Abstract
The Kv4.3 gene is believed to encode a large proportion of the transient outward current (Ito), responsible for the early phase of repolarization of the human cardiac action potential. There is evidence that this current is involved in the dispersion of refractoriness which develops during myocardial ischaemia and which predisposes to the development of potentially fatal ventricular tachyarrhythmias.
Epidemiological, clinical, animal, and cellular studies indicate that these arrhythmias may be ameliorated in myocardial ischaemia by n-3 polyunsaturated fatty acids (n-3 PUFA) present in fish oils.
We describe stable transfection of the Kv4.3 gene into a mammalian cell line (Chinese hamster ovary cells), and using patch clamp techniques have shown that the resulting current closely resembles human Ito.
The current is rapidly activating and inactivating, with both processes being well fit by double exponential functions (time constants of 3.8±0.2 and 5.3±0.4 ms for activation and 20.0±1.2 and 96.6±6.7 ms for inactivation at +45 mV at 23°C). Activation and steady state inactivation both show voltage dependence (V1/2 of activation=−6.7±2.5 mV, V1/2 of steady state inactivation=−51.3±0.2 mV at 23°C). Current inactivation and recovery from inactivation are faster at physiologic temperature (37°C) compared to room temperature (23°C).
The n-3 PUFA docosahexaenoic acid blocks the Kv4.3 current with an IC50 of 3.6 μmol L−1. Blockade of the transient outward current may be an important mechanism by which n-3 PUFA provide protection against the development of ventricular fibrillation during myocardial ischaemia.
Keywords: Potassium current, fatty acid, arrhythmia, transient outward current, Kv4.3
Introduction
The transient outward current is responsible for early repolarization (phase 1) of the cardiac action potential (Campbell et al., 1995). It is a rapidly activating and inactivating current, which both modulates action potential duration (Rose et al., 1998; Barry et al., 1998) and is important for setting the voltage of the action potential plateau. This in turn influences the activity of other currents, such as the inward calcium current (Campbell et al., 1995). There are two components of the transient outward current. Ito1 is Ca2+-independent and sensitive to inhibition by 4-aminopyridine (4-AP). It is responsible for the majority of the human transient outward current. Ito2 is dependent on intracellular calcium and is 4-AP-insensitive but suppressed by caffeine and drugs which block the inward calcium current (Escande et al., 1987; Tseng & Hoffman, 1989; Wang et al., 1997). There is evidence that the transient outward current is of fundamental importance to the development of the dispersion of repolarization which occurs during acute myocardial ischaemia and which predisposes to the development of ventricular tachyarrhythmias (Antzelevitch et al., 1991; Lukas & Antzelevitch, 1993). Antzelevitch et al. (1991) have shown in a model of myocardial ischaemia that blocking the transient outward current with 4-aminopyridine (4-AP) markedly reduced the development of dispersion of refractoriness and prevented tachyarrhythmias.
In Dixon et al., 1996 performed a transient transfection of rat Kv4.3 into Xenopus oocytes and demonstrated that, like native mammalian Ito, the resultant current showed rapid voltage-dependent activation and inactivation and relatively rapid voltage-dependent recovery from inactivation. They demonstrated that of the K+ channels expressed in canine heart which are known to encode rapidly inactivating channels (Kv1.4, Kv3.4 and Kv4.3), only Kv4.3 demonstrates pharmacological properties in response to H2O2, TEA and flecainide which match those of native mammalian Ito. The expression of Kv4 genes in human heart is similar to that of canine heart, and this group concluded that the Kv4.3 gene contributed a significant proportion of human Ito. We have performed a stable transfection of the rat Kv4.3 gene into mammalian cells (CHO-K1) and characterized the kinetics of this current in this model, both at room temperature and at 37°C.
There is evidence that n-3 long chain polyunsaturated fatty acids (n-3 PUFA) present in fish oils are protective against the development of ventricular fibrillation (VF) associated with myocardial ischaemia (Kromhoutt et al., 1985; Burr et al., 1989; Siscovick et al., 1995; Albert et al., 1998; McLennan et al., 1988; 1992; Billman et al., 1994), although the mechanism(s) have not yet been established. In view of the finding by Antzelevitch et al. (1991) that blockade of Ito by 4-AP prevents tachyarrhythmias in a model of myocardial ischaemia, we proposed that n-3 PUFA may modulate the Kv4.3 current.
Methods
Molecular biology
The CHO-K1 cells (American Type Culture Collection, Bethesda, MD, U.S.A.) used in the following experiments were maintained in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (Ham) medium (Gibco BRL, Gaithersburg, MD, U.S.A.) supplemented with 5% foetal calf serum. The Kv4.3 cDNA (gift from Dr David McKinnon, State University of New York at Stony Brook, New York, U.S.A.) was cloned into the pRc/CMV eukaryotic expression vector (Invitrogen, San Diego, CA, U.S.A.), which also carries the G418-resistance gene. This construct was then transfected into CHO-K1 cells using the Lipofectamine Reagent (Gibco BRL). Stable transfectants were then selected by prolonged culture with 1000 μg mL−1 G418 (Boehringer, Mannheim). Individual subclones were isolated, expanded and maintained long term in tissue culture. Cells were grown on sterile glass coverslips for 1 day and then used for patch clamping experiments.
Solutions
All experiments were performed in bath solution containing (mmol L−1): NaCl 130, KCl 4.8, MgCl2 1.2, NaH2PO4 1.2, CaCl2 1.0, N-2-hydroxylethylpiperazine-N1-2-ethanesulphonic acid (HEPES) 10 and glucose 12.5 buffered to pH 7.4 with KOH. The pipette solution contained (mmol L−1): K-gluconate 120, KCl 20, ethyleneglycol-bis-(β-aminoethyl ether)N,N,N′,N′-tetraacetic (EGTA) 5, HEPES 10 and MgATP 1.5. The n-3 PUFA Docosahexaenoic acid (DHA) was purchased from ICN Biomedicals Australasia (Sydney, Australia) and prepared as a stock solution in 100% ethanol (maximum final concentration of ethanol in bath solution=0.1%). Experiments were performed at room temperature (20–23°C), unless otherwise stated.
Electrophysiology
Cells plated on coverslips were placed at the bottom of a 2 ml perfusion chamber mounted on the stage of an inverted phase contrast microscope (Nikon Diaphot, Tokyo, Japan) and superfused with bath solution at 1 ml min−1. A constant fluid level was maintained by continuous suction. Electrodes were positioned using a micromanipulator (Narishige WB 90, Tokyo, Japan). A silver/silver-chloride reference electrode was placed directly in the perfusion chamber. Whole cell currents were recorded from round CHO-K1 cells using a single electrode voltage-clamp technique. Junction potentials were calculated using commercial software (Barry, 1994) and all potentials reported below have been corrected accordingly. Cells were patch-clamped using micropipettes fabricated from thin-walled borosilicate glass (Vitrex Microhematocrit Tubes, Modulohm I/S, Denmark) using a Vertical Pipette Puller (Model 720, David Kopf Instruments, California, U.S.A.). Pipettes had resistances between 2 and 6 MΩ. Currents were amplified and filtered on line at 2 kHz with a four pole Bessel filter (−3 dB point) using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, U.S.A.). Currents were digitized at a sampling frequency of 1 kHz for activation and inactivation protocols and 0.33 kHz for repetitive stimuli protocols. Stimulus control and data acquisition were carried out via a microcomputer (IBM Pentium), using commercial software and hardware (pClamp 6.0.1/Digidata 1200, Axon Instruments Inc.).
Linear passive leak currents and capacitance transients were corrected by the P/4 leak subtraction protocol. Whole cell capacitance was determined from transient decay in currents elicited by voltage steps of ±10 mV and ranged between 10 and 78 pF (mean 35±5.0 pF). At least 80% series resistance compensation was achieved in all experiments reported.
Experiments with DHA were performed between 5 and 8 min after its addition to the superfusate. Preliminary experiments (n=3) showed that the vehicle used with these studies (0.1% ethanol) had no effect on the amplitude or kinetics of the Kv4.3 current.
The reversal potential (Vrev) for Kv4.3 current was calculated by tail current measurement (Nabauer et al., 1993). From a holding potential of −90 mV a depolarizing step to 65 mV was applied for 15 ms to fully activate the Kv4.3 current. A 490 ms repolarizing step was then applied to the membrane, to voltages between −35 and −95 mV in 5 mV increments. A plot of observed tail currents versus the repolarizing potential demonstrated a mean Vrev of current reversal of −72.1±1.2 mV (n=7).
Current analysis and statistics
Current analysis was performed using the Clampfit module of the pClamp software. Data are expressed as mean±s.e.mean. Statistical analyses were performed using Prism 2.0 (Graphpad Software, San Diego, CA, U.S.A.). Unpaired t-tests were used for comparisons of control and drug study data. A P value <0.05 was considered significant.
The channel conductance was determined according to the formula
where G is the conductance of potassium, Vt is the test potential at which current is measured, and Vrev is the reversal potential for Kv4.3 current. The instantaneous current-voltage relation was assumed to be linear. The voltage dependence of current activation was determined by fitting the normalized conductance for a given test voltage to the Boltzmann function:
where Gt represents the conductance at test potential Vt, V1/2 the voltage at which the current is half activated and k the slope factor. The voltage dependence of steady state inactivation was also well fit with a Boltzmann function, with V1/2 the voltage at which the channel was at 50% of steady state inactivation. The relationship between drug concentration and current blockade with DHA was determined by fit to the Hill equation:
where ID represents current after exposure to drug, IC control current, D the concentration of drug, IC50 the concentration required for 50% current blockade and n the Hill coefficient.
Results
Characterization of Kv4.3 current
Non-transfected CHO-K1 cells produced outward currents of the order of 100 pA on depolarization from a holding potential of −105 mV to a test potential of +85 mV. This current was negligible compared to that elicited by the same protocol in Kv4.3 transfected CHO-K1 cells, which was of the order 4–10 nA (current density 200 pA pF−1). We have therefore disregarded this endogenous current in our analysis. The Kv4.3 current displayed rapid voltage-dependent activation and rapid inactivation. The time course of inactivation was well fit by a double exponential function and was only weakly voltage dependent. The current was sensitive to inhibition by 4-aminopyridine (complete inhibition at 10 mmol L−1). Only a small amount of current rundown was observed at 20 min, and the average rundown at 8 min was 7.7±12.4% (n=6). Peak current amplitudes for dose-response studies for DHA as described below have been adjusted accordingly.
Current activation: voltage dependence and time course
The Kv4.3 current was elicited by stepping from a holding potential of −105 mV to test potentials ranging from −75 to +125 mV (in 20 mV increments) for 490 ms. The current was measured as the difference between the peak current and the steady state current at the end of the 490 ms step (Figure 1a). Elicited current was maximal at steps to +85 or +105 mV in all cells. From these currents conductances were calculated using equation (1) above. A plot of normalized conductance versus test potential followed a Boltzmann distribution (Figure 1b), with V1/2 of current activation being −6.9±2.5 mV, and slope factor +26.9±2.9 mV (n=10). The time course of activation of Kv4.3 current was well fit with a double exponential function, with τ1=3.8±0.2 ms and τ2=5.3±0.4 ms at +45 mV. Time constants of activation could only be fit reliably for test potentials in the range +5 to +65 mV. Within this voltage range there was no significant dependence of time constants of activation on test potential, although there was a trend to more rapid activation at more depolarized potentials.
Figure 1.
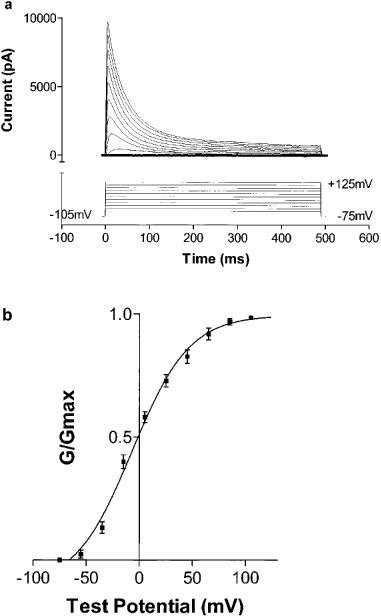
(a) Activation of Kv4.3 current. Cells were held at −105 mV and then stepped to test potentials ranging from −75 to +125 mV (in 20 mV increments) for 490 ms. The lower portion of this Figure illustrates this stimulation protocol, and the upper portion shows the resulting current traces. (b) The normalized conductance was plotted as a function of test potential, and the resulting curve was well fit by a Boltzmann sigmoidal function, with V1/2 of current activation=−6.9±2.9 and slope factor +26.9±2.9 mV (n=10).
Time course of inactivation
Current inactivation was rapid and was well fit by a double exponential function, with time constants τf and τs, with the majority of current inactivation proceeding as the fast process. There was weak dependence upon voltage for τf (25.1±1.5 ms at +5 mV, 18.8±1.0 ms at +65 mV and 18.4±1.2 ms at +85 mV, P<0.01, one-way ANOVA), but τs showed no voltage-dependence. At a test potential of +45 mV τf=20.0±1.2 ms and τs=96.6±6.7 ms (n=9).
Steady state inactivation
The voltage dependence of steady state inactivation was determined by a two step protocol. The first step was of 400 ms duration from a holding potential of −105 mV to a conditioning potential ranging from −105 to +25 mV in 10 mV increments, followed by a step to a fixed test potential of +65 mV for 300 ms (Figure 2a). Currents elicited on stepping to +65 mV were normalized to the maximum current elicited (i.e. at conditioning potential of −105 mV), and plotted against conditioning potential (Figure 2b). The resultant curve was well fit by a Boltzmann distribution, with V1/2 of −51.3±0.2 mV and slope factor of −7.2±0.2 mV (n=11).
Figure 2.
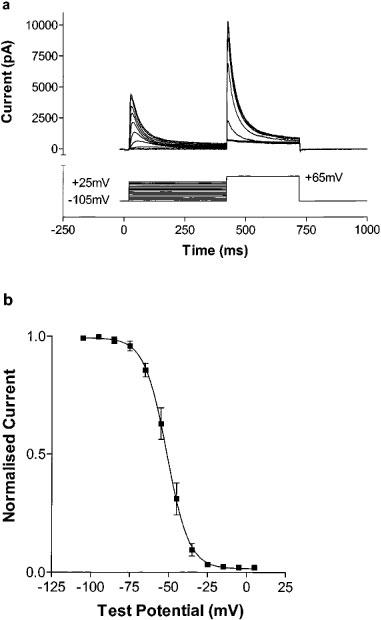
(a) Steady state inactivation of Kv4.3 current. Cells were subjected to a conditioning potential of 400 ms duration ranging from −105 to +25 mV (in 10 mV increments) prior to a 300 ms step to +65 mV. (b) Normalized current as a function of conditioning potential. The resultant curve is well fit by a Boltzmann sigmoidal function, with V1/2 of steady state inactivation of −51.3±0.2 mV and slope factor of −7.2±0.2 mV (n=11).
Time course of recovery from inactivation
The time course of recovery from inactivation was determined by a double stimulus protocol (Figure 3a). The first stimulus (S1) was from a negative holding potential to a test potential of +45 mV for 500 ms, followed by a return to the holding potential for a variable time interval (5–905 ms), and then a second 500 ms test stimulus (S2), also to +45 mV. The amplitude of the current elicited by S2 was normalized to the current elicited by the corresponding S1, and the ratio plotted as a function of the interpulse interval duration. The resultant recovery curves were well fit by monoexponential functions (n=6, Figure 3b). The time course of recovery from inactivation was assessed in this way at five different holding potentials, ranging from −125 to −75 mV. Recovery was markedly slower at less negative holding potentials, with the time constant ranging from 49 ms at −125 mV to 510 ms at −75 mV.
Figure 3.
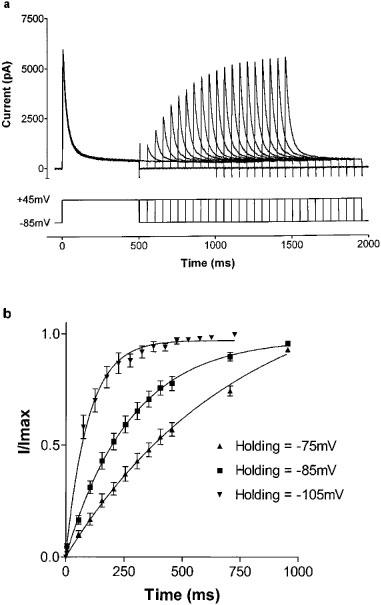
(a) Time course of recovery from inactivation of Kv4.3 current. Cells were held at a polarized membrane potential and then a 500 ms depolarization to +45 mV was applied (S1). The cell was then returned to the polarized holding potential for a variable time interval (5–905 ms) before a second 500 ms duration stimulus, also to +45 mV was applied (S2). Currents elicited by the second depolarization show exponential recovery. (b) Time course of recovery from inactivation of Kv4.3 current. Current elicited by S2 is normalized to that elicited by S1 and plotted as a function of the interpulse interval duration. Recovery from inactivation follows a monoexponential course, and is slower at more depolarized holding membrane potentials (n=6).
Frequency dependence
Frequency dependence of current was assessed by a train of 30 consecutive test pulses to +65 mV, each of 300 ms duration, from a holding potential of −95 mV, at frequencies of 0.33, 0.65, 1.30, 2.0 and 2.60 Hz. The amplitude elicited by the thirtieth stimulus of the train was normalized to that elicited by the first stimulus and plotted against the stimulating frequency. A use-dependent decrease in current became evident only at a frequency of ⩾2 Hz, with steady state current amplitude at 2 Hz being 24.3±3.7% less than the baseline current (n=6).
Effects of temperature
A series of experiments was performed at 37°C. Current inactivation was accelerated at 37°C compared to 20–23°C, with the τf of inactivation decreasing from 20.0±1.2 to 7.6±1.4 ms (n=9, P=0.0002) and τs of inactivation decreasing from 96.6±6.7 to 44.8±6.0 ms (n=9, P=0.0013). The time course of recovery from inactivation was also accelerated at 37°C compared to room temperature (Figure 4), with the time constant of recovery from inactivation at −85 mV decreasing from 276 ms (95% C.I. 245–317 ms, n=8) to 94 ms (95% C.I. 77–120 ms, n=6).
Figure 4.
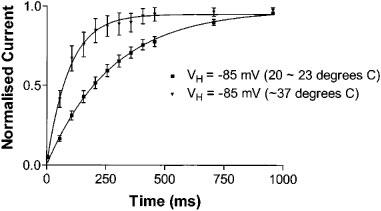
The time course of recovery from inactivation is accelerated at 37°C compared to room temperature, with the time constant of inactivation at −85 mV decreasing from 276 ms (95% C.I. 245–317 ms) to 94 ms (95% C.I. 77–120 ms); n=8, P<0.001.
Effects of the N-3 polyunsaturated fatty acid docosahexaenoic acid
Docosahexaenoic acid blocked the Kv4.3 current in a concentration-dependent manner, with an IC50 of 3.6 μmol L−1, with Hill slope of −0.95±0.31 (Figure 5a and b). To ensure that current blockade was not merely a consequence of a shift in voltage dependence of current activation, measurement of current for all concentrations of DHA were made at a test potential at which the normalized conductance had plateaued at 100%, indicating full channel activation. Washout of ⩾70% of current blockade was consistently demonstrated except at the highest concentration studied (30 μmol L−1), at which washout was more typically between 25 and 50%.
Figure 5.
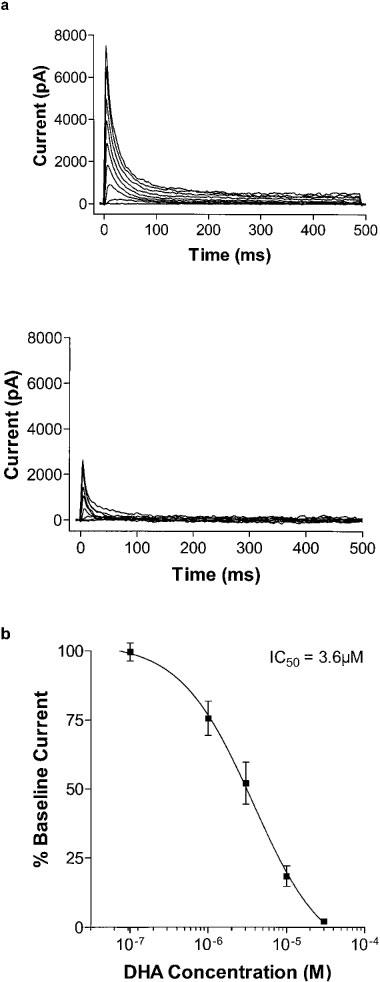
(a) Raw traces showing block of Kv4.3 current with DHA. In this example, 3 μmol L−1 DHA results in 65% block. (b) Dose-response curve for DHA inhibition of Kv4.3 current by DHA. The IC50 is 3.6 μmol L−1, and the Hill slope is −0.95±0.31 (n=5).
There was no significant shift in the V1/2 of current activation. As is the case in the control state, the time course of current inactivation followed a course well fit by a double exponential, with the majority of inactivation proceeding via the faster process. DHA produced a modest acceleration in the time course of current inactivation, with τf at +45 mV decreasing from 20.0±1.2 to 11.2±2.3 ms with 3 μmol L−1 DHA (n=9, P<0.001, Figure 6).
Figure 6.
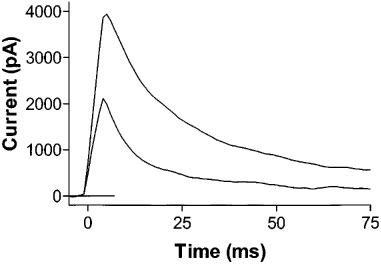
DHA produces a modest acceleration in current inactivation: raw traces from a single cell of current in response to a depolarization to +45 mV showing acceleration of inactivation with 3 μmol L−1 DHA. The τf of inactivation decreased from 20.0±1.2 to 11.2±2.3 ms with 3 μmol L−1 DHA (n=6).
The steady state inactivatioan curve was shifted to more hyperpolarized potentials by DHA, with a statistically significant shift in V1/2 of steady state inactivation from −48.4±1.0 mV in control to −67.1±2.2 mV with 3 μmol L−1 DHA (n=6, P<0.001, Figure 7). However, the shift in voltage dependency of steady state inactivation does not account for the current blockade observed, since at 3 μμol L−1 DHA there is still no steady state inactivation at the holding potential used for the current activation protocol (−105 mV).
Figure 7.
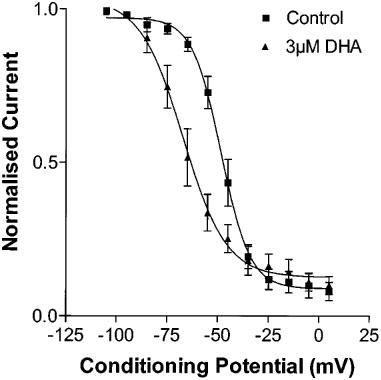
Change in the voltage-dependence of Kv4.3 current steady state inactivation with 3 μmol L−1 DHA. The V1/2 is shifted to the left, from −48.4±1.0 to −67.1±2.2 mV (n=6, P<0.001).
Neither the time course of recovery from inactivation at a membrane potential of −85 mV, nor the response of the Kv4.3 current to repetitive stimuli at 2.0 Hz was significantly altered by 3 μmol L−1 DHA.
Discussion
Characterization of Kv4.3 current stably expressed in CHO-K1 cells
We report stable transfection of rat Kv4.3 into a mammalian cell line and have found that it encodes a current which closely resembles human Ito. It is 4-AP sensitive, and shows rapid, voltage-dependent activation and steady state inactivation and relatively rapid, weakly voltage-dependent inactivation. Recovery from inactivation is also voltage-dependent, being slower at more depolarized membrane potentials. There is a resultant modest rate-dependent reduction of current at stimulation rates above 2 Hz.
These properties of the Kv4.3 current are similar to those reported for the human transient outward current by Nabauer et al. (1993). This group used the patch clamp technique to record currents from enzymatically isolated human ventricular myocytes obtained from the explanted hearts of 22 patients with terminal heart failure. The transient outward current was sensitive to 4-AP, with an IC50 of 1.15 mmol L−1. These investigators found the V1/2 of Ito activation to be +16.7±1.6 mV and the V1/2 of steady state inactivation to be −34.5±2.3 mV. The current inactivated rapidly and was fit with a monoexponential function, with time constant of 54.8±3.7 ms at +40 mV. Recovery from inactivation was voltage dependent and followed a biexponential time course, although the faster process accounted for the vast majority of total current (τfast=41.0±6.5 ms at −80 mV). The ‘frequency-dependent' decrease of peak Ito current was 29.8% at 2 Hz.
Dixon et al. (1996) performed a transient transfection of the K+ channel gene Kv4.3 into Xenopus oocytes and found that the resultant current had biophysical and pharmacological properties similar to human Ito. The Kv4.3 current was rapidly activating and inactivating, with V1/2 of steady state inactivation of −59±0.6 mV. The time course of recovery from inactivation was also voltage dependent, and was best fit with a monoexponential process of time constant of 204 ms at −90 mV. Neither frequency dependent properties nor voltage-dependence of Kv4.3 current activation were reported by Dixon et al. (1996).
Faivre et al. (1999) have performed transient transfection of rat Kv4.3 into the HEK293 mammalian cell line and demonstrated that the resulting current has similar biophysics to rat Ito. We have confirmed these findings with stable transfection of the rat Kv4.3 gene in a mammalian cell line.
Althouth it is established that Ito inactivation and recovery from inactivation are accelerated at 37°C compared to room temperature (Nabauer et al., 1993), to our knowledge the effect of temperature on Kv4.3 current has not been previously reported. We found that the τf of inactivation was accelerated by a factor of 1.8, and that recovery from inactivation (at holding potential of −85 mV) was accelerated by a factor of 2.9. This latter finding is of particular significance, because with relative membrane depolarization (as seen in ischaemia) the recovery from inactivation becomes comparatively slow at room temperature (τ=510 ms at −75 mV). At heart rates above 100 min−1 there would be considerable rate-dependent decrease in current amplitude. However, at physiologic temperature, with more rapid recovery from inactivation, even with relative membrane depolarization there would be virtually no rate-dependent decrease in current amplitude even at heart rates as rapid as 200 min−1. More pronounced membrane depolarization would be required for rate dependent decrease in current magnitude to become apparent.
Effects of docosahexaenoic acid
Epidemiologic studies by Bang et al. (1976) among Greenland Inuits demonstrated a low mortality rate from coronary heart disease despite a total dietary fat intake similar to that of Danes and Americans. The Inuit diet is high in the n-3 PUFAs eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA). Research on the cardiovascular effects of n-3 PUFA initially concentrated on a potential anti-atherogenic role. However, although n-3 PUFA have beneficial effects on platelet aggregation and alter production of vasoactive substances in favour of net vasodilation (Leaf & Weber, 1988), a clinically significant protective effect against atherogenesis has not been demonstrated (Leaf & Weber, 1988; Ascherio et al., 1995; Leaf et al., 1994; Jacobs et al., 1994). Epidemiologic studies have shown a decrease of death from coronary artery disease with fish oil consumption rather than a decrease in the incidence of coronary artery disease (Kromhoutt et al., 1985; Burr et al., 1989; Albert et al., 1998; Ascherio et al., 1995). It has been suggested that this represents a decrease in sudden cardiac death due to an antiarrhythmic effect, and this has been strongly supported by the results of a recent prospective observational study of fish consumption within the US Physicians Health Study (Albert et al., 1998). This study found that for men who consumed at least one fish meal per week the multivariate relative risk of sudden death was 0.48 (95% confidence interval, 0.24–0.96, P=0.04) compared with men who consumed fish less than monthly. The estimated dietary n-3 fatty acid intake from seafood was also associated with a reduced risk of sudden cardiac death. The results of the GISSI-Prevention trial, a randomized clinical trial which includes fish oil supplementation as one of its arms, are expected in the near future (GISSI Investigators, 1993).
Evidence supporting an antiarrhythmic effect as the mechanism of protection has been provided by animal and cellular electrophysiologic studies. It has been demonstrated that fish oil feeding in rats and marmoset monkeys prevents ventricular tachycardia and ventricular fibrillation during both coronary artery occlusion and reperfusion, whereas saturated fat was proarrhythmic (McLennan et al., 1988; 1992). In a dog model of acute myocardial ischaemia-induced ventricular fibrillation, Billman et al. (1994) demonstrated that infusion of an emulsion of n-3 PUFA immediately prior to the acute ischaemic insult prevented ventricular fibrillation in seven out of eight dogs. Cellular studies have reported blockade of Ito (Bogdanov et al., 1998). We proposed that n-3 PUFA may block the Kv4.3 current and set out to test this hypothesis in our model of Kv4.3 stably expressed in CHO-K1.
Xiao et al. (1995) have shown in cultured neonatal rat ventricular myocytes that n-3 PUFA shift the steady state inactivation curve for INa to more hyperpolarized potentials. The implication of this is that the relatively depolarized ischaemic cells have a greater degree of steady state inactivation of INa if exposed to n-3 PUFA with resultant ‘blockade' of INa. These workers suggest that n-3 PUFA act to prolong the duration of the inactivated state of the Na+ channels, analogous to the action of class I antiarrhythmics. However, the class IC antiarrhythmic agents flecainide and encainide were found to increase cardiac mortality post myocardial infarction in the CAST study (Echt et al., 1991). It seems unlikely that the INa blocking effect of n-3 PUFA is responsible for their clinically observed antifibrillatory effect in states of ischaemia.
Hallaq et al. (1992) suggest that the protective effect of n-3 PUFA result from their modulatory effects on nitrendipine-sensitive L-type calcium channels. They showed in neonatal rat cardiac myocytes that DHA and EPA prevented high levels of cytosolic free calcium from developing in response to ouabain due to a 30% reduction in calcium influx rate. DHA was also shown to block the inhibitory effect on calcium influx of the slow calcium channel blocker, nitrendipine. Thus the n-3 PUFA appear to have a dual modulatory role on nitrendipine-sensitive calcium channels, preventing both excessive or deficient influx of calcium. Arachidonic acid [(C20:4(n-6)] did not display this behaviour. Pepe et al. (1994) confirmed these findings in studies of the effects of DHA on L-type Ca2+ channel current (ICa) in isolated rat cardiac myocytes. However, they also found that DHA alone at 5 μmol L−1 had no effect on ICa or on β-adrenergic induced increases in ICa. They concluded that DHA specifically binds to Ca2+ channels at or near dihydropyridine binding sites and thereby interferes with ICa modulation.
There is evidence that the transient outward current is important in the development of dispersion of repolarization which occurs with acute myocardial ischaemia. Antzelevitch et al. (1991) and Lukas & Antzelevitch (1993) have shown that blockade of Ito with 4-AP can abolish this dispersion of repolarization, and thereby prevent tachyarrhythmias. It is proposed that in myocardial ischaemia the inward Na current and the plateau inward currents are reduced and can become totally overwhelmed by the early repolarizing Ito, particularly in epicardial cells where Ito is stronger, with a resultant marked shortening of action potential duration. If this process occurs in a heterogeneous fashion then dispersion of repolarization would occur, and uniform blocking of Ito would be expected to attenuate this development of dispersion of repolarization.
We tested the hypothesis that the n-3 PUFA DHA would block the human Ito, encoded by Kv4.3, and have found the IC50 for inhibition of the Kv4.3 current by DHA to be 3.6 μmol L−1. There was no significant change in the voltage-dependence of current activation, indicating that changes in current magnitude are due to direct inhibition of channel opening by DHA. There was a shift of steady state inactivation to more polarized potentials by DHA (Figure 7), which would mean that in conditions of relative membrane depolarization (as in myocardial ischaemia) there would be a greater degree of steady state inactivation of Kv4.3 current in the presence of DHA. This effect would be additive to the decrease in current resulting from direct inhibition of channel opening by DHA.
In conclusion, we report a stable transfection of the Kv4.3 gene into a mammalian cell line, and have shown that the resulting current closely resembles the human transient outward current. We have shown that the n-3 polyunsaturated fatty acid docosahexaenoic acid blocks this current, and propose that this effect may contribute to the observed protective effects of n-3 PUFA against the development of ventricular fibrillation in myocardial ischaemia.
Acknowledgments
This work was supported by the National Heart Foundation of Australia (Grant-in Aid to Dr Campbell; Research Scholarship to Dr Singleton). C.B. Singleton and S.M. Valenzuela contributed equally to this work.
Abbreviations
- 4-AP
4-aminopyridine
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- Ito
transient outward current
- n-3 PUFA
n-3 polyunsaturated fatty acid
References
- ALBERT C.M., HENNEKENS C.H., O'DONNELL C.J., AJANI U.A., CAREY V.J., WILLETT W.C., RUSKIN J.N., MANSON J.E. Fish consumption and risk of sudden cardiac death. J. Am. Med. Assoc. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- ANTZELEVITCH C., SICOURI S., LITOVSKY S.H., LUKAS A. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ. Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- ASCHERIO A., RIMM E.B., STAMPFER M.J., GIOVANNUCCI E.L., WILLETT W.C. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N. Engl. J. Med. 1995;332:977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- BANG H.O., DYERBERG J., HORNE N. The composition of food consumed by Greenland Eskimos. Acta. Med. Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- BARRY D.M., XU H., SCHUESSLER R.B., NERBONNE J.M. Functional knockout of the transient outward current, long-QT syndrome and cardiac remodelling in mice expressing a dominant-negative Kv4 alpha subunit. Circ. Res. 1998;83:560–567. doi: 10.1161/01.res.83.5.560. [DOI] [PubMed] [Google Scholar]
- BARRY P.H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- BILLMAN G.E., HALLAQ H., LEAF A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGDANOV K.Y., SPURGEON H.A., VINOGRADOVA T.M., LAKATTA E.G. Modulation of the transient outward current in adult rat ventricular myocytes by polyunsaturated fatty acids. Am. J. Physiol. 1998;274:H571–H579. doi: 10.1152/ajpheart.1998.274.2.H571. [DOI] [PubMed] [Google Scholar]
- BURR M.L., GILBERT J.F., HOLLIDAY R.M., ELWOOD P.C., FEHILY A.M., ROGERS S., SWEETNAM P.M., DEADMAN N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial. Lancet. 1989;8666:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- CAMPBELL D.L., RASMUSSON R.L., COMER M.B., STRAUSS H.C.The cardiac calcium independent transient outward potassium: kinetics, molecular properties and role in ventricular repolarisation Cardiac electrophysiology: from cell to bedside 1995Philadelphia, PA: Saunders and Co. Ltd; 83–96.(Second Edition) ed. Zipes, D.P. Jalife, J. pp [Google Scholar]
- DIXON J.E., SHI W., MCDONALD C., YU H., WYMORE R.S., COHEN I.S., MCKINNON D. Role of the Kv4.3 K+ channel in Ventricular Muscle. A molecular correlate for the Transient Outward Current. Circ. Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- ECHT D.S., LIEBSON P.R., MITCHELL B., PETERS R.W., OBIAS-MANNO D., BARKER A.H., ARENSBERG D., BAKER A., FRIEDMAN L., GREENE H.L., HURTHER M.L., RICHARDSON D.W., THE CAST INVESTIGATORS Mortality and morbidity in patients receiving encainide, flecainide, or placebo: the Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- ESCANDE D., COULOMBE A., FAIVRE J.-F., DEROUBAIX E., CORABOEUF E. Two types of transient outward currents in adult human atrial cells. Am. J. Physiol. 1987;252:H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- FAIVRE J.-F., CALMELS T., ROUANET S., JAVRE J.-L., CHEVAL B., BRIL A. Characterisatiaon of Kv4.3 in HEK293 cells: comparison with the rat ventricular transient outward potassium current. Cardiovascular Res. 1999;41:188–199. doi: 10.1016/s0008-6363(98)00215-6. [DOI] [PubMed] [Google Scholar]
- GISSI INVESTIGATORS Protocollo dello studio GISSI-Prevenzione. Studio di intervento sulle componenti atersclerotica e trombotica del rischio post-infarcto. G. Ital. Cardiol. 1993;23:1053–1061. [PubMed] [Google Scholar]
- HALLAQ H., SMITH T.W., LEAF A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS A.K., WEINER B.H., RAIZNER A., SCHOENFELD D.A., SCHEER J., MAHRER P., COTE G., SLACK J., KELLETT M.A., JORGENSEN M., LEAF A. The impact of fish oil on restenosis following coronary angioplasty: the fish oil restonosis trial (FORT) [abstract] J. Am. Coll. Cardiol. 1994;23:59A. [Google Scholar]
- KROMHOUTT D., BOSSCHIETER E.B., COULANDER C. The inverse relation between fish consumption and 20-year mortality from coronary artery disease. N. Engl. J. Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- LEAF A., JORGENSEN M.B., JACOBS A.K., COTE G., SCHOENFELD D.A., SCHEER J., WEINER B.H., SLACK J.D., KELLETT M.A., RAIZNER A.E., WEBER P.C., MAHRER P.R., ROSSOUW J.E. Do fish oils prevent restonosis after coronary angioplasty. Circulation. 1994;90:2248–2257. doi: 10.1161/01.cir.90.5.2248. [DOI] [PubMed] [Google Scholar]
- LEAF A., WEBER P.C. Cardiovascular effects of n-3 fatty acids. N. Engl. J. Med. 1988;318:549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- LUKAS A., ANTZELEVITCH C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischaemia. Role of the transient outward current. Circulation. 1993;88:2903–2915. doi: 10.1161/01.cir.88.6.2903. [DOI] [PubMed] [Google Scholar]
- MCLENNAN P.L., ABEYWARDENA M.Y., CHARNOCK J.S. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am. Heart J. 1988;116:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- MCLENNAN P.L., BRIDLE T.M., ABEYWARDENA M.Y., CHARNOCK J.S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am. Heart J. 1992;123:1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- NABAUER M., BEUCKELMANN D.J., ERDMANN E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993;73:386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- PEPE S., BOGDANOV K., HALLAQ H., SPURGEON H., LEAF A., LAKATTA E. ω3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE J., WINSLOW R., JAFRI S., MARBAN E. Role of Ito in determining action potential duration in rabbit ventricular myocytes. Circulation. 1998;98:I–126. [Google Scholar]
- SISCOVICK D.S., RAGHUNATHAN T.E., KING I., WEINMANN S., WICKLUND K.G., ALBRIGHT J., ARBOGAST P., SMITH H., KUSHI L.H., COBB L.A., COPASS M.K., PSATY B.M., LEMAITRE R., RETZLAFF B., CHILDS M., KNOPP R.H. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. J. Am. Med. Assoc. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- TSENG G.-N., HOFFMAN B.F. Two components of transient outward current in canine ventricular myocytes. Circ. Res. 1989;64:633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- WANG H., DIXON J.E., MCKINNON D. Unexpected and differential effects of Cl (24/Mar/2006 13:54 24/Mar/2006 13:54channel blockers on the Kv4.3 and Kv4.2 K+ channels. Implications for the study of the Ito2 current. Circ. Res. 1997;81:711–718. doi: 10.1161/01.res.81.5.711. [DOI] [PubMed] [Google Scholar]
- XIAO Y., KANG J.X., MORGAN J.P., LEAF A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]


