Abstract
The effects of spermine and methoctramine, a selective M2 muscarinic receptor antagonist, were studied on the high-affinity GTPase activity of G proteins, and on ligand binding to M2 muscarinic receptors in pig heart sarcolemma.
The spontaneous GTP hydrolysis by pig heart sarcolemma and its stimulation by mastoparan or carbachol were prevented by pertussis toxin and inhibited by methoctramine (IC50s: 21, 13 and 0.005 μM, respectively), and spermine (IC50s: 967, 278 and 11 μM). Spermine and methoctramine also inhibited spontaneous GTP hydrolysis by rat peritoneal mast cell membranes which do not respond to carbachol.
The neutral muscarinic antagonists, AF-DX 116 and atropine, did not modify the inhibitory effect of high concentrations of methoctramine, indicating that this effect was not related to the antagonist binding site of muscarinic receptors. We suggest that methoctramine behaves as a receptor antagonist at nanomolar concentrations and interacts with G proteins at micromolar concentrations.
Spermine did not modify the binding of the tritiated muscarinic antagonist [3H]-NMS, but decreased the binding of the agonist [3H]-Oxo-M. Spermine elicited a rightward shift of the carbachol/[3H]-NMS binding isotherm with a decrease in the proportion of sites with high-affinity for carbachol, suggesting that polyamines uncouple Gi proteins from receptors.
The inhibition of GTPase activity by polyamines, preventing the re-association of α and βγ subunits of Gi proteins, might sustain the regulatory effect of Gi subunits on downstream effectors. The level of intracellular polyamines might be important for the control of the transduction of extracellular signals through Gi protein-coupled receptors.
Keywords: G protein, GTPase activity, mastoparan, methoctramine, muscarinic M2 acetylcholine receptor, spermine, rat mast cells, pig sarcolemma, polyamines
Introduction
The three polyamines, spermine, spermidine and putrescine are found in every eukaryotic cell up to millimolar concentrations (Seiler, 1991). Their first recognized physiological function has been their requirement for proliferative processes (Fozard et al., 1980). In biochemical studies, polyamines have been shown to modulate many enzymatic systems, but little physiological correlation has been demonstrated (Seiler, 1991; Seiler et al., 1996). The polycationic property of polyamines led to the assumption that they could affect physiological systems by binding to anionic sites of membranes and nucleic acids (Schuber, 1989) and by modulating ion channels (Scott et al., 1993). The most widely studied effects of polyamines with physiological relevance has been the modulation (inhibition or stimulation) of NMDA receptors and of inwardly rectifying potassium channels (KIR) (Johnson, 1996, for review).
Only limited experimental evidence has been obtained to suggest a role for intracellular polyamines in the control of the transduction of extracellular signals through membrane receptors. Natural polyamines have been shown to decrease the basal and PGE1-stimulated adenylyl cyclase activity of cultured cardiac cells (Clo et al., 1988; Pignatti et al., 1990). The inhibition of basal adenylyl cyclase activity by polyamines has also been observed in erythrocyte membranes (Khan et al., 1990). Polyamines also induced phospholipase C activation in rat peritoneal mast cells, leading to histamine secretion (Bueb et al., 1991; 1992). The effects of polyamines on cardiomyocytes and mast cells were completely prevented by pretreating cells with pertussis toxin, which is known to ADP-ribosylate Gi and Go proteins. These observations suggested that the modulation of the activity of adenylyl cyclase or phospholipase C by polyamines was related to the interaction of polyamines with receptors or G proteins, rather than with enzymatic effectors.
Interestingly, receptor-independent effects have been reported for the synthetic polyamine, methoctramine. This polymethylene tetramine is a highly potent and selective antagonist at M2 muscarinic acetylcholine receptors (Melchiorre et al., 1987). Lee et al. (1989) showed that methoctramine, at concentrations over 5 μM, is a powerful activator of phosphoinositide hydrolysis in dissociated rat cerebral cortex. Muscarinic receptor antagonists were unable to block methoctramine-stimulated phosphoinositide hydrolysis but the underlying mechanism was not determined. Methoctramine also induced concentration-dependent (0.003–1 mM) histamine release and phosphoinositide breakdown from rat peritoneal mast cells which do not express muscarinic receptors (Chahdi et al., 1998a). The effects of methoctramine on rat cerebral cortex and peritoneal mast cells were prevented by pertussis toxin-pretreatment indicating the possible involvement of G proteins. These observations suggested that the receptor-independent effects of methoctramine might be related to its polyamine structure and might involve the regulation of G protein activity.
The aim of the present paper was to study the effect of spermine and methoctramine on pertussis toxin-sensitive G proteins. Pig atrial sarcolemma offered the opportunity to study the spontaneous GTPase activity of this class of G protein constitutively coupled with muscarinic M2 receptors in the absence of agonist (Hilf & Jakobs, 1992; Daeffler et al., 1999). This model also allows us to activate the G protein cycle with muscarinic agonists or with mastoparan, a peptide interacting with G proteins and mimicking agonist-liganded receptors (Higashijima et al., 1988). Alternatively, rat peritoneal mast cells were also used as these cells do not express muscarinic receptors, but contain Gi proteins involved in their secretory response to peptides and polyamines (Bueb et al., 1990; Aridor et al., 1993).
Methods
Preparation of membranes
Pig atrial sarcolemma were prepared according to the method of Peterson & Schimerlik (1984) as described previously (Daeffler et al., 1999). Membranes from bovine and rat brain cortex, were prepared similarly. Peritoneal rat mast cells were collected and purified as previously described (Chahdi et al., 1998c). Cells from 20 rats were pooled (17–20×106; purity >95%), disrupted by sonication and centrifuged at 4°C for 8 min at 1000×g. The supernatant was centrifuged for 40 min at 40,000×g and the final membrane pellet was suspended in triethanolamine hydrochloride 50 mM-NaOH pH 7.4, including (in mM): ATP 1, EDTA 0.1, dithiothreitol 1, NaCl 150 and MgCl2 1.
GTPase activity measurements
GTPase activity was determined by a method adapted from Hilf & Jakobs (1989) in 50 mM triethanolamine hydrochloride-NaOH, pH 7.4, including (in mM): NaCl 150, ATP 1, EDTA 0.1, dithiothreitol 1 and MgCl2 1. Samples of membranes (10 μg of protein) were first preincubated for 30 min at 25°C with drugs. The reaction was started by addition of 20 μl [γ-32P]-GTP (30 Ci mmol−1) to the samples, so as to reach a final concentration of 0.1 μM in a final volume of 100 μl. Alternatively this concentration was varied from 0.03 to 0.8 μM to determine KM and Vmax. GTP hydrolysis was stopped after 15 min of incubation at 25°C, by the addition of 0.7 ml of an ice-cold 5% (w v−1) charcoal suspension in 50 mM KH2PO4-HCl, pH 7.4. The mixture was centrifuged at 800×g for 20 min at 4°C. A fraction of the supernatant (0.4 ml) was put into counting vials containing 3.6 ml scintillation solution. The high affinity GTPase activity was calculated by subtracting the 32Pi released in the presence of 50 μM unlabelled GTP, from total 32Pi accumulation (Cassel & Selinger, 1976; Hilf & Jakobs, 1989). Spontaneous high-affinity GTPase activity was determined as the activity measured in the absence of ligands.
Pertussis toxin-catalyzed ADP-ribosylation of the sarcolemma membranes
Pertussis toxin was preactivated for 30 min at 37°C in 100 μl of 50 mM Tris-HCl, pH 7.5 including 5 mM dithiothreitol. Pig atrial sarcolemma (0.8 mg) was resuspended after centrifugation at 4°C for 20 min at 44,000×g, in 900 μl of ADP-ribosylation buffer 50 mM Tris-HC, pH 7.5, including (in mM): thymidine 10, EDTA 1, MgCl2 5, ATP 0.4, GTP 0.4 and NAD+ 0.5. Preactivated pertussis toxin solution was then added to each 900 μl membrane suspension, to reach a final concentration of 10, 20 or 40 μg ml−1, and incubated for 2 h at 30°C. The reaction was stopped by addition of 1 ml ice-cold 50 mM triethanolamine pH 7.4 including (in mM): NaCl 150, ATP 1, EDTA 0.1, dithiothreitol 1 and MgCl2 1. The membrane suspension was centrifuged at 4°C for 20 min at 44,000×g. The pellets were washed twice to remove toxin, and resuspended with a glass-teflon tissue homogenizer in the same buffer and used immediately for GTPase activity determination.
SDS–PAGE and immunoblotting
Electrophoresis was performed using a minigel system (Mini-PROTEAN II; Bio-Rad, Ivry sur Seine, France) in discontinuous gel system described by Laemmli (1970) with minor modifications. Briefly, stacking and resolving gels contained 4 and 12% acrylamide, respectively. Membrane samples (10 μg protein) were solubilized by boiling for 5 min in Laemmli's sample buffer containing 350 mM dithiothreitol. Gels were run at 200 V until the dye front reached the bottom of the resolving gel (about 2 h). Electrophoretic transfer of resolved proteins to nitrocellulose membranes (Hybond ECL, Amersham, U.K.) was carried out for 2 h at 100 V using a mini Trans-Blot transfert cell (Bio-Rad). Before exposure to antibodies, nitrocellulose membranes were saturated by incubation overnight in a blocking solution containing 100 mM NaCl, casein 0.1% (w v−1). Nitrocellulose membranes were then incubated for 1 h with primary rabbit polyclonal (Gi3α) or mouse monoclonal (Gi1α, Gi2α and Goα) antibodies at a final dilution of 1/2000, 1/2000, 1/3000 and 1/3000, respectively. The nitrocellulose membrane was finally, after incubation with secondary antibodies for 1 h, incubated for 2 min in ECL reagents (Amersham, U.K.). The bound antibodies were visualized by autoradiography for 2 min with Kodak X-OMAT films.
Radioligand binding studies
Competition experiments, were carried out with 2 nM [3H]-oxotremorine-M ([3H]-Oxo-M, 86.6 Ci mmol−1) and with 0.2 nM [3H]-N-methylscopolamine ([3H]-NMS, 80.4 Ci mmol−1). All binding studies were carried out at 25°C in 10 mM Tris-HCl, pH 7.4 including 150 mM NaCl and 1 mM MgCl2. Incubation mixture (0.5 ml) contained 20 and 40 μg of protein, for [3H]-NMS and [3H]-Oxo-M binding, respectively. Samples were incubated for 1 h to reach the equilibrium. Non-specific binding was determined in the presence of 1 μM atropine (4–5% of total binding) as described previously (Daeffler et al., 1999). Saturation experiments with the radiolabelled ligands demonstrated saturability in each condition used and Scatchard analysis of the saturation isotherms was used to calculate the density of binding sites (Bmax), 0.41±0.10 ([3H]-Oxo-M), 1.45±0.39 ([3H]-NMS) and 1.48±0.35 pmol. (mg of protein)−1 ([3H]-NMS+GppNHp) and dissociation constants (KD), 1.20±0.37, 0.24±0.08 and 0.16±0.06 nM, respectively (means±s.e.mean, n=3).
Data analysis
Experimental data for the saturation and the inhibition binding studies were analysed using the non-linear regression analysis described by Munson & Rodbard (1980) (LIGAND program, Elsevier-Biosoft, Cambridge, U.K.) to yield equilibrium dissociation constants and receptor densities. The precision of the fit to a one- or two-site model was determined with an F test (P<0.01), by comparing the residual sum of squares for fitting data to a one- or two-site model. Data were weighted with the reciprocal of the variance. Ki values were calculated from IC50s according to Cheng & Prusoff (1973) for competitive interactions, taking into account the concentration and the KD of tritiated ligands. Values for binding experiments are expressed as geometric means±s.e.mean to decrease the errors associated with estimation of the means from logarithmic curves (Fleming et al., 1972).
Materials
Atropine, carbachol, spermine, Tween 20 and guanylylimidodiphosphate (GppNHp) were purchased from Sigma Chemical (St. Louis, MO, U.S.A). Methoctramine and pirenzepine were obtained from RBI (Natick, U.S.A.). AF-DX 116 (11-[[2-[(diethyl-amino)-methyl]-1-piperidinyl] acety]-5,11-dihydro-6H-pyrido[2,3-b] [1,4] benzodiazepin-6-one) was a gift from Boehringer-Ingelheim (Ingelheim, Germany). The substance-P related peptide GPAnt-2 also referred to as [P-Glu5,D-Trp7,9,10]-substance P5–11, (pGlu-Gln-D-Trp-Phe-D-Trp-D-Trp-Met-NH2) was purchased from Bachem (Paris, France) and mastoparan from Neosystem (Strasbourg, France). [3H]N-methylscopolamine ([3H]-NMS, 80.4 Ci mmol−1), [3H]-oxotremorine-M ([3H]-Oxo-M, 86.6 Ci mmol−1), and [γ-32P]-GTP (30 Ci mmol−1) were purchased from New England Nuclear (Boston, MA, U.S.A.). Glass fibre filters (GF/C) were obtained from Whatmann (Clifton, U.S.A.). Casein was purchased from Tropix (Bedford, MA, U.S.A.). Acrylamide, bis-acrylamide, Laemmli's sample buffer and horseradish peroxidase-conjugated goat anti-rabbit IgG antiserum were from Bio-Rad (Ivry sur Seine, France). Horseradish peroxidase-conjugated anti-mouse IgG antiserum was from Jackson ImmunoResearch (West Grove, U.S.A.). Nitrocellulose (Hybond ECL) membranes and ECL detection reagents were obtained from Amersham (U.K.). Anti-Giα3 subunit, rabbit polyclonal antibody was purchased from Upstate (Lake Placid, U.S.A.). Anti-Giα1, -Giα2, -Goα mouse monoclonal antibodies were obtained from Chemicon (Temecula, U.S.A.). All drugs were prepared in the different buffers described above, with the exception of AF-DX 116 and GPAnt-2 which were dissolved in 0.1 N HCl and 80% CH3COOH, respectively, and diluted with buffers described above.
Results
Inhibition of high-affinity GTPase activity by spermine and methoctramine
Figure 1 shows that the muscarinic receptor agonist, carbachol, and the G protein ligand, mastoparan, increased the spontaneous GTP hydrolysis by pig atrial sarcolemma at concentrations corresponding to their affinity for their respective targets. Carbachol and mastoparan did not modify KM for GTP, but increased Vmax (Table 1). The spontaneous and stimulated GTP hydrolysis were prevented by pertussis toxin pretreatment (Table 2). The neutral muscarinic antagonists, AF-DX 116 and atropine did not modify the spontaneous GTPase activity (Figure 1). Unexpectedly, the M2 selective antagonist, methoctramine, inhibited the spontaneous GTP hydrolysis (Figure 1), with an increase in KM for GTP and a decrease in Vmax (Table 1). The active concentrations of methoctramine ranged from micro to millimolar levels. Millimolar concentrations of the natural polyamine, spermine, also inhibited the spontaneous GTP hydrolysis by pig atrial sarcolemma. GPAnt-2, a substance P analogue which prevents the muscarinic receptor-G protein interaction (Mukai et al., 1992), also inhibited GTP hydrolysis. This effect was related to a decreased Vmax, with no modification in KM for GTP (Table 1). The non-hydrolysable analogue of GTP, GppNHp, increased the KM value without modifying Vmax. The inverse agonist of muscarinic M2 receptors, pirenzepine (Daeffler et al., 1999), also decreased Vmax for GTP, but did not modify KM (Table 1). In contrast to pirenzepine, the inhibitory effect of methoctramine on the spontaneous GTP hydrolysis was not inhibited by the neutral antagonists, AF-DX 116 and atropine (Figure 2).
Figure 1.
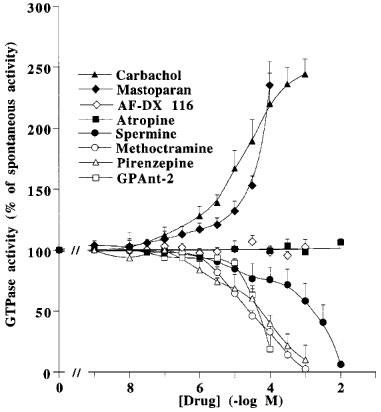
Modulation of high affinity GTPase activity of pig atrial sarcolemma by various drugs. Spontaneous high-affinity GTPase activity (100%) was 925±155 fmol Pi. min−1. pmole of receptor−1. Values are geometric means±s.e.mean of three independent experiments.
Table 1.
Michaelis-Menten constants, KM and Vmax, for high affinity GTPase activity of pig atrial sarcolemma, in the presence of different drugs
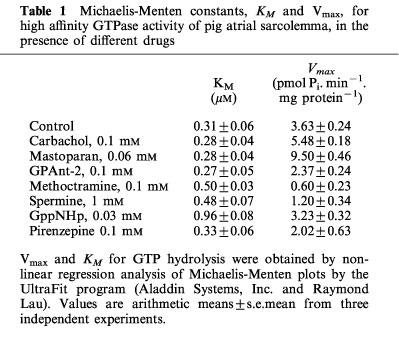
Table 2.
Modulation by pertussis toxin-pretreatment of the high-affinity GTPase activity of pig atrial sarcolemma
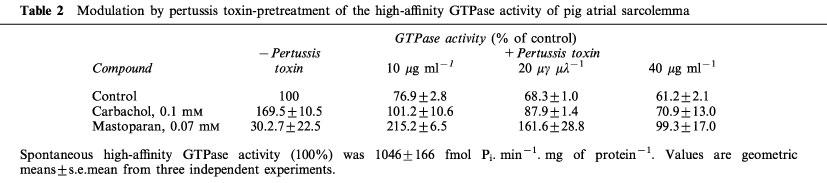
Figure 2.
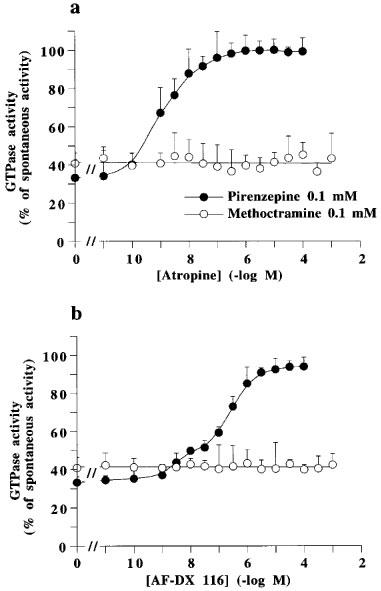
Concentration-dependent effect of atropine and AF-DX 116 on the high affinity GTPase activity of pig atrial sarcolemma observed in the presence of 0.1 mM methoctramine or pirenzepine. Spontaneous high affinity GTPase activity (100%) was 596±77 fmol Pi. min−1. mg of protein−1. In the presence of methoctramine or pirenzepine, the GTPase activity was 243±37 or 196±25 fmol Pi. min−1. mg of protein−1, respectively. Results are geometric means±s.e.mean from four experiments.
We also studied the effect of these various drugs on the rates of GTP hydrolysis stimulated by carbachol or mastoparan. AF-DX 116 (Figure 3b) and atropine (not shown) did not modify mastoparan-induced GTP hydrolysis. AF-DX 116 and methoctramine inhibited the carbachol-induced effect, at concentrations in the range of their known antagonist activity at muscarinic receptors (Figure 3a, Table 3). Methoctramine also inhibited mastoparan-induced GTP hydrolysis, but this effect was observed only at millimolar concentrations (Figure 3b), i.e. similar to that required for the inhibition of spontaneous GTP hydrolysis (Table 3). Spermine inhibited the carbachol-induced GTPase activity in the micromolar range of concentrations, but higher concentrations of spermine were required to inhibit the spontaneous or mastoparan-induced GTPase activity. GPAnt-2 similarly inhibited both spontaneous and induced activities (Figure 3, Table 3).
Figure 3.
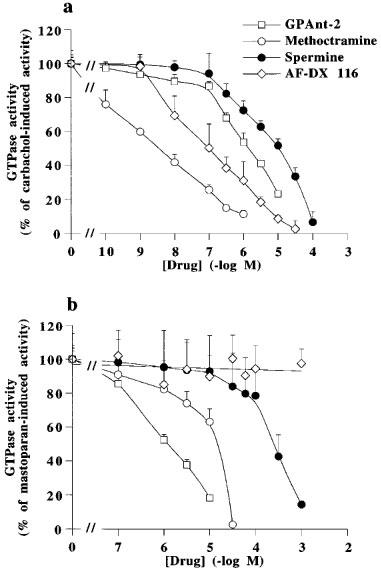
Concentration-dependent effect of methoctramine, spermine, GPAnt-2 and AF-DX 116 on 0.1 mM carbachol- (a) or 0.06 mM mastoparan-stimulated (b) high affinity GTPase activity of pig atrial sarcolemma. Spontaneous GTPase activity was 722±84 fmol Pi. min−1. mg of protein−1. Carbachol- and mastoparan-induced activities (100%) reached 1432±109 and 1408±109 fmol Pi. min−1. mg of protein−1, respectively. Results are geometric means±s.e.mean from three independent experiments.
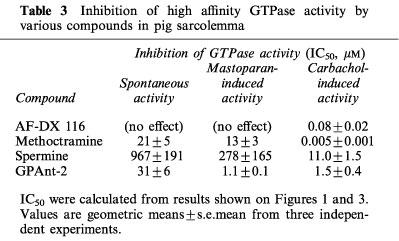
Table 3.
Inhibition of high affinity GTPase activity by various compounds in pig sarcolemma
Tissue selectivity of the effect of spermine and methoctramine
Figure 4 shows that carbachol, unlike mastoparan, did not modify the spontaneous rate of GTP hydrolysis in membranes from rat peritoneal mast cells. Spermine and methoctramine inhibited spontaneous GTP hydrolysis by mast cell membranes with IC50s of 141.3±9.2 and 23.1±4.8 μM, respectively, i.e. similar to those observed for pig atrial sarcolemma (Table 3). Comparative experiments were also performed with rat and bovine brain membranes (Table 4). Spermine (10 mM) inhibited totally the spontaneous GTP hydrolysis in rat mast cell membranes, about 94% in pig atrial membranes, 44% in rat brain, and only 20% in bovine brain membranes. Similar results were observed in the presence of 1 mM methoctramine (Table 4). The various pertussis toxin-sensitive G proteins present in these membrane preparations were characterized by immunoblotting detection of their alpha subunits in membrane samples containing 10 μg of protein per lane (Figure 5). Gi1α was not rervealed in rat mast cell membranes, but was observed in pig atria, rat and bovine brain membranes. Gi2α was mainly revealed in pig atria with traces in the other tissues. Gi3α was observed in all membrane preparations. Goα was revealed mainly in rat and bovine brain. A lower amount of Goα was observed in pig atria, but Goα was not detected in rat mast cell membranes.
Figure 4.
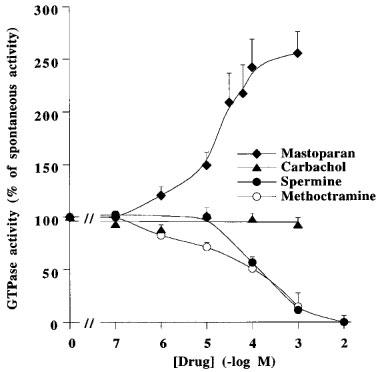
Modulation of high affinity GTPase activity of rat peritoneal mast cells by various drugs. Spontaneous GTPase activity (100%) was 425±32 fmol Pi. min−1. mg of protein−1. Values are geometric means±s.e.mean from three independent experiments.
Table 4.
Inhibitory activities of spermine and methoctramine on high affinity GTPase activity of various membranes
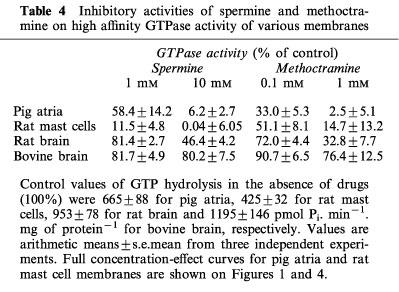
Figure 5.
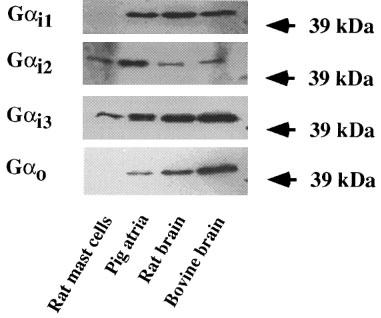
Immunoblot analysis of the expression of Giα and Goα proteins in various membrane preparations. Results shown are representative of three independent experiments.
Displacement of tritiated muscarinic agonist and antagonist binding by spermine and methoctramine
Figure 6 shows binding curves for increasing concentrations of methotramine and spermine performed with the agonist [3H]-Oxo-M and with the antagonist [3H]-NMS. Methoctramine fully displaced the specific binding of both radioligands. However, the apparent affinity of methoctramine for [3H]-NMS binding sites was higher than its affinity for [3H]-Oxo-M sites (Ki, 0.97±0.4 and 2.0±0.3 nM) with Hill coefficients (nH) of 0.97±0.03 and 1.01±0.04, respectively. Spermine, up to 1 mM, did not modify [3H]-NMS binding, but partly decreased, from 0.1 μM, the specific binding of [3H]-Oxo-M. Controls were performed with mastoparan and GPAnt-2 (Figure 7). Both G protein ligands also decreased [3H]-Oxo-M specific binding without affecting [3H]-NMS binding. Figure 8 shows that spermine induced a concentration-dependent rightward shift of the carbachol/[3H]-NMS specific binding isotherm. The Hill coefficient for carbachol displacing [3H]-NMS binding was significantly different from unity and was best fitted by a two-site binding model in each case (Table 5). The righward shift induced by spermine was accompanied by a decrease in the proportion of high affinity binding sites (Rh) (Table 5).
Figure 6.
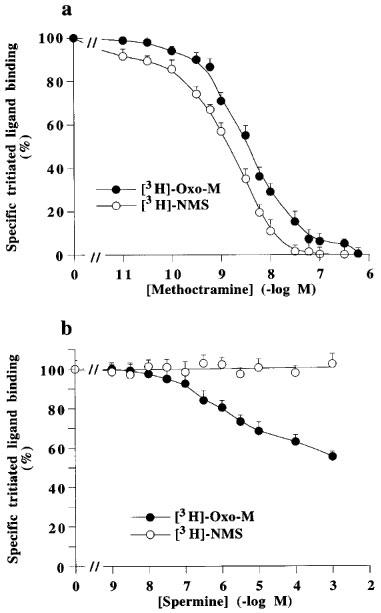
Inhibition of specific binding of [3H]-Oxo-M and [3H]-NMS on pig atrial sarcolemma by methoctramine (a) or spermine (b). The binding of [3H]-NMS was performed in the presence of 100 μM GppNHp. Total specific binding in the absence of drugs (100%) correspond to 720±70 ([3H]-Oxo-M) and 1627±131 ([3H]-NMS) d.p.m. per assay. Values are geometric means±s.e.mean from three independent experiments.
Figure 7.
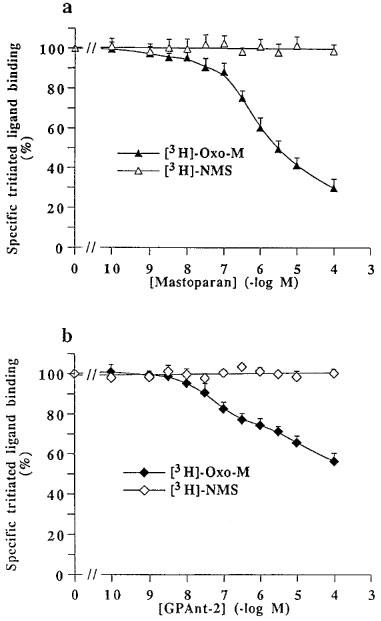
Inhibition of specific binding of [3H]-oxotremorine-M and [3H]-N-methylscopolamine on pig atrial sarcolemma by mastoparan (a) or GPAnt-2 (b). Total specific bindings in the absence of drugs (100%) corresponded to 716±66 ([3H]-Oxo-M) and 1690±176 ([3H]-NMS) d.p.m. per assay. Values are geometric means±s.e.mean of three independent experiments.
Figure 8.
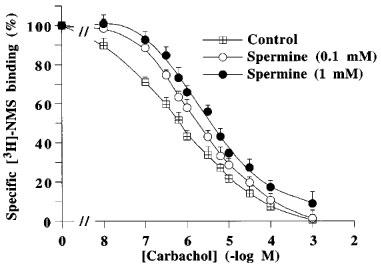
Inhibition of specific [3H]-N-methylscopolamine binding to pig atrial sarcolemma by carbachol in the absence (control) or presence of 1 or 10 mM spermine. Values are geometric means±s.e.mean of three experiments performed in triplicate.
Table 5.
Two-site analysis of the inhibition of [3H]-NMS binding by carbachol in the presence of spermine in pig atria sarcolemma shown in Figure 8

Discussion
The function of heterotrimeric G proteins is regulated cyclically by exchange of GDP to GTP on the α subunit, dissociation of α and βγ subunits leading to the activation of effectors and hydrolysis of GTP to GDP allowing the reassociation of α and βγ subunits (Gilman, 1987).
The effect of polyamines on Gi and Go proteins
The present results show that spermine inhibits the high-affinity and pertussis toxin-sensitive GTPase activity of membrane preparations from pig atria and rat mast cells (Figures 1 and 4). These properties indicate that the GTP hydrolysis measured in these preparations is relevant, at least partly, of Gi and/or Go proteins, considering the property of pertussis toxin to uncouple receptors from G proteins through ADP-ribosylation of a cysteine residue of the C terminus of Gi and Go (Gilman, 1987, for review). Go was not detected in mast cell membranes which contained Gi3 and Gi2, confirming previous observations (Aridor et al., 1993). In pig atria sarcolemma, Go was detected besides Gi1, Gi2 and Gi3. This indicates that the GTP hydrolysis measured was entirely (mast cells) or predominantly (atria) relevant of Gi proteins. Thus, the inhibitory effect of spermine for GTP hydrolysis by G proteins can be ascribed to the Gi subfamily.
We observed previously that natural polyamines increase GTP hydrolysis by G proteins purified from bovine cortex containing mostly Go (Bueb et al., 1991; 1992). In line with this observation, we show here (Table 4) that concentrations of spermine which fully inhibited GTP hydrolysis in mast cells or atria membranes, decreased only partially the hydrolysis in brain membranes characterized by presence of Go (Figure 5). The maximal inhibitory effect of polyamines in membranes might be limited by their Go content. Different effects of drugs on G protein subfamilies has been previously observed (Chahdi et al., 1998b, for review). For instance, mellitin activates Gi and Go proteins (Higashijima et al., 1990), but inhibits Gs (Fukushima et al., 1998).
Alterations of effector activity, a common consequence of the activation of the G protein cycle and of the inhibition of GTPase activity
We show here that mastoparan stimulates the GTPase activity of Gi proteins, whereas spermine and methoctramine inhibit this activity. Both types of drug activate phospholipase C promoting exocytosis in mast cells (Mousli et al., 1990a; Bueb et al., 1992; Chahdi et al., 1998a). These properties of spermine and methoctramine are shared with non-hydrolyzable analogues of GTP which also activate phospholipase C and trigger secretion when introduced into mast cells (Cockcroft & Gomperts, 1985; Howell et al., 1987). Thus, two groups of mast cell ‘triggers' activating phospholipase C can be distinguished. Activators of the Gi protein cycle include mastoparan and putatively other cationic peptides (Mousli et al., 1990b). Inhibitors of GTPase activity comprise non-hydrolyzable analogues of GTP, natural polyamines and methoctramine.
Both the C- and the N-termini of Gα proteins have been proposed to contribute to the binding site of cationic peptides (Mousli et al., 1990a; Higashijima & Ross, 1991; Conklin & Bourne, 1993; Chahdi et al., 1998b). Concerning inhibitors, non-hydrolysable analogues of GTP such as GppNHp, are considered to compete for GTP binding as confirmed here by the increase of KM with no modification of Vmax (Table 1). In contrast, spermine and methoctramine modified both Vmax and KM for GTP, suggesting some allosteric process leading to the inhibition of GTP hydrolysis. Further studies are required to locate their putative binding sites. Nevertheless, the present results show that the opposite modulation of the GTPase activity of Gi proteins can correlate with the activation of phospholipase C. Similarly, the activation of adenylyl cyclase or cyclic GMP-phosphodiesterase can be achieved both through the receptor-dependent activation of the functional cycle of Gs and Gt, respectively, or through the inhibition of GTPase activity by cholera toxin which ADP-ribosylates Gs and Gt in the vicinity of their GTP binding site, promoting the dissociation of α and βγ subunits (Cassel & Selinger, 1997; Abood et al., 1982; Kahn & Gilman, 1984). We propose that the inhibition of GTPase activity of Gi by spermine and methoctramine, at high concentrations, prevent the reassociation of α and βγ subunits, maintaining the regulation of effectors by Gi subunits. This proposal fits with the activation of phospholipase C observed in brain cortical cells and in mast cells (Lee et al., 1989; Bueb et al., 1992; Chahdi et al., 1998a) and with the inhibition of adenylyl activity observed in cardiomyocytes and erythrocytes (Melchiorre et al., 1987; Clo et al., 1988; Pignatti et al., 1990; Khan et al., 1990), these two types of regulation involving βγ and α subunits, respectively (Clapham & Neer, 1997, for review).
The decreased affinity of muscarinic agonists, a consequence of the polyamine-G protein interaction
Binding experiments performed with β-adrenoceptor and muscarinic receptor ligands revealed that guanylyl nucleotides shifted the [3H] antagonist/agonists binding curves to lower affinities, with a decreased proportion of high-affinity binding sites for agonists (Maguire et al., 1976; Lefkowitz et al., 1976; Birdsall et al., 1978; Hulme et al, 1978). We observe here a guanylyl nucleotide-like effect for spermine (Figure 8, Table 5). The spermine-induced decrease in agonist affinity was observed directly in [3H]-Oxo-M binding assays (Figure 6). These results strongly suggest that spermine, by decreasing GTPase activity as observed with GppNHp, might uncouple Gi proteins from M2 muscarinic receptors, increasing the proportion of uncoupled receptors with low affinity for agonists (referred to as R; see for review Leff, 1995) and decreasing the proportion of M2 receptors spontaneously coupled to Gi with high affinity for agonists (referred to as R*).
The inhibitory effect of spermine on spontaneous and mastoparan-induced GTP hydrolysis was observed at millimolar concentrations (Figure 1, Table 3). The potency of spermine decreased to the micromolar concentration range when observed on carbachol-induced GTP hydrolysis (Figure 3, Table 3). These results agree with the proposal that spontaneous GTP hydrolysis is related to G proteins coupled to R* in the absence of agonist, and with the property of the mastoparan-G protein interaction to mimick the receptor-G protein interaction (Higashijima et al., 1988). The high potency of spermine to decrease the agonist-dependent GTPase activity might arise from the spermine-induced decrease of receptor affinity for agonists which abolishes the stimulation of the G protein cycle by the agonist-liganded receptor. Such a feedback effect could not arise for mastoparan-dependent and for spontaneous, R*-dependent, activations which are both agonist-independent, leading to a lower potency of spermine to decrease corresponding GTPase activities.
The dual effect of methoctranine
Nanomolar concentrations of methoctramine decreased the carbachol-dependent GTP hydrolysis (Figure 3, Table 3), and displaced [3H]-NMS binding (Figure 6), suggesting that the decrease of carbachol-dependent GTP hydrolysis was related to the antagonist activity of methoctramine at M2 receptors. The observation of an apparent higher affinity of methoctramine for [3H]-antagonist (NMS) sites compared to the [3H]-agonist (Oxo-M) sites, might suggest inverse agonist activity for methoctramine, as shown before for pirenzepine (Daeffler et al., 1999). However, the inhibitory effect of methoctramine on GTPase activity was not prevented by neutral muscarinic antagonists, (Figure 2), excluding this hypothesis.
Micromolar concentrations of methoctramine were required to inhibit spontaneous and mastoparan-induced GTP hydrolysis, both in pig atria and mast cell membranes, the latter lacking muscarinic receptors (Table 4). Receptor-independent, but pertussis toxin-sensitive activation of phosphoinositide hydrolysis by micromolar concentrations of methoctramine have been reported in dissociated rat cerebral cortex and peritoneal mast cells (Lee et al., 1989; Chahdi et al., 1998a). These observations can be explained by the above proposal that high concentrations of methoctramine prevent the reassociation of α and βγ subunits of Gi proteins allowing the activation of phospholipase C. It is conceivable that the interaction of methoctramine with G proteins decreases the affinity of the receptor for agonists, as proposed for spermine, lowering the apparent affinity for [3H]-Oxo-M binding sites compared to [3H]-NMS sites (Figure 6). Due to the potent antagonist activity of low concentrations of methoctramine at M2 receptors, its putative potency to induce, at high concentrations, a decrease of receptor affinity for agonists was not demonstrable through the shift of carbachol / [3H]-NMS curves as was observed for spermine (Figure 8).
In conclusion, spermine and methoctramine inhibit the GTPase activity of Gi proteins, preventing the reassociation of α and βγ subunits, maintaining the activation of effectors and decreasing the affinity of receptors for agonist. The effects of spermine were observed at concentrations within physiological limits for mammalian tissues (Seiler, 1991). The level of intracellular polyamines might be important for the regulation of the interaction of receptors and their related G proteins and for the modulation of downstream transduction events.
Abbreviations
- GPAnt-2 or [pGlu5,D-Trp7,9,10] SP5–11
pGlu-Gln-D-Trp-Phe-D-Trp-D-Trp-Met-NH2
- GppNHp
guanylyl-imido-diphosphate
- NMS
N-methylscopolamine
- Oxo-M
oxotremorine-M
References
- ABOOD M.E., HURLEY J.B., PAPPONE M.C., BOURNE H.R., STRYER L. Functional homology between signal-coupling proteins. Cholera toxin inactivates the GTPase activity of transducin. J. Biol. Chem. 1982;257:10540–10543. [PubMed] [Google Scholar]
- ARIDOR M., RAJMILEVICH G., BEAVEN M.A., SAGI-EISENBERG R. Activation of exocytosis by the heterotrimeric G protein G13. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- BIRDSALL N.J.M., BURGEN A.S.V., HULME E.C. The binding of agonists to brain muscarinic receptors. Mol. Pharmacol. 1978;14:723–736. [PubMed] [Google Scholar]
- BUEB J.L., DA SILVA A., MOUSLI M., LANDRY Y. Natural polyamines stimulate G proteins. Biochem. J. 1992;282:545–550. doi: 10.1042/bj2820545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUEB J.L., MOUSLI M., BRONNER C., ROUOT B., LANDRY Y. Activation of Gi-like, proteins, a receptor-independent effect of kinins in mast cells. Mol. Pharmacol. 1990;38:816–822. [PubMed] [Google Scholar]
- BUEB J.L., MOUSLI M., LANDRY Y. Molecular basis for cellular effects of naturally occuring polyamines. Agents Actions. 1991;33:84–87. doi: 10.1007/BF01993133. [DOI] [PubMed] [Google Scholar]
- CASSEL D., SELINGER Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim. Biophys. Acta. 1976;452:538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- CASSEL D., SELINGER Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAHDI A., DAEFFLER L., BUEB J.L., GIES J.P., LANDRY Y. The M2 muscarinic receptor antagonist methoctramine activates mast cells via a pertussis toxin-sensitive G protein. Naunyn-Schmiedeberg Arch. Pharmacol. 1998a;357:357–362. doi: 10.1007/pl00005179. [DOI] [PubMed] [Google Scholar]
- CHAHDI A., DAEFFLER L., GIES J.P., LANDRY Y. Drugs interacting with G proteins α subunits: selectivity and perspectives. Fund. Clin. Pharmacol. 1998b;12:121–132. doi: 10.1111/j.1472-8206.1998.tb00932.x. [DOI] [PubMed] [Google Scholar]
- CHAHDI A., MOUSLI M., LANDRY Y. Substance P-related inhibitors of mast cell exocytosis act on G-proteins or on the cell surface. Eur. J. Pharmacol. 1998c;341:329–335. doi: 10.1016/s0014-2999(97)01480-5. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3118. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E., NEER E.J. G protein βγ subunits. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- CLO C., TANTINI B., SACCHI P., CALDARERA C.M. Spermine inhibition of basal and stimulated adenylate cyclase is mediated by the inhibitory GTP-binding protein (Gi) Adv. Exp. Med. Biol. 1988;250:535–543. doi: 10.1007/978-1-4684-5637-0_48. [DOI] [PubMed] [Google Scholar]
- COCKCROFT S., GOMPERTS B.D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985;314:534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- CONKLIN B.R., BOURNE H.R. Structural elements of Gα-subunits that interact with βγ-subunits, receptors and effectors. Cell. 1993;73:631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- DAEFFLER L., SCHMIDLIN F., GIES J.P., LANDRY Y. Inverse agonist activity of pirenzepine at M2 muscarinic acetylcholine receptors. Br. J. Pharmacol. 1999;126:1246–1252. doi: 10.1038/sj.bjp.0702407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P., DE LA LANDE I.S., JELLETT L.B. Log-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. Exp. Ther. 1972;181:339–345. [PubMed] [Google Scholar]
- FOZARD J.R., PART M.L., PRAKASH N.J., GROVE J., SCHECHTER P.J., SJOERDSMA A., KOCH-WESER J. L-ornithine decarboxylase: an essential role in early mammalian embryogenesis. Science. 1980;208:505–508. doi: 10.1126/science.6768132. [DOI] [PubMed] [Google Scholar]
- FUKUSHIMA N., KOHNO M., KATO T., KAWAMOTO S., OKUDA K., MISU Y., UEDA H. Melittin, a metabostatic peptide inhibiting Gs activity. Peptides. 1998;19:811–819. doi: 10.1016/s0196-9781(98)00027-8. [DOI] [PubMed] [Google Scholar]
- GILMAN A.G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- HIGASHIJIMA T., BURNIER J., ROSS E.M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. J. Biol. Chem. 1990;265:14176–14186. [PubMed] [Google Scholar]
- HIGASHIJIMA T., ROSS E.M. Mapping of the mastoparan-binding site on G proteins. Cross-linking of (125I-Tyr3, Cys11) mastoparan to Go. J. Biol. Chem. 1991;266:12655–12661. [PubMed] [Google Scholar]
- HIGASHIJIMA T., UZU S., NAKAJIMA T., ROSS E.M. Mastoparan, a peptide toxin from wasp venom, mimics receptor by activating GTP-binding proteins (G proteins) J. Biol. Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- HILF G., JAKOBS K.H. Activation of cardiac G proteins by muscarinic acetylcholine receptors: regulation by Mg2+ and Na+ ions. Eur. J. Pharmacol. 1989;172:155–163. doi: 10.1016/0922-4106(89)90006-0. [DOI] [PubMed] [Google Scholar]
- HILF G., JAKOBS K.H. Agonist-independent inhibition of G protein activation by muscarinic acetylcholine receptor antagonists in cardiac membranes. Eur. J. Pharmacol. 1992;225:245–252. doi: 10.1016/0922-4106(92)90026-r. [DOI] [PubMed] [Google Scholar]
- HOWELL T.W., COCKCROFT S., GOMPERTS B.D. Essential synergy between Ca2+ and guanine nucleotides in exocytosis secretion from permeabilized mast cells. J. Cell Biol. 1987;105:191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULME E.C., BIRDSALL N.J.M., BURGEN A.S.V., METHA P. The binding of antagonists to brain muscarinic receptors. Mol. Pharmacol. 1978;14:737–750. [PubMed] [Google Scholar]
- JOHNSON T.D. Modulation of channel function by polyamines. Trends Pharmacol. Sci. 1996;17:22–27. doi: 10.1016/0165-6147(96)81566-5. [DOI] [PubMed] [Google Scholar]
- KAHN R.A., GILMAN A.G. ADP-ribosylation of GS promotes the dissociation of its alpha and beta subunits. J. Biol. Chem. 1984;259:6235–6240. [PubMed] [Google Scholar]
- KHAN N.A., QUEMENER V., MOULINOUX P. Inhibition of adenylate cyclase activity by polyamines in human erythrocytes plasma membranes. Life Sci. 1990;46:43–47. doi: 10.1016/0024-3205(90)90055-v. [DOI] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LEE N.H., FORRAY C., EL-FAKAHANY E.E. Methoctramine, a cardioselective muscarinic antagonist, stimulates phosphoinositide hydrolysis in rat cerebral cortex. Eur. J. Pharmacol. 1989;176:295–298. doi: 10.1016/0014-2999(89)90591-8. [DOI] [PubMed] [Google Scholar]
- LEFF P. The two-state model of receptor activation. Trends Pharmacol. Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., MULLIKIN D., CARON M.G. Regulation of beta-adrenergic receptors by guanyl-5′-yl imidodiphosphate and other purine nucleotides. J. Biol. Chem. 1976;251:4686–4692. [PubMed] [Google Scholar]
- MAGUJIRE M.E., VAN ARSDALE P.M., GILMAN A.G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol. Pharmacol. 1976;12:335–339. [PubMed] [Google Scholar]
- MELCHIORRE C., CASSINELLI A., QUAGLIA W. Differential blockade of muscarinic receptor subtypes by polymethylene tetraamines. J. Med. Chem. 1987;30:201–204. doi: 10.1021/jm00384a034. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BRONNER C., LANDRY Y., BOCKAERT J., ROUOT B. Direct activation of GTP-binding regulatory proteins (G proteins) by substance P and compound 48/80. FEBS Lett. 1990a;259:260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BUEB J.L., BRONNER C., ROUOT B., LANDRY Y. G proteins activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol. Sci. 1990b;11:358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- MUKAI H., MUNEKATA E., HIGASHIJIMA T. G protein antagonists. J. Biol. Chem. 1992;267:16237–16243. [PubMed] [Google Scholar]
- MUNSON J., RODBARD D. Ligand: a versatile computerized approach to characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- PETERSON G.L., SCHIMERLIK M.I. Large scale preparation and characterization of membrane-bound and detergent-solubilized muscarinic acetylcholine receptor from pig atria. Prep. Biochem. 1984;14:33–74. doi: 10.1080/10826068408070612. [DOI] [PubMed] [Google Scholar]
- PIGNATTI C., TANTINI B., SACCHI P., ZANFANTI M.L., CLO C. Effect of bacterial toxins on spermine-induced inhibition of adenylate cyclase activity of cultured rat heart cells. Cardioscience. 1990;1:209–212. [PubMed] [Google Scholar]
- SCHUBER F. Influence of polyamines on membrane functions. Biochem. J. 1989;260:1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT R.H., SUTTON K.G., DOLPHIN A.C. Interactions of polyamines with neuronal ion channels. Trends Neurosci. 1993;16:153–160. doi: 10.1016/0166-2236(93)90124-5. [DOI] [PubMed] [Google Scholar]
- SEILER N. Pharmacological properties of the natural polyamines and their depletion by biosynthesis inhibitors as a therapeutic approach. Prog. Drug. Res. 1991;37:107–159. doi: 10.1007/978-3-0348-7139-6_3. [DOI] [PubMed] [Google Scholar]
- SEILER N., HARDY A., MOULINOUX J.P. Aminoglycosides and polyamines: targets in the mammalian organism of two important groups of natural aliphatic polycations. Prog. Drug Res. 1996;46:183–241. doi: 10.1007/978-3-0348-8996-4_5. [DOI] [PubMed] [Google Scholar]


