Abstract
In a guinea-pig model of allergic asthma, we investigated the involvement of the tachykinin NK2 receptors in allergen-induced early (EAR) and late (LAR) asthmatic reactions, airway hyperreactivity (AHR) after these reactions and inflammatory cell influx in the airways, using the selective non-peptide NK2 receptor antagonist SR48968.
On two different occasions, separated by a 1 week interval, ovalbumin (OA)-sensitized guinea-pigs inhaled either vehicle (3 min) or SR48968 (100 nM, 3 min) at 30 min before as well as at 5.5 h after OA provocation (between the EAR and LAR) in a random crossover design.
SR48968 had no significant effect on the EAR, but significantly attenuated the LAR by 44.2±16.4% (P<0.05) compared to saline control.
The NK2 receptor antagonist did not affect the OA-induced AHR to histamine after the EAR at 5 h after OA challenge (3.59±0.59 fold increase in histamine reactivity vs 3.79±0.61 fold increase in the controls, NS), but significantly reduced the AHR after the LAR at 23 h after OA challenge (1.59±0.24 fold increase vs 1.93±0.15 fold increase, respectively, P<0.05).
Bronchoalveolar lavage studies performed at 25 h after the second OA provocation showed that SR48968 significantly inhibited the allergen-induced infiltration of neutrophils (P<0.05) and lymphocytes (P<0.01) in the airways.
These results indicate that NK2 receptor activation is importantly involved in the development of the allergen-induced late (but not early) asthmatic reaction and late (but not early) AHR to histamine, and that NK2 receptor-mediated infiltration of neutrophils and lymphocytes in the airways may contribute to these effects.
Keywords: Tachykinins, NK2 receptor, SR48968, histamine, allergic asthma, airway hyperreactivity, airway inflammation, guinea-pig
Introduction
Tachykinins are a group of neuropeptides, including substance P (SP), neurokinin A (NKA) and neurokinin B (NKB). These tachykinins are widely distributed in various species, and are found in the central nervous system and peripheral tissues, including the respiratory tract. In the airways, tachykinins are localized in capsaicin-sensitive sensory C-fibres, which are found beneath and within the epithelium, around blood vessels and submucosal glands, and within the bronchial smooth muscle layer (Lundberg et al., 1984). These nerves can be stimulated by a wide variety of agents, generating local axon reflex responses and release of tachykinins, which leads to a series of events, referred to as ‘neurogenic inflammation' (Barnes et al., 1991). This process includes smooth muscle contraction, submucosal gland secretion, vasodilatation, increase in vascular permeability, facilitation of cholinergic neurotransmission, and recruitment and activation of inflammatory leukocytes (recently reviewed by Advenier et al., 1997). These actions are mediated mainly through tachykinin NK1 and NK2 receptors, which are preferentially activated by SP and NKA, respectively. NK2 receptors and, to a lesser extent NK1 receptors, have been shown to be involved in bronchoconstriction, whereas NK1 receptors were found to be mainly involved in mucus secretion, microvascular leakage, and inflammatory cell responses (Frossard & Advenier, 1991; Maggi, 1993; Advenier et al., 1997). Nevertheless, NK2 receptors may also be involved in microvascular leakage (Qian et al., 1993; Tousignant et al., 1993) and in the activation of mast cells (Lilly et al., 1995) and alveolar macrophages (Kudlacz & Knippenberg, 1994).
Since several allergic mediators may induce sensory nerve stimulation (Bertrand & Geppetti, 1996; Advenier et al., 1997) and responses of neurogenic inflammation are cardinal features of allergic asthma, mechanisms underlying neurogenic inflammation may be involved in the pathophysiology of this disease (Barnes et al., 1991). This hypothesis is supported by various observations in asthmatic patients. Thus, immunohistochemistry revealed evidence of an increase in SP-containing nerves in these patients (Ollerenshaw et al., 1991), while enhanced levels of SP have been observed in bronchoalveolar lavage (BAL) fluid (Nieber et al., 1992) and in induced sputum (Tomaki et al., 1995). In addition, an enhanced expression of mRNA for the NK1 receptor (Adcock et al., 1993) as well as the NK2 receptor (Bai et al., 1995) has been observed in lung tissue from asthmatic patients, while both SP and NKA were shown to cause enhanced bronchoconstriction in such patients (Joos et al., 1987; Cheung et al., 1994).
Direct evidence for the involvement of endogenous tachykinins in human allergic airway disease, however, has thus far not been obtained. In guinea-pigs, some controversy exists about the contribution of tachykinins to acute airway responses to allergen challenge (Manzini et al., 1987; Ingenito et al., 1991; Lai, 1991; Matsuse et al., 1991; Bertrand et al., 1993; Kudlacz et al., 1996). In the same species, we very recently demonstrated that NK1 receptors are not involved in the late asthmatic reaction (Schuiling et al., 1999); however, the role of NK2 receptors in this response has thus far not been established.
Using different tachykinin receptor antagonists, some studies have indicated that endogenous tachykinins may be involved in the development of allergen-induced airway hyperreactivity (AHR). Thus, allergen-induced AHR to cholinergic agonists in guinea pigs was abolished by the selective NK2 receptor antagonist SR48968 (Boichot et al., 1995), or the dual NK1NK2 receptor antagonists MDL 105,212 (Kudlacz et al., 1996) and FK224 (Mizuguchi et al., 1996), whereas the selective NK1 receptor antagonists SR140333 (Boichot et al., 1995) or FK888 (Mizuguchi et al., 1996), after oral administration of the drugs, were ineffective. In contrast, by administration of SR140333 via the inhalational route, we have recently demonstrated that NK1 receptors are involved in the development of AHR to histamine after both the allergen-induced early (EAR) and late (LAR) asthmatic reaction (Schuiling et al., 1999). The attenuation of allergen-induced AHR SR140333 was paralleled with a reduction of eosinophils, lymphocytes and neutrophils in the bronchoalveolar lavage (BAL), indicating that NK1 receptor-mediated infiltration of pro-inflammatory cells may contribute (Schuiling et al., 1999). The involvement of NK2 receptors in allergen-induced airway inflammation is still unknown. Therefore, to establish the role of NK2 receptors in allergic airway disease, we investigated the effects of inhaled SR48968 on the development of allergen-induced EAR and LAR, the AHR after these reactions, and airway inflammation.
Methods
Animals
Specified pathogen free Dunkin Hartley guinea-pigs of either sex (Harlan, Heathfield, U.K.) were used in this study. All animals were sensitized to ovalbumin (OA) at 4 weeks of age as described previously (Van Amsterdam et al., 1989). To obtain a shift to IgE class antibodies, an allergen solution containing 100 μg OA and 100 mg Al(OH)3 per ml saline was used. The mixture of allergen solution and Al(OH)3 was gently rotated for 60 min to obtain an alu-gel, and 0.5 ml was injected intraperitoneally, while another 0.5 ml was divided over seven intracutaneous injection sites in the proximity of lymphnodes in the paws, lumbar regions and neck. Animals were operated on 3 weeks after sensitization and used experimentally 4–8 weeks after sensitization. The animals were housed in individual cages in climate-controlled animal quarters and were given water and food ad libitum. All protocols described were approved by the University of Groningen Animal Health Committee.
Measurement of airway function
Airway function was assessed by measurement of pleural pressure (Ppl) as described previously (Santing et al., 1992). In short, a small saline-filled latex balloon, connected to a saline-filled cannula, was surgically implanted inside the thoracic cavity. The free end of the cannula was driven subcutaneously to the neck of the animal, where it was exposed and attached permanently. The pleural balloon was connected to a pressure transducer (Ohmeda DTX™, SpectraMed, Bilthoven, The Netherlands) via an external saline-filled cannula. Ppl (in cmH2O) was continuously measured using an on-line computer system. Ppl data were sampled for 10 s every minute. Breath by breath variation was normally less than 10%, incidental large variations caused by sudden movements or deep sights were rejected from the calculation. We have previously shown that changes in Ppl are linearly correlated to changes in airway resistance and hence can be used as a sensitive index for allergic and non-allergic bronchoconstriction (Santing et al., 1992).
During the experimental protocol (1–5 weeks after surgery) baseline Ppl-measurements remained stable and no signs of inflammation were observed at the sites of surgery. Airway function can be monitored repeatedly and continuously for periods of at least 24 h, while the animals are unaware of the measurements being taken.
Vagal nerve stimulation
In order to study vagally-induced eNANC bronchoconstriction, some animals were also provided with a stimulation electrode around the right cervical vagal nerve, using a surgical procedure described by Ten Berge et al. (1996). In short, an incision was made on the head, and the skull membranes in the vicinity of the bregma were removed; three small screws were inserted into the skull. Two stainless steel electrodes, bent into a circle (inner diameter (ID) 1.5 mm) with a slight opening, were each soldered to 10 cm of flexible, isolated electrode wire (Biomed Wire, Cooner Wire Company, Los Angeles, CA, U.S.A.), and fixed at 2 mm distance by the use of dental acrylic glue. At the other end of the wires a microplug was soldered. The circular electrodes were embedded in a small piece of longitudinally opened silicone tubing (ID 0.64 mm, OD 1.19 mm; Silastic, Dow Corning, Michigan, U.S.A.) to provide isolation from surrounding tissue. In the vicinity of the trachea the right cervical vagal nerve was cautiously exposed using an operation microscope. The bipolar electrode and the silicone tubing were carefully placed around the exposed vagus nerve, the tubing was closed at both ends, and the electrode was anchored to the connective tissue covering the trachea. The electrode wire connected to the microplug was driven subcutaneously to emerge at the crown of the head of the animal, where the microplug was placed between the three microscrews and permanently attached to the skull of the animal with dental acrylic glue.
Stimulation of the right vagal nerve, applied via a constant-current biphasic pulse generator (Journée Systemen, Bedum, The Netherlands), resulted in an immediate increase in Ppl. Stimulation periods of 10 s were applied to obtain a plateau increase in Ppl, which lasted for at least 5 s; pulse duration was 0.1 ms. Prior to each experiment, the current intensity was selected so as to obtain a 1.5 fold enhancement of Ppl at 8 Hz, and was kept constant throughout the experiment. An average current intensity of 2.77±0.18 mA was obtained; this value was not altered in subsequent experiments within the same animals.
Stimulation-response curves were made with doubling frequencies (1-2-4-8-16-32 Hz). All stimulation episodes were performed in duplicate, separated by a short interval during which the respiration amplitude returned to basal levels for at least 10 s; average values obtained at each frequency were used.
Provocation procedures
OA, histamine, and SR48968 provocations were performed by inhalation of aerosolized solutions. These provocations were carried out in a specially designed perspex cage of 9 1, in which the guinea-pigs could move freely. A DeVilbiss nebulizer (type 646, DeVilbiss, Somerset, PA, U.S.A.) driven by an airflow of 8 l min−1, provided the aerosol with an output of 0.33 ml min−1.
The animals were habituated to the experimental conditions and the provocation procedures on 2 sequential days at least 1 week after surgery, when preoperative weight was restored. On the first day, the animals were placed in the provocation cage unconnected to the pressure transducer. After an adaptation period of at least 30 min, three consecutive provocations with saline were performed – each provocation lasting 3 min – separated by a 7 min interval. The next day, this procedure was repeated with the animals connected to the measurement system.
On the experimental days following the habituation procedure, allergen and histamine provocations were performed as indicated below. All provocations were preceded by an adaptation period of at least 30 min, followed by two consecutive control provocations with saline as described above. A baseline Ppl-value was calculated by averaging the Ppl-values from the last 20 min of the adaptation period.
In order to assess the airway reactivity for histamine, provocations with the agonist were performed starting with a 25 μg ml−1 solution in saline, followed by increasing dosage steps of 25 μg ml−1. Histamine provocations lasted 3 min and were separated by 7 min intervals. Animals were challenged until Ppl was increased by more than 100% above baseline for at least 3 consecutive min. The provocation concentration, causing a 100% increase of Ppl (PC100) was derived by linear intrapolation of the concentration-Ppl response curve and was used as an index for airway reactivity towards histamine. Ppl returned to baseline within 15 min after the last histamine provocation.
Allergen provocations were performed by inhalation of increasing concentrations of 1.0, 3.0 and 5.0 mg ml−1 OA in saline for 3 min each, separated by 7 min intervals. Allergen inhalations were discontinued when an increase in Ppl of more than 100% was observed. Using these conditions, none of the animals developed anaphylactic shock after allergen provocation.
SR48968 administration was performed by inhalation. To this aim, SR48968 was dissolved in 10% ethanol and diluted 1 : 10,000 with saline to obtain the appropriate concentration of 0.1 μM. Accordingly, saline containing 0.001% ethanol was used as a vehicle.
Experimental protocols
In a separate group of animals provided with the vagal electrode, the effect of various doses of inhaled SR48968 (50–1000 nM, 3 min) on the vagally-induced eNANC bronchoconstriction was assessed to establish an effective and selective dose to inhibit NK2 receptor stimulation by endogenous tachykinins. For this purpose, a vagal nerve stimulation-response curve was made, which was followed by inhalation of an aerosolized solution of SR48968 (3 min). Thirty minutes after inhalation of the antagonist, a subsequent stimulation-response curve was recorded. Within each animal, different concentrations of the antagonist were tested on separate days.
In another group of animals, the effect of SR48968 on allergen-induced EAR and LAR, AHR to histamine after these responses, and airway inflammation was assessed. On two different occasions, separated by a 1 week interval, the guinea-pigs inhaled either vehicle or SR48968 (100 nM) during 3 min, at 30 min before and 5.5 h after OA provocation (i.e. between the EAR and LAR). Vehicle and SR48968 inhalations were alternated using a random crossover design. In a control group of animals, vehicle was inhaled both in the first and in the second week, at the time points indicated above.
Subsequent allergen provocations performed at week 1 and week 2 were identical with respect to the allergen concentration. Twenty-four hours before each allergen provocation, the basal histamine PC100 was determined. Thirty minutes later, either vehicle (3 min) or SR48968 (100 nM, 3 min) was inhaled and a subsequent histamine PC100 measurement was performed after another 30 min, to assess the effects of vehicle and SR48968 on baseline histamine reactivity. During the next day, histamine PC100 values were assessed at 5 h after allergen provocation (after the EAR) and 23 h (after the LAR) after OA provocation in the vehicle or SR48968 treated animals, to assess allergen-induced AHR at these time points.
For the quantitative assessment of the EAR (between 0 h and 5 h after allergen provocation) and LAR (between 8 h and 23 h after allergen provocation), airway function was continuously measured during the whole procedure. Between the measurements of histamine PC100-values at 5 and 23 h, the animals were placed in their homecage (0.16 m2), in which water and food were freely accessible and where they could move around freely. During this transfer the animals remained connected to the measurement system.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed at 25 h after the second OA provocation in the group of animals receiving vehicle at 30 min before and at 5.5 h after OA provocation in both week 1 and week 2, and in the group receiving vehicle in week 1 and SR48968 in week 2. In a control group, OA-sensitized animals were lavaged 25 h after a provocation with saline instead of OA.
BAL was performed as described previously (Santing et al., 1994b). In short, the animals were anaesthetized with 20 mg kg−1 Brietal®, 35 mg kg−1 Ketalar® and 6 mg kg−1 Rompun®, administered intraperitoneally. The trachea was exposed and cannulated, and the lungs were lavaged gently using 5 ml of sterile saline at 37°C, followed by three subsequent aliquots of 8 ml of saline. The recovered lavage samples were cooled on ice, and centrifuged at 200×g for 10 min at 4°C. The pellets were combined and resuspended to a final volume of 1.0 ml in phosphate-buffered saline and total cell numbers were counted using a Coulter Counter. For cytological examination, cytospin-preparations were stained with May-Grünwald and Giemsa stain. A cell differentiation was performed by counting at least 400 cells in duplicate.
Data analysis
Effects of nerve stimulation, at all frequencies applied, are expressed as percentage increase in basal respiration amplitude.
The magnitudes of the allergen-induced EAR and LAR are expressed as the area under the Ppl time-response curve (AUC) between 0 and 5 h after allergen provocation for the EAR, and between 8 and 23 h after provocation for the LAR. Ppl was expressed as percentage change from baseline and AUC was calculated by trapezoid integration over discrete (5 min) time-periods. Based on saline control provocations, threshold values of AUC (mean+2×s.d.; 99% confidence interval) were defined as 1185%×5 min for a positive EAR and 2790%×5 min for a positive LAR, respectively (Santing et al., 1994b). Using these criteria, animals were characterized as single early responders and dual responders (i.e. animals expressing an early as well as late asthmatic response). Inherent to the research question, only dual responding animals (16 out of 20 animals; 80%) were included in this study.
Changes in nerve stimulation-response curves were statistically evaluated by a two-way analysis of variance (ANOVA, treatment×concentration) with repeated measure on the two factors, whereas changes in airway reactivity towards histamine, and changes in allergen-induced asthmatic reactions were statistically analysed by a one-way ANOVA. When significance was observed (P<0.05), a complementary analysis was undertaken (Student Newman-Keuls test) to identify differences between groups. Changes in allergen-induced cell infiltration were statistically analysed by a Kruskal-Wallis ANOVA. When a significance was found (P<0.05), a Mann-Whitney U-test was performed to determine the significance between different groups. All data are presented as mean±s.e.mean.
Chemicals
Histamine hydrochloride, OA (grade III), May-Grünwald stain and Giemsa stain were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Al(OH)3 was obtained from JT Baker Chemical Co. (Phillipsburg NJ, U.S.A.). Brietal® (methohexital sodium) was purchased from Eli Lilly (Nieuwegein, The Netherlands), Ketalar® (ketamine hydrochloride) from Parke-Davis (Hoofddorp, The Netherlands), Rompun® (2-(2, 6-xylidino)-5, 6- dihydro- 4H-1, 3-thiazine-hydrochloride, methylparaben) from Bayer (Leverkusen, Germany).
SR48968 ((S)-N-methyl-N-[4-acetyl-amino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)butyl] benzamide) was a kind gift of Sanofi Recherche (Montpellier, France).
Results
Effect of SR 48968 on vagally-induced bronchoconstriction
Stimulation of the right vagus nerve resulted in a frequency-dependent increase in the respiration amplitude, reaching a maximum of approximately 180% of control at 32 Hz (Figure 1). Inhalation of the NK2 receptor antagonist SR48968 resulted in a dose-dependent attenuation of the respiration amplitude at all frequencies of stimulation. A maximal inhibition by 29.2±5.5% at 32 Hz (P<0.001) was observed after inhalation of 1 μM SR48968, while a submaximal effect (24.7±2.4% inhibition; P<0.001) was obtained in the presence of 100 nM of the antagonist. The latter concentration was selected to analyse the role of NK2 receptor activation in the allergen-induced responses. SR48968 had no effect on basal Ppl (not shown).
Figure 1.
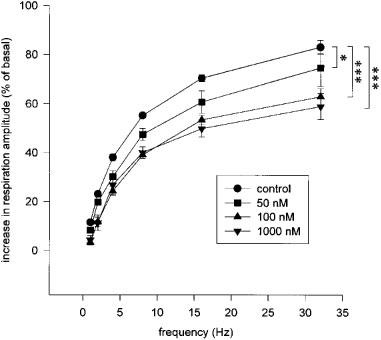
Effect of inhalations (3 min) of the selective NK2 receptor antagonist SR48968 on the vagally-induced eNANC bronchoconstriction in OA-sensitized guinea-pigs. A nerve stimulation-response curve was performed before (control) and 30 min after inhalation of various concentrations of SR48968. Data represent mean values±s.e.mean of six animals. Statistical analysis by two-way repeated measures ANOVA followed by a Newman-Keuls test, *P<0.05, ***P<0.001.
Effect of SR 48968 on allergen-induced early and late asthmatic reactions
Allergen provocation of OA-sensitized guinea-pigs induced a pronounced EAR and LAR in all animals used (Table 1). Comparable early and late reactions were found after two subsequent allergen challenges when the animals were treated with vehicle at both occasions (first and second week). Inhalation of SR48968 did not affect the EAR, but significantly attenuated the LAR by 44.2±16.4% (P<0.05) as compared to vehicle inhalation by the same animals (control week).
Table 1.
Allergen-induced early (EAR) and late (LAR) asthmatic reactions in dual responding guinea-pigs inhaling saline or the selective NK2 antagonist SR48968 (100 nM, 3 min) 30 min before and 5.5 h after allergen challenge

Effect of SR 48968 on allergen-induced airway hyperreactivity to histamine
Both vehicle and SR48968 inhalations had no significant effect on the basal histamine PC100 (Figure 2). In vehicle-treated animals, exposure to OA aerosol induced a marked 3.79±0.61 fold increase (P<0.01) in airway reactivity to histamine after the EAR (at 5 h after allergen provocation), while a significant 1.93±0.15 fold increase (P<0.05) in airway reactivity was found after the LAR (at 23 h after allergen provocation; Figure 3A). Inhalation of SR48968 by these animals at 30 min before and 5.5 h after allergen challenge did not affect the observed AHR to histamine after the EAR (3.59±0.59 fold increase in airway reactivity; NS), but significantly attenuated the AHR after the LAR (1.59±0.24 fold increase in airway reactivity; P<0.05; Figure 3A). No changes in allergen-induced AHR were observed when animals were treated with vehicle at two subsequent occasions (Figure 3B).
Figure 2.
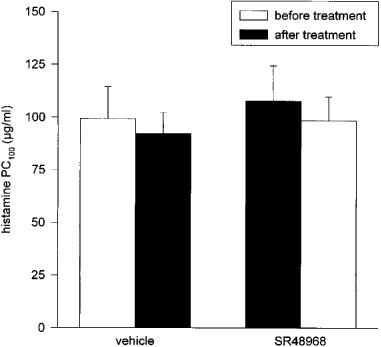
Effect of inhalations (3 min) of vehicle and the selective NK2 receptor antagonist SR48968 (100 nM) on basal airway reactivity to histamine. Two subsequent histamine PC100-measurements were performed 30 min before and 30 min after vehicle or SR48968 inhalation. Data represent mean values±s.e.mean of 7–9 animals. No statistical differences were found between groups (one-way ANOVA).
Figure 3.
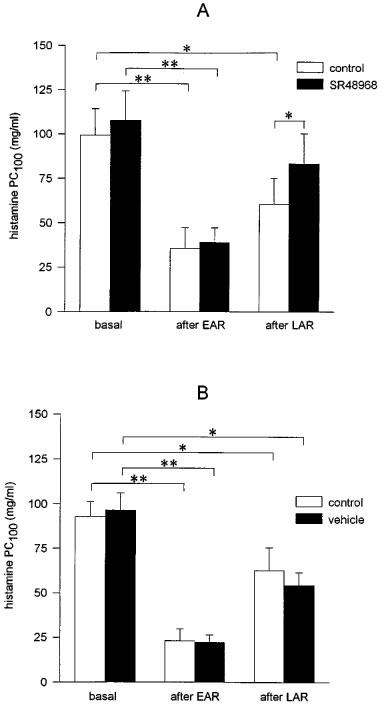
Effects of inhalations of the selective NK2 receptor antagonist SR48968 (100 nM, 3 min) (A) and vehicle (B) on ovalbumin (OA)-induced airway hyperreactivity to histamine after the allergen-induced early (EAR) and late (LAR) asthmatic reactions. (A) Vehicle (control) or SR48968 were inhaled at 30 min before and 5.5 h after OA provocation on two subsequent occasions with 1 week interval. (B) Vehicle was inhaled at 30 min before and 5.5 h after OA provocation in both week 1 and week 2. Data represent mean values±s.e.mean of 7–9 animals. Statistical analysis by one-way ANOVA followed by a Newman-Keuls test: *P<0.05, **P<0.01.
Effect of SR 48968 on allergen-induced airway inflammation
Total and differential BAL cell counts from saline (control) and OA-challenged guinea-pigs are presented in Figure 4A and B. In all groups measured, the recovery of the lavage fluid was high, with an overall average of 80.6±0.7% (n=17). Compared to a control challenge with saline, OA provocation caused a significant increase in total BAL cells (P<0.01), eosinophils (P<0.001), macrophages (P<0.05), lymphocytes (P<0.01) and neutrophils (P<0.01) in animals which were treated with vehicle at 30 min before and 5.5 h after the second allergen challenge. In the animals treated with SR48968 at these time points; the number of infiltrated eosinophils and macrophages were not significantly different from the vehicle treated group, while the numbers of neutrophils (P<0.05) and lymphocytes (P<0.01) were significantly reduced. In addition, the number of epithelial cells in the BAL fluid tended to be decreased in the SR48968 treated animals (P=0.10).
Figure 4.
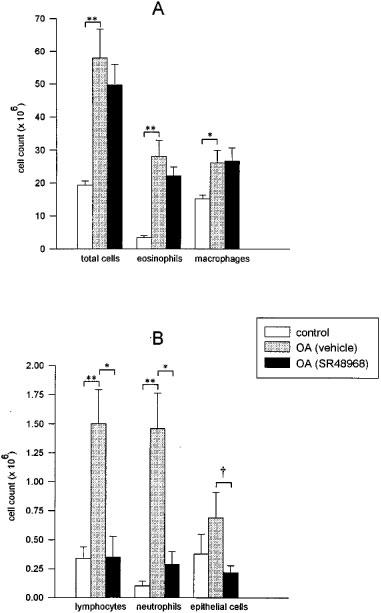
Bronchoalveolar lavage cell counts at 25 h after inhalation of saline (control) or ovalbumin (OA). The OA-challenged animals received vehicle (3 min) at 30 min before and 5.5 h after OA challenge in week 1, and either vehicle (3 min) or SR48968 (100 nM, 3 min) at 30 min and 5.5 h after OA challenge in week 2. (A) Effects on total cells, eosinophils and macrophages; (B) Effects on lymphocytes, neutrophils and epithelial cells. Data represent mean values±s.e.mean of 5–7 animals. Statistical analysis by Kruskal-Wallis one way ANOVA, followed by a Mann-Whitney U-test: *P<0.05, **P<0.01, †P=0.10.
Discussion
In this study, we have demonstrated that the selective NK2 receptor antagonist SR48968 attenuated the allergen-induced LAR as well as the AHR after the LAR, whereas the EAR and the AHR after this early reaction were not affected. The SR48968-induced attenuation of the LAR and the AHR after this reaction was paralleled by a reduction of neutrophils and lymphocytes in the BAL collected after the LAR, whereas the influx of eosinophils had not significantly changed.
To investigate the involvement of NK2 receptors in the development of allergen-induced EAR and LAR, the AHR after both reactions, and the airway inflammation, SR48968 was used. In previous studies, this compound has been demonstrated to be highly selective towards the NK2 receptor. Thus, in radioligand binding experiments for the NK2 receptor, SR48968 competitively inhibited the binding of [125I]-NKA with an inhibition constant (Ki) of 0.51 nM, while in classical binding assays for NK1 and NK3 receptors, SR48968 was ineffective (Ki>5000 nM) (Emonds Alt et al., 1992). In vitro, SR48968 potently antagonized [β-Ala8]-NKA(4-10)-induced contraction of guinea-pig tracheal preparations with a pA2 value of 10.51 (Advenier et al., 1992), while in functional assays for NK1 or NK3 receptors SR48968 was inactive up to 1 μM (Emonds Alt et al., 1992).
In guinea-pigs, stimulation of the vagus nerve produces a cholinergic and a non-cholinergic bronchospastic response, the latter component being caused by the endogenous release of tachykinins from sensory nerves (Lundberg & Saria, 1982), which is predominantly mediated by tachykinin NK2 receptors (Boni et al., 1995; Yuan et al., 1996). In this study, inhalation of the selective NK2 receptor antagonist SR48968 dose-dependently inhibited the total vagally-induced bronchoconstriction in guinea-pigs, and a submaximal effect of about 25% inhibition was achieved at a concentration of 100 nM. This is totally in line with observations of Chan et al. (1994), who demonstrated in vivo that inhalation of 100 nM SR48968 caused almost complete inhibition of NKA-induced bronchoconstriction in awake guinea-pigs. Therefore, the inhaled dose of SR48968 (100 nM solution, nebulized during 3 min in a 9 l provocation cage) is both selective and effective.
Effect of SR 48968 on allergen-induced early and late asthmatic reactions
The allergen-induced EAR was not altered by inhalation of SR48968, indicating that NK2 receptors are not importantly involved in the allergen-induced immediate bronchoconstriction. This is in line with a previous observation in guinea-pigs that i.v. administration of the same antagonist did not affect the acute OA-induced bronchoconstriction (Yoshihara et al., 1996). In addition, depletion of tachykinins in capsaicinized guinea-pigs did not prevent the allergen-induced immediate bronchoconstriction (Ingenito et al., 1991; Lai, 1991). However, in phosphoramidon-pretreated guinea-pigs, the allergen-induced bronchoconstriction was attenuated by SR48968 (Bertrand et al., 1993), indicating that a role for NK2 receptors in the EAR can only be visualized when degradation of tachykinins by neutral endopeptidase (NEP) is inhibited.
In contrast to the EAR, the LAR was effectively diminished by SR48968 in the absence of a NEP inhibitor, indicating the involvement of NK2 receptors. The mechanism of this inhibition is presently unknown, and several mechanisms may be envisaged. First, increased airway levels of tachykinins in response to allergen provocation could contribute to the severity of the LAR via enhanced NK2 receptor-mediated bronchoconstriction. Thus, in guinea-pig airways an increase of tissue NKA and SP was observed at 24 h, but not 12 h, after single allergen challenge, which was paralleled by an increase in the number of tachykinin-immunoreactive nerves (Fischer et al., 1996). In addition, airway tachykinin levels may be increased as a consequence of decreased degradation by NEP. This is supported by the observation that attenuation of NEP activity induced by repeated allergen exposure contributed to increased contractility to SP in tracheally perfused guinea-pig lungs (Lilly et al., 1994). Decreased tachykinin metabolism could be due to epithelial damage (Nadel, 1991), which may be induced by eosinophil-derived cytotoxic mediators during the LAR (Gleich et al., 1988). Infiltration and activation of eosinophils during the LAR has also been demonstrated in our model (Santing et al., 1994a; 1994b), while the present study showed a tendency to an enhanced number of epithelial cells in the BAL after the LAR, which appeared to be inhibited by SR48968. Furthermore, eosinophil-derived cationic proteins have also been implicated in the activation of sensory C-fibres in the airways (Coyle et al., 1994).
Secondly, NK2 receptor-mediated infiltration and activation of pro-inflammatory cells may also contribute to the LAR, as indicated by the observation that SR48968 attenuated the number of lymphocytes and neutrophils in the BAL collected after the LAR. It is widely accepted that lymphocyte-derived cytokines, such as interleukin 5, are involved in the activation of eosinophils (Corrigan & Kay, 1992), and eosinophil-derived mediators may subsequently be involved in the LAR (O'Byrne et al., 1987).
Effect of SR 48968 on allergen-induced airway hyperreactivity to histamine
SR48968 attenuated the allergen-induced AHR to histamine after the LAR, but not after the EAR, indicating the involvement of the NK2 receptors. This is in line with observations from other investigators, who found that allergen-induced AHR to cholinergic agonists was reduced by SR48968 (Boichot et al., 1995) or the NK1/NK2 receptor antagonist MDL105,212 (Kudlacz et al., 1996) at 48 and 24 h after allergen provocation, respectively.
Although SR48968 did not diminish the influx of macrophages, a number of observations suggest that NK2 receptor-mediated activation of alveolar macrophages may be involved in allergen-induced AHR. First, tachykinins may activate guinea-pig alveolar macrophages to enhanced production of superoxide anion (O2−) via NK2 receptors (Brunelleschi et al., 1990), and the responsiveness of macrophages to NK2 receptor stimulation was even increased in macrophages obtained from OA-sensitized guinea-pigs (Brunelleschi et al., 1992). Moreover, fMLP- or zymosan-induced O2− production of guinea-pig alveolar macrophages was enhanced at 24 h after in vivo exposure to tachykinins or capsaicin (Boichot et al., 1993; Kudlacz & Knippenberg, 1994), which was paralleled by AHR to both acetylcholine and histamine (Boichot et al., 1993). Reactive oxygen species in the lung may enhance airway reactivity by provoking mediator release and chemotaxis, inhibition of epithelial NEP activity, airway secretion and enhanced vascular permeability (reviewed by Barnes, 1990). In addition, macrophage-derived O2− could rapidly react with (inducible nitric oxide synthase (iNOS)-derived) nitric oxide (NO) to produce the highly cytotoxic metabolite peroxynitrite (Huie & Padmaja, 1993), which has been demonstrated to cause epithelial damage and hyperreactivity of guinea-pig airways (Sadeghi Hashjin et al., 1996). Moreover, peroxynitrite has been demonstrated to increase the release of major basic protein from eosinophils in tracheal preparations (Sadeghi Hashjin et al., 1996), which may similarly cause epithelial damage in the airways (Gleich et al., 1988). In our model, we indeed demonstrated development of iNOS activity during the LAR, which was involved in both inflammatory cell influx and AHR after the LAR (Schuiling et al., 1998).
Direct evidence that O2− may be involved in allergen-induced AHR was reported by Ikuta et al. (1992), who showed inhibition of OA-induced AHR to acetylcholine by a polyoxyethylene-modified long-acting superoxide dismutase in guinea-pigs after repeated allergen challenge.
Since part of the histamine-induced bronchoconstriction occurs through stimulation of vagal afferents (Chapman & Danko, 1990; Ellis & Undem, 1992; Costello et al., 1998), allergen-induced changes in excitatory nonadrenergic noncholinergic (eNANC) neural function could also be involved in the AHR to inhaled histamine. As indicated above, allergen provocation increased the number of tachykinin-immunoreactive nerves in the airways of guinea-pigs (Fischer et al., 1996) and augmented the mechanical sensitivity of sensory nerve fibers located in the tracheal wall (Riccio et al., 1996). In addition, the accessibility of these afferents for stimuli may be increased after allergen challenge, as a consequence of epithelial damage caused by cytotoxic mediators derived from infiltrated inflammatory cells (Gleich et al., 1988; Sadeghi Hashjin et al., 1996). Therefore, increased density, excitability, and accessibility of eNANC nerves caused by allergen provocation may contribute to the allergen-induced AHR to histamine after the LAR.
Finally, since NKA-induced plasma extravasation was attenuated by SR48968 (Qian et al., 1993; Tousignant et al., 1993), NK2 receptor-induced airway oedema may additionally contribute to allergen-induced AHR due to thickening of the airway wall (Hogg, 1982).
Effect of SR 48968 on allergen-induced airway inflammation
In a previous study, we demonstrated that NK1 receptors are involved in the allergen-induced migration of eosinophils, neutrophils and lymphocytes into the airway lumen, and in some epithelial shedding, since inhalation of the selective NK1 antagonist SR140333 reduced the BAL numbers of these cells 25 h after allergen provocation and tended to reduce the accumulation of ciliated epithelial cells (Schuiling et al., 1999). In the present study, we demonstrated that SR48968 reduced the numbers of neutrophils and lymphocytes, but not eosinophils in the BAL, while there was again a tendency to reduced epithelial cells in the airway lumen. This indicates that NK2 receptor-induced inflammatory cell infiltration and subsequent epithelial damage may similarly be involved in development of allergen-induced AHR. These results suggest that the two NK receptors are differentially involved in the allergen-induced infiltration of proinflammatory cells into the airways in vivo. Evidence for such a differential involvement of NK receptors in vivo was previously also found by Kudlacz & Knippenberg (1994), who found that inhalation of SP elicited eosinophil influx, whereas inhalation of NKA caused neutrophil recruitment into the airways at 24 h after inhalation of the tachykinins.
The observation that the selective NK1 receptor antagonist SR140333 significantly reduced both eosinophil and neutrophil influx and inhibited AHR both after the EAR and LAR, whereas the NK2 receptor antagonist SR48968, which reduced neutrophil but not eosinophil influx, diminished the AHR after the LAR only, would suggest that the early AHR is mainly eosinophil-driven, whereas in the late AHR the neutrophil could have an important function. In a previous study using the same animal model, we found a linear relationship between eosinophil peroxidase activity in the BAL after the EAR and the number of eosinophils infiltrated; after the LAR the eosinophil number had significantly increased further whereas the activation state per cell had diminished, however (Santing et al., 1994a). It has been reported that neutrophils may potentiate mediator release by eosinophils when both cells are stimulated together in vitro (Kloprogge et al., 1989). Hence, it could be envisaged that neutrophils may prolong the activation state of the infiltrated mucosal eosinophils, which process would gain importance during and after the LAR. ‘Selective' inhibition of neutrophil influx by NK2 receptor blockade could therefore reduce eosinophil activation during/after the LAR further, whereas during/after the EAR the activation state of the freshly infiltrated eosinophils would be less sensitive for diminished neutrophil influx. It should be born in mind, however, that the neutrophil itself has a large potential to generate tissue injury by its release among others of lysosomal enzymes (Weiss & Peppin, 1986) and O2− (Sedgwick et al., 1988). As indicated above, formation of peroxynitrite from O2− and NO could contribute to epithelial damage, which was indicated by the tendency of an increased number of epithelial cells in the BAL fluid after allergen challenge.
In conclusion, the present data indicate that a NK2 receptor-mediated mechanism is involved in the development of the allergen-induced LAR and AHR to histamine after this reaction. Although further investigations are necessary to elucidate the actual mechanism, NK2 receptor-mediated inflammatory responses may be involved. Furthermore, this study indicates that also tachykinin NK2 receptor antagonists may be useful in the treatment of allergic asthma.
Acknowledgments
The authors wish to thank the Netherlands Asthma Foundation for the financial support of this work (grant number 92.72).
Abbreviations
- AHR
airway hyperreactiviy
- BAL
bronchoalveolar lavage
- EAR
early asthmatic reaction
- eNANC
excitatory nonadrenergic noncholinergic
- iNOS
inducible nitric oxide synthase
- LAR
late asthmatic reaction
- NEP
neutral endopeptidase
- NKA
neurokinin A
- NKB
neurokinin B
- NO
nitric oxide
- O2−
superoxide anion
- OA
ovalbumin
- Ppl
pleural pressure
- PC100
provocation concentration causing 100% increase in pleural pressure
- SP
substance P
- SR 48968
(S)-N-methyl-N-[4-acetyl-amino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)butyl] benzamide
References
- ADCOCK I.M., PETERS M., GELDER C., SHIRASAKI H., BROWN C.R., BARNES P.J. Increased tachykinin receptor gene expression in asthmatic lung and its modulation by steroids. J. Mol. Endocrinol. 1993;11:1–7. doi: 10.1677/jme.0.0110001. [DOI] [PubMed] [Google Scholar]
- ADVENIER C., LAGENTE V., BOICHOT E. The role of tachykinin receptor antagonists in the prevention of bronchial hyperresponsiveness, airway inflammation and cough. Eur. Respir. J. 1997;10:1892–1906. doi: 10.1183/09031936.97.10081892. [DOI] [PubMed] [Google Scholar]
- ADVENIER C., ROUISSI N., NGUYEN Q.T., EMONDS ALT X., BRELIERE J.C., NELIAT G., NALINE E., REGOLI D. Neurokinin A (NK2) receptor revisited with SR 48968, a potent non-peptide antagonist. Biochem. Biophys. Res. Commun. 1992;184:1418–1424. doi: 10.1016/s0006-291x(05)80041-5. [DOI] [PubMed] [Google Scholar]
- BAI T.R., ZHOU D., WEIR T., WALKER B., HEGELE R., HAYASHI S., MCKAY K., BONDY G.P., FONG T. Substance P (NK1)- and neurokinin A (NK2)-receptor gene expression in inflammatory airway diseases. Am. J. Physiol. 1995;269:L309–L317. doi: 10.1152/ajplung.1995.269.3.L309. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Reactive oxygen species and airway inflammation. Free Radic. Biol. Med. 1990;9:235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., BARANIUK J.N., BELVISI M.G. Neuropeptides in the respiratory tract. Part II. Am. Rev. Respir. Dis. 1991;144:1391–1399. doi: 10.1164/ajrccm/144.6.1391. [DOI] [PubMed] [Google Scholar]
- BERTRAND C., GEPPETTI P., GRAF P.D., FORESI A., NADEL J.A. Involvement of neurogenic inflammation in antigen-induced bronchoconstriction in guinea pigs. Am. J. Physiol. 1993;265:L507–L511. doi: 10.1152/ajplung.1993.265.5.L507. [DOI] [PubMed] [Google Scholar]
- BERTRAND C., GEPPETTI P. Tachykinin and kinin receptor antagonists: therapeutic perspectives in allergic airway disease. Trends Pharmacol. Sci. 1996;17:255–259. doi: 10.1016/0165-6147(96)10027-4. [DOI] [PubMed] [Google Scholar]
- BOICHOT E., GERMAIN N., LAGENTE V., ADVENIER C. Prevention by the tachykinin NK2 receptor antagonist, SR 48968, of antigen-induced airway hyperresponsiveness in sensitized guinea-pigs. Br. J. Pharmacol. 1995;114:259–261. doi: 10.1111/j.1476-5381.1995.tb13220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOICHOT E., LAGENTE V., PAUBERT BRAQUET M., FROSSARD N. Inhaled substance P induces activation of alveolar macrophages and increases airway responses in the guinea-pig. Neuropeptides. 1993;25:307–313. doi: 10.1016/0143-4179(93)90048-f. [DOI] [PubMed] [Google Scholar]
- BONI P., BALLATI L., EVANGELISTA S. Tachykinin NK1 and NK2 receptors mediate the non-cholinergic bronchospastic response to capsaicin and vagal stimulation in guinea-pigs. J. Auton. Pharmacol. 1995;15:49–54. doi: 10.1111/j.1474-8673.1995.tb00347.x. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., PARENTI A., CENI E., GIOTTI A., FANTOZZI R. Enhanced responsiveness of ovalbumin-sensitized guinea-pig alveolar macrophages to tachykinins. Br. J. Pharmacol. 1992;107:964–969. doi: 10.1111/j.1476-5381.1992.tb13392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNELLESCHI S., VANNI L., LEDDA F., GIOTTI A., MAGGI C.A., FANTOZZI R. Tachykinins activate guinea-pig alveolar macrophages: involvement of NK2 and NK1 receptors. Br. J. Pharmacol. 1990;100:417–420. doi: 10.1111/j.1476-5381.1990.tb15821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN C.C., TOUSIGNANT C., HO E., BRIDEAU C., SAVOIE C., RODGER I.W. Evaluation of bronchoconstriction induced by neurokinins and its inhibition by selective nonpeptide antagonists in conscious guinea pigs, using a double-chamber plethysmograph technique. Can. J. Physiol. Pharmacol. 1994;72:11–18. doi: 10.1139/y94-003. [DOI] [PubMed] [Google Scholar]
- CHAPMAN R.W., DANKO G. Role of cholinergic, vagal reflexes on the bronchoconstrictor responses to histamine during carbon dioxide inhalation in conscious guinea-pigs. Pharmacol. Res. 1990;22:133–141. doi: 10.1016/1043-6618(90)90709-m. [DOI] [PubMed] [Google Scholar]
- CHEUNG D., VAN DER VEEN H., DEN HARTIGH J., DIJKMAN J.H., STERK P.J. Effects of inhaled substance P on airway responsiveness to methacholine in asthmatic subjects in vivo. J. Appl. Physiol. 1994;77:1325–1332. doi: 10.1152/jappl.1994.77.3.1325. [DOI] [PubMed] [Google Scholar]
- CORRIGAN C.J., KAY A.B. Asthma. Role of T-lymphocytes and lymphokines. Br. Med. Bull. 1992;48:72–84. doi: 10.1093/oxfordjournals.bmb.a072543. [DOI] [PubMed] [Google Scholar]
- COSTELLO R.W., FRYER A.D., BELMONTE K.E., JACOBY D.B. Effects of tachykinin NK1 receptor antagonists on vagal hyperreactivity and neuronal M2 muscarinic receptor function in antigen challenged guinea-pigs. Br. J. Pharmacol. 1998;124:267–276. doi: 10.1038/sj.bjp.0701822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COYLE A.J., PERRETTI F., MANZINI S., IRVIN C.G. Cationic protein-induced sensory nerve activation: role of substance P in airway hyperresponsiveness and plasma protein extravasation. J. Clin. Invest. 1994;94:2301–2306. doi: 10.1172/JCI117594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Antigen-induced enhancement of noncholinergic contractile responses to vagus nerve and electrical field stimulation in guinea pig isolated trachea. J. Pharmacol. Exp. Ther. 1992;262:646–653. [PubMed] [Google Scholar]
- EMONDS ALT X., VILAIN P., GOULAOUIC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., LE FUR G., BRELIERE J.C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50:PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- FISCHER A., MCGREGOR G.P., SARIA A., PHILIPPIN B., KUMMER W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J. Clin. Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROSSARD N., ADVENIER C. Tachykinin receptors and the airways. Life Sci. 1991;49:1941–1953. doi: 10.1016/0024-3205(91)90636-p. [DOI] [PubMed] [Google Scholar]
- GLEICH G.J., FLAVAHAN N.A., FUJISAWA T., VANHOUTTE P.M. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J. Allergy Clin. Immunol. 1988;81:776–781. doi: 10.1016/0091-6749(88)90931-1. [DOI] [PubMed] [Google Scholar]
- HOGG J.C. Bronchial mucosal permeability and its relationship to airways hyperreactivity. Eur. J. Respir. Dis. Suppl. 1982;122:17–22. [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- IKUTA N., SUGIYAMA S., TAKAGI K., SATAKE T., OZAWA T. Implication of oxygen radicals on airway hyperresponsiveness after ovalbumin challenge in guinea pigs. Am. Rev. Respir. Dis. 1992;145:561–565. doi: 10.1164/ajrccm/145.3.561. [DOI] [PubMed] [Google Scholar]
- INGENITO E.P., PLISS L.B., MARTINS M.A., INGRAM R.H., JR Effects of capsaicin on mechanical, cellular, and mediator responses to antigen in sensitized guinea pigs. Am. Rev. Respir. Dis. 1991;143:572–577. doi: 10.1164/ajrccm/143.3.572. [DOI] [PubMed] [Google Scholar]
- JOOS G., PAUWELS R., VAN DER STRAETEN M. Effect of inhaled substance P and neurokinin A on the airways of normal and asthmatic subjects. Thorax. 1987;42:779–783. doi: 10.1136/thx.42.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOPROGGE E., DE LEEUW A.J., DE MONCHY J.G., KAUFFMAN H.F. Cellular communication in leukotriene C4 production between eosinophils and neutrophils. Int. Arch. Allergy Appl. Immunol. 1989;90:20–23. doi: 10.1159/000234994. [DOI] [PubMed] [Google Scholar]
- KUDLACZ E.M., KNIPPENBERG R.W. In vitro and in vivo effects of tachykinins on immune cell function in guinea pig airways. J. Neuroimmunol. 1994;50:119–125. doi: 10.1016/0165-5728(94)90037-x. [DOI] [PubMed] [Google Scholar]
- KUDLACZ E.M., KNIPPENBERG R.W., LOGAN D.E., BURKHOLDER T.P. Effect of MDL 105, 212, a nonpeptide NK-1/NK-2 receptor antagonist in an allergic guinea pig model. J. Pharmacol. Exp. Ther. 1996;279:732–739. [PubMed] [Google Scholar]
- LAI Y.L. Endogenous tachykinins in antigen-induced acute bronchial responses of guinea pigs. Exp. Lung. Res. 1991;17:1047–1060. doi: 10.3109/01902149109064334. [DOI] [PubMed] [Google Scholar]
- LILLY C.M., HALL A.E., RODGER I.W., KOBZIK L., HALEY K.J., DRAZEN J.M. Substance P-induced histamine release in tracheally perfused guinea pig lungs. J. Appl. Physiol. 1995;78:1234–1241. doi: 10.1152/jappl.1995.78.4.1234. [DOI] [PubMed] [Google Scholar]
- LILLY C.M., KOBZIK L., HALL A.E., DRAZEN J.M. Effects of chronic airway inflammation on the activity and enzymatic inactivation of neuropeptides in guinea pig lungs. J. Clin. Invest. 1994;93:2667–2674. doi: 10.1172/JCI117280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG J.M., HOKFELT T., MARTLING C.R., SARIA A., CUELLO C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res. 1984;235:251–261. doi: 10.1007/BF00217848. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., SARIA A. Bronchial smooth muscle contraction induced by stimulation of capsaicin-sensitive sensory neurons. Acta Physiol. Scand. 1982;116:473–476. doi: 10.1111/j.1748-1716.1982.tb07170.x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinin receptors and airway pathophysiology. Eur. Respir. J. 1993;6:735–742. [PubMed] [Google Scholar]
- MANZINI S., MAGGI C.A., GEPPETTI P., BACCIARELLI C. Capsaicin desensitization protects from antigen-induced bronchospasm in conscious guinea-pigs. Eur. J. Pharmacol. 1987;138:307–308. doi: 10.1016/0014-2999(87)90451-1. [DOI] [PubMed] [Google Scholar]
- MATSUSE T., THOMSON R.J., CHEN X.R., SALARI H., SCHELLENBERG R.R. Capsaicin inhibits airway hyperresponsiveness but not lipoxygenase activity or eosinophilia after repeated aerosolized antigen in guinea pigs. Am. Rev. Respir. Dis. 1991;144:368–372. doi: 10.1164/ajrccm/144.2.368. [DOI] [PubMed] [Google Scholar]
- MIZUGUCHI M., FUJIMURA M., AMEMIYA T., NISHI K., OHKA T., MATSUDA T. Involvement of NK2 receptors rather than NK1 receptors in bronchial hyperresponsiveness induced by allergic reaction in guinea-pigs. Br. J. Pharmacol. 1996;117:443–448. doi: 10.1111/j.1476-5381.1996.tb15210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NADEL J.A. Neutral endopeptidase modulates neurogenic inflammation. Eur. Respir. J. 1991;4:745–754. [PubMed] [Google Scholar]
- NIEBER K., BAUMGARTEN C.R., RATHSACK R., FURKERT J., OEHME P., KUNKEL G. Substance P and beta-endorphin-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J. Allergy Clin. Immunol. 1992;90:646–652. doi: 10.1016/0091-6749(92)90138-r. [DOI] [PubMed] [Google Scholar]
- O'BYRNE P.M., DOLOVICH J., HARGREAVE F.E. Late asthmatic responses. Am. Rev. Respir. Dis. 1987;136:740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- OLLERENSHAW S.L., JARVIS D., SULLIVAN C.E., WOOLCOCK A.J. Substance P immunoreactive nerves in airways from asthmatics and nonasthmatics. Eur. Respir. J. 1991;4:673–682. [PubMed] [Google Scholar]
- QIAN Y., EMONDS ALT X., ADVENIER C. Effects of capsaicin, (+/−)-CP-96,345 and SR 48968 on the bradykinin-induced airways microvascular leakage in guinea-pigs. Pulm. Pharmacol. 1993;6:63–67. doi: 10.1006/pulp.1993.1009. [DOI] [PubMed] [Google Scholar]
- RICCIO M.M., MYERS A.C., UNDEM B.J. Immunomodulation of afferent neurons in guinea-pig isolated airway. J. Physiol. Lond. 1996;491.2:499–509. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADEGHI HASHJIN G., FOLKERTS G., HENRICKS P.A., VERHEYEN A.K., VAN DER LINDE H.J., VAN ARK I., COENE A., NIJKAMP F.P. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am. J. Respir. Crit. Care Med. 1996;153:1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., HOEKSTRA Y., PASMAN Y., ZAAGSMA J., MEURS H. The importance of eosinophil activation for the development of allergen-induced bronchial hyperreactivity in conscious, unrestrained guinea-pigs. Clin. Exp. Allergy. 1994a;24:1157–1163. doi: 10.1111/j.1365-2222.1994.tb03322.x. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., MEURS H., VAN DER MARK T.W., REMIE R., OOSTEROM W.C., BROUWER F., ZAAGSMA J. A novel method to assess airway function parameters in chronically instrumented, unrestrained guinea-pigs. Pulm. Pharmacol. 1992;5:265–272. doi: 10.1016/0952-0600(92)90069-s. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., OLYMULDER C.G., ZAAGSMA J., MEURS H. Relationships among allergen-induced early and late phase airway obstructions, bronchial hyperreactivity, and inflammation in conscious, unrestrained guinea pigs. J. Allergy Clin. Immunol. 1994b;93:1021–1030. doi: 10.1016/s0091-6749(94)70051-6. [DOI] [PubMed] [Google Scholar]
- SCHUILING M., MEURS H, , ZUIDHOF A.B., ZAAGSMA J. Dual action of iNOS-derived nitric oxide in allergen-induced airway hyperreactivity in conscious, unrestrained guinea pigs. Am. J. Respir. Crit. Care Med. 1998;158:1442–1449. doi: 10.1164/ajrccm.158.5.9803027. [DOI] [PubMed] [Google Scholar]
- SCHUILING M., ZUIDHOF A.B., ZAAGSMA J., MEURS H. Involvement of the NK1-receptor in the development of allergen-induced airway hyperreactivity and airway inflammation in conscious, unrestrained guinea pigs. Am. J. Respir. Crit. Care Med. 1999;159:423–430. doi: 10.1164/ajrccm.159.2.9804125. [DOI] [PubMed] [Google Scholar]
- SEDGWICK J.B., VIRTIS R.F., GOURLEY M.F., BUSSE W.W. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J. Allergy Clin. Immunol. 1988;81:876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- TEN BERGE R.E.J., KRIKKE M., TEISMAN A.C.H., ROFFEL A.F., ZAAGSMA J. Dysfunctional muscarinic M2 autoreceptors in vagally induced bronchoconstriction of conscious guinea pigs after the early allergic reaction. Eur. J. Pharmacol. 1996;318:131–139. doi: 10.1016/s0014-2999(96)00820-5. [DOI] [PubMed] [Google Scholar]
- TOMAKI M., ICHINOSE M., MIURA M., HIRAYAMA Y., YAMAUCHI H., NAKAJIMA N., SHIRATO K. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 1995;151:613–617. doi: 10.1164/ajrccm.151.3.7533601. [DOI] [PubMed] [Google Scholar]
- TOUSIGNANT C., CHAN C.C., GUEVREMONT D., BRIDEAU C., HALE J.J., MACCOSS M., RODGER I.W. NK2 receptors mediate plasma extravasation in guinea-pig lower airways. Br. J. Pharmacol. 1993;108:383–386. doi: 10.1111/j.1476-5381.1993.tb12813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN AMSTERDAM R.G., BROUWER F., ZAAGSMA J. Analysis of the beta-adrenoceptor mediated inhibition of IgG1 and IgE dependent guinea-pig anaphylactic tracheal smooth muscle contraction. Agents Actions. 1989;26:48–51. doi: 10.1007/BF02126559. [DOI] [PubMed] [Google Scholar]
- WEISS S.J., PEPPIN G.J. Collagenolytic metalloenzymes of the human neutrophil. Characteristics, regulation and potential function in vivo. Biochem. Pharmacol. 1986;35:3189–3197. doi: 10.1016/0006-2952(86)90412-0. [DOI] [PubMed] [Google Scholar]
- YOSHIHARA S., GEPPETTI P., LINDEN A., HARA M., CHAN B., NADEL J.A. Tachykinins mediate the potentiation of antigen-induced bronchoconstriction by cold air in guinea pigs. J. Allergy Clin. Immunol. 1996;97:756–760. doi: 10.1016/s0091-6749(96)80152-7. [DOI] [PubMed] [Google Scholar]
- YUAN L., BURCHER E., NAIL B. Tachykinin receptors and non-cholinergic bronchoconstriction in the anaesthetized guinea-pig. Clin. Exp. Pharmacol. Physiol. 1996;23:119–124. doi: 10.1111/j.1440-1681.1996.tb02582.x. [DOI] [PubMed] [Google Scholar]


