Abstract
Activated T-cells constitute a target for treatment of autoimmune diseases. We have found that the antitumour ether phospholipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3; edelfosine) induced dose- and time-dependent apoptosis in human mitogen-activated peripheral blood T-lymphocytes, but not in resting T-cells. T-lymphocytes were stimulated with phytohemagglutinin and interleukin-2 or with concanavalin A. Apoptosis was assessed by DNA fragmentation through cell cycle and TUNEL analyses, as well as through visualization of internucleosomal DNA fragmentation in agarose gels.
The ET-18-OCH3-mediated apoptotic response in activated T-lymphocytes was less intense than in human leukaemic T cell lines, such as Jurkat cells and Peer cells; namely about 25% apoptosis in activated T-cells versus about 46–61% apoptosis in T leukaemic cells after 24 h treatment with 10 μM ET-18-OCH3.
The ET-18-OCH3 thioether analogue BM 41.440 (ilmofosine) showed a similar apoptotic capacity to that found with ET-18-OCH3 in activated T-cells, whereas the phospholipid analogue hexadecylphosphocholine (miltefosine) failed to promote this response.
The uptake of [3H]-ET-18-OCH3 was much larger in activated T-cells than in resting lymphocytes.
Using a cytofluorimetric approach we have found that ET-18-OCH3 induced disruption of the mitochondrial transmembrane potential and production of reactive oxygen species in activated T-cells, but not in resting lymphocytes.
ET-18-OCH3 induced an increase in Fas (APO-1/CD95) ligand mRNA expression in activated T-cells, and incubation with a blocking anti-Fas (APO-1/CD95) antibody partially inhibited the ET-18-OCH3-induced apoptosis of activated T-lymphocytes.
These results demonstrate that mitogen-activated T-cells, unlike resting lymphocytes, are able to take up significant amounts of ET-18-OCH3, and are susceptible to undergo apoptosis by the ether lipid via, in part, the Fas (APO-1/CD95) receptor/ligand system. This ET-18-OCH3 apoptotic action can be of importance in the therapeutic action of this ether lipid in certain autoimmune diseases.
Keywords: Antitumour ether lipids, ET-18-OCH3, edelfosine, ilmofosine, miltefosine, apoptosis, activated T-lymphocytes, Fas/FasL system, mitochondrial transmembrane potential, autoimmune disease
Introduction
Antitumour ether lipids and alkylphosphocholines constitute a novel class of promising cancer chemotherapeutic drugs (Munder & Westphal, 1990; Lohmeyer & Bittman, 1994; Houlihan et al., 1995). Two classes of antitumour lipids can be distinguished: (a) the synthetic ether lipid analogues of platelet-activating factor (PAF) featuring a glycerol backbone which are referred collectively as antitumour ether lipids, exemplified by the ether phospholipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3, edelfosine), and by the thioether phospholipid 1-S-hexadecyl-2-methoxymethyl-rac-glycero-3-phosphocholine (BM 41.440, ilmofosine); and (b) the alkylphosphocholines, such as hexadecylphosphocholine (HPC, miltefosine). Extensive studies using cell lines, fresh tumour explants, and leukaemic cells have confirmed that several of these antitumour lipids are active in vitro against a broad spectrum of tumour cells (Munder & Westphal, 1990; Lohmeyer & Bittman, 1994; Houlihan et al., 1995; Mollinedo et al., 1997). Several of these compounds are scheduled for, or currently undergoing, phase I/II clinical evaluation (Berdel et al., 1987; Unger & Eibl, 1991; Winkelmann et al., 1992; Planting et al., 1993; Lohmeyer & Bittman, 1994; Houlihan et al., 1995; von Mehren et al., 1995; Vogler et al., 1996). ET-18-OCH3 is being used as a purging agent in autologous bone marrow transplantation (Koenigsmann et al., 1996; Glasser et al., 1996; Yamazaki & Sieber, 1997), and HPC is employed for topical applications in cutaneous breast cancer (Unger et al., 1992). The ether phospholipid ET-18-OCH3 is a synthetic analogue of 2-lysophosphatidylcholine, shows a selective cytotoxic action against transformed cells (Munder & Westphal, 1990; Houlihan et al., 1995; Mollinedo et al., 1997), and it has become the effective standard and prototype of the antitumour ether lipids. An important finding in the elucidation of the processes involved in the antineoplastic action of ET-18-OCH3 was its action as a potent inducer of apoptosis in tumour cells (Mollinedo et al., 1993; 1997; Diomede et al., 1993; 1994), and this apoptotic effect seems to account for the previously reported cytotoxic effects exerted by this ether phospholipid (Mollinedo et al., 1997). ET-18-OCH3 is able to induce apoptosis in a broad spectrum of tumour cells, whereas spares normal cells (Mollinedo et al., 1997). The apoptotic response induced by ET-18-OCH3 seems to be mediated by JNK activation and c-Jun (Gajate et al., 1998). Subsequent reports have indicated that HPC is also able to induce apoptosis in several tumour cells (Boggs et al., 1998; Konstantinov et al., 1998).
Andreesen et al. (1979) have previously reported that ET-18-OCH3 killed selectively mitogen-activated peripheral blood lymphocytes (PBLs) in vitro, while resting PBLs were not affected in their viability. Some in vivo studies have shown that ET-18-OCH3 and its cyclic analogue SRI 62-834 inhibited the onset of clinical signs of chronic relapsing experimental allergic encephalomyelitis in mice and rats (Baker et al., 1991; Klein-Franke & Munder, 1992; Chabannes et al., 1992; Kovarik et al., 1995), suggesting a potential beneficial effect for ET-18-OCH3 in the treatment of ongoing autoimmune diseases of the central nervous system. Experimental allergic encephalomyelitis (EAE) is generally considered to be an instructive animal model for multiple sclerosis in humans, sharing numerous clinical and pathological features (Zamvil et al., 1985; Satoh et al., 1987; Acha-Orbea et al., 1988). EAE is an experimental autoimmune disease of the central nervous system white matter, characterized by a T-cell response to myelin basic protein (Ben-Nun et al., 1981). To further assess a putative protective action of ET-18-OCH3 on autoimmune diseases, we have investigated the apoptotic action of antitumour lipids, especially ET-18-OCH3, on resting and activated human T-lymphocytes. In the present study, we have found that ET-18-OCH3 and BM 41.440, but not HPC, are able to induce apoptosis in mitogen-activated T-lymphocytes, sparing resting T-cells. The ET-18-OCH3-induced apoptosis in activated T-lymphocytes, involving disruption of the mitochondrial transmembrane potential, correlated with an increase in ether lipid incorporation into the cell and was mediated in part by the Fas/FasL system.
Methods
Chemicals and reagents
1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3, edelfosine) was from Laboratorios INKEYSA (Barcelona, Spain). 1-S-hexadecyl-2-methoxymethyl-rac-glycero-3-phosphocholine (BM41.440, ilmofosine) was a kind gift from Dr D.B.J. Herrmann (Boehringer Mannheim, Mann-heim, Germany). Hexadecylphosphocholine (HPC, miltefosine) was from Alexis (San Diego, CA, U.S.A.). [3H]-ET-18-OCH3 (specific activity 42 mCi ml−1) was synthesized by tritiation of the 9-octadecenyl derivative (Amersham Buchler, Braunschweig, Germany). ET-18-OCH3, BM 41.440 and HPC were dissolved at 1 mM (ET-18-OCH3 and BM41.440) or 1.25 mM (HPC) as stock solutions in RPMI-1640 containing 10% (v v−1) heat-inactivated foetal calf serum (FCS) by heating at 50°C for 30 min. The clear solutions were sterilized by filtration through a sterile filter (pore size 0.22 μm) and stored at 4°C. RPMI-1640 culture medium, L-glutamine, antibiotics and FCS were purchased from Gibco-BRL (Grand Island, NY, U.S.A.). Cycloheximide (CHX) and concanavalin A (Con A) were from Sigma Chemical Co. (St Louis, MO, U.S.A.). Cytotoxic anti-human Fas IgM monoclonal antibody (mAb) (clone CH-11) was from Upstate Biotechnology Inc. (Lake Placid, NY, U.S.A.). Anti-human Fas IgG2a mAb (clone SM1/1) and non-cytotoxic blocking anti-human Fas IgG2b mAb (clone SM1/23) were from Bender MedSystems (Vienna, Austria). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin was from Dakopatts (Glostrup, Denmark). Ficoll-Paque was from Pharmacia-LKB Biotechnology (Uppsala, Sweden). M-MLV reverse transcriptase and the Fluorescein Apoptosis Detection System kit were purchased from Promega (Madison, WI, U.S.A.). Taq DNA polymerase was from ECOGEN (Barcelona, Spain). TRIZOL reagent was from Gibco-BRL. Water miscible liquid scintillator was from Ultrafluor (National Diagnostics, Manville, NJ, U.S.A.). All other chemicals were from Sigma or Merck (Darmstadt, Germany).
Isolation of peripheral blood lymphocytes (PBLs) and mitogen activation of T-cells
Mononuclear cells were isolated from fresh human peripheral blood by dextran sedimentation and centrifugation on Ficoll-Paque density gradients. Mononuclear cells at the interface were saved, washed twice with phosphate-buffered saline (PBS), and resuspended in RPMI-1640 culture medium containing 10% (v v−1) heat-inactivated FCS, 2 mM L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 24 μg ml−1 gentamicin, and incubated overnight at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Monocytes were depleted by culture dish adherence. After overnight incubation at 37°C, the nonadherent cells (lymphocytes) were washed with PBS and collected. Lymphocyte preparations were typically 67–73% CD3+, 25–28% CD19+, and <0.4% CD14+. To further purify T-cells, the non-adherent cells were washed with PBS and passed twice through a nylon wool column to deplete residual B cells and monocytes. T-cell purity was checked by flow cytometry analysis. These purified T-cell preparations were typically >95% CD3+, <0.3% CD14+ and <4% CD25+. Similar results were obtained with both T-cell preparations.
Proliferation of T-lymphocytes was induced by incubation of peripheral blood lymphocytes for 4 days with 0.5 μg ml−1 PHA, which primarily stimulates T-cell proliferation, followed by one day treatment with both 0.5 μg ml−1 PHA and 50 U ml−1 interleukin-2 (IL-2) in RPMI-1640 culture medium containing 10% (v v−1) heat-inactivated FCS, 2 mM L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 24 μg ml−1 gentamicin. In some cases, PBLs were activated with Con A (5 μg ml−1) in complete culture medium for 3 days and then passed through a nylon wool column to deplete residual B cells. Both PHA/IL-2- and Con A-activated T-cells were more than 80% CD25+.
ET-18-OCH3, BM 41.440 and HPC were added to the cell cultures in complete culture medium at the concentrations and for the incubation times indicated in the respective figures.
Cell lines and culture conditions
The human leukaemia T lymphoid Jurkat and Peer cell lines, derived from human acute T-cell leukaemias, kindly provided by Dr F. Sanchez-Madrid (Hospital de la Princesa, Madrid, Spain), were grown in RPMI-1640 culture medium containing 10% (v v−1) heat-inactivated FCS, 2 mM L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 24 μg ml−1 gentamicin. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Incorporation of ET-18-OCH3
Ether lipid uptake was measured as described previously (Mollinedo et al., 1997). Briefly, cells (106) were incubated in 1 ml of complete culture medium containing 5 μg of ET-18-OCH3 and 0.05 μCi of [3H]-ET-18-OCH3. At the times indicated in the corresponding figure, the cells were washed three times with 1 ml of PBS and mixed with 2 ml of water-miscible liquid scintillator to measure the incorporated radioactivity.
Analysis of DNA fragmentation in agarose gels
Fragmented DNA was isolated as described previously (Mollinedo et al., 1993; 1998). Briefly, 2.5×106 cells were washed with PBS and then lysed with 200 μl hypotonic detergent buffer (Tris-HCl (pH 7.5) 10 mM, EDTA 1 mM and 0.2% Triton X-100) for 30 min at 4°C. Nuclear and subcellular organelles were removed by centrifugation in a microfuge for 20 min and the supernatant, containing the DNA released into the cytosol due to DNA fragmentation, was incubated with RNase A (75 μg ml−1) for 1 h at 37°C, and then with 200 μg ml−1 proteinase K in the presence of 0.5% SDS for an additional 1 h at 37°C. The DNA was extracted, precipitated and analysed by electrophoresis on 1% (w v−1) agarose as described previously (Mollinedo et al., 1993; 1998). DNA was visualized after electrophoresis by ethidium bromide staining.
Detection of DNA fragmentation by TUNEL method
Cells (2×106) were also analysed for DNA fragmentation in situ by the TUNEL technique using the Fluorescein Apoptosis Detection System kit (Promega), according to the manufacturer's instructions. This method measures the fragmented DNA by catalitically incorporating fluorescein-12-dUTP at the 3′-OH ends generated by DNA fragmentation using the TdT enzyme (Gavrieli et al., 1992). Cells were fixed on microscope slides, permeabilized with 0.2% Triton X-100 and fragmented DNA was stained following manufacturer's instructions. Then, 0.2 μg ml−1 propidium iodide in PBS was added for 15 min to stain both apoptotic and non-apoptotic cells. Thus, propidium iodide stains both apoptotic and non-apoptotic cells red throughout the cytoplasm, whereas fluorescein-12-dUTP is incorporated at the 3′-OH ends of fragmented DNA, resulting in localized green fluorescence within the nucleus of apoptotic cells. Samples were analysed with a Zeiss LSM 310 laser scan confocal microscope.
Analysis of DNA fragmentation by flow cytometry
Cells (5×105) were centrifuged and fixed overnight in 70% ethanol at 4°C. Cells were then washed three times with PBS and incubated for 1 h with 1 mg ml−1 RNase A and 20 μg ml−1 propidium iodide at room temperature. Cells were then analysed with Becton Dickinson (San Jose, CA, U.S.A.) FACScan or FACStar-Plus flow cytometers as previously described (Nicoletti et al., 1991; Ormerod et al., 1992; Pérez-Sala et al., 1995). The induction of apoptosis was monitored as the appearance of the sub-G1 peak in cell cycle analysis (Nicoletti et al., 1991; Ormerod et al., 1992; Pérez-Sala et al., 1995).
The possible implication of the Fas/FasL system in ET-18-OCH3-induced apoptosis in mitogen-activated T-lymphocytes was evaluated by using the blocking anti-Fas SM1/23 mAb as previously described (Gamen et al., 1997). Cells (2.5×105 ml−1) were preincubated at 4°C for 30 min in PBS+1% BSA in the absence (control) and in the presence of 100 ng ml−1 of the blocking SM1/23 mAb. The cells were resuspended at 5×105 ml−1 in complete RPMI-1640 culture medium containing the same amount of blocking anti-Fas mAb, and treated with 50 ng ml−1 of cytotoxic IgM anti-Fas antibody (CH-11) or 10 μM ET-18-OCH3 at 37°C for 24 h. Cells were collected by centrifugation and treated with RNase A and propidium iodide as described above for flow cytometry analysis. The same experiment was also performed preincubating the cells for 10 min with 0.1 μg ml−1 CHX before treatment.
Phosphatidylserine exposure
Phosphatidylserine exposure at the external surface of the cell was measured by the binding of FITC-labelled annexin V according to the protocol outlined by the manufacturers in the Annexin-V-FLUOS reagent (Boehringer-Mannheim). Cells were analysed with a FACScan flow cytometer as previously described (Gajate et al., 1998).
Cytofluorimetric analysis of mitochondrial transmembrane potential and generation of superoxide anion
To evaluate the mitochondrial transmembrane potential and the generation of reactive oxygen species, cells (106 ml−1) were incubated in PBS with DiOC6(3) (green fluorescence) (20 nM) (Molecular Probes Europe, Leiden, The Netherlands) and dihydroethidine (HE) (red fluorescence after oxidation) (2 μM) (Sigma) for 20 min at 37°C, followed by analysis on an EPICS Profile II Analyzer cytofluorimeter (Coulter, Hialeah, FL, U.S.A.).
Analysis of Fas membrane expression by flow cytometry
Cell surface antigen expression was analysed by flow cytometry as previously described (Mollinedo et al., 1992). Briefly, cells (2.5×105 in 100 μl) were incubated with 0.5 μg ml−1 of SM1/1 anti-human Fas mAb in PBS for 1 h at 4°C, washed with cold PBS, and incubated for 1 h at 4°C with 100 μl of FITC-labelled goat anti-mouse immunoglobulin, previously diluted 1 : 50 in PBS. Cells were washed in PBS and fixed with 1.5% formaldehyde in PBS and subjected to immunofluorescence flow cytometry in a FACScan or FACStar-Plus cytofluorometer. Fluorescence data were collected on log scale. Per cent of Fas-positive cells was estimated using the P3X63 myeloma supernatant as a negative control.
RT–PCR
Total RNA from 107 cells was extracted using the TRIZOL reagent following the manufacturer's instructions. RNA preparations were carefully checked by gel electrophoresis and found to be free of DNA contamination. Total RNA (3 μg) was primed with oligo-dT and reverse transcribed into cDNA with 50 units of M-MLV reverse transcriptase in a 20-μl volume. The mixture was incubated at 37°C for 1 h followed by incubation at 95°C for 10 min, and stored at −20°C until use. The generated cDNA was used for semiquantitative RT–PCR analyses to assess mRNA expression as previously described (Mollinedo et al., 1997), and the β-actin gene was used as an internal control. A 25 μl-PCR mixture contained 1 μl of the RT reaction, 10 pmol of each primer, 0.2 mM of each deoxynucleotide triphosphate, Tris-HCl (pH 8.3) 10 mM, KCl 50 mM, MgCl2 1.5 mM (except for fas: MgCl21 mM) and 2.5 units of EcoTaq polymerase derived from Thermus aquaticus. PCR-reactions were performed in a GenAmp PCR System Model 9600 (Perkin Elmer, Norwalk, CT, U.S.A.). The primers used are listed below, where the nucleotide numbers indicate the primer location in the corresponding human sequences obtained from the GenBank/EMBL database:
β-actin (accession number: X00351)
(forward; nt 936–955) 5′-CTGTCTGGCGGCACCACCAT-3′
(reverse; nt 1170–1189) 5′-GCAACTAAGTCATAGTCCGC-3′
bcl-2 (accession number: M13994)
(forward; nt 1799–1823) 5′-AGATGTCCAGCCAGCTGCACCTGAC-3′
(reverse; nt 2139–2165) 5′-AGATAGGCACCCAGGGTGATGCAAGCT-3′
c-myc (accession number: V00568)
(forward; nt 948–967) 5′-CCAGGACTGTATGTGGAGCG-3′
(reverse; nt 1433–1452) 5′-CTTGAGGACCAGTGGGCTGT-3′
bax (accession number: L22473)
(forward; nt 172–195) 5′-AAGCTGAGCGAGTGTCTCAAGCGC-3′
(reverse; nt 516–537) 5′-TCCCGCCACAAAGATGGTCACG-3′
fas (accession number: M67454)
(forward; nt 373–392) 5′-ATAAGCCCTGTCCTCCAGGT-3′
(reverse; nt 1023–1042) 5′-TGATGCCAATTACGAAGCAG-3′
fasL (accession number: D38122)
(forward; nt 483–503) 5′-CTGGGGATGTTTCAGCTCTTC-3′
(reverse; nt 693–713) 5′-CTTCACTCCAGAAAGCAGGAC-3′
Primers were designed using the PCgene program for DNA analysis from Intelligenetics (Mountain View, CA, U.S.A.) and the Primer3 program (Roze, S. and Skaletsky, H.J., Whitehead Institute for Biomedical Research, MIT Center for Genome Research, MA, U.S.A.). The PCR reaction profile was as follows: denaturation step at 95°C for 30 s; annealing step for 30 s (except for fas: 1 min) at 62°C (c-myc), 65°C (fas) or 69°C (β-actin, bcl-2, bax and fasL); extension step at 72°C for 90 s (except for fas: 2 min). After 18 cycles (β-actin), 22 cycles (bax and fas), 25 cycles (bcl-2 and c-myc) and 35 cycles (fasL), shown to be at the linear phase of amplification, the expected PCR products (231 bp for fasL, 254 bp for β-actin, 366 for bax, 367 bp for bcl-2, 505 bp for c-myc, and 670 bp for fas) were size fractionated onto a 2% agarose gel and stained with ethidium bromide.
Statistical analyses
The results given are mean values±s.e.mean from at least three independent experiments.
Results
Induction of apoptosis by antitumour ether lipids in mitogen-activated T-lymphocytes, but not in resting T-cells
We have analysed the effect of the antitumour lipids ET-18-OCH3 (edelfosine), BM 41.440 (ilmofosine) and HPC (miltefosine) in the induction of apoptosis in resting and mitogen-activated human T-lymphocytes. Resting human peripheral blood T-lymphocytes (Figure 1) were found to be resistant to the apoptotic action of the antitumour lipids. As it has been previously reported that ET-18-OCH3 killed selectively mitogen-activated PBLs in vitro, while resting PBLs were not affected in their viability (Andreesen et al., 1979), we examined the effect of the antitumour lipids in both resting and mitogen-activated T-cells. Activation of T-lymphocytes isolated from peripheral blood was rendered upon stimulation with PHA (0.5 μg ml−1) for 4 days and subsequent incubation with both 0.5 μg ml−1 PHA and 50 U ml−1 IL-2 in complete culture medium for 1 additional day. T-cell preparations were >95% CD3+, <0.3% CD14+, and <4% CD25+ in resting state, and >80% CD25+ after mitogen-activation. Furthermore, PHA/IL-2-treated T-cells were fully activated as assessed by morphological and proliferation patterns (data not shown). Resting human T-cells were resistant to these lipids, whereas human PHA/IL-2-stimulated T-lymphocytes underwent apoptosis after treatment for 24 h with 10 μM of ET-18-OCH3 or BM 41.440, as assessed by an increase in the propidium iodide fluorescence sub-G1 region in flow cytometric analysis, indicating the appearance of fragmented DNA that is detected as less than 2 N DNA content in cell cycle analysis (Figure 1). In contrast, HPC failed to induce any apoptotic response in activated T-cells (Figure 1D). As shown in Figure 1, ET-18-OCH3 and BM 41.440 induced about 25% apoptosis in PHA/IL-2-activated T-lymphocytes when used at 10 μM for 24 h. Similar results were found when apoptosis was analysed by visualization of internucleosomal DNA fragmentation in agarose gel electrophoresis (Figure 2). Thus, ET-18-OCH3 and BM 41.440, but not HPC, induced apoptosis in activated T-cells when incubated at 10 or 25 μM for 24 h (Figure 2). Similar results were obtained when T-cells were stimulated with ConA (data not shown). TUNEL analysis also demonstrated the selective induction of apoptosis in activated T-lymphocytes by ET-18-OCH3, but not in resting PBLs (Figure 3). Similar TUNEL results were also obtained with BM 41.440 (data not shown). Time-course and dose-response analyses of the effects of the above antitumour lipids, ET-18-OCH3, BM 41.440 and HPC, on the induction of apoptosis in mitogen-activated T-lymphocytes are shown in Figure 4. ET-18-OCH3 and BM 41.440 started to induce apoptosis in human activated T-cells when used at 10 or 25 μM after 9–12 h of incubation; this apoptotic response being higher after 24 h treatment (Figure 4). Ether lipid incubations for more than 24 h did not significantly promote a further enhancement in the induction of apoptosis (data not shown). Prolonged incubations of activated T-lymphocytes in complete culture medium in the absence of ether lipids induced a progressive increase in the percentage of apoptotic cells, namely 21±4% after 24 h, 39±4% after 48 h and 64±5% after 72 h incubation. This hampered the analysis of the effect of protracted incubations of activated T-lymphocytes with ether lipids. On the other hand, we found that HPC did not induce apoptosis in activated T-cells, even at high lipid concentrations and after prolonged incubation times (Figure 4). These results demonstrated conclusively that both ET-18-OCH3 and BM 41.440 were able to induce apoptosis in mitogen-activated T-lymphocytes and spared resting lymphocytes. Resting PBLs were resistant to undergo apoptosis upon treatment with these lipids and very prolonged incubation times were required to promote a very weak apoptotic response. ET-18-OCH3 (10 μM) induced 0.5±0.1, 3±1.5 and 6.5±2% apoptosis in resting PBLs after 24, 48 and 72 h treatment, respectively, whereas BM 41.440 (10 μM) induced 0.2±0.1, 2±1 and 5±2% apoptosis in resting PBLs after 24, 48 and 72 h treatment, respectively. These results are in agreement with previous reports indicating that resting human lymphocytes were resistant to the ET-18-OCH3 action (Andreesen et al., 1979; Mollinedo et al., 1994; 1997). HPC did not induce apoptosis in resting PBLs even after 48 h treatment, and only a 1.5±0.5% apoptosis induction could be detected after 72 h treatment.
Figure 1.
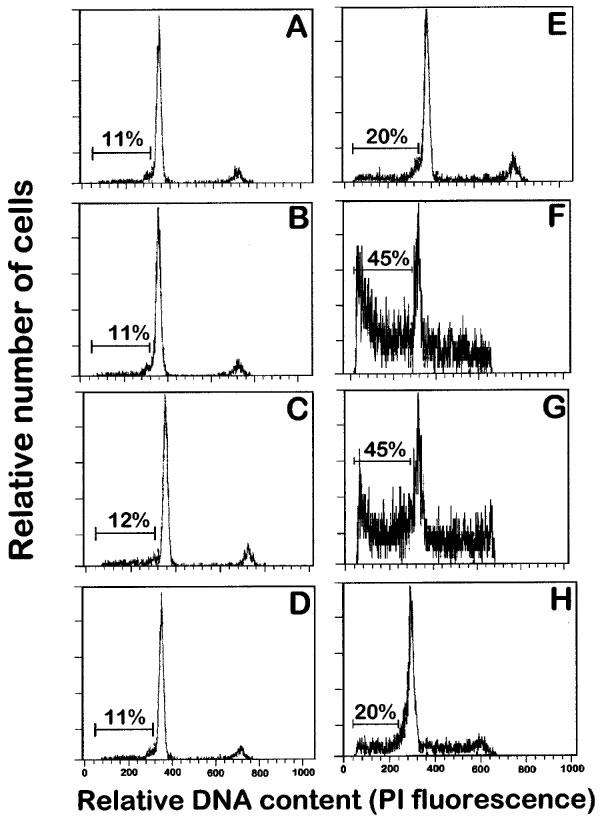
Effects of ET-18-OCH3, BM 41.440 and HPC on the induction of apoptosis in human resting and mitogen-activated T-cells. Resting T-lymphocytes (A, B, C and D) and T-lymphocytes activated with 0.5 μg ml−1 PHA for 4 days and with 0.5 μg ml−1 PHA and 50 U ml−1 IL-2 for 1 additional day (E, F, G and H) were incubated in complete culture medium in the absence (A and E) and in the presence of 10 μM ET-18-OCH3 (B and F), 10 μM BM 41.440 (C and G) and 10 μM HPC (D and H) for 24 h, and then analysed for apoptotic cells by flow cytometry analysis as described in the Methods section. Apoptotic cells distribute at the sub-G1 region of the cell cycle phases. Percentages of apoptotic cells are shown in each histogram. Data shown are representative of four experiments performed.
Figure 2.
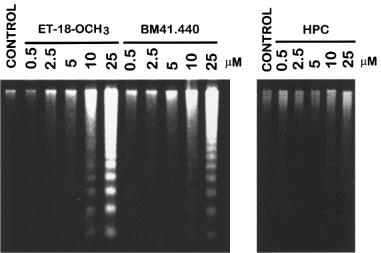
Visualization of DNA fragmentation in activated human T-lymphocytes upon treatment with ET-18-OCH3, BM 41.440 and HPC. Human PBLs were activated for 4 days with 0.5 μg ml−1 PHA and for 1 additional day with 0.5 μg ml−1 PHA and 50 U ml−1 IL-2. Activated T-lymphocytes (2.5×106) were incubated in complete culture medium for 15 h in the absence (control) and in the presence of increasing concentrations of ET-18-OCH3, BM 41.440 and HPC. Then, fragmented DNA was extracted and run onto agarose gels as described in the Methods section. DNA loaded in each lane was from 8×105 cells. Results shown are representative of three independent experiments performed.
Figure 3.
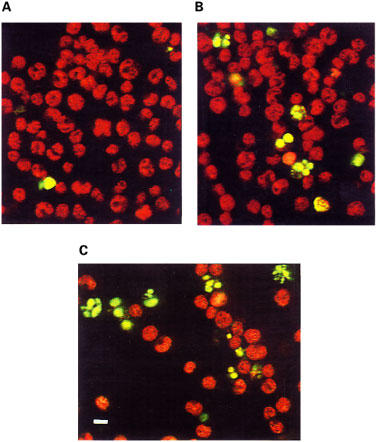
TUNEL analysis of resting and mitogen-activated T-cells upon treatment with ET-18-OCH3. Resting (A) and PHA/IL-2-activated (B and C) T-lymphocytes were treated for 9 h with 10 μM (B) or 25 μM (C) ET-18-OCH3, and analysed by the TUNEL technique through confocal microscopy as described in the Methods section. Data shown are representative of four experiments performed. Bar, 10 μm.
Figure 4.
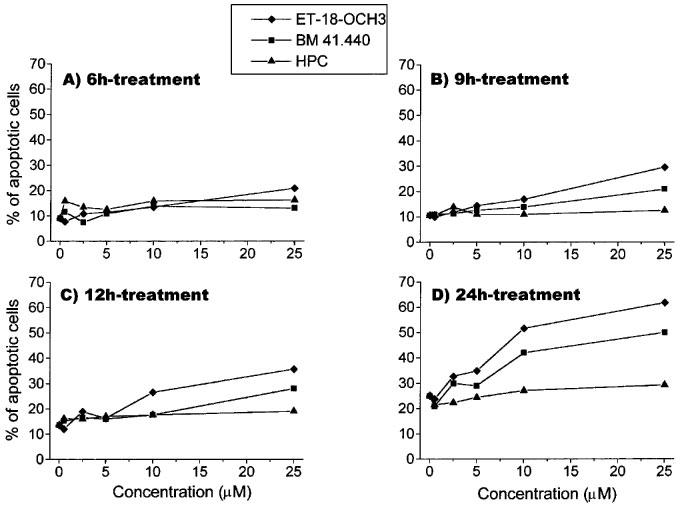
Dose-response and time-course of the effects of ET-18-OCH3, BM 41.440 and HPC on the induction of apoptosis in activated T-lymphocytes. Human PBLs were activated with 0.5 μg ml−1 PHA for 4 days and with 0.5 μg ml−1 PHA and 50 U ml−1 IL-2 for 1 additional day as described in the Methods section. Then, activated T-lymphocytes were incubated with increasing concentrations of ET-18-OCH3 (solid diamonds), BM 41.440 (solid squares) and HPC (solid triangles) for 6 h (A), 9 h (B), 12 h (C) and 24 h (D). Percentages of apoptotic cells were determined by propidium iodide staining of ethanol-fixed cells as described in the Methods section. Control untreated activated T-lymphocytes were also run in parallel. Data are representative of four experiments performed.
Incorporation of ET-18-OCH3 in activated versus resting T-cells
Previous data have shown the existence of a close relationship between ET-18-OCH3 uptake by sensitive tumour cells and subsequent induction of apoptosis (Mollinedo et al., 1997), leading to the hypothesis that ether lipid uptake is an essential requirement for ET-18-OCH3-induced apoptosis in sensitive tumour cells (Mollinedo et al., 1997). In this regard, we found that PHA/IL-2-activated T-cells incorporated high levels of ET-18-OCH3, whereas resting PBLs took up only small amounts of the ether lipid (Figure 5). The kinetics of ET-18-OCH3 uptake in mitogen-activated T-cells was very different from that of resting T-cells (Figure 5). Mitogen-activated T-lymphocytes incorporated rapidly high amounts of ET-18-OCH3, and this ether lipid incorporation was slowed down after 9 h incubation (Figure 5). In contrast, the small ET-18-OCH3 uptake into resting T-cells proceeded slowly (Figure 5). ET-18-OCH3-induced apoptosis in activated T-lymphocytes was clearly evident after 24 h incubation, and at this incubation time, mitogen-activated T-cells incorporated about 3.5 times more ether lipid than resting cells (Figure 5).
Figure 5.
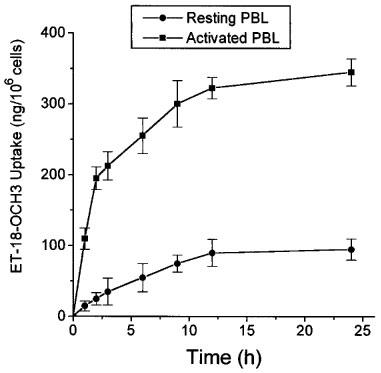
Incorporation of ET-18-OCH3 in human resting and mitogen-activated T-lymphocytes. Human resting T-lymphocytes (solid circles) and T-cells activated for 4 days with 0.5 μg ml PHA and for 1 additional day with 0.5 μg ml−1 PHA and 50 U ml−1 IL-2 (solid squares) were incubated with 5 μg ml−1 ET-18-OCH3 and 0.05 μCi ml−1 of [3H]-ET-18-OCH3 for the times indicated to measure incorporation of the ether lipid into the cell as described in the Methods section. Data are shown as mean values±s.e.mean. from three independent experiments.
ET-18-OCH3 uptake in activated T-cells was evidenced long before phosphatidylserine exposure on the outer leaflet of the plasma membrane, which seems to play an important role in the recognition and removal of the apoptotic cells by macrophages. Using immunofluorescence flow cytometry analysis to detect FITC-labelled annexin V binding to phosphatidylserine, we found that phosphatidylserine exposure to the cell outer leaflet of activated T-cells occurred after 15 h treatment with ET-18-OCH3 (data not shown). Thus, phosphatidylserine exposure took place after the onset of DNA fragmentation in ET-18-OCH3-treated mitogen-activated T-cells. This is in agreement with previous data indicating that the appearance of internucleosomal DNA degradation preceded the exposure of phosphatidylserine to the cell outer leaflet in ET-18-OCH3-treated HL-60 cells (Gajate et al., 1998).
ET-18-CH3 induces a more potent apoptotic response in leukaemic T lymphoid cells than in activated T-lymphocytes
We have previously reported that human leukaemic T lymphoid Jurkat cells undergo rapidly apoptosis upon ET-18-OCH3 treatment (Mollinedo et al., 1997). Other human leukaemic T lymphoid cell lines, such as Peer cells, were also very sensitive to undergo ET-18-OCH3-induced apoptosis, as assessed by the increase in the percentage of hypodiploid sub-G1 apoptotic cells (Figure 6). A comparative study showed that Jurkat and Peer T leukaemia cell lines were much more sensitive to undergo ET-18-OCH3-induced apoptosis than mitogen-activated human T-lymphocytes (Figure 7). About 46 and 55% of Jurkat cells, and about 61 and 75% of Peer cells underwent apoptosis after 24 and 48 h, respectively, of ET-18-OCH3 treatment, whereas only about 25% of mitogen-activated T-lymphocytes underwent an apoptotic response under identical experimental conditions (Figure 7).
Figure 6.
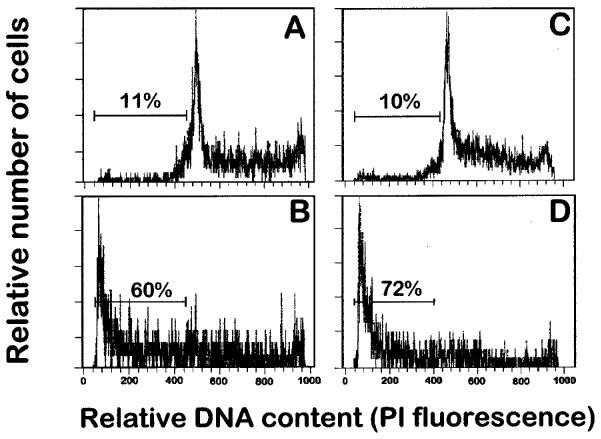
Effect of ET-18-OCH3 on the induction of apoptosis in human leukaemic T cell lines. Human leukaemic T lymphoid Jurkat (A and B) and Peer (C and D) cells were incubated in the absence (A and C) and in the presence of 10 μM ET-18-OCH3 (B and D) for 24 h in complete culture medium, and then analysed for apoptotic cells by flow cytometry as described in the Methods section. Apoptotic cells distribute at the sub-G1 region of the cell cycle phases. Percentages of apoptotic cells are shown in each histogram. Data shown are representative of four experiments performed.
Figure 7.
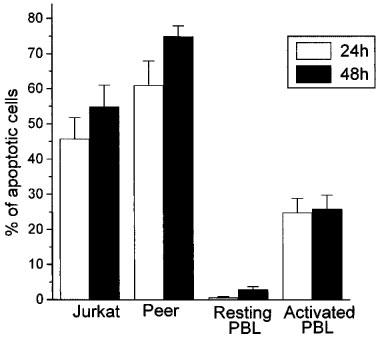
Comparison of the apoptotic action of ET-18-OCH3 on human leukaemic T cell lines versus resting and activated human peripheral blood T-cells. The human leukaemic T lymphoid Jurkat and Peer cell lines, as well as resting and PHA/IL-2-activated PBLs were treated with 10 μM ET-18-OCH3 for 24 h (open histograms) and 48 h (solid histograms) in complete culture medium, and the percentages of apoptotic cells were determined by flow cytometry as described in the Methods section. The percentage of apoptotic cells after ET-18-OCH3 incubation was calculated after subtracting the percentage of spontaneous apoptosis obtained in the corresponding untreated control cells. Data are shown as mean values±s.e.mean from four independent experiments.
ET-18-OCH3 induces disruption of mitonchondrial transmembrane potential and generation of reactive oxygen species in human activated T-lymphocytes, but not in resting T-cells.
Mitochondrial transmembrane potential disruption and reactive oxygen species generation have been widely shown to be invariant features of early apoptosis induced by many different agents or experimental conditions (Petit et al., 1995; Zamzami et al., 1995; Marchetti et al. 1996; Kroemer et al., 1997). As shown in Figure 8A, addition of 10 μM ET-18-OCH3 to mitogen-activated T-cells induced a reduction in the mitochondrial transmembrane potential, as well as induced the generation of reactive oxygen species, as detected by double staining experiments, using DiOC6(3) (green fluorescent), a cationic probe that accumulates into mitochondria as a function of its potential (Petit et al., 1990), and hydroethidine (HE, non-fluorescent) that becomes ethidium (Eth, red fluorescence) after its oxidation via reactive oxygen species. This mitochondrial transmembrane potential disruption and reactive oxygen species generation in ET-18-OCH3-treated activated T-cells (Figure 8A) was accompanied by the appearance of cells with a DNA content less than G1, characteristic of early apoptotic cells (sub-G1 peak, Figure 8B). The increase in the percentage of DiOC6(3)low mitogen-activated T-lymphocytes at short incubation times with 10 μM ET-18-OCH3 (about 5% after 9 h of ether lipid treatment) (Figure 8A) correlated well with the percentage of cells undergoing apoptosis (about 6% after 9 h of ether lipid treatment) (Figure 4). The disruption of the mitochondrial transmembrane potential was increased after prolonged incubations of activated T-cells with ET-18-OCH3 (data not shown). In contrast, purified resting T-cells neither underwent apoptosis nor changed the mitochondrial transmembrane potential after ET-18-OCH3 treatment (Figure 8).
Figure 8.
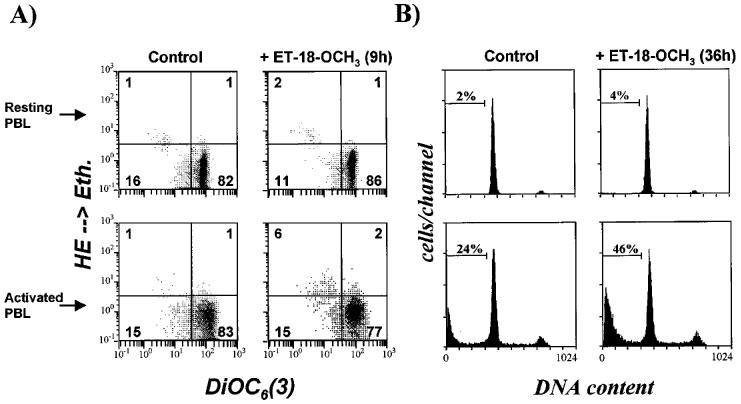
ET-18-OCH3 induces disruption of the mitochondrial transmembrane potential, generation of reactive oxygen species and apoptosis in human mitogen-activated T-cells, but not in resting T-lymphocytes. Purified resting T-cells (resting PBL) and mitogen-activated T-cells (activated PBL) were incubated for either 9 h (A) or 36 h (B) in the absence (control) or in the presence of 10 μM ET-18-OCH3 as described in the Methods section. (A) Effects of ET-18-OCH3 in the disruption of mitochondrial transmembrane potential and in the generation of reactive oxygen species, assessed as described in the Methods section. (B) Determination of apoptosis by cell cycle analysis as described in the Methods section.
Participation of the Fas/FasL system in the induction of apoptosis in activated T-lymphocytes by ET-18-OCH3
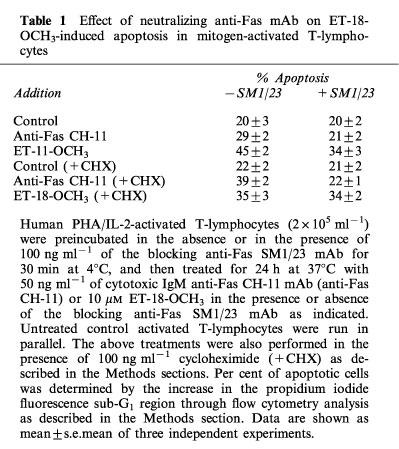
The Fas (APO-1/CD95) receptor/ligand system has been implicated in the induction of apoptosis under different conditions, including neutrophil spontaneous apoptosis, cytotoxic drug-induced apoptosis and T-cell killing by cancer cells (Liles et al., 1996; Friesen et al., 1996; O'Connell et al., 1996). Fas/FasL interactions, either through an autocrine or paracrine mode, have also been shown to be required for programmed cell death after T-cell activation (Dhein et al., 1995; Brunner et al., 1995; Ju et al., 1995), and play a key role in the homeostatic regulation of immune responses (Lynch et al., 1995). Thus, we examined the putative role of Fas/FasL system in the ET-18-OCH3-induced apoptosis of mitogen-activated T-lymphocytes. Both resting and activated T-lymphocytes contained Fas antigen in their respective cell surfaces, but mitogen-activated T-lymphocytes showed a significantly higher Fas cell surface expression, namely about 90% Fas-positive cells in activated T-cells versus about 60% Fas-positive cells in resting T-cells (Figure 9). Upon ET-18-OCH3 treatment, no significant changes in Fas cell surface expression were observed in both resting and activated PBLs (Figure 9). These data were in agreement with semiquantitative RT–PCR analysis indicating that ET-18-OCH3 treatment had no any significant effect on fas mRNA expression in activated T-lymphocytes (Figure 10). However, the amount of fasL transcripts was increased after incubation of activated T-lymphocytes with the ether lipid (Figure 10). We also found that ET-18-OCH3 induced an increase in the mRNA levels of c-myc in activated T-lymphocytes, but had no effect on the levels of expression of the anti-apoptotic gene bcl-2 and of the pro-apoptotic gene bax (Figure 10). No changes in the expression of all of these genes assayed, including fas and fasL, were detected in ET-18-OCH3-treated resting PBLs (data not shown). As activated T-lymphocytes displayed a higher content of Fas at the cell surface, and ET-18-OCH3 induced an increase in fasL expression, we next examined whether the Fas/FasL system could play a role in the ET-18-OCH3-induced apoptosis of mitogen-activated T-lymphocytes. We found that co-incubation with an anti-Fas blocking mAb (SM1/23), inhibited significantly (about 44% inhibition), but not blocked completely, the apoptotic response elicited by ET-18-OCH3 in activated T-lymphocytes (Table 1). In spite of containing a high level of cell surface Fas, activated T-lymphocytes showed a low capacity to undergo apoptosis upon addition of the agonistic cytotoxic anti-human Fas IgM mAb (clone CH-11), about 9% apoptosis (Table 1). This weak CH-11-induced apoptosis was blocked upon co-incubation with the anti-Fas SM1/23 blocking mAb (Table 1). The CH-11-induced apoptosis (about 9% apoptosis) was less potent than that induced by ET-18-OCH3 (about 25% apoptosis) in activated T-lymphocytes (Table 1). Interestingly, as shown in Table 1 from the SM1/23 experiments, ET-18-OCH3 induced about 14% apoptosis independently of the Fas/FasL system, and about 11% apoptosis via Fas/FasL interaction in activated T-cells. This latter percentage of apoptotic cells correlated well with the 9% apoptotic cells found upon CH-11 mAb addition (Table 1). As ET-18-OCH3 induced FasL expression in activated T-lymphocytes, and part of the apoptotic response exerted by this ether lipid on activated T-cells required Fas/FasL interaction, we performed the above experiments also in the presence of cycloheximide (CHX) which abrogated FasL induction. When activated T-lymphocytes were incubated in the presence of CHX (0.1 μg ml−1) for 12 h, both cytotoxic anti-Fas CH-11 antibody and ET-18-OCH3 induced a similar percentage of apoptosis in activated T-cells (Table 1), namely about 17% and 13% apoptosis in CH-11- and ET-18-OCH3-treated cells, respectively (Table 1). Interestingly, in the presence of CHX, the CH-11-induced apoptosis was prevented by the anti-Fas blocking SM1/23 mAb, but the ET-18-OCH3-induced apoptosis was not affected by the presence of the anti-Fas blocking SM1/23 mAb (Table 1). These results, together with the lack of de novo FasL protein synthesis in the presence of CHX, indicate that there are two pathways in the induction of apoptosis in activated T-lymphocytes by ET-18-OCH3, one dependent on the interaction of FasL with its cognate receptor and another one independent of the Fas/FasL system. The results shown in Table 1 indicate that in the absence of CHX we obtained a higher induction of apoptosis in activated T-lymphocytes (about 25%), which is in part dependent on the Fas/FasL system, whereas in the presence of CHX, we observed a lower induction of apoptosis (about 13%), which is independent of Fas/FasL interactions. An examination of the percentages of apoptosis detected in ET-18-OCH3-treated activated T-lymphocytes in the presence and in the absence of anti-Fas blocking mAb as well as of CHX indicate that the apoptotic response induced by ET-18-OCH3 in activated T-lymphocytes is mediated about 45% through a Fas/FasL system, requiring de novo synthesis of FasL, and about 55% through a Fas/FasL independent system (Table 1).
Figure 9.
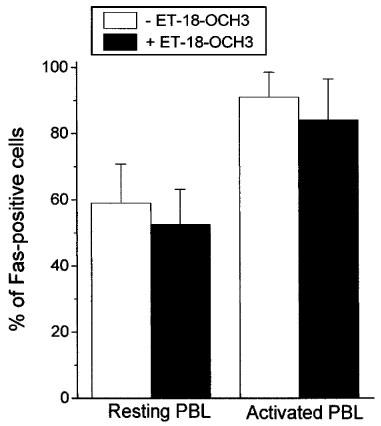
Cell surface expression of Fas in resting and activated T-cells. Resting (Resting PBL) and PHA/IL-2-activated (Activated PBL) T-cells were incubated in the absence (open histograms) and in the presence (solid histograms) of 10 μM ET-18-OCH3 for 24 h, and then were analysed for Fas cell surface expression by flow cytometry as described in the Methods section. The percentages of Fas-positive cells are expressed as mean values±s.e.mean from three independent experiments.
Figure 10.
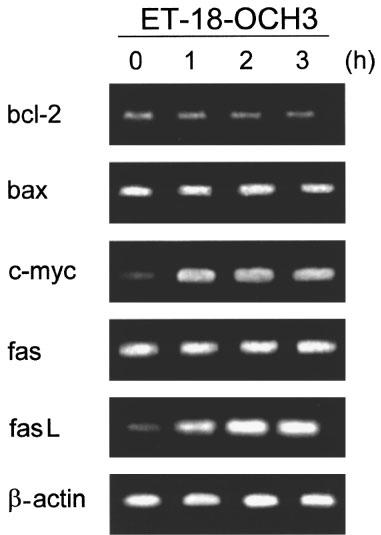
Expression of bcl-2, bax, c-myc, fas and fasL in ET-18-OCH3-treated mitogen-activated T-lymphocytes. Human T-cells activated with PHA and IL-2 as described in the Methods section were incubated with 10 μM ET-18-OCH3 for the indicated times, and then total RNA was purified and subjected to semiquantitative RT–PCR analysis with oligonucleotide primers specific to bcl-2, bax, c-myc, fas and fasL. PCR amplification of β-actin was used as an internal control. After 18 cycles (β-actin), 22 cycles (bax and fas), 25 cycles (bcl-2 and c-myc) and 35 cycles (fasL), shown to be at the linear phase of amplification, the PCR products were electrophoresed onto a 2% agarose gel and stained with ethidium bromide.
Table 1.
Effect of neutralizing anti-Fas mAb on ET-18-OCH3-induced apoptosis in mitogen-activated T-lymphocytes
Discussion
Previous findings showed that ET-18-OCH3 induced apoptosis selectively in tumour cells, but spared normal cells (Mollinedo et al., 1997). As this selectivity for the apoptotic effect of this ether lipid seems to reside at the level of ET-18-OCH3 uptake (Mollinedo et al., 1997), we have previously proposed that tumour cells, unlike normal cells, express or modify a cellular structure that is able to bind ET-18-OCH3 in order to promote its cellular uptake and subsequently apoptosis (Mollinedo et al., 1997). Leukaemic cells, including T leukaemia cells, were especially sensitive to undergo ET-18-OCH3 apoptosis (Mollinedo et al., 1997). We report here that normal human mitogen-activated T-lymphocytes, but not resting T-lymphocytes, undergo apoptosis upon treatment with the antitumour ether phospholipids ET-18-OCH3 and BM 41.440, as assessed by DNA fragmentation through cell cycle and TUNEL analyses, as well as through visualization of internucleosomal DNA fragmentation in agarose gels. We have found that the ether lipid ET-18-OCH3 and its thioether analogue BM 41.440 showed similar apoptotic actions against mitogen-activated T-cells. However, the antitumour alkylphosphocholine HPC failed to induce an apoptotic response in these cells even after 24 h incubation.
ET-18-OCH3 induced in mitogen-activated T-cells, but not in resting T-cells, dissipation of the mitochondrial transmembrane potential and generation of reactive oxygen species which have been shown to be invariant features of early apoptosis (Petit et al., 1995; Zamzami et al., 1995; Marchetti et al. 1996; Kroemer et al., 1997). The results herein reported indicate that resting peripheral blood T-cells incorporate very low amounts of ET-18-OCH3 and are resistant to undergo apoptosis upon treatment with the antitumor lipids ET-18-OCH3 and BM 41.440, even after prolonged incubations. This result is in agreement with previous reports indicating that resting human lymphocytes were resistant to the ET-18-OCH3 action (Andreesen et al., 1979; Mollinedo et al., 1994; 1997). However, after mitogen activation, T-cells were able to incorporate high amounts of ET-18-OCH3 and became sensitive to the apoptotic action of the ether lipids, suggesting that normal mitogen-activated peripheral blood T-cells share some features with tumour cells that make them susceptible to incorporate ET-18-OCH3 and to undergo apoptosis by ET-18-OCH3. This apoptotic effect on activated T-cells, but not in resting cells, does not seem to be due to a specific action of the ether lipid on proliferating cells, as other normal proliferating cell types, including normal bone marrow cells, show resistance to undergo apoptosis by ET-18-OCH3 (Mollinedo et al., 1997; Gajate et al., in preparation). Interestingly, normal human mitogen-activated peripheral blood T-lymphocytes were less sensitive than T leukaemia cells to undergo ET-18-OCH3-induced apoptosis, namely about 25% apoptosis in activated T-lymphocytes versus about 46–61% apoptosis in T leukaemia cells after 24 h of ET-18-OCH3 treatment. These different sensitivities are more prominent after 48 h of ether lipid treatment (Figure 7). This suggests that normal activated T-lymphocytes share some characteristics displayed by T leukaemia cells which make normal T lymphocytes sensitive to the ether lipid ET-18-OCH3, but the lack of a tumour transformed state in activated T-cells make them less sensitive to the antitumour ether lipid than T leukaemic cells. These results further support that the main cellular target of ET-18-OCH3 apoptotic action is the tumour cell (Mollinedo et al., 1997), but mitogen-activated T-cells, in a lesser extent, can be considered as another target of ET-18-OCH3. Alternatively, it could also be envisaged that the relatively low percentage of apoptosis observed in activated T-cells upon ET-18-OCH3 treatment could be due to the presence of distinct activated T-cell subsets showing different sensitivities to undergo ether lipid-induced apoptosis. Thus, it cannot be ruled out the putative existence of a specific T-cell subset population susceptible to undergo ET-18-OCH3-induced apoptosis. This putative ET-18-OCH3-sensitive T-cell subset population could be present at very reduced levels in resting PBLs, but it could be largely increased upon mitogen activation in order to account on for the percentage of the total activated T-cell population sensitive to the ether lipid.
On the other hand, the induction of apoptosis by ET-18-OCH3 in activated T-cells is rather slow compared to the induction of apoptosis by the ether lipid in T leukaemic cells. Thus, apoptosis was clear-cut after 12–24 h of 10 μM ET-18-OCH3 treatment in activated T-cells, whereas a 3–6 h of 10 μM ET-18-OCH3 treatment was sufficient to induce a strong apoptotic response in human leukaemic cells (Mollinedo et al., 1997).
The herein reported apoptotic effect of ET-18-OCH3 on activated T-cells can account on for the previously reported cytotoxic effect exerted by this ether lipid on lymphoblasts (Andreesen et al., 1979). Previous reports have indicated that ET-18-OCH3 prevents induction of chronic relapsing EAE in rats and mice (Baker et al., 1991; Klein-Franke & Munder, 1992; Chabannes et al., 1992; Kovarik et al., 1995). EAE is an experimental autoimmune disease of the central nervous system white matter, characterized by a T-cell response to myelin basic protein, sharing clinical and pathological features with human multiple sclerosis, and is therefore regarded as a good experimental model for human demyelinating disease (Zamvil et al., 1985; Satoh et al., 1987; Acha-Orbea et al., 1988). Furthermore, a phase I/II study with a short number of multiple sclerosis patients showed that ET-18-OCH3 treatment improved their clinical symptons (Munder & Westphal, 1990; Klein-Franke & Munder, 1992). Although EAE in animals and multiple sclerosis in humans cannot be fully compared, it has been assumed that in both diseases autoaggressive T-lymphoblasts play a key role (Engelhardt et al., 1989). Thus, as multiple sclerosis is thought to be immunologically mediated, the results herein reported could explain the beneficial effects of ET-18-OCH3 found in multiple sclerosis patients. In this regard, it can be envisaged that the capacity of ET-18-OCH3 to induce apoptosis in mitogen-activated T-cells, but not in resting T-cells, shows that this antitumour ether lipid may have a potential in the therapy of autoimmune diseases, including those involving the central nervous system. In this regard, it is worthwhile to note that ET-18-OCH3 is able to cross the blood brain barrier (Arnold et al., 1978).
As previously reported in human leukaemic HEL and HL-60 cell lines (Mollinedo et al., 1997), ET-18-OCH3 induced an increase in the mRNA levels of c-myc, but had no effect on the expression of the anti-apoptotic gene bcl-2 or of the pro-apoptotic gene bax, in mitogen-activated T-lymphocytes. However, additional experiments will be required to elucidate a putative role for c-myc, if any, in the mechanism of action of ET-18-OCH3.
Apoptosis plays an important role in shaping the repertoire of lymphocytes, in regulating the size of the mature lymphocyte pool, and in the deletion of autoreactive T and B lymphocytes, thus limiting immune responses (Osborne, 1996). Fas/FasL interactions are involved in activation-induced suicide of T cells (Dhein et al., 1995; Brunner et al., 1995; Ju et al., 1995; Nagata, 1997). Fas has been shown to be involved in controlling autoreactivity, as deficiency in Fas results in the accumulation of autoreactive T cells in peripheral lymphoid tissues (Parijs et al., 1998). In addition, it has been suggested that the Fas/FasL system is involved in the increased apoptosis of T cell subsets in aging humans (Aggarwal & Gupta, 1998). The data herein reported indicate that the apoptotic response induced by ET-18-OCH3 in mitogen-activated T-lymphocytes is mediated about 45% through a Fas/FasL system, requiring de novo synthesis of FasL, and about 55% through a Fas/FasL-independent system. This is evidenced by the fact that the ether lipid is able to induce fasL expression and to promote about 25% apoptosis in activated T-cells, which is partially inhibited (44% inhibition) upon incubation with the blocking anti-Fas SM1/23 mAb. In the presence of CHX, there is no synthesis of FasL, and ET-18-OCH3 induces about 13% apoptosis in activated T-cells, which is not affected by incubation with the blocking anti-Fas SM1/23 mAb. The inhibition of Fas/FasL signalling through receptor-specific mAbs was evidenced by complete prevention of the cytotoxic anti-Fas IgM CH-11-induced apoptosis by the neutralizing anti-Fas SM1/23 mAb. The cytotoxic anti-Fas IgM CH-11 mAb, which mimics Fas L, promoted a greater response in activated T-cells after treatment with CHX. This could be due to the putative presence of rapid turnover anti-apoptotic proteins that may regulate programmed cell death in activated T-cells.
Overall, the results herein reported indicate that the antitumour ether lipid ET-18-OCH3 is able to induce apoptosis in normal human mitogen-activated T-lymphocytes, sparing resting T-lymphocytes. This fact may be of importance in the treatment of autoimmune diseases, including those involving the central nervous system, and it can explain the beneficial effects of ET-18-OCH3 in multiple sclerosis patients and in chronic relapsing EAE, considered as a good experimental model for human multiple sclerosis.
Acknowledgments
This work was supported in part by Grant CDTI97-0355 from INKEYSA and Ministerio de Industria y Energia of Spain, Grant 1FD97-0622 from the European Commission and comisión Interministerial de ciencià y Tecnologia (CICYT), Grant VA32/99 from Junta de Castilla y León, Grant PB95-0713 from Dirección General de Investigación Científica y Técnica (DGICYT), Grant SAF98/0047 from the Comisión Interministerial de Ciencia y Tecnologia (CICYT), and Grants HA1996-0118 and AI-40/96 from Acciones Integradas Hispano-Alemanas. C. Cabaner. is a recipient of a fellowship from the Ministerio de Educación y Cultura of Spain. C. Gajate is a recipient of a fellowship associated to a CDTI project. We are thankful to Enrique Barbosa and Sagrario Callejo for their help in the confocal microscopy experiments. We thank Antonio de la Hera and Javier Naval for helpful discussions. We also thank Antonio Iglesias for critically reading the manuscript. We thank the Blood Banks of the Hospital Clínico Universitario and Hospital Rio Hortega of Valladolid for blood supply.
Abbreviations
- BM 41.440
1-S-hexadecyl-2-methoxymethyl-rac-glycero-3-phosphocholine
- CHX
cycloheximide
- ConA
concanavalin A
- DiOC6(3)
3,3′-dihexyloxacarbocyanine iodide
- EAE
Experimental allergic encephalomyelitis
- ET-18-OCH3
1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine
- Eth
ethidium
- FasL
Fas ligand
- FCS
foetal calf serum
- FITC
fluorescein isothiocyanate
- HE
hydroethidine
- HPC
hexadecylphosphocholine
- IL-2
interleukin-2
- mAb
monoclonal antibody
- PBL
peripheral blood lymphocyte
- PBS
phosphate-buffered saline
- PHA
phytohemagglutinin
- RT–PCR
reverse transcription-PCR
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling
References
- ACHA-ORBEA H., MITCHELL D.J., TIMMERMANN L., WRAITH D.C., TAUSCH G.S., WALDOR M.K., ZAMVIL S.S., MCDEVITT H.O., STEINMAN L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988;54:263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- AGGARWAL S., GUPTA S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J. Immunol. 1998;160:1627–1637. [PubMed] [Google Scholar]
- ANDREESEN R., MODOLELL M., WELTZIEN H.U., MUNDER P.G. Alkyl-lysophospholipid induced suppression of human lymphocyte response to mitogens and selective killing of lymphoblasts. Immunobiology. 1979;156:498–508. doi: 10.1016/S0171-2985(80)80083-0. [DOI] [PubMed] [Google Scholar]
- ARNOLD B., REUTHER R., WELTZIEN H.U. Distribution and metabolism of synthetic alkyl analogs of lysophosphatidylcholine in mice. Biochim. Biophys. Acta. 1978;530:47–55. doi: 10.1016/0005-2760(78)90125-x. [DOI] [PubMed] [Google Scholar]
- BAKER D., O'NEILL J.K., AMOR S., KHAMASHTA M.A., TURK J.L. Inhibition of chronic relapsing experimental allergic encephalomyelitis in the mouse by the alkyl-lysophospholipid ET-18-OCH3. Int. J. Immunopharmacol. 1991;13:385–392. doi: 10.1016/0192-0561(91)90008-u. [DOI] [PubMed] [Google Scholar]
- BEN-NUN A., WERKERLE H., COHEN I.R. The rapid isolation of clonable antigen specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur. J. Immunol. 1981;11:195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- BERDEL W.E., FINK U., RASTETTER J. Clinical phase I pilot study of the alkyl lysophospholipid derivative ET-18-OCH3. Lipids. 1987;22:967–969. doi: 10.1007/BF02535566. [DOI] [PubMed] [Google Scholar]
- BOGGS K., ROCK C.O., JACKOWSKI S. The antiproliferative effect of hexadecylphosphocholine toward HL60 cells is prevented by exogenous lysophosphatidylcholine. Biochim. Biophys. Acta. 1998;1389:1–12. doi: 10.1016/s0005-2760(97)00145-8. [DOI] [PubMed] [Google Scholar]
- BRUNNER T., MOGIL R.J., LAFACE D., YOO N.J., AMAHBOUBU A., ECHEVERRI F., MARTIN S.J., FORCE W.R., LYNCH D.H., WARE C.F., GREEN D.R. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- CHABANNES D., RYFFEL B., BOREL J.-F. SRI 62-834, a cyclic ether analogue of the phospholipid ET-18-OCH3, displays long-lasting beneficial effect in chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. Comparison with cyclosporin and (Val2)-dihydrocyclosporin effects in clinical, functional and histological studies. J. Autoimmunity. 1992;5:199–211. doi: 10.1016/0896-8411(92)90200-a. [DOI] [PubMed] [Google Scholar]
- DHEIN J., WALCZAK H., BÄUMLER C., DEBATIN K.-M., KRAMMER P.H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- DIOMEDE L., COLOTTA F., PIOVANI B., RE F., MODEST E.J., SALMONA M. Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine: a possible basis for its selective action. Int. J. Cancer. 1993;53:124–130. doi: 10.1002/ijc.2910530123. [DOI] [PubMed] [Google Scholar]
- DIOMEDE L., PIOVANI B., RE F., PRINCIPE P., COLOTTA F., MODEST E.J., SALMONA M. The induction of apoptosis is a common feature of the cytotoxic action of ether-linked glycerophospholipids in human leukemic cells. Int. J. Cancer. 1994;57:645–649. doi: 10.1002/ijc.2910570506. [DOI] [PubMed] [Google Scholar]
- ENGELHARDT B., DIAMANTSTEIN T., WERKERLE H. Immunotherapy of experimental autoimmune encephalomyelitis (EAE): Differential effect of anti-IL-2 receptor antibody therapy on actively induced and T-line mediated EAE of the Lewis rat. J. Autoimmun. 1989;2:61–73. doi: 10.1016/0896-8411(89)90108-x. [DOI] [PubMed] [Google Scholar]
- FRIESEN C., HERR I., KRAMMER P.H., DEBATIN K.-M. Involvement of CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- GAJATE C., SANTOS-BENEIT A., MODOLELL M., MOLLINEDO F. Involvement of c-Jun NH2-terminal kinase activation and c-Jun in the induction of apoptosis by the ether phospholipid 1-O?-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine. Mol. Pharmacol. 1998;53:602–612. doi: 10.1124/mol.53.4.602. [DOI] [PubMed] [Google Scholar]
- GAMEN S., ANEL A., LASIERRA P., ALAVA M.A., MARTINEZ-LORENZO M.J., PIÑEIRO A., NAVAL J. Doxorubicin-induced apoptosis in human T-cell leukemia is mediated by caspase-3 activation in a Fas-independent way. FEBS Lett. 1997;417:360–364. doi: 10.1016/s0014-5793(97)01282-9. [DOI] [PubMed] [Google Scholar]
- GAVRIELI Y., SHERMAN Y., BEN-SASSON S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell. Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASSER L., DALTON W.S., FIEDERLEIN R.L., COOK P., POWIS G., VOGLER W.R. Response of human multiple myeloma-derived cell lines to alkyl-lysophospholipids. Exp. Hematol. 1996;24:253–257. [PubMed] [Google Scholar]
- HOULIHAN W., LOHMEYER M., WORKMAN P., CHEON S.H. Phospholipid antitumor agents. Med. Res. Rev. 1995;15:157–223. doi: 10.1002/med.2610150302. [DOI] [PubMed] [Google Scholar]
- JU S.-T., PANKA D.J., CUI H., ETTINGER R., EL-KHATIB M., SHERR D.H., STANGER B.Z., MARSHAK-ROTHSTEIN A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- KLEIN-FRANKE A., MUNDER P.G. Alkyllysophospholipid prevents induction of experimental allergic encephalomyelitis. J. Autoimmunity. 1992;5:83–91. doi: 10.1016/s0896-8411(05)80053-8. [DOI] [PubMed] [Google Scholar]
- KOENIGSMANN M.P., NOTTER M., KNAUF W.U., PAPADIMITRIOU C.A., OBERBERG D., REUFI B., MÜCKE C., Thiel E., BERDEL W.E. Chemopurging of peripheral blood-derived progenitor cells by alkyl-lysophospholipid and its effect on haematopoietic rescue after high-dose therapy. Bone Marrow Transplant. 1996;18:549–557. [PubMed] [Google Scholar]
- KONSTANTINOV S.M., EIBL H., BERGER M.R. Alkylphosphocholines induce apoptosis in HL-60 and U-937 leukemic cells. Cancer Chemother. Pharmacol. 1998;41:210–216. doi: 10.1007/s002800050730. [DOI] [PubMed] [Google Scholar]
- KOVARIK J., CHABANNES D., BOREL J.F. Immunoregulation and drug treatment in chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. Int. J. Immunopharmacol. 1995;17:255–263. doi: 10.1016/0192-0561(95)00012-q. [DOI] [PubMed] [Google Scholar]
- KROEMER G., ZAMZAMI N., SUSIN S.A. Mitochondrial control of apoptosis. Immunol. Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- LILES W.C., KIENER P.A., LEDBETTER J.A., ARUFFO A., KLEBANOFF S.J. Differential expression of Fas(CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J. Exp. Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOHMEYER M., BITTMAN R. Antitumor ether lipids and alkylphosphocholines. Drugs Future. 1994;19:1021–1037. [Google Scholar]
- LYNCH D.H., RAMSDELL F., ALDERSON M.R. Fas and FasL in the homeostatic regulation of immune responses. Immunol. Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- MARCHETTI P., CASTEDO M., SUSIN S.A., ZAMZAMI N., HIRSCH T., MACHO A., HAEFFNER A., HIRSCH F., GEUSKENS M., KROEMER G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLINEDO F., BURGALETA C., VELASCO G., ARROYO A.G., ACEVEDO A., BARASOAIN I. Enhancement of human neutrophil functions by a monoclonal antibody directed against a 19-kDa antigen. J. Immunol. 1992;149:323–330. [PubMed] [Google Scholar]
- MOLLINEDO F., FERNANDEZ-LUNA J.L., GAJATE C., MARTIN-MARTIN B., BENITO A., MARTINEZ-DALMAU R., MODOLELL M. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (Edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-XL. Cancer Res. 1997;57:1320–1328. [PubMed] [Google Scholar]
- MOLLINEDO F., GAJATE C., MODOLELL M. The ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine induces expression of fos and jun proto-oncogenes and activates AP-1 transcription factor in human leukaemic cells. Biochem. J. 1994;302:325–329. doi: 10.1042/bj3020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLINEDO F., MARTINEZ-DALMAU R, MODOLELL M. Early and selective induction of apoptosis in human leukemic cells by the alkyl-lysophospholipid ET-18-OCH3. Biochem. Biophys. Res. Commun. 1993;192:603–609. doi: 10.1006/bbrc.1993.1458. [DOI] [PubMed] [Google Scholar]
- MOLLINEDO F., SANTOS-BENEIT A.M., GAJATE C.The human leukemia cell line HL-60 as a cell culture model to study neutrophil functions and inflammatory cell responses Animal Cell Culture Techniques 1998Heidelberg, Germany: Springer-Verlag; 264–297.ed. Clynes, M. pp [Google Scholar]
- MUNDER P.G., WESTPHAL O. Antitumoral and other biomedical activities of synthetic ether lysophospholipids. Chem. Immunol. 1990;49:206–235. [PubMed] [Google Scholar]
- NAGATA S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- NICOLETTI I., MIGLIORATI G., PAGLIACCI M.C., RICCARDI C. A rapid simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Meth. 1991;139:271–280. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- O'CONNELL J., O'SULLIVAN G.C., COLLINS J.K., SHANAHAN F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J. Exp. Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD M.G., COLLINS M.K.L., RODRIGUEZ-TARDUCHY G., ROBERTSON D. Apoptosis in interleukin-3-dependent hemopoietic cells. Quantification by two cytometric methods. J. Immunol. Meth. 1992;153:57–65. doi: 10.1016/0022-1759(92)90305-d. [DOI] [PubMed] [Google Scholar]
- OSBORNE B.A. Apoptosis and the maintenance of homeostasis in the immune system. Curr. Opin. Immunol. 1996;8:245–254. doi: 10.1016/s0952-7915(96)80063-x. [DOI] [PubMed] [Google Scholar]
- PARIJS L.V., BIUCKIANS A., ABBAS A.K. Functional roles of Fas and Bcl-2-regulated apoptosis in T lymphocytes. J. Immunol. 1998;160:2065–2071. [PubMed] [Google Scholar]
- PEREZ-SALA D., COLLADO-ESCOBAR D., MOLLINEDO F. Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J. Biol. Chem. 1995;270:6235–6242. doi: 10.1074/jbc.270.11.6235. [DOI] [PubMed] [Google Scholar]
- PETIT P.X., LECOEUR H., ZORN E., DAUGUET C., MIGNOTTE B., GOUGEON M.L. Alterations of mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell. Biol. 1995;130:157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETIT P.X., O'CONNOR J.E., GRUNWALD D., BROWN S.C. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur. J. Biochem. 1990;194:389–397. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- PLANTING A.S., STOTER G., VERWEI J. Phase II study of daily oral miltefosine (hexadecylphosphocholine) in advanced colorectal cancer. Eur. J. Cancer. 1993;29A:518–519. doi: 10.1016/s0959-8049(05)80142-x. [DOI] [PubMed] [Google Scholar]
- SATOH J., SAKAI K., ENDOH M., KOIKE F., KUNISHITA T., NAMIKAWA T., YAMAMURA T., TABIRA T. Experimental allergic encephalomyelitis mediated by murine encephalitogenic T-cell lines specific for myelin proteolipid apoprotein. J. Immunol. 1987;138:179–184. [PubMed] [Google Scholar]
- UNGER C., EIBL H. Hexadecylphosphocholine: preclinical and the first clinical results of a new antitumor drug. Lipids. 1991;26:1412–1417. doi: 10.1007/BF02536578. [DOI] [PubMed] [Google Scholar]
- UNGER C., SINDERMANN H., PEUKERT M., HILGARD P., ENGEL J., EIBL H. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Prog. Exp. Tumor Res. 1992;34:153–159. doi: 10.1159/000420840. [DOI] [PubMed] [Google Scholar]
- VOGLER W.R., BERDEL W.E., GELLER R.B., BROCHSTEIN J.A., BEVERIDGE R.A., DALTON W.S., MILLER K.B., LAZARUS H.M. A phase II trial of autologous bone marrow transplantation (ABMT) in acute leukemia with edelfosine purged bone marrow. Adv. Exp. Med. Biol. 1996;416:389–396. doi: 10.1007/978-1-4899-0179-8_62. [DOI] [PubMed] [Google Scholar]
- VON MEHREN M., GIANTONIO B.J., MCALEER C., SCHILDER R., MCPHILLIPS J., O'DWYER P.J. Phase I trial of ilmofosine as a 24 h infusion weekly. Invest. New Drugs. 1995;13:205–210. doi: 10.1007/BF00873801. [DOI] [PubMed] [Google Scholar]
- WINKELMANN M., EBELING K., STROHMEYER G., HOTTENROTT G., MECHL Z., BERGES W., SCHOLTEN T., WESTERHAUSEN M., SCHLIMOK G., STERZ R. Treatment results of the thioether lipid ilmofosine in patients with malignant tumours. J. Cancer Res. Clin. Oncol. 1992;118:405–407. doi: 10.1007/BF01629421. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI T., SIEBER F. The alkyl-lysophospholipid, ET-18-OCH3 synergistically enhances the merocyanine 540-mediated photoinactivation of leukemia cells: implications for the extracorporeal purging of autologous hematopoietic stem cells. Bone Marrow Transplant. 1997;19:113–119. doi: 10.1038/sj.bmt.1700625. [DOI] [PubMed] [Google Scholar]
- ZAMVIL S., NELSON P., TROTTER J., MITCHELL D., KNBLER R., FRITZ R., STEINMAN L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- ZAMZAMI N., MARCHETTI P., CASTEDO M., ZANIN C., VAYSSIERE J.L., PETIT P.X., KROEMER G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]


