubes (Heikkila, 1984). Results were expressed as a percentage of the remaining activity.
Ex vivo assays
FA-73 and L-deprenyl compound were dissolved in 4% di-methyl-formamide (v v−1) which was also the vehicle administerd to the control group.
C57B1/6 mice were injected i.p. with either MAO-B FA-73 or L-deprenyl inhibitors at a dose of 4 mg kg−1. After 1, 24 and 96 h the mice were sacrificed by decapitation and their brains were removed and homogenized in 5 ml of 50 mM potassium phosphate buffer, pH 7.2, at 4°C. MAO-A and MAO-B activities were determined at 37°C using 5-HT and PEA as specific substrates, respectively.
Results were expressed as a percentage of remaining activity, with respect to vehicle-treated controls.
Drugs and radiochemicals
The series of acetylenic and allenic derivatives of 2-(5-benzyloxyindolyl)methylamine were obtained from CSIC (Madrid, Spain) and were synthesized according to previously published methods (Cruces et al., 1991). The [14C]-5-hydroxytryptamine (5-HT) and [3H]-dopamine (DA) radiochemicals were purchased from Amersham (U.K.), [14C]-phenylethylamine (PEA) was purchased from New England Nuclear (Boston, MA, U.S.A.). Kynuramine, benzylamine, L-deprenyl and clorgyline came from Sigma Chemical Co. (St. Louis, MO, U.S.A.). GBR-12909 was obtained from Research Biochemicals International (Natick, MA, U.S.A.).
Statistical analysis
The ex vivo effect of FA 73 on MAO A and MAO B activities in brain of C57/BL mice was expressed as mean±s.d. of three separate experiments in duplicate. One-way analysis of variance (ANOVA) followed by Scheffé's test was used for the statistical evaluation. Correlation coefficients were calculated by the method of least squares. The level of significance was chosen as P<0.05.
Results
MAO assays in the rat-liver mitochondrial fraction
In order to establish the selectivity of inhibition towards both MAO isoforms, the MAO activity remaining was measured after preincubating the mitochondrial fraction with the inhibitors for 30 min at 37°C in the presence of different inhibitor concentrations. All of the compounds studied were shown to be selective inhibitors of MAO-B. Figure 1 shows data for FA-73 which is as representative of all the benzyloxy-derivatives.
Figure 1.
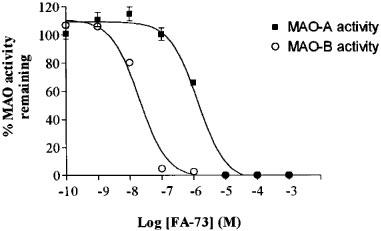
Inhibition of MAO-A and MAO-B activity by FA-73. Data is representative of all benzyloxy derivatives studied. Rat liver mitochondrial fraction was pre-incubated in presence of different concentrations of inhibitors for 30 min at 37°C. MAO-A activity was measured with 100 μM [14C]-5-hydroxytryptamine as substrate and MAO-B activity was measured with 20 μM [14C]-phenylethylamine as substrate. The results are the mean±s.d. of three separate experiments.
The IC50 values are shown in Table 1. The selectivity as inhibitors of MAO-B, expressed as IC50 MAO-A / IC50 MAO-B ratio, decreased in all cases in which the H atom attached to N of the indole ring was substituted by a CH3 group (compounds FA-75 and FA-97, FA-88 and FA-66, and FA-87 and FA-65). Similar results were obtained when the same substitution was performed at the N atom of the side-chain. Thus, when compounds FA-74 and FA-88 or FA-73 and FA-87 were compared, the MAO-B selectivity of the inhibition was also decreased.
Table 1.
IC50 values for MAO inhibition by 5-benzyloxy indolalquilamine derivatives
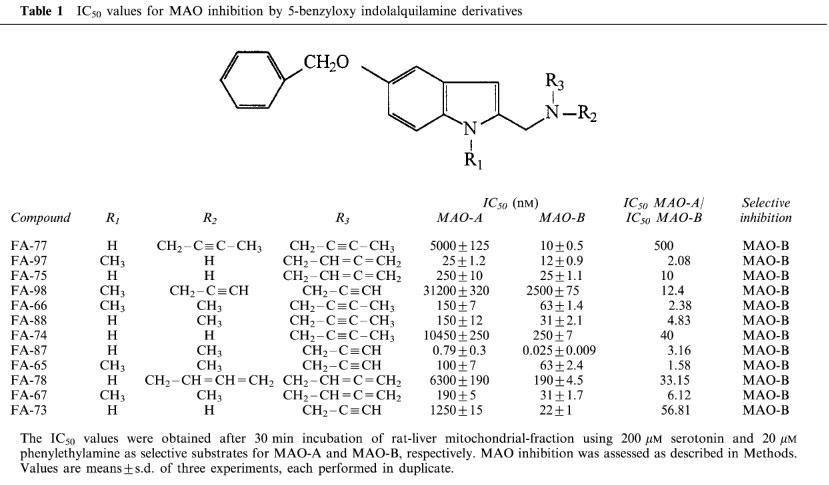
In all cases, when a second allenic or acetylenic reactive group was attached at the N atom of the side-chain, the selectivity of inhibition increased (e.g. FA-65 and FA-98, FA-75 and FA-78 or FA-74 and FA-77). Furthermore, when the mitochondrial fraction was preincubated with each benzyloxy derivative, all of the compounds were found to be time-dependent inhibitors of both MAO-A and MAO-B (Figure 2).
Figure 2.
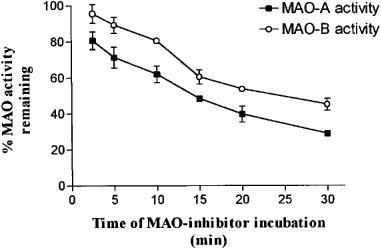
Time dependence of MAO inhibition by FA-73. Data is representative of all benzyloxy derivatives studied, at concentrations of 10 μM for MAO-A and 100 nM for MAO-B. The results are expressed as the mean±s.d. of three experiments, each performed in duplicate.
The monoamine oxidase inhibition by this series of benzyloxy derivatives was irreversible. After three successive centrifugations and washings following incubation of the enzyme with inhibitor, there was no significant recovery (Student t-test analysis) of MAO-A or MAO-B activity (Figure 3).
Figure 3.
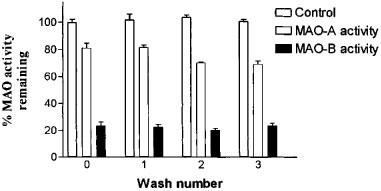
Reversibility of MAO-A and MAO-B inhibition by compound FA-73. This compound was used at concentrations that produced 80 and 25% of MAO-A and MAO-B inhibition, respectively. All the benzyloxy derivatives showed similar behaviour as irreversible inhibitors (data not shown). Results are presented as the mean±s.d. of three experiments, each performed in duplicate.
Table 2 shows the Ki and Kcat kinetic parameters. From the Ki values it can be deduced that the substitution of a benzyloxy group at position 5 of the indole ring resulted in increased selectivity for non-covalent inhibition of MAO-B. These data are in agreement with the IC50 values obtained.
Table 2.
Kinetic parameters of MAO-A and -B inhibition by 5-benzyloxy derivatives
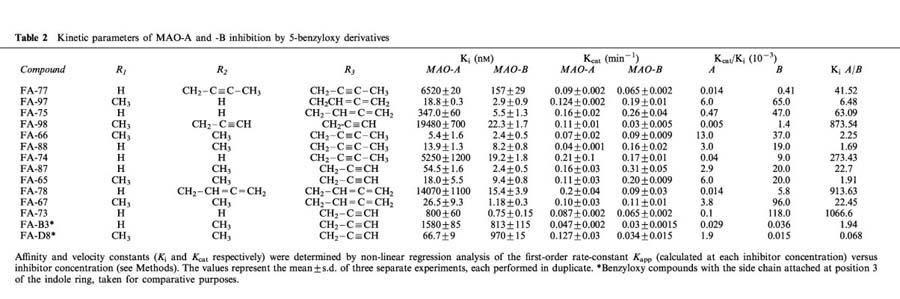
When the hydrogen atom from the N of the indole ring was replaced by a methyl group, the affinity for MAO-B was not greatly affected (Ki: from 5.5–2.9 nM for FA-75, when compared with FA-97; from 8.2–2.4 nM for FA-88, when compared with FA-66, and from 13.6–9.4 nM for FA-87, when compared with FA-65). In contrast, the corresponding Ki values for inhibition of MAO-A were disminished for the same compounds (from 347–18.8 nM; from 13.9–5.4 nM; from 54.5–18.0 nM, respectively). This effect on the affinity-constant values is correlated with the loss of selectivity observed concerning MAO-B.
When a methyl group was attached to the N of the side-chain, changes in the respective Ki values reflected those seen in the IC50 values. The affinity constant for MAO-A increased slightly for the allenic derivatives (from 18.8–26.5 nM for FA-97, when compared with FA-67) and decreased considerably for the N-but-2-ynyl-N derivatives (from 5250–13.9 nM for FA-74, when compared with FA-88). The corresponding Ki values for MAO-B slightly decreased (from 2.9–1.18 nM, and from 19.2–8.2 nM, respectively). In contrast, the affinity constants showed the opposite behaviour when FA-73 and FA-87 were compared. In this case, the Ki value for MAO-A decreased 14 times (from 800–54.5 nM), whereas the corresponding Ki value for MAO-B increased 3.2 times (from 0.75–2.4 nM). This effect was correlated with the loss of selectivity (Ki MAO-A/ Ki MAO-B), which disminished approximately 47 fold.
A second allenic, N-but-2-ynyl, or a second acetylenic reactive group attached at the N of the side-chain was associated with a decrease in the corresponding affinities for non-covalent binding to both MAO-A and MAO-B. The Ki value increased 1082 times for MAO-A and 2.37 times for MAO-B when a second acetylenic group was attached at the N of the side-chain (compare compounds FA-65 and FA-98). When an allenic group was attached at the N of the side-chain, the Ki values for MAO-A and MAO-B increased 40 times and three times, respectively (compare: FA-75 and FA-78). A N-but-2-ynyl second reactive group attached at this position resulted in an increase in Ki values of 469 and 19 times for MAO-A and MAO-B respectively (e.g. FA-88 and FA-77).
For all compounds tested, the corresponding rate constant Kcat for the irreversible step was in the range of 10−1–102 min−1 (see Table 2).
Catalytic efficacy, expressed by the ratio Kcat/Ki, was higher for MAO-B than for MAO-A. These effects are dominated by the changes in the Ki values. For example, the most potent and selective MAO-B inhibitor of this series, turned out to be FA-73, which showed MAO-B selectivity, in terms of the ratio Ki MAO-A / Ki MAO-B, of 1066 and 1180 times a more potent inhibitor towards MAO-B, in terms of catalytic efficacy (expressed in MAO-B Kcat/Ki/MAO-A Kcat/Ki values).
The kinetic parameters for the MAO-B inhibitor L-deprenyl (Table 3) showed a higher selectivity for reversible interaction with MAO-B (Ki MAO-A/Ki MAO-B) (22 times) and a greater selectivity in terms of catalytic efficacy of 6.4 fold. When these results were compared, compound FA-73 was about 47 times more selective than L-deprenyl, whereas the catalytic efficiency was 18 times higher for FA-73 than for L-deprenyl with MAO-B.
Table 3.
Comparative kinetic parameters between the L-deprenyl and FA-73

Dopamine uptake
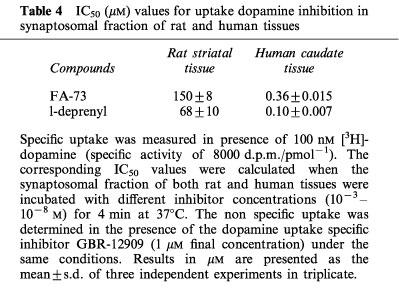
Dopamine uptake was assayed in rat and human caudate synaptosomal fractions. The MAO-B inhibitors FA-73 and L-deprenyl reduced [3H]-DA uptake. The corresponding IC50 values indicated that L-deprenyl showed approximately three times more affinity than FA- 73 in both tissues (Table 4).
Table 4.
IC50 (μM) values for uptake dopamine inhibition in synaptosomal fraction of rat and human tissues
Ex vivo assays
In order to determine whether FA-73 was able to cross the brain-blood barrier, either this compound or L-deprenyl (4 mg kg−1) was injected i.p., and the recovery of MAO activity was assayed at different times in whole brain homogenates of C57 Bl/ mice.
As observed in vitro assays, MAO-A activity was unaffected after ex vivo administration of FA 73 MAO-B selective inhibitor to C57/mice (Figure 4). Thus FA-73, like L-deprenyl, crosses the blood-brain barrier. Furthermore, when brain MAO-B activity was determined at different times after (i.p.) administration of these compounds, similar time-courses of recovery, consistent with de novo enzyme synthesis, were observed.
Figure 4.
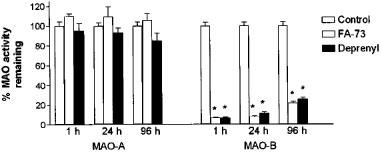
Recovery of MAO activity. The remaining MAO activity was calculated in ex vivo assays in C57B1/6 mice as described in Methods. Three groups of mice (five mice in each group) were injected i.p. with FA-73 or L-deprenyl (at a single dose of 4 mg kg−1) or vehicle (controls). The mice were sacrified 4, 24 and 96 h after injection and the MAO A and B specific activities in whole brain homogenates measured. The results are presented as the mean±s.d. of three separate experiments in duplicate. *Significantly different from control, as analysed using Sheffé test after ANOVA. P<0.05.
Discussion
MAO assays in the rat liver mitochondrial fraction
The specific and irreversible inhibitory behaviour towards MAO-A and MAO-B by this series of 5-benzyloxyindolyl derivatives can take place in two different stages, a first and reversible process, associated with an affinity-constant Ki, and a second step associated with a velocity-constant Kcat, responsible for covalent, enzyme-inhibitor complex formation. This mechanism is in agreement with that previously reported for other propargylamine derivatives (Fowler et al., 1982). This suggests that the compounds in this series act as enzyme-activated irreversible inhibitors (‘suicide' or Kcat inhibitors) of monoamine oxidase (Fowler et al., 1982).
Substitution of the hydrogen atom, hydroxy group or methoxy group, compounds FA-27, FA-69 and FA-43, respectively (Avila et al., 1993), at the 5-position on the indole ring of these acetylenic derivatives by a benzyloxy group (FA-65) gave rise to MAO-B selective inhibition. These three compounds were found to be irreversible and selective MAO-A inhibitors (Ki MAO-A/MAO-B ratio 0.12, 0.0048 and 0.026, respectively), whereas a ratio of 1.91 was obtained for compound FA-65 (Table 5).
Table 5.
MAO-A and MAO-B kinetic parameters in 5-benzyloxy-indole derivatives compared with some 5-substituents
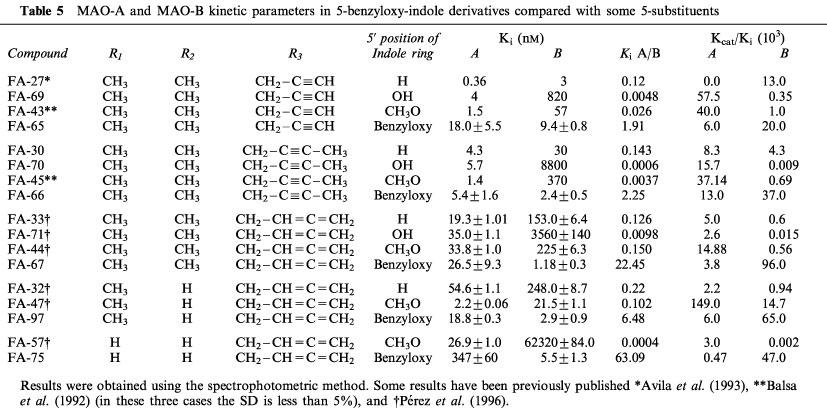
Similarly, the substitution of the hydrogen atom, hydroxy group or methoxy group at the 5-position of the indole ring of these N-but-2-ynyl derivatives, in the MAO-A selective inhibitors FA-30, FA-70 and FA-45, respectively (Avila et al., 1993), by a benzyloxy group (FA-66) changed the selective inhibition towards monoamine oxidase B. The lower Ki values of FA-30, FA-70 and FA-45 for MAO-A were changed to a lower Ki for MAO-B in the benzyloxy derivative FA-66 (Table 5). This change in the selectivity towards MAO-B was also shown in the 5-benzyloxy derivatives with an allenic group attached at the N atom of the side-chain. In these allenic compounds the substitution of a hydrogen atom, a hydroxy group or a methoxy group (compounds FA-33, FA-71 and FA-47, respectively (Pérez et al., 1996)), by a benzyloxy group (compound FA-67) gave rise to the selectivity towards MAO-B (Table 5). As can be seen in Table 5, similar changes in selectivity also occurred in other allenic derivatives of these same series of benzyloxy compounds.
For the FA-75 allenic compound, the presence of the benzyloxy group attached at the 5-position of the indole ring also appears to be critical for inhibitor selectivity towards monoamine oxidase B. In comparison with the corresponding indole derivative, which has a methoxy group in this position, i.e. compound FA-57 (Pérez et al., 1996), its affinity constant changed from being 2316 times lower for MAO-A (compound FA-57) to being 63 times lower for MAO-B (compound FA-75) (Table 5).
A benzyloxy group attached in the 5-position of the indole ring increases the molecular hydrophobicity in this series of compounds, consistent with the environment of the active site being an important factor in MAO-B selectivity (Naoi & Yagi, 1980). Benzyloxy substituents have previously been shown to give rise to selective MAO-B inhibition in a series of oxadizone derivatives (Mazouz et al., 1993).
This effect of the benzyloxy group in the 5-position of the indole derivatives in giving rise to MAO-B selective inhibition is only observed when the side-chain is attached at position 2 of the indole ring. The selectivity and affinity for MAO-B change when the side-chain is attached at position 3 of the indole ring (Table 5). These data are in agreement with those observed for MAO-B selective inhibition by para-, meta- and ortho-substituents of analogues of N-(2-aminoethyl)-4-cholorobenzamide (Ro 16-6491) (Nikoi & Silverman, 1993) and for the phenyl-substituted MPTP derivatives (Palmer et al., 1997).
In contrast to earlier suggestions (McDonald et al., 1991), inhibitory selectivity towards MAO-B is not simply related to the distance between the aromatic ring and the N-propargyl terminal, or the allylamine moiety. Although the increase of 2, 3 and 4 C atoms in side-chain length has been suggested to be related to increased potency for MAO-A inhibition (O'Brien et al., 1994), these changes in the inhibitory potency could result from lipophilic, electronic and steric properties of the different compounds (Kneubühler et al., 1995).
The Ki and Kcat values for the acetylenic derivative L-deprenyl (a specific, irreversible, ‘suicide' inhibitor of MAO-B (Fowler et al., 1982)) were compared with the same values obtained for the compound FA-73 (the most potent and selective MAO-B inhibitor of this series of 5 benzyloxy derivatives). The Ki MAO-A / Ki MAO-B ratio for FA-73 was 1066 : 1, whereas the ratio for the L-deprenyl was 23 : 1. This indicates that FA-73 is roughly 45 times more selective in the non-covalent phase of inhibition. Moreover, the Ki value of FA-73 for MAO-B was 22 times lower than that of L-deprenyl. In terms of catalytic efficacy, this benzyloxy derivative is also more potent by a factor of almost 26.
Dopamine uptake
Moreover, FA-73 was also found to be a dopamine uptake inhibitor in rat striatum and human caudate synaptosomal preparations, with an IC50 value similar to that reported for L-deprenyl. Inhibitors of dopamine uptake have been shown to protect against the effects of the dopaminergic neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Javitch et al., 1985; Sánchez-Ramos et al., 1992). Thus, both of these compounds may protect MPTP-dopaminergic toxicity in vivo by inhibiting uptake into nerve terminals as well as by inhibiting MAO-B.
Ex vivo assays
When FA-73 and L-deprenyl were injected i.p. in C57 mice, a selective MAO-B inhibition was obtained in whole brain homogenates. This effect shows that these compounds are able to cross the blood-brain barrier. Moreover, differences between FA-73 and L-deprenyl with respect to in vivo MAO B inhibitor potency were not observed, probably due to the high inhibitor concentration used. Such properties are essential for effective therapeutic application.
In summary, we conclude that the presence of the benzyloxy group at the 5-position in this series of indole derivatives results in a change from MAO-A to MAO-B selective inhibition. This effect should aid in gaining better knowledge of the monoamine oxidase active site and serve as a starting point for the design of further selective non-amphetamine-like MAO-B inhibitors with potential values as therapeutic agents for the treatment of Parkinson's disease.
Acknowledgments
This work has been supported by a collaboration contract between the Pharmaceutical Company PRODESFARMA, S.A. (Barcelona) and the Universitat Autonoma de Barcelona. We are very grateful to Professor Keith F. Tipton for his critical comments and review of this manuscript.
Abbreviations
- DSP-4
N-2-(chloroethyl)-N-ethyl-2-bromobenzylamine
- DA
dopamine
- 5-HT
serotonin
- MAO
monoamine oxidase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-pyridine ion
- MDMA
3,4-methylendioxy-methylamphetamine
- PEA
phenylethylamine
References
- AVILA M., BALSA M.D., FERNÁNDEZ-ÁLVAREZ E., TIPTON K.F., UNZETA M. The effect of side-chain substitution at Positions 2 and 3 of the heterocyclic ring of N-acetylenic analogues of tryptamine as monoamine oxidase inhibitors. Biochem. Pharmacol. 1993;45:2231–2237. doi: 10.1016/0006-2952(93)90194-2. [DOI] [PubMed] [Google Scholar]
- BACK A.W., LAN N.C., JOHNSON D.L., ABELL C.W., BEMBENEK M.E., KWAN S.W., SEEBURG P.H., SHIH J.C. c-DNA cloning of human liver monoamine oxidases A and B. Molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALSA D., FERNÁNDEZ-ÁLVAREZ E., TIPTON K.F., UNZETA M. Inhibition of MAO by substituted tryptamine analogues. J. Neural. Transm. 1990;32 suppl:103–105. doi: 10.1007/978-3-7091-9113-2_13. [DOI] [PubMed] [Google Scholar]
- BALSA D., FERNÁNDEZ-ÁLVAREZ E., TIPTON K.F., UNZETA M. Monoamine oxidase inhibitory potencies and selectivities of 2-[N-(2-propynyl)-aminomethyl]-1-methyl indole derivatives. Biochem. Soc. Trans. 1991;19:215–218. doi: 10.1042/bst0190215. [DOI] [PubMed] [Google Scholar]
- BALSA D., FERNÁNDEZ-ÁLVAREZ E., UNZETA M. Structure-activity relationship studies on some indolealkylamine acetylenic derivatives as selective MAO-A inhibitors. Behav. Pharmacol. 1992;3 suppl 1:40. [Google Scholar]
- BALSA D., PÉREZ V., FERNÁNDEZ-ÁLVAREZ E., UNZETA M. Kinetic behaviour of some acetylenic indolalkylamine derivatives and their corresponding parent amines. J. Neural. Transm. 1994;41 suppl:281–285. doi: 10.1007/978-3-7091-9324-2_36. [DOI] [PubMed] [Google Scholar]
- CRUCES M.A., ELORRIAGA C., FERNÁNDEZ-ÁLVAREZ E., LÓPEZ M.T., NIETO O. Acetylenic and allenic derivatives of 2-(1-methylindolyl)methylamine as selective inhibitors of the monoamine oxidases A and B. Il Farmaco De Sci. 1988;43:567–573. [PubMed] [Google Scholar]
- CRUCES M.A., ELORRIAGA C., FERNÁNDEZ-ÁLVAREZ E., NIETO O. Acetylenic and allenic derivatives of 2-(5-methoxyindolyl)methylamine synthesis and evaluation as selective inhibitors of monoamine oxidases A and B. Eur. J. Med. Chem. 1990;25:257–265. [Google Scholar]
- CRUCES M.A., ELORRIAGA C., FERNÁNDEZ-ÁLVAREZ E. Acetylenic and allenic derivatives of 2-(5-methoxyindolyl) and 2-(5-hydroxyindolyl)methylamines: synthesis and in vitro evaluation as monoamine oxidase inhibitors. Eur. J. Med. Chem. 1991;26:33–41. [Google Scholar]
- DODD P.R., HARDY J.A., OAKLEY A.E., EDWARDSON J.A., PERRY E.K., DELAUNOY J.P. A rapid method for preparing synaptosomes: Comparison with alternative procedures. Br. Res. 1981;226:107–118. doi: 10.1016/0006-8993(81)91086-6. [DOI] [PubMed] [Google Scholar]
- DOSTERT P., STROLIN-BENEDETTI M., TIPTON K.F. Interactions of monoamine oxidase with substrates and inhibitors. Med. Res. Rev. 1989;9:45–48. doi: 10.1002/med.2610090104. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., TIPTON K.F. Concentration dependence of the oxidation of tyramine by the two forms of rat liver mitochondrial monoamine oxidase. Biochem. Pharmacol. 1981;30:3329–3332. doi: 10.1016/0006-2952(81)90607-9. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., MANTLE T.J., TIPTON K.F. The nature of inhibition of rat liver monoamine oxidase types A and B by the acetylenic inhibitors clorgyline. l-deprenil and pargyline. Biochem. Pharmacol. 1982;31:3555–3561. doi: 10.1016/0006-2952(82)90575-5. [DOI] [PubMed] [Google Scholar]
- GÓMEZ N., BALSA M.D., UNZETA M. A comparative study of some kinetic and molecular properties of microsomal and mitochondrial monoamine oxidases. Biochem. Pharmacol. 1988;37:3407–3413. doi: 10.1016/0006-2952(88)90689-2. [DOI] [PubMed] [Google Scholar]
- HARFENIST M., HEUSER D.J., JOYNER C.T., BATCHELOR J.F., WHITE H.L. Selective inhibitors of monoamine oxidase. Structure-activity relationship of tricyclics bearing imidazoline, oxadiazole, or tetrazole groups. J. Med. Chem. 1996;39:1857–1863. doi: 10.1021/jm950595m. [DOI] [PubMed] [Google Scholar]
- HEIKKILA R.E. Behavioral properties of GBR-12909, GBR-13069 and GBR-13098: specific inhibitors of dopamine uptake. Eur. J. Pharmacol. 1984;103:241–248. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- HEIKKILA R.E., MANZINO L., CABBAT F.S., DUVOISIN R.C. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984;311:467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- HEINONEN E., MYLLYLA V., SOTANIEMI K., LAMINTAUSTA R., SALONEN J., ANTTILA M., SAVIJARVI M., KOTILA M., RONNE U. Pharmacokinetics and metabolism of selegiline. Act. Neurol. Scand. 1989;126:93–99. doi: 10.1111/j.1600-0404.1989.tb01788.x. [DOI] [PubMed] [Google Scholar]
- JAVITCH J.A., D'AMATO R.J., STRITTMATTER S.M., SYNDER S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON J.P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem. Pharmacol. 1968;17:1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- KALGUTKAR A.S., CASTAGNOLI N., JR Selective inhibitors of monoamine oxidase (MAO-A and MAO-B) as probes of its catalytic site and mechanism. Medicinal Res. Rev. 1995;15:325–388. doi: 10.1002/med.2610150406. [DOI] [PubMed] [Google Scholar]
- KNEUBÜHLER S., THULL U., ALTOMARE C., CARTA V., GAILLARD P., CARRUPT P.A., CAROTTI A., TESTA B. Inhibition of monoamine oxidase B by 5H-indenol[1,2-c]pyridazines: Biological activities, quantitative structure-activity relationships (QSARs) and 3D-QSARs. J. Med. Chem. 1995;38:3874–3883. doi: 10.1021/jm00019a018. [DOI] [PubMed] [Google Scholar]
- MAZOUZ F., GUEDDARI S., BURSTEIN C., MANSUY D., MILCENT R. 5-[4-(Benzyloxy)phenyl]-1,3,4-oxadiazol-2(3H)-one derivatives and related analogues: new reversible, highly potent, and selective monoamine oxidase type B inhibitors. J. Med. Chem. 1993;36:1157–1167. doi: 10.1021/jm00061a006. [DOI] [PubMed] [Google Scholar]
- MCDONALD I.A., BEY P., ZREIKA M., PALFREYMAN M.G. MDL 72,974A. Drugs Future. 1991;16:428–431. [Google Scholar]
- MYLLYLÄ V.V., HEINONEN E.H., VUORINEN J.A., KIKKU O.I., SOTANIEMI K.A. Early selegiline therapy reduces levodopa dose requirement in Parkinson's disease. Acta Neurol. Scand. 1995;91:177–182. doi: 10.1111/j.1600-0404.1995.tb00429.x. [DOI] [PubMed] [Google Scholar]
- NAOI M., YAGI K. Effects of phospholipids on beef heart mitochondrial monoamine oxidase. Arch. Biochem. Biophys. 1980;205:18–26. doi: 10.1016/0003-9861(80)90079-x. [DOI] [PubMed] [Google Scholar]
- NIKOI A., SILVERMAN R.B. New analogues of N-(2-aminoethyl)-4-chlorobenzamide (Ro-16-6491). Some of the most potent monoamine oxidase-B inactivators. J. Med. Chem. 1993;36:3968–3970. doi: 10.1021/jm00076a026. [DOI] [PubMed] [Google Scholar]
- O'BRIEN E.M., TIPTON K.F., MERONI M., DOSTERT P. Inhibition of monoamine oxidase by clorgyline analogues. J. Neural. Transm. 1994;41 suppl:295–305. doi: 10.1007/978-3-7091-9324-2_39. [DOI] [PubMed] [Google Scholar]
- PALMER S.L., MABIC S., CASTAGNOLI N., JR Probing the active sites of monoamine oxidases A and B with 1,4-Disubstituted tetrahydropyridine substrates and inactivators. J. Med. Chem. 1997;40:1982–1989. doi: 10.1021/jm970079r. [DOI] [PubMed] [Google Scholar]
- PÉREZ V., MARCO J.L., FERNÁNDEZ-ÁLVAREZ E., UNZETA M. Kinectic studies of N-allenic analogues of tryptamine as monoamine oxidase inhibitors. J. Pharm. Pharmacol. 1996;48:718–722. doi: 10.1111/j.2042-7158.1996.tb03958.x. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-RAMOS J.R., SONG S., MASH D.C., WEINER W.J. 21-Aminosteroides interact with the dopamine transporter to protect against 1-methyl-4-phenylpyridinium-induced neurotoxicity. J. Neurochem. 1992;58:328–334. doi: 10.1111/j.1471-4159.1992.tb09314.x. [DOI] [PubMed] [Google Scholar]
- SINGER T.P., RAMSAY R.R.New aspects of the substrate specificities, kinetic mechanisms and inhibition of monoamine oxidases Monoamine Oxidase: basic and clinical aspects 1993Utrecht, Holland: VSP; 23–43.Yasuhara H, Parvez S.H., Oguchi K, Sandler M and Nagatsu T. (eds). pp [Google Scholar]
- SPRAGUE J.E., NICHOLS D.E. Inhibition of MAO-B protects against MDMA-induced neurotoxicity in the striatum. Psychopharmacology. 1995;118:357–359. doi: 10.1007/BF02245967. [DOI] [PubMed] [Google Scholar]
- TETRUD J.W., LANGSTON J.W. The effect of deprenyl (selegiline) on the natural history of Parkinson's disease. Science. 1989;245:519–522. doi: 10.1126/science.2502843. [DOI] [PubMed] [Google Scholar]
- TIPTON K.F., BALSA D., UNZETA M.Newer approaches to the study of monoamine oxidase inhibition Monoamine oxidase: basic and clinical aspects 1993Utrech, Holland: VSP; 1–13.Yasuhara H, Parvez S.H., Oguchi K, Sandler M and Nagatsu T. (eds). pp [Google Scholar]
- WALDEMEIR P.C., FELNER A.E., TIPTON K.F. The monoamine oxidase inhibitory properties of CGP 1135A. Euro. J. Pharmacol. 1983;94:73–83. doi: 10.1016/0014-2999(83)90443-0. [DOI] [PubMed] [Google Scholar]
- YOUDIM M.B.H., FINBERG J.M.P.Monoamine oxidase inhibitors Psychopharmacology, Preclinical Psychopharmacology 1983Amsterdam: Elsevier Science; 37–51.Grahame-Smith D.G. and Cowen P.E. (eds). pp [Google Scholar]
- YOUDIM M.B.H., FINBERG J.P.M., TIPTON K.F.Monoamine oxidase Handbook of experimental pharmacology, Catecholamines I. 1988Berlin: Springer-Verlag; 119–192.Trendelenburg U and Weiner N. (eds). pp [Google Scholar]
- YU P.H., DAVIS B.A., BOULTON A.A. Aliphatic propargylamines: potent, selective, irreversible monoamine oxidase B inhibitors. J. Med. Chem. 1992;35:3705–3713. doi: 10.1021/jm00098a017. [DOI] [PubMed] [Google Scholar]
- YU P.H., DAVIS B.A., FANG J., BOULTON A.A. Neuroprotective effects of some monoamine oxidase-B inhibitors against DSP-4 induced noradrenaline depletion in the mouse hippocampus. J. Neurochem. 1994;63:1820–1828. doi: 10.1046/j.1471-4159.1994.63051820.x. [DOI] [PubMed] [Google Scholar]


