Abstract
The enteric nervous system (ENS) is activated when exposing the intestinal mucosa to cholera toxin or certain bile salts. Cholera toxin stimulates ENS, at least in part, by the release of 5-hydroxytryptamine (5-HT) from the enterochromaffin cells. Calcium channel blockers of the L-type markedly attenuate the fluid secretion and the luminal release of 5-HT caused by cholera toxin.
The objective of the present study was to elucidate if sodium deoxycholate activated ENS in a similar manner as cholera toxin. Furthermore, the effect of several calcium channel blockers was tested on the fluid secretion caused by cholera toxin or bile salt.
Sodium deoxycholate (4 mM) caused a release of 5-HT into the intestinal lumen, which was inhibited by calcium channel blockade. Granisetron, a 5-HT3 receptor blocker, partly inhibited the fluid secretion caused by bile salt.
The effects of nifedipine, felodipine, R-felodipine, H186/86 (t-butyl analogue of felodipine) on the fluid secretion caused by cholera toxin or sodium deoxycholate were studied. Both secretory states were markedly attenuated in a dose dependent manner by all calcium channel blockers tested regardless of their effects on arterial pressure.
It is concluded that both cholera toxin and bile salt activate ENS, at least in part, via a release of 5-HT from the enterochromaffin cells. The antisecretory effect calcium channel blockers is partly explained by an inhibition of this release of 5-HT.
Keywords: 5-Hydroxytryptamine, cholera toxin, bile salt, calcium channel blocker, small intestine, fluid transport, enteric nervous system, enterochromaffin cells (rat)
Introduction
Earlier studies performed in this laboratory on the mechanisms underlying the intestinal fluid secretion evoked by cholera toxin strongly support the hypothesis that reflex(es) of the enteric nervous system (ENS) play an important role in cholera secretion (Cassuto et al., 1981; 1983; Jodal et al., 1993a). The current model for such an involvement of ENS in cholera is illustrated in Figure 1. This model is mainly based on electrophysiological observations reported by Bornstein and collaborators (Bornstein et al., 1986; 1988; 1994), on pharmacological investigations performed in our laboratory, on immunohistochemical studies by Kirchgessner et al. (1992) and on chemical ablation experiments by Jodal et al. (1993a). The studies suggest that the intestinal fluid secretion caused by most secretagogues, including certain bile salts (Karlström, 1986a), the heat stable toxin produced by Escherichia coli (Eklund et al., 1989) and by inflammation (Brunsson, 1987; Jodal et al., 1993b; Castagliuolo et al., 1994; Nellgård et al., 1996), to a large extent is mediated via the ENS. For a recent survey of this research field the reader is referred to a review by Jodal & Lundgren (1995).
Figure 1.
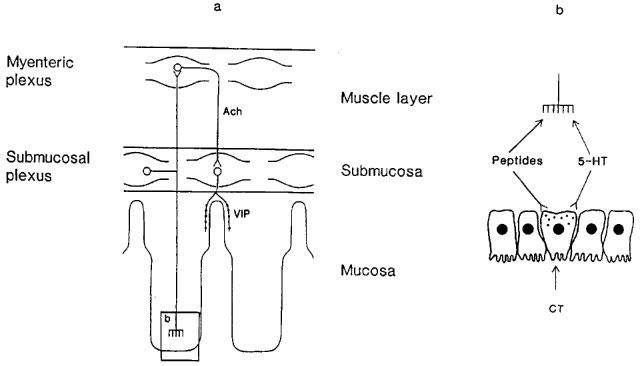
Schematic figure of the model proposed for the secretory nervous reflex activated by cholera toxin (CT). (a) illustrates the intramural reflex activated by the toxin. The reflex arch consists of at least three neurons. The transmitter of the afferent neuron is presently unknown. The efferent neuron releases vasoactive intestinal polypeptide (VIP) and there is a cholinergic interneuron (Ach=acetylcholine). (b) depicts how cholera toxin may activate the enteric nerves by causing the release of peptides and/or 5-hydroxytryptamine (5-HT) from the endocrine cells of the mucosa. It should be underlined that the figure is highly schematic. The nerves activated by peptides and/or 5-HT are situated just underneath the intestinal epithelium.
According to model of Figure 1 ENS is activated by the release of amines/peptides from the endocrine cells of the intestinal epithelium. This has been particularly well studied with regard to the release of serotonin from the enterochromaffin (EC) cells when exposing the intestinal mucosa to cholera toxin. Such a release has been demonstrated by electron microscopy (Osaka et al., 1975) and histochemistry (Nilsson et al., 1983). Furthermore, a release of 5-HT into the intestinal lumen accompanies cholera secretion both in rats and humans (Beubler et al., 1989; Peterson et al., 1993; Peterson & Whipp, 1995; Bearcroft et al., 1996; Timar Peregrin et al., 1997a) and 5-HT3 receptor antagonists diminish cholera secretion (Beubler & Horina, 1990; Sjöqvist et al., 1992) at least in rats. In humans the observations regarding the effect of 5-HT3 receptor blockade are conflicting (Eherer et al., 1994; Turvill & Farthing 1997). Finally, placing a 5-HT solution in the intestinal lumen or giving 5-HT at a close location intra-arterially evokes a fluid secretion, which is almost completely blocked by agents interfering with the functions of ENS (Cassuto et al., 1982, Sjöqvist et al., 1992). Together these observations strongly suggest that 5-HT released from EC cells participate in the cholera toxin induced fluid secretion.
Karlström and coworkers (Karlström, 1986a,1986b; Karlström et al., 1983; 1986a,1986b) showed that the bile salt sodium deoxycholate stimulates intestinal fluid secretion and motility to a large extent via enteric nervous reflex(es). Only a small part of the change in the net fluid transport evoked by the bile salt could be explained by a reduced absorptive capacity of the villus epithelium (Karlström et al., 1986b). Observations reported by Eklund et al. (1989) suggested that, in the case of the bile salt induced secretion, ENS may not be activated by the release of compounds from the endocrine cells of the intestinal epithelium. Bile salt may diffuse into the villus lamina propria to directly or indirectly activate the afferent limb of the secretory reflex. One of the aims of the present study was to elucidate if 5-HT participated in the fluid secretion caused by sodium deoxycholate.
Several studies have reported that it is possible to inhibit cholera toxin induced secretion by calcium channel blockers of the L-type (Beubler et al., 1989, Timar Peregrin et al., 1997a,1997b). One aim of this investigation was to elucidate if the bile salt induced secretion is also attenuated by calcium channel blockers. The antisecretory potency of nifedipine and two stereoisomeric forms of felodipine, was investigated. Both drugs are used clinically to treat arterial hypertension implying that they lower blood pressure. This may be of importance in explaining their effect on intestinal fluid transport. A fourth blocker, H 186/86, was therefore also tested. This drug, which is the t-butyl analogue of felodipine, is devoid of the blood pressure lowering effect of felodipine (Berntsson et al., 1987). Thus, experiments with this compound made it possible to elucidate if lowering of arterial pressure per se may explain the effects of calcium channel blockade on intestinal fluid secretion.
Methods
Operative procedures
Experiments were performed on adult male Sprague-Dawley rats, weighing 300–500 g (Alab AB, Stockholm, Sweden or Charles River, Margate, U.K.). The animals were kept under standardized environmental conditions (22°C, 60% humidity, artificial light from 0600–1800 h) in the animal quarters for at least 7 days prior to the experiments. The study was approved by the Animal ethics committee at Göteborg university.
In all animals anaesthesia was induced with pentobarbitone i.p. (Mebumal, 60 mg kg−1 body wt). The anaesthetized animals were placed on a operating table. Body temperature was monitored by a rectal thermistor and was kept at 37°C by means of a heated operation table and a heating lamp. A tracheal cannula was inserted to assure free airways, and a femoral vein was cannulated for the administration of drugs. Arterial pressure was recorded by means of a pressure transducer (DPT-6000 Single-Use Transducer, Peter von Berg Medizinteknik Gmbh, Englhartin, Germany) via a catheter in the femoral artery. This catheter was also used to maintain anaesthesia by continuous infusion of chloralose (2–4 mg ml−1; 0.02 ml min−1) which was given in a solution containing glucose (138 mM) and NaHCO3 (33 mM) to prevent dehydration and acidosis during and after surgery.
After tracheotomy, a midline abdominal incision was performed. One or two jejunal segments, 5–7 cm long, were isolated with intact vascular supply. The rest of the small intestine and the colon were extirpated. In order to interrupt the extrinsic autonomic nervous supply to the intestinal segment, all nerves along the superior mesenteric artery were cut.
Gravimetric recording of intestinal net fluid transport
Net intestinal fluid transport was measured by a gravimetric technique developed in the laboratory and described in detail by Cassuto et al. (1981; 1982; 1983). In short, the intestinal segment was placed on a specially designed plastic balance suspended 3–5 mm above the abdominal wall and connected to a force displacement transducer (Grass FT03C), allowing a continuous recording of changes of intestinal weight. In the bile salt experiments the jejunal segment was connected to a recirculating system containing a reservoir (25 ml) to minimize recirculation of the fluid (Karlström, 1986a). The intestinal segment was perfused throughout the experiment at a rate of 0.1 ml min−1 by means of pump (Ismatec, type Minimicro 2/6; Ismatec, Zurich, Switzerland).
Net fluid transport across the intestinal epithelium (reflected as changes in intestinal weight) was continuously recorded by connecting the force displacement transducer to a Grass polygraph. To minimize evaporation, the intestinal segment was carefully covered by a plastic film, and the rat was placed in a plastic cage in which the temperature was kept constant at 37°C. The head of the rat was placed outside the cage wall to allow the animal to breathe air of room temperature. The rate of net fluid transport measured during 15 min periods was related to the serosal surface area of the investigated segment.
Simultaneous measurements of intestinal net fluid transport, luminal release of serotonin and its main metabolite and transepithelial potential difference
In some experiments two isolated intestinal segments were perfused at a constant rate of 5.66 ml h−1 with a modified Krebs-Henseleit solution (see below). Net intestinal fluid transport was estimated from the weight of the fluid leaving the intestinal segment collected during 30 min periods and the known perfusion rate, assuming that the volume of the intestinal lumen was maintained at a constant level.
The concentration of the 5-hydroxytryptamine (5-HT) and its main metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in the collected intestinal perfusate was determined by HPLC with electrochemical detection as described in detail by Timar Peregrin et al. (1997a).
The potential difference (PD) across the jejunal epithelium was measured between two agar bridge electrodes, one in contact with the luminal fluid via a plastic tubing and the other in contact with the fluid in the peritoneal cavity. The electrodes were connected via calomel half cells (Calomel electrode K401, Radiometer, Copenhagen, Denmark) to an amplifier whose output was displayed on a Grass polygraph.
Experimental protocol
Serotonin experiments
Two types of experiments was performed. In one series of experiments the effects of bile salt on net fluid transport, transepithelial PD and luminal release of 5-HT and 5-HIAA were investigated. Two intestinal segments were perfused as described above. After a 1 h period of perfusion with a modified Krebs-Henseleit solution devoid of bile salt, the intestines were perfused with a solution containing sodium deoxycholate (4 mM). In one series of experiments (nifedipine series) samples of the luminal solution leaving the intestine were collected every 30 min throughout the experiments. The drug was given 1 h after starting the perfusion with the bile salt solution. In two other series (felodipine and H186/86 series) samples were only collected 30 min before and 30–60 min after giving the drugs. In all experiments PD was monitored throughout the experiments.
In another type of experiment the effects of granisetron, a 5-HT3 receptor blocker (0.11 μmol (40 μg) per kg body wt.; n=5), given i.v. was investigated with regard to its effect on the bile salt induced fluid secretion. Net fluid transport was recorded with the gravimetric method described above. The intestinal segment was first perfused with an isotonic solution devoid of bile salt for 15–30 min during which net fluid transport was constant. The segment was then perfused with a solution containing sodium deoxycholate (4 mM) which within 30 min evoked a net fluid secretion. After recording a constant net fluid secretion for at least 20–30 min granisetron was administered.
Calcium channel blockade experiments
Two types of experiments were performed. In one type pure crystalline cholera toxin (20 μg) dissolved in 0.5 ml physiological saline was introduced into the intestinal lumen. Within 3 h all animals developed a steady intestinal net fluid secretion. After a control period of constant fluid secretion lasting for at least 30 min the drugs to be tested were given into the femoral vein, implying that the drugs were administered between 2 and 3.5 h after exposing the intestinal mucosa to cholera toxin. The following drugs were used: nifedipine (n=7), two stereoisomers of felodipine (felodipine n=6; R-felodipine n=7) and H186/86 (t-butyl analogue of felodipine (Berntsson et al., 1987); n=5), a calcium channel blocker synthesized by AB Astra-Hässle, Sweden.
In another type of experiments net fluid secretion was evoked by sodium deoxycholate. Net fluid transport was monitored with the gravimetric technique described earlier. A jejunal segment was perfused with a modified Krebs-Henseleit solution until net fluid transport had been maintained at a steady state level for at least 30 min. After this control period, the intestines were perfused with a solution containing sodium deoxycholate, 4 mM. After 1 h of bile salt perfusion a calcium channel blocker was administered i.v. while the bile salt perfusion continued. The following drugs were tested: nifedipine, the two stereoisomers of felodipine and H186/86. All drugs were tested in five experiments.
Solutions and drugs
In the bile salt experiments the lumen of the intestinal segment was perfused with a modified Krebs-Henseleit solution containing (in mM): NaCl, 122; NaHCO3, 25; KCl, 4.7; KH2PO4, 1.2; mannitol 20. The bile salt solution also contained 4 mM sodium deoxycholate. The osmolality of the solutions ranged between 290 and 310 mosm kg−1 H2O.
The modified KrebsHenseleit solution used in all cholera toxin experiments contained (mM): NaCl, 122; NaHCO3, 25; KCl, 4.7; KH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; mannitol 30. The osmolality ranged between 305 and 315 mosm kg−1 H2O.
The following drugs were used in the experiments: pentobarbitone, cholera toxin (List Biological Laboratories Inc., Campbell; U.S.A.), granisetron (SmithKline Beecham Pharmaceuticals, Brentford; Middelsex, U.K.), nifedipine (Sigma Chemical Co., St Louis, MO, U.S.A.), H 186/86, R-felodipine, felodipine (Astra-Hässle AB, Mölndal, Sweden).
Statistics
Statistical analyses were performed using sign test and Wilcoxon's signed rank test. Differences resulting in P values of 0.05 or less were considered significant. Values are given as mean±s.e. mean.
Results
Serotonin experiments
The possible involvement of 5-HT in the bile salt induced secretion was studied in two types of experiments. In one series the release of 5-HT and 5-HIAA into the intestinal lumen was followed in two perfused segments. The results from the experiments with nifedipine are illustrated in Figure 2. In these experiments samples of the fluid leaving the two segments were collected every 30 min throughout the experiments. Exposing the intestinal mucosa to sodium deoxycholate (4 mM) caused a statistically significant increase of the luminal release of 5-HT (from 0.10±0.05 to 1.20±0.51 nmol 30 min−1 100 cm−2 serosal surface; P<0.05), whereas 5-HIAA could not be detected in any luminal sample. Concomitantly, net fluid transport turned from absorption (139±69 μl min−1 100 cm−2) to secretion (437±73 μl min−1 100 cm−2; P<0.05) and PD increased in four out of five experiments (data not shown). After giving nifedipine i.v. (5.75 μmol kg−1 body wt) all studied variables decreased to control (Figure 2). No significant changes in the recorded variables were observed in the control segment not exposed to bile salt.
Figure 2.
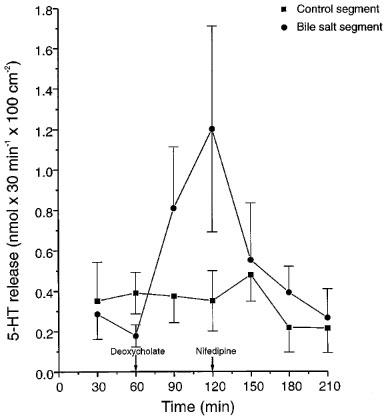
Release of 5-HT into the intestinal lumen in control segments and in segments exposed to sodium deoxycholate (4 mM) before and after giving nifedipine (5.75 μmol (2 mg) per kg body wt). n=5. Means±s.e.mean.
In two other series of experiments the calcium channel blockers felodipine (2.6 μmol kg−1 body wt.) and H186/86 (2.2 μmol kg−1 body wt.) were administered to the animals. The experiments were in principle performed as illustrated on Figure 2 but samples from the intestinal segments were collected only 30 min before and 30–60 min after giving the drugs. The results are given in Table 1. Only a release of 5-HT was detected. It was significantly larger in the intestinal segments exposed to bile salt. The increased amine release was accompanied by an increased PD and intestinal fluid secretion. All three calcium channel blockers tested significantly attenuated the increased 5-HT release (Table 1) as well as the increase of PD and fluid secretion evoked by sodium deoxycholate. No significant changes in the recorded variables were observed in the control segments not exposed to bile salt (data not shown).
Table 1.
The effect of three different calcium channel blockers on the release of 5-hydroxytryptamine (pmol 30 min−1 100 cm−2) into the intestinal lumen
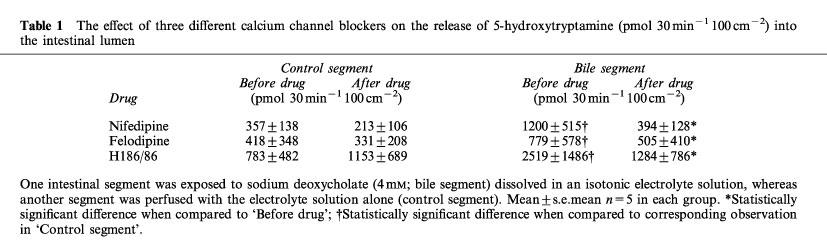
It is evident from Figure 2 and Table 1 that there was a large variation in 5-HT release among the experiments. It reflects the fact that the measured luminal 5-HT concentrations fell roughly into two groups, the concentration in one group being about ten times higher than that in the other group. This was seen in one third of the 15 experiments performed. The reason for this large variation between animals is not known.
In another type of experiment (n=5) granisetron, a 5-HT3-receptor blocker, was given i.v. in a dose of 0.11 μmol (40 μg) per kg. The use of this dose was based on dose-response curve for granisetron determined with regard to its inhibitory effect on the duodenal bicarbonate secretion (Jaup et al., 1998) and on the intestinal fluid secretion caused by cholera toxin (Sjöqvist et al., 1992). Furthermore, in the latter study the dose used attenuated the bradycardia evoked by i.v. injection of 5-HT. Net fluid transport was recorded with the gravimetric technique. The bile salt induced secretion (154± 30 μl min−1 100 cm−2) was decreased to 43± 7 μl min−1 100 cm−2 (P<0.05) by granisetron. In no experiment was fluid secretion totally abolished. In control experiments the drug was shown not to influence basal net fluid transport in the intestine (data not shown).
Calcium channel blockade experiments
All the experiments reported below and illustrated in Figures 3–6 were performed using the gravimetric technique for estimating net fluid transport. Cholera toxin placed in the intestinal lumen evoked within 2–3 h a pronounced net fluid secretion in all experiments. Similarly, exposing the intestinal mucosa to an electrolyte solution containing 4 mM sodium deoxycholate caused net fluid secretion within 30 min. Four different calcium channel blockers were investigated with regard to their effects on the fluid secretion. The effect on cholera secretion of one of them, nifedipine, has been reported elsewhere (Timar Peregrin et al., 1997a,1997b).
Figure 3.
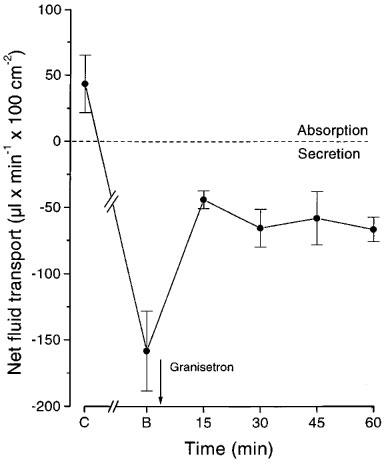
The effect of granisetron (0.11 μmol (40 μg) per kg body wt i.v) on the fluid secretion (gravimetric technique) caused by sodium deoxycholate (4 mM). C: control recording; B: perfusion with bile salt. n=5. Means±s.e.mean.
Nifedipine
The effect of nifedipine (5.75 μmol (2 mg) per kg body wt. i.v.) on the bile salt induced secretion was studied in five experiments. The dose was chosen based on the dose response curve earlier reported for cholera secretion (Timar Peregrin et al., 1997b). In all experiments the net fluid secretion evoked by bile salt (166±12 μl min−1 100 cm−2) was completely abolished within 15–30 min and turned into a net fluid absorption (134±54 μl min−1 100 cm−2; P<0.05). The drug decreased arterial pressure by 20–40 mmHg.
Nifedipine at the dose used in this study has been shown not to influence fluid transport in control intestinal segments (Timar Peregrin et al., 1997b).
Felodipine
In six experiments with cholera toxin felodipine was given i.v. in doses of. 0.26 μmol (0.1 mg), 1.3 μmol (0.5 mg) and 2.6 μmol (1 mg) per kg body wt. (Figure 4). In the lower dose range the drug caused a blood pressure drop of about 25 mmHg. Increasing the dose did not lower blood pressure further. Net fluid secretion was reduced dose-dependently (Figure 4). The highest dose used decreased net fluid secretion from 207±13 to 31±49 μl min−1 100 cm−2 serosa (n=6; P<0.05) within 10–25 min. The decreased rate of secretion was maintained for at least 30 min.
Figure 4.
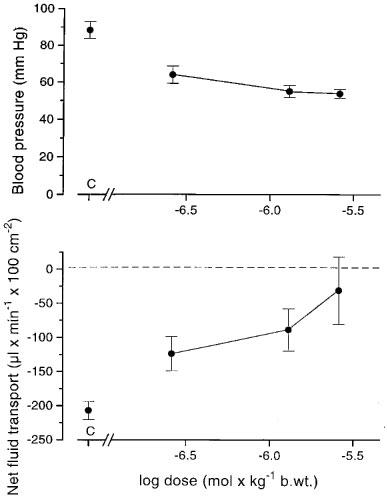
The effect of increasing doses of felodipine on arterial pressure (upper panel) and on the intestinal net fluid secretion (lower panel; gravimetric technique) evoked by exposing the intestinal mucosa to cholera toxin. The drug was administered i.v. C: control recording. n=6. Means±s.e.mean.
In five experiments with sodium deoxycholate we measured the effect of felodipine in a dose of 2.6 μmol (1 mg) per kg body wt. i.v. on bile salt induced secretion. Net fluid secretion (166±22 μl min−1 100 cm−2) turned to a net fluid absorption (44±4 μl min−1 100 cm−2; P<0.05) within 30 min after giving the drug. The effect on fluid transport remained unchanged for at least 75 min. Blood pressure was decreased 40–50 mmHg.
R-felodipine
R-felodipine was given i.v. in the same doses as felodipine in seven experiments with cholera toxin. The drug lowered the systemic arterial pressure and net fluid secretion dose-dependently (Figure 5). The lowering of arterial pressure was less pronounced than when giving felodipine particularly in the lower dose range. At the highest dose a decrease of the net fluid secretion from 199±27 to 34±22 μl min−1 100 cm−2 (P<0.01) was observed (Figure 5)
Figure 5.
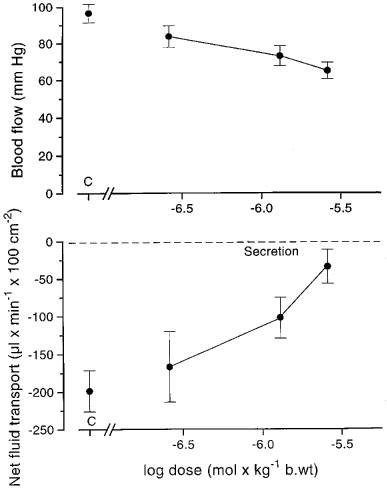
Dose-response curves for R-felodipine given i.v. with regard to the effects on arterial pressure (upper panel) and on net fluid secretion caused by cholera toxin (lower panel; gravimetric technique). C: control recording. n=7. Means±s.e.mean.
In five experiments the effects of R-felodipine on bile salt induced secretion was investigated using a dose of 2.6 μmol (1 mg) per kg body wt. Net fluid secretion (166± 28 μl min−1 100 cm−2) was effectively inhibited and turned into net fluid absorption (104±56 μl min−1 100 cm−2; P<0.05). Arterial pressure was lowered 25–35 mmHg.
H 186/86
H 186/86 in a dose of 2.2 μmol (1 mg) per kg body wt. administered i.v. caused a decrease of cholera toxin induced fluid secretion within 15 min. Secretion decreased from 168±35 to 36±41 μl min−1 cm−2 (n=5; P<0.05; Figure 6) and remained around that level during the whole registration period (60 min). Systemic arterial pressure was hardly influenced by this drug compared with the other calcium channel blockers tested.
Figure 6.
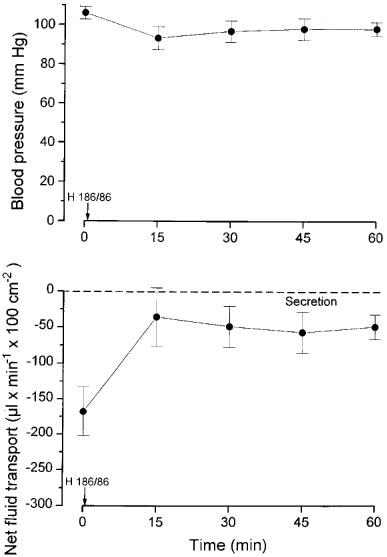
The effects of giving H186/86 (2.2 μmol (1 mg) per kg body wt i.v.) on blood pressure (upper panel) and on intestinal net fluid transport induced by cholera toxin (lower panel; gravimeteric technique). The drug was administered at time 0. Means±s.e.mean. n=5.
Similarly, in five experiments with bile salt net fluid secretion was reduced from 250±37 to 104± 33 μl min−1 100 cm−2 (P<0.05) within 15 min by H 186/86 (2.2 μmol (1 mg) per kg body wt.). The secretion further decreased to reach a value of zero within 60 min. Concomitantly, arterial pressure decreased 5–15 mmHg.
Discussion
5-HT and bile salt induced fluid secretion
There are, as reviewed briefly in the introduction, several types of observations that support the proposal that 5-HT plays an important role in the pathophysiology of cholera toxin induced secretion. Electron microscopy and histochemical studies have revealed that the enterochromaffin (EC) cells release their contents of 5-HT when exposed to cholera toxin (Osaka et al., 1975; Nilsson et al., 1983). An increased release of 5-HT into the intestinal lumen is evoked by cholera toxin (Beubler et al., 1989; Peterson et al., 1993; Peterson & Whipp 1995; Bearcroft et al., 1996; Timar Peregrin et al., 1997a), an effect that is blocked by L-type of calcium blocker (Timar Peregrin et al., 1997a,1997b). Concomitantly, fluid secretion is markedly attenuated. Furthermore, 5-HT receptor antagonists decrease the fluid secretion caused by cholera toxin at least in rats (Beubler & Horina 1990; Sjöqvist et al., 1992). As pointed out in the introduction the observations in humans regarding the effect of 5-HT3 receptor blockade are conflicting (Eherer et al., 1994; Turvill & Farthing, 1997). We have suggested that the 5-HT released from the EC cells activate neural dendrites located just underneath the intestinal epithelium (Cassuto et al., 1981; 1982; Nilsson et al., 1983; see Figure 1) to evoke a fluid secretion.
In the present study we investigated if the bile salt sodium deoxycholate also released 5-HT from the EC cells by following its release into the intestinal lumen before and after perfusing with a solution containing 4 mM sodium deoxycholate. It was shown that the bile salt evoked an increased release of 5-HT into the intestine. Furthermore, granisetron, a blocker of 5-HT3 receptors, partly inhibited the bile salt induced fluid secretion. Thus, these observations suggest that 5-HT is released from the EC cells not only by cholera toxin but also by bile salts (c.f. Figure 1). Furthermore, it was demonstrated that three different calcium channel blockers (nifedipine, felodipine, H186/86) significantly attenuated the 5-HT release. This may partly explain their inhibitory action on the bile induced fluid secretion as discussed below.
It might be argued that the serotonin released into the intestinal lumen does not emanate from the EC cells but from other sources. Two other sources seem possible: serotonergic neurons and/or mast cells (Furness & Costa, 1982; Wingren et al., 1983). It should be underlined that more than 90% of the 5-HT in the intestinal wall is contained in the EC cells. Furthermore, granules containing 5-HT are located not only in the basal part of the EC cells but also in the apical parts facing the intestinal lumen (Ahlman et al., 1978). Measurements of 5-HT in rat and guinea-pig intestine show similar values (Penttilä, 1969; Juorio & Gabella, 1974). From these it can be calculated that the increased release of 5-HT during 30 min evoked by bile salt in the present study represents about 20% of the total amount of 5-HT found in the muscle layers where the cell somas and many of the axons of the serotonergic neurons are located (Furness & Costa, 1982). It is unlikely that such a large proportion of a transmitter in a group of neurons is released during such a comparatively short time as half an hour. Hence, the observed release of 5-HT did not emanate from serotonergic neurons in the outer layers of the intestine but was mainly released from the mucosa.
There are observations that suggest that mast cells may be involved in the secretory response to sodium deoxycholate (Gelbmann et al., 1995). It is not possible on the basis of the present results to exclude that part of the 5-HT released into the intestinal lumen is emanated from mast cells. However, it seems less likely considering that the amount of 5-HT in the EC cells is so much larger than that contained in the mast cells.
Calcium channel blockade and intestinal fluid secretion
A major part of the present study was devoted to the study of the effect of different calcium channel blockers on the fluid secretion caused by cholera toxin and bile salt. Both these secretory agents have been shown to elicit their effects to a large extent via an activation of the ENS (Cassuto et al., 1981; 1982; 1983; Karlström, 1986a). The blockers used were all of the dihydropyridine type influencing calcium channels of the L-type. All blockers markedly inhibited the induced fluid secretion in a dose dependent fashion when tested. In the case of bile salt the blockers turned fluid secretion into absorption in most cases. The present observations made on cholera toxin induced fluid secretion confirm those of other studies that calcium channel blockers of the L-type markedly diminish cholera secretion in jejunal segments of rats (Beubler et al., 1989; Timar Peregrin et al., 1997a, 1997b).
It might be argued that the decreased fluid transport evoked by calcium channel blockade is related to the fall of arterial pressure induced by the drugs. The lowered blood pressure may decrease mucosal blood flow and/or mean hydrostatic pressure in the crypt capillaries. However, mean capillary hydrostatic pressure in the intestine and villus plasma flow are autoregulated and kept at a constant, normal level when lowering arterial pressure to 60 mmHg at least in cats (Haglund & Lundgren, 1972; Lundgren & Svanvik, 1973). Furthermore, comparing the effects of the different calcium channels blockers in this study made it unlikely that the observed effect on fluid transport was an indirect haemodynamic effect. Thus, H 186/86 which was inefficient in lowering arterial pressure decreased fluid secretion to the same extent as felodipine, a drug that caused a marked arterial hypotension.
Sites of action for calcium channel blockade
In a recent study we attempted to localize the site of action for a calcium channel blocker nifedipine with regard to its effect on cholera toxin induced fluid secretion (Timar Peregrin et al., 1997b). It was shown that nifedipine inhibits the release of serotonin from the enterochromaffin cells caused by the toxin as was also shown in this study for bile salt. On the other hand, nifedipine did not influence the electrolyte secretion evoked by stimulating electrically the submucosal efferent neurons in vitro. We concluded that the effect of nifedipine on cholera secretion was at least in part mediated by decreasing the release of amines and possibly also peptides from the endocrine cells of the intestinal epithelium (Figure 1). The present results extend these conclusions also to the fluid secretion evoked by sodium deoxycholate. These observations obviously imply that the release of serotonin from the enterochromaffin cells is dependent on voltage gated calcium channels of the L-type.
Electrophysiological investigations have shown that there are at least two types of neurons in the ENS, the so called S and AH cells. The AH neurons are characterized, among other things, by action potentials of their somas that are resistant to tetrodotoxin. This implies that the ion ‘carrying' this type of action potential is not sodium, but may well be calcium. Furthermore, there is evidence that the AH neurones are afferent with dendrites located in the mucosa (Bornstein et al., 1994). Calcium ions may thus be important also for the generation of action potentials of the afferent neurons of the secretory reflex arch. Our earlier studies, briefly summarized above, did not exclude that nifedipine also inhibited action potentials of AH neurons.
When this study was initiated we thought it might be possible to elucidate if calcium channel blockade also influenced the AH neurons using bile salt induced fluid secretion as an experimental model. There were indirect observations reported by Eklund et al. (1989) suggesting that sodium deoxycholate did not evoke a release of 5-HT from the EC cells. However, the observations of this study clearly indicate that bile salt indeed causes a serotonin release. Hence, it is not possible from the present experiments to draw any certain conclusions concerning possible effects of calcium channel blockade on the action potentials of AH neurons.
It is obvious from this study and earlier investigations that calcium channel blockers of the L-type efficiently attenuate secretory states in the small intestine evoked via the release of e.g. 5-HT from intestinal endocrine cells. In another study (Timar Peregrin et al. to be published) we have shown that placing acid in the intestinal lumen causes a fluid secretion that is not is not mediated via 5-HT. This type of intestinal fluid secretion is also significantly attenuated by nifedipine. Hence, it seems possible that calcium channel blockers of the L-type can inhibit fluid secretion evoked by a wide variety of luminal secretory agents. A calcium channel blocker of the L-type devoid of effects on arterial pressure may therefore turn out to be an efficient antisecretory compound.
Acknowledgments
This research was supported by grants from the Swedish Medical Research Council (2855, 5220), Åke Wiberg's foundation, Knut and Alice Wallenberg's foundation and AB Astra-Hässle, Mölndal, Sweden.
Abbreviations
- Ach
acetylcholine
- EC
enterochromaffin
- ENS
enteric nervous system
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
5-hydroxytryptamine
- PD
potential difference
- VIP
vasoactive intestinal polypeptide
References
- AHLMAN H., BHARGAVA H.N., DONAHUE P.E., NEWSON B., DAS GUPTA T.K., NYHUS L.M. The vagal release of 5-hydroxytryptamine from enterochromaffin cells in the cat. Acta Physiol. Scand. 1978;104:262–270. doi: 10.1111/j.1748-1716.1978.tb06278.x. [DOI] [PubMed] [Google Scholar]
- BEARCROFT C.P., PERRETT D., FARTHING M.J. 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut. 1996;39:528–531. doi: 10.1136/gut.39.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNTSSON P., JOHANSSON E., WESTERLUND C. Felodipine analogs: structure-activity relationships. J. Cardiovasc. Pharmacol. 1987;10 suppl:60–65. doi: 10.1097/00005344-198710001-00011. [DOI] [PubMed] [Google Scholar]
- BEUBLER E., HORINA G. 5-HT2 and 5-HT3 receptor subtypes mediate cholera-toxin-induced intestinal fluid secretion in the rat. Gastroenterology. 1990;99:83–89. doi: 10.1016/0016-5085(90)91233-v. [DOI] [PubMed] [Google Scholar]
- BEUBLER E., KOLLAR F., SARIA A., BUKHAVE K., RASK-MADSEN J. Involvement of 5-hydroxytryptamine, prostaglandin-E2, and cyclic adenosine monophosphate in cholera-toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989;96:368–376. doi: 10.1016/0016-5085(89)91560-6. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN J.C., COSTA M., FURNESS J.B. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J. Physiol. 1986;381:465–482. doi: 10.1113/jphysiol.1986.sp016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSTEIN J.C., COSTA M., FURNESS J.B. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J. Physiol. 1988;398:371–390. doi: 10.1113/jphysiol.1988.sp017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSTEIN J.C., FURNESS J.B., KUNZE W.A.A. Electrophysiological characterization of myenteric neurons: how do classification schemes relate. J. Auton. Nerv. Syst. 1994;48:1–17. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- BRUNSSON I. Enteric nerves mediate the fluid secretory response due to Salmonella typhimurium R5 infection in the rat small intestine. Acta Physiol. Scand. 1987;131:609–617. doi: 10.1111/j.1748-1716.1987.tb08282.x. [DOI] [PubMed] [Google Scholar]
- CASSUTO J., JODAL M., TUTTLE R., LUNDGREN O. On the role of intramural nerves in the pathogenesis of cholera toxin-induced intestinal secretion. Scand. J. Gastroenterol. 1981;16:377–384. doi: 10.3109/00365528109181984. [DOI] [PubMed] [Google Scholar]
- CASSUTO J., JODAL M., TUTTLE R., LUNDGREN O. 5-Hydroxytryptamine and cholera secretion. Scand. J. Gastroenterol. 1982;17:695–703. doi: 10.3109/00365528209181081. [DOI] [PubMed] [Google Scholar]
- CASSUTO J., SIEWERT A., JODAL M., LUNDGREN O. The involvement of intramural nerves in cholera toxin induced intestinal secretion. Acta Physiol. Scand. 1983;117:195–202. doi: 10.1111/j.1748-1716.1983.tb07197.x. [DOI] [PubMed] [Google Scholar]
- CASTAGLIUOLO I., LAMONT J.T., LETOURNEAU R., KELLY C., O'KEANE J.C., JAFFER A., THEOHARIDES T.C., POTHOULAKIS C. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology. 1994;107:657–665. doi: 10.1016/0016-5085(94)90112-0. [DOI] [PubMed] [Google Scholar]
- EHERER A.J., HINTERLEITNER T.A., PETRITSCH W., HOLZER-PETSCHE U., BEUBLER E., KREJS G.J. Effect of 5-hydroxytryptamine antagonists on cholera toxin-induced secretion in the human jejunum. Eur. J. Clin. Invest. 1994;24:664–668. doi: 10.1111/j.1365-2362.1994.tb01058.x. [DOI] [PubMed] [Google Scholar]
- EKLUND S., KARLSTRÖM L., RÖKAEUS A., THEODORSSON E., JODAL M., LUNDGREN O. Effects of cholera toxin, Escherichia coli heat stable toxin and sodium deoxycholate on neurotensin release from the ileum in vivo. Regul. Pept. 1989;26:241–252. doi: 10.1016/0167-0115(89)90192-4. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B., COSTA M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric system: their projections in the guinea-pig small intesteine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- GELBMANN C.M., SCHTEINGART C.D., THOMPSON S.M., HOFMANN A.F., BARRETT K.E. Mast cells and histamine contribute to bile acid-stimulated secretion in the mouse colon. J. Clin. Invest. 1995;95:2831–2839. doi: 10.1172/JCI117988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGLUND U., LUNDGREN O. Reactions within consecutive vascular sections of the small intestine of the cat during prolonged hypotension. Acta Physiol. Scand. 1972;84:151–163. doi: 10.1111/j.1748-1716.1972.tb05166.x. [DOI] [PubMed] [Google Scholar]
- JAUP E.A., TIMAR PEREGRIN A., JODAL M., LUNDGREN O. Nervous control of alkaline secretion in the duodenum as studied by the use of cholera toxin in the anaesthetized rat. Acta Physiol. Scand. 1998;162:165–174. doi: 10.1046/j.1365-201X.1998.0290f.x. [DOI] [PubMed] [Google Scholar]
- JODAL M., HOLMGREN S., LUNDGREN O., SJÖQVIST A. Involvement of the myenteric plexus in the cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology. 1993a;105:1283–1293. [Google Scholar]
- JODAL M., LUNDGREN O.Neural reflex modulation of intestinal epithelial transport Regulatory mechanisms in gastrointestinal function 1995Boca Raton: CRC Press; 99–144.ed. T.S. Gaginella. pp [Google Scholar]
- JODAL M., WINGREN U., JANSSON M., HEIDEMANN M., LUNDGREN O. Nerve involvement in fluid transport in the inflamed rat jejunum. Gut. 1993b;34:1526–1530. doi: 10.1136/gut.34.11.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUORIO A.V., GABELLA G. Noradrenaline in the guinea pig alimentary canal: regional distribution and sensitivity to denervation and reserpine. J. Neurochem. 1974;22:851–858. doi: 10.1111/j.1471-4159.1974.tb04304.x. [DOI] [PubMed] [Google Scholar]
- KARLSTRÖM L. Mechanisms in bile salt-induced secretion in the small intestine of the rat. Acta Physiol. Scand. 1986a;126 suppl:549. [PubMed] [Google Scholar]
- KARLSTRÖM L. Evidence of involvement of the enteric nervous system in the effects of sodium deoxycholate on small intestinal transepithelial fluid transport and motility. Scand. J. Gastroenterol. 1986b;21:321–330. doi: 10.3109/00365528609003082. [DOI] [PubMed] [Google Scholar]
- KARLSTRÖM L., CASSUTO J., JODAL M., LUNDGREN O. The importance of the enteric nervous system for the bile salt-induced secretion in the small intestine of the rat. Scand. J. Gastroenterol. 1983;18:117–123. doi: 10.3109/00365528309181570. [DOI] [PubMed] [Google Scholar]
- KARLSTRÖM L., CASSUTO J., JODAL M., LUNDGREN O. Involvement of the enteric nervous system in the intestinal secretion induced by sodium deoxycholate and sodium ricinoleate. Scand. J. Gastroenterol. 1986a;21:331–340. doi: 10.3109/00365528609003083. [DOI] [PubMed] [Google Scholar]
- KARLSTRÖM L., JODAL M., LUNDGREN O. Blood flow distribution, lymph flow, villus tissue osmolality and fluid and electrolyte transport after exposing the cat small intestine to sodium deoxycholate. Acta Physiol. Scand. 1986b;128:83–96. doi: 10.1111/j.1748-1716.1986.tb07953.x. [DOI] [PubMed] [Google Scholar]
- KIRCHGESSNER A.L., TAMIR H., GERSHON M.D. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: Activity-induced expression of fos immunoreactivity. J. Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDGREN O., SVANVIK J. Mucosal hemodynamics in the small intestine of the cat during reduced perfusion pressure. Acta Physiol. Scand. 1973;88:551–563. doi: 10.1111/j.1748-1716.1973.tb05484.x. [DOI] [PubMed] [Google Scholar]
- NELLGÅRD P., JONSSON A., BOJO L., TARNOW P., CASSUTO J. Small-bowel obstruction and the effects of lidocaine, atropine and hexamethonium on inflammation and fluid losses [see comments] Acta Anaesthesiol. Scand. 1996;40:287–292. doi: 10.1111/j.1399-6576.1996.tb04435.x. [DOI] [PubMed] [Google Scholar]
- NILSSON O., CASSUTO J., LARSSON P.-A., JODAL M., LIEDBERG P., AHLMAN H., DAHLSTRÖM A., LUNDGREN O. 5-hydroxytryptamine and cholera secretion: a histochemical and physiological study in cats. Gut. 1983;24:542–548. doi: 10.1136/gut.24.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSAKA M., FUJITA T., YANATORI Y. On the possible role of intestinal hormones as the diarrhoeagenic messenger in cholera. Virchows Arch. B. Cell Pathol. 1975;18:287–296. doi: 10.1007/BF02889255. [DOI] [PubMed] [Google Scholar]
- PENTTILÄ A. The effect of reserpine on the regional 5-hydroxytryptamine content of the rat gastrointestinal tract. Ann. Med. Exp. Fenn. 1969;47:99–104. [PubMed] [Google Scholar]
- PETERSON J.W., CANTU J., DUNCAN ., CHOPRA A.K. Molecular mediators formed in the small intestine in response to cholera toxin. J. Diarrhoeal Dis. Res. 1993;11:227–234. [PubMed] [Google Scholar]
- PETERSON J.W., WHIPP S.C. Comparison of the mechanisms of action of cholera toxin and the heat-stable enterotoxins of Escherichia coli. Infect. Immun. 1995;63:1452–1461. doi: 10.1128/iai.63.4.1452-1461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJÖQVIST A., CASSUTO J., JODAL M., LUNDGREN O. Actions of serotonin antagonists on cholera-toxin-induced intestinal fluid secretion. Acta Physiol. Scand. 1992;145:229–237. doi: 10.1111/j.1748-1716.1992.tb09360.x. [DOI] [PubMed] [Google Scholar]
- TIMAR PEREGRIN A., AHLMAN H., JODAL M., LUNDGREN O. Effects of calcium channel blockade on intestinal fluid secretion: sites of action. Acta Physiol. Scand. 1997a;160:379–386. doi: 10.1046/j.1365-201X.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- TIMAR PEREGRIN A., SVENSSON M., JODAL M., LUNDGREN O. Calcium channels and intestinal fluid secretion. Acta Physiol. Scand. 1997b;160:371–378. doi: 10.1046/j.1365-201X.1997.00174.x. [DOI] [PubMed] [Google Scholar]
- TURVILL J.L., FARTHING M.J. Effect of granisetron on cholera toxin-induced secretion. Lancet. 1997;349:1293. doi: 10.1016/S0140-6736(97)24018-3. [DOI] [PubMed] [Google Scholar]
- WINGREN U., ENERBÄCK L., AHLMAN H., ALLENMARK S., DAHLSTRÖM A. Amines of the mucosal mast cell of the gut in normal and nematode infected rats. Histochemistry. 1983;77:145–158. doi: 10.1007/BF00506557. [DOI] [PubMed] [Google Scholar]


