Abstract
The importance of Ca2+ release from the sarcoplasmic reticulum (SR) in excitation contraction (EC) coupling in human detrusor muscle remains controversial. In this paper the contribution of Ca2+ release to agonist induced contraction is assessed.
Dose response curves to carbachol (0.01–10 μM) were constructed before and after exposure to 200 nM Thapsigargin (Tg). Tg pre-treatment reduced the force of contraction at all agonist concentrations however, the reduction was dose dependent. At 0.1 μM the contractions were reduced to 14.5±7% (mean±s.e.mean) of controls (n=8) while at 10 μM the contractions were only reduced to 92±3% of controls (n=10).
The role of external Ca2+ was examined by measuring the magnitude of contraction to low and high doses of agonist in the presence and absence of external Ca2+. With (0.1–0.3 μM) carbachol the contractions in nominally Ca2+ free media were 4±4% of controls (n=7) whilst with (1–10 μM) carbachol the contractions were 36±8% of controls (n=7) suggesting that at low agonist concentrations the release of Ca2+ has a requirement for external Ca2+.
Pre-treatment of muscle strips with the Ca2+ channel blocking agent diltiazem reduced the contractile responses to carbachol. Contractions induced by 0.1 μM were reduced to 29±11% (P<0.05) of controls while those activated by 10 μM were reduced to 86±6% (P=0.1) of controls (n=4) suggesting the Ca2+ influx needed to activate internal store release at low agonist stimulation is through L-type Ca2+ channels.
These observations confirm the importance of thapsigargin sensitive intracellular Ca2+ store release in the activation of contraction of detrusor smooth muscle and suggest the overall contribution of this store depends upon the magnitude of the agonist stimulation.
Keywords: Human bladder, Ca2+ stores, Ca2+ dependent, Ca2+ release, Ca2+ influx
Introduction
For many smooth muscle cell types, including detrusor smooth muscle, the cellular components involved in excitation contraction (EC) coupling have been identified and described. Information is available on the surface membrane receptors, voltage operated Ca2+ channels, receptor-linked phospholipase C, inositol trisphophate (IP3) production, IP3, Ca2+ activated channels on the sarcoplasmic reticulum, calmodulin and activation of the contractile proteins (Andersson, 1995; Chambers et al., 1996; Fry & Wu, 1997; Iacovou et al., 1990; Mostwin, 1985). The key event in the initiation of a contraction is the rise in cytoplasmic Ca2+. Two sources of Ca2+ have been identified which could contribute to this change; an influx of Ca2+ across the surface membrane and a release of Ca2+ stored within the sarcoplasmic reticulum (SR). The influx could involve at least three types of ion channel; voltage gated Ca2+ channels, receptor operated channels (Andersson, 1995), and Ca2+ release activated Ca2+ channels (Berridge, 1995). Two molecularly distinct intracellular Ca2+ release mechanisms have been identified in smooth muscle; an inositol trisphosphate (IP3) and a Ca2+ and ryanodine sensitive, Ca2+ induced Ca2+ release (RyR-CICR) process (Somlyo & Somlyo, 1994). With respect to human detrusor the relative importance of the contribution of the different Ca2+ influx mechanisms and the release of Ca2+ from the IP3 and RyR-CICR dependent release processes on the SR remains controversial. This paper describes experiments to identify the contribution of Ca2+ influx mechanisms and the release of intracellular Ca2+ release to carbachol induced contractions.
In the normal human bladder the major neurotransmitter is acetylcholine (ACh). However, ATP and numerous neuropeptides are also effective in initiating detrusor contractions (Andersson, 1995; Hasan & Neal, 1995). The current concept is that several neurotransmitters are co-released from the nerve terminals to initiate contraction (Burnstock, 1985). ACh activated contractions do not appear to be the result of membrane depolarization, action potential generation and Ca2+ influx (Brading & Inoue, 1991; Fry & Wu, 1997). Rather, the membrane hyperpolarizes, there is a Ca2+ influx via non selective cation channels and the release of intracellular Ca2+ release via an IP3 dependent mechanism. On the other hand ATP can depolarize the detrusor smooth muscle and initiate Ca2+ dependent action potentials (Fry & Wu, 1997; Inoue & Brading, 1990, 1991). Such complex and varied effects of different neurotransmitters will add to the complexity of EC coupling in the human detrusor in vitro.
In order to assess the contribution of intracellular Ca2+ release to acetylcholine induced contraction one approach is to remove the contribution of Ca2+ stores. Thapsigargin (Tg), at low doses, is known to be a potent irreversible inhibitor of the sarcoplasmic reticulum Ca2+ pump (Thastrup et al., 1994). Concern has been raised about the specificity of Tg when micromolar concentrations are used as at these concentrations it may also block the L-type Ca2+ channels, (Buryi et al., 1995; Shmigol et al., 1995). However at nanomolar concentrations whilst inhibition of Ca2+ uptake into the SR can be demonstrated there does not appear to be an affect on the L-type Ca2+ channel in smooth muscle cells (Buryi et al., 1995). In the intact cell, inhibition of the pump allows Ca2+ to leak from the SR often resulting in a rise in cytosolic Ca2+ with an associated contraction. With prolonged exposure to Tg the Ca2+ content of the SR is reduced and consequently the effectiveness of the SR to contribute Ca2+ during EC coupling is diminished. In many and varied smooth muscle cell types Tg has been reported to decrease the magnitude of agonist induced contractions (Bian et al., 1991; Mikkelson et al., 1988; Neusser et al., 1993). This approach was used in the experiments described in this paper. The data confirm the importance of intracellular Ca2+ release in the activation of contraction of detrusor smooth muscle but reveal a level of complexity not previously reported.
Methods
Isolation of muscle strips
Detrusor muscle biopsies were obtained from patients during open and endoscopic bladder surgery with prior informed consent and with ethical committee approval from Newcastle Area Health Authority. Biopsy specimens were obtained mainly from the posterior and postero-lateral bladder walls during endoscopic surgery and the bladder dome following open surgical approach. In the present study no differences were detected between tissue sample obtained from the different anotomical locations or when the samples were obtained by endoscopy or open bladder surgery. No biopsies were taken from the bladder neck or the trigone area. Samples were collected in sterile RPMI 1640 medium (Life Technologies Ltd, Renfrewshire, U.K.), containing penicillin (100 μg ml−1) and streptomycin (100 g ml−1) and maintained at 4°C during transport from theatre. The detrusor was isolated by careful excision of the mucosal and serosal layers. Muscle strips approximately 5 mm in length and 1 mm in diameter were prepared. Experiments were carried out at 35–37°C in an organ bath containing oxygenated (95% O2 and 5% CO2) Tyrodes solution (mM): NaCl 120, NaHCO3 24, Glucose 6.1, Na Pyruvate 5, KCl 4, CaCl2 1.8, MgCl2 1 and NaH2PO4 0.4 (pH 7.36), supplied by a continuously fed irrigation system (5–7 ml per min). The force generated by the muscle strips was recorded on a computer based data acquisition system (Sensonor and Newcastle Photometric Systems). In all experiments the strips were pre-tensioned to about 11 mN and then allowed to equilibrate for 60–90 min.
Solutions
Carbachol (Sigma) was made up as a 1 mM stock solution in distilled water and further diluted in the experimental solutions to the desired concentration at the time of the experiment. Thapsigargin (Sigma) was dissolved in DMSO and frozen in 2 mM aliquots and stored at −20°C. At the time of the experiment this was thawed and added to the experimental solutions to give a concentration of 200 nM. This concentration of thapsigargin was chosen based on a preliminary series of experiments to determine the effectiveness of low doses of thapsigargin to inhibit the uptake of 45Ca2+ into the SR. Saponin permeabilized detrusor smooth muscle cells maintained in vitro were used as previously described (Chambers et al., 1996, 1999). Tg concentrations between 3–200 nM progressively inhibited the uptake of 45Ca2+, the inhibition reaching a plateau at 200 nM which represented an 85% inhibition of total uptake. Higher concentration had no effect on this residual uptake which may represent non-specific binding of residual accumulation into organelles. On the assumption that cultured human bladder cells will behave as cells in intact tissue this concentration of Tg was chosen for all subsequent experiments. Nominally Ca2+ free solutions were made by excluding Ca2+ and adding the Ca2+ chelator EGTA (Sigma), (2 mM). Diltiazem (Sigma), an L-type Ca2+ channel antagonist was dissolved in 1 ml of DMSO and then made up to 10 ml of 10 mM stock with distilled water before further dilution in the experimental solutions to give a final concentration of 10 μM.
Statistical analysis
Mean data±s.e.mean are shown. Significance was tested using Student's t-test. P values <0.05 were considered significant. Where stated n values represent the number of subjects.
Results
The first series of experiments was done to determine the effect of removal of intracellular Ca2+ release on agonist (carbachol) induced contractions. In each experiment contractions were activated by brief exposure to increasing concentrations of agonist (0.01–10 μM; Figure 1A). Thapsigargin (Tg) was then added to the superfusion medium. In eight out of ten experiments Tg (200 nM) did not cause a contraction (see also Figure 2). This may be interpreted to suggest that, despite Ca2+ pump inhibition, the leak of Ca2+ from the SR is low and insufficient to raise cytosolic Ca2+ enough to initiate a contraction. In order to be certain that the internal stores were depleted of Ca2+ the muscle strips were exposed to a maximal dose of agonist. This will release stored Ca2+ but in the presence of Tg the stores will not refill. The strips were then re-exposed to the same range of concentrations of agonist to initiate contraction (Figure 1A). Tg pre-treatment reduced the amplitude of contraction at both high and low carbachol concentrations. Figure 1B shows the accumulated data from a total of ten experiments from different subjects illustrating the force produced before and after Tg treatment. Over the entire agonist concentration range Tg reduced the force of contraction supporting the idea that intracellular Ca2+ release contributes to contraction (P<0.05 for all concentrations).
Figure 1.
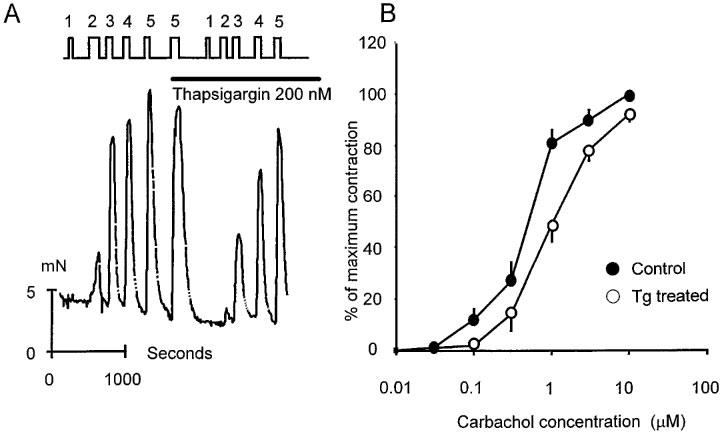
The effect of thapsigargin (Tg; 200 nM) on carbachol induced contractions of human detrusor smooth muscle. (A) Illustrates original data from a single experiment. Ordinate shows force produced by the muscle strip (mN) and the abscissa, time in s. Where indicated carbachol, was added to the superfusing solution. (The concentrations at points 1, 2, 3, 4 and 5 were 0.1, 0.3, 1, 3 and 10 μM respectively). Tg was added where indicated and subsequent exposure to carbachol carried out in the presence of Tg. All experiments were done at 35–37°C. (B) Shows combined data from ten separate experiments. The dose response curve to carbachol is shown for each strip before and after exposure to Tg. Mean values are shown±s.e. mean.
Figure 2.
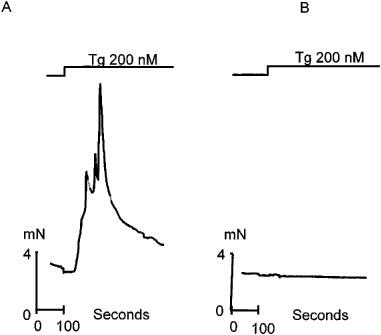
The effects of thapsigargin on resting tension in isolated strips of human detrusor smooth muscle. (A) Shows one of three recordings in which a contraction was observed in response to Tg (200 nM). (B) Illustrates one of 33 recordings in which no overt contraction was seen. All experiments were carried out in gassed Tyrodes solution pH 7.36. Temperature 35–37°C.
Two series of control experiments were done in which either repetitive doses of carbachol were given over the entire dose range or two successive dose response curves were determined. There was no difference seen between the successive sets of responses in either of these series of control experiments (data not shown). Therefore the effects of Tg were not influenced by run down or alterations to the integrity of the preparations.
In other smooth muscle preparations exposure to Tg results in a transient contraction as a consequence of SR pump inhibition and the leakage of Ca2+ from the SR. In a total of 36 experiments on isolated human detrusor smooth muscle an overt contraction was only seen in three strips. Figure 2A illustrates one such contraction. In all of the other muscle strips no contraction was seen (Figure 2B).
There is a clear effect of Tg on subsequent agonist evoked contractions (Figure 1). Therefore, these data suggest that either the leakage of Ca2+ from the SR in human detrusor must be low and that the Ca2+ homeostatic mechanisms are sufficient to cope with an increased cytoplasmic Ca2+ load or that any leaked Ca2+ is successfully buffered away from the myofilaments.
Examination of Figure 1 suggests that Tg treatment has a relatively greater effect on small contractions compared to that on large contractions. This is illustrated directly in Figure 3A where the amplitude of the contraction in the presence of Tg is expressed as a percentage of the contraction in the absence of Tg and plotted against the agonist concentration. With low doses of carbachol, Tg treatment causes a near complete abolition of the contraction. With progressively greater doses of agonist the effect of Tg becomes less so that at the highest agonist concentrations used there is only an 8% reduction in the force of contraction. These data can be re-plotted to illustrate the relationship between the amplitude of contraction and the effectiveness of Tg. Figure 3B shows this relationship. It is clear that for small contractions there is almost a total dependence on intracellular Ca2+ release to activate a contraction. For large contractions there is less reliance on store release. These data can be interpreted to suggest that detrusor smooth muscle relies predominantly on intracellular Ca2+ release to activate small contractions but less so for large contractions. Thus, for large contractions a Ca2+ influx must contribute nearly all the Ca2+ needed to activate the contractile apparatus.
Figure 3.
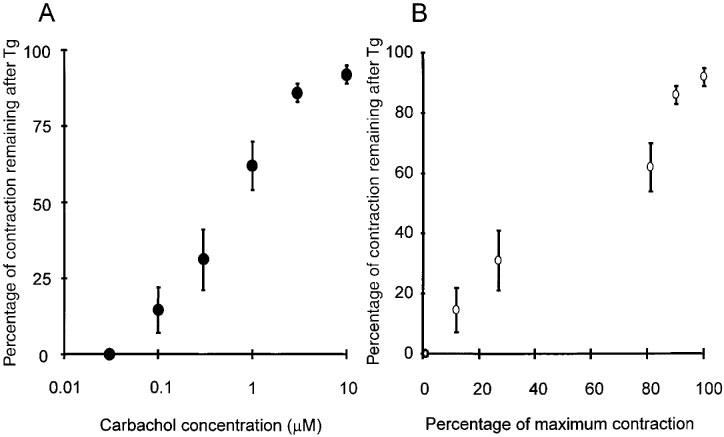
The ratio of amplitudes of contractions in the absence and presence of Tg, expressed as percentages, are plotted against (A) the carbachol concentration and (B) the amplitude of the control contraction in the absence of Tg. Temperature 35–37°C. Mean values are shown±s.e. mean.
If the conclusions drawn from the above experiments are true, then, contractions activated by low doses of agonist should be of similar amplitude in the presence and absence of extracellular Ca2+. Figure 4 illustrates an experiment designed to examine this possibility. The original records in Figure 4A show first a contraction to 0.3 μM carbachol in the presence of external Ca2+ followed by a wash in nominally Ca2+ free medium and exposure to the same carbachol concentration. In the absence of Ca2+ there was no contraction. This was not what was expected from the previous analysis on the effects of Tg. On exposure to high agonist, 10 μM carbachol, a contraction was recorded in both Ca2+ containing and Ca2+ free solutions. Thus, the absence of any response at the low concentration cannot be a consequence of the stores being empty since they can be accessed by the higher concentration. The combined data from seven experiments are shown in Figure 4B.
Figure 4.
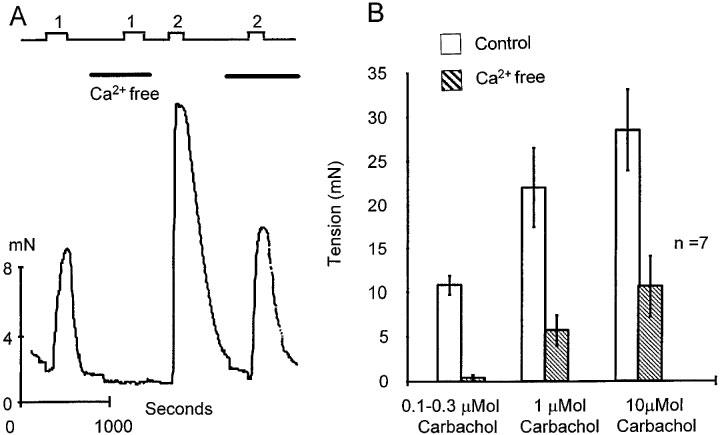
The amplitude of agonist induced contractions in the presence and absence of extracellular Ca2+. (A) Shows original data from a single experiment. Ordinate shows force produced by the muscle strip (mN) and the abscissa, time in s. Where indicated carbachol, at (1), 0.3 μM and (2), 10 μM was added to the superfusing solution. The superfusion solution was changed to a nominally Ca2+ free solution where indicated. (B) Illustrates data accumulated from seven separate experiments. Contractions induced by low (0.1–0.3 μM) moderate (1 μM) and high (10 μM) doses of carbachol, in the presence (open bars) and absence (filled bars) of extracellular Ca2+ are shown. Values are means±s.e.mean and all experiments were done at 35–37°C.
The response to 10 μM carbachol in nominally Ca2+ free was 38±11% (n=4) of the contraction in the presence of external Ca2+. This appears to indicate that the stores contribute a significant amount of Ca2+ to the contraction activated by 10 μM carbachol. This conclusion is in contrast to that derived from the Tg experiments where, at this agonist concentration, only 8% of the Ca2+ used for contraction is derived from the stores. One possible interpretation of these data is that the influx activated at high agonist concentrations reduced the amount of Ca2+ released from the store. This is in keeping with the published data on the effects of Ca2+ in IP3 induced Ca2+ release (Bezprozvanny et al., 1991; Chambers et al.,1996; Hirose et al., 1998; Iino & Endo 1992; Missiaen et al., 1994).
The data in Figure 4 demonstrate that extracellular Ca2+ is important for the initiation of the agonist induced release from the Tg sensitive internal stores. This could suggest that a Ca2+ influx is essential during agonist stimulation in order to trigger intracellular release. Such an influx could occur via voltage operated Ca2+ channels or receptor operated Ca2+ channels or both. An insight into which type of channel might be involved was obtained using diltiazem, a blocker of voltage operated Ca2+ channels (Figure 5). Contractions were activated either by exposure to a low dose of carbachol (0.1 μM) in order to activate a store dependent contraction or by a high dose of carbachol (10 μM) where the contraction is more dependent on an influx of Ca2+. After treating the tissue with dilitiazem (10 μM), low and high doses of agonist were reapplied. (A single high dose of diltiazem was chosen since preliminary experiments showed that this dose produced a maximum effect on contraction). Contractions induced by low doses of carbachol (0.1 μM) were reduced to 29±11% (P<0.05) of controls while those activated by high concentrations (10 μM) were reduced to 86±6% (P=0.1) of controls (mean±s.e.mean; n=4). Thus, if an influx is essential to facilitate intracellular Ca2+ release at low doses of agonist it is likely to occur by diltiazem sensitive voltage operated Ca2+ channels. The absence of any significant effect of diltiazem at high agonist concentrations is consistent with the idea that in this dose range voltage operated channels do not contribute to the influx and that receptor operated channels or possibly other channels are primarily responsible. However at doses of 10 μM carbachol it has been suggested that carbachol may itself inhibit the inward Ca2+ current subsequent to depolarization and if so this could mask any effect of diltiazem (Yoshino & Yabu, 1995).
Figure 5.
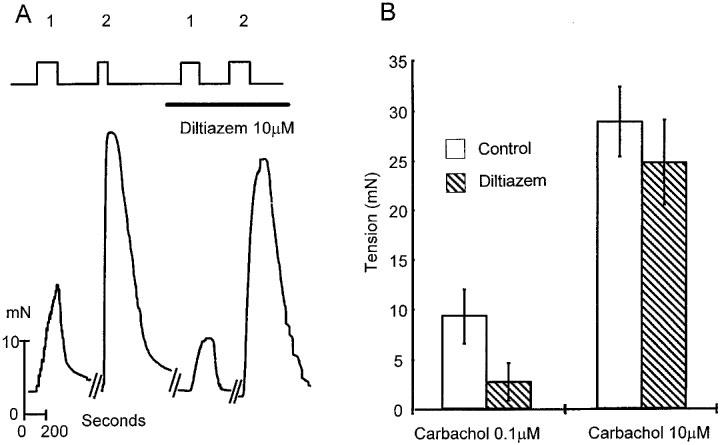
The effect of the Ca2+ channel antagonist diltiazem on agonist induced contractions of human detrusor human smooth muscle. (A) Shows sections of original records from a single experiment. Where indicated carbachol at a concentration of either 0.1 μM (1) or 10 μM (2) was added to the superfusion solution. Also, where shown, diltiazem (10 μM) was added. Ordinate shows force produced by the muscle strip (mN) and the abscissa, time in s. All experiments were done at 35–37°C. (B) Illustrates data from four separate experiments presenting mean data of the amplitudes of contractions at two doses of carbachol in the absence (open bars) and presence (filled bars) of diltiazem (10 μM). Error bars show s.e. mean.
The results presented so far reflect the possibility that there is an absolute requirement for a rise in cytoplasmic Ca2+ in order to facilitate intracellular store release by IP3 at low agonist concentrations. Such a co-operativity has been documented in many cell systems but it is not clear how it operates in human detrusor smooth muscle. For example, is Ca2+ required to facilitate IP3 action or does IP3 act to sensitize the release channel to Ca2+? In the latter mode the IP3 receptor channel complex operates like an IP3 modulated Ca2+ induced Ca2+ release mechanism. The data shown in Figure 6 support the idea that in the human detrusor the IP3 receptor can operate as an IP3 regulated CICR mechanism. Muscle strips were stimulated electrically to produce sub maximal contractions. Carbachol was then added to the superfusion solution at a concentration (0.03 μM) which did not by itself activate a contracture. In the presence of carbachol the amplitude of the electrically evoked contractions increased. This effect was seen in three out of four experiments. This augmentation of contraction was reversed on washing. These data are consistent with the idea that IP3 is a co-factor in the process elevating Ca2+ and activating contraction.
Figure 6.
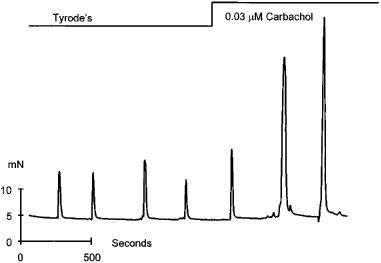
The effect of low dose agonist on the amplitude of electrically evoked contractions in human detrusor human smooth muscle. Ordinate shows force produced by the muscle strip (mN) and the abscissa, time in seconds. Where indicated, electrical stimuli (20 Hz; 20 V; 5 msec pulse width) was applied for periods of 2 s and carbachol (0.03 μM) was added to the superfusing solution. All experiments were done at 35–37°C.
Discussion
The major observation reported in this paper is that the intracellular Ca2+ stores contribute significantly to the rise in cytosolic Ca2+ needed to initiate contractions in human detrusor smooth muscle. This result on human detrusor smooth muscle is consistent with reports on a variety of smooth muscle types (Maggi et al., 1989; Neusser et al., 1993), and also on rabbit bladder smooth muscle which show that thapsigargin (Tg) treatment reduces the magnitude of agonist induced contraction (Damaser et al., 1997). An unexpected and new observation was that the effectiveness of Tg appeared to depend on the concentration of agonist used. One way to interpret these observations is to suggest that small contractions are activated using Ca2+ derived predominantly from the internal stores while large contractions rely almost entirely on an influx of Ca2+ from the external media. Therefore, if care is not taken to explore the effects of Tg over a suitable range of stimuli then it is possible that a false conclusion may be reached regarding the role and importance of intracellular store release and influx mechanisms. This also adds a further complication in the analysis of EC coupling in vivo where the precise stimulus and concentration of neurotransmitter may not be known.
At low concentrations of agonist the Tg data indicate that the internal stores are the predominant source of Ca2+ for contraction. However, the data presented in Figures 4 and 5 strongly suggest that this Tg sensitive intracellular release is highly dependent on an influx of external Ca2+. There are several possibilities which might account for this observation. It has been suggested that in some cell types the internal stores are depleted of Ca2+ at rest and it is only when the cells are stimulated resulting in an influx of Ca2+ that the stores fill and are subsequently able to release Ca2+ (Berridge, 1997). This may be the case here but the observation that high doses of agonist can release Ca2+ would argue against this. Alternatively, it may be that an influx of Ca2+ and a rise in cytoplasmic Ca2+ concentration is essential in order to facilitate IP3 dependent release. This is in keeping with the positive feedback of Ca2+ on the IP3 receptor channel complex. In the concentration range 100–300 nM, Ca2+ exerts a positive feed back on the release channels such that Ca2+ release is promoted. At cytoplasmic Ca2+ concentrations greater than 10 μM the IP3 activated release channel is inhibited and Ca2+ cannot be released from the store (Bezprozvanny et al., 1991; Chambers et al., 1996; Iino & Endo 1992; Missiaen et al., 1994). Thus, the Ca2+ dependence of the IP3 dependent Ca2+ release process can be described as ‘bell-shaped'. A rise in cytoplasmic Ca2+ is essential to sensitize the IP3 receptor channel complex to low concentrations of IP3 in order to initiate release. The data shown in Figure 6, demonstrating that low doses of agonist augment electrically induced contractions, are consistent with this mode of operation of the IP3 Ca2+ release system. Electrical activity gives rise to a Ca2+ influx which can activate CICR and the combined changes in Ca2+ trigger contraction. In the presence of low levels of IP3, the IP3 receptor channel complex is primed but not yet activated. When the cytoplasmic Ca2+ rises this allows the IP3 channel to open and Ca2+ to be released. The additional increase in Ca2+ from the stores augments the rise in cytoplasmic Ca2+ and increases the force of contraction.
The observations that, with Tg at high agonist concentrations, 8% of the Ca2+ comes from the stores while the experiments with nominally Ca2+ free solutions indicates that the stores can contribute 36% deserve comment. It would appear that in the presence of external Ca2+ the influx reduces the effective release of stored Ca2+. This is in keeping with the published data on the Ca2+ dependent inhibition of IP3 dependent Ca2+release (Bezprozvanny et al., 1991; Chambers et al., 1996; Iino & Endo 1992; Missiaen et al., 1994).
It is clear from this analysis that an influx of Ca2+ is essential in order to activate the human detrusor smooth muscle. There are reports using L-type Ca2+ channel blockers, that cholinergic stimulation does not involve activation of voltage dependent Ca2+ channels and an influx of Ca2+ via this route (Fry & Wu, 1997). However, there are contrary reports suggesting that Ca2+ channel blockade does reduce contractions activated by agonists (Damaser et al., 1997; Fovaeus et al., 1987; Maggi et al., 1989). In the present experiments a distinct difference was noted in the action of Ca2+ channel blockers depending on the agonist concentration and the magnitude of the induced contraction. Small contractions are blocked by diltiazem but not large contractions. This implies that L-type Ca2+ channels are activated at low agonist concentrations. Additional Ca2+ influx pathways, possibly receptor operated Ca2+ channels or store regulated Ca2+ entry mechanisms, are activated at higher agonist concentrations. It is not possible, at this stage, to identify which might be responsible for this additional Ca2+ influx. At present, there are no estimates of the in vivo concentrations of agonist. Therefore, it is not possible to determine the events contributing to EC coupling in vivo. The situation may be more complex if more than one transmitter substance is co-released from nerve terminals in the bladder (Burnstock, 1985).
The interpretation of these data presented here are based upon the assumption that the magnitude of contraction is only related to the intracellular Ca2+ concentration. In rabbit detrusor when the contractile force and the intracellular Ca2+ have been measured simultaneously, then the increasing contractile response correlated with a nearly proportional increase in the magnitude of the free intracellular Ca2+ (Levin et al., 1991). However, in this study bethanechol stimulated contractions were completely blocked, a result which differs from that reported here. The reasons for these experimental differences are not clear but may be related to species differences. Other results have been described in rat detrusor where contractile responses to bethancol in various degrees of obstruction correlated with a nearly proportional increase in the magnitude of the free intracellular Ca2+ (Saito et al., 1994). No data are published for human detrusor and therefore we have presumed the results from these animal studies are applicable and that contraction in response to application of muscarininc agonists is dependent upon a proportional rise in the intracellular Ca2+ and proportional production in force. This is clearly an over simplification since it is now well recognized that there are a large number of complex intracellular signalling events between agonist binding and force development. However, the data presented in this paper do not totally rely on a detailed appreciation of these complexities. The major point to be made from this work is that the apparent effectiveness of Tg and hence the apparent contribution of intracellular Ca2+ release to contraction is related to the magnitude of the stimulus and correlates with the magnitude of contraction.
In addition, in smooth muscle, agonist stimulation can give rise to phenomenon of ‘Ca2+ sensitization' of the contractile apparatus (Somlyo & Somlyo, 1994). In relation to the data described in this paper of the effects of Tg the results cannot be explained in relation to Ca2+ sensitization. This is because, the carbachol doses are the same in both the absence and presence of Tg. However, the increase in contraction seen on the application of low dose agonist may also occur if the agonist were to sensitize the contractile apparatus to Ca2+ being elevated by the electrical activity. Subsequent to these interesting preliminary experiments, there is clearly a need to measure intracellular Ca2+ and tension simultaneously in human detrusor whilst repeating the experiments and this will be the focus of a future study.
Acknowledgments
We would like to thank the Royal College of Surgeons of England, the Freeman Hospital Trustees and the Northern Region Health Authority for financial support for this project. We would also like to thank Drs H.A. Otun and P. Chambers for constructive discussions throughout the work.
Abbreviations
- ACh
acetylcholine
- EC
excitation contraction
- IP3
inositol trisphophate
- RyR-CICR
ryanodine sensitive, Ca2+ induced Ca2+ release
- SR
sarcoplasmic reticulum
- Tg
thapsigargin
References
- ANDERSSON K-E.Smooth Muscle Physiology The Bladder 1995Edinburgh, U.K.: Churchill Livingstone; 17–46.ed. Fitzpatrick J.M. Krane J.K. pp [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE M.J. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- BEZPROZVANNY I., WATRAS J., EHRLICH B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- BIAN J.H., GHOSH T.K., WANG J.C., GILL D.L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J. Biol. Chem. 1991;15:8801–8806. [PubMed] [Google Scholar]
- BRADING A.F., INOUE R. Ion channels and excitatory transmission in the smooth muscle of the urinary bladder. Zeitschrift fur Kardiologie. 1991;80:47–53. [PubMed] [Google Scholar]
- BURNSTOCK G. Nervous control of smooth muscle by transmitters, co-transmitters and modulators. Experientia. 1985;41:869–874. doi: 10.1007/BF01970003. [DOI] [PubMed] [Google Scholar]
- BURYI V., MOREL N., SALOMONE S., KERGER S., GODFRAIND T. Evidence for a direct interaction of thapsigargin with voltage-dependent Ca2+ channel. Naunyn-Schmiedebergs Archives of Pharmacology. 1995;351:40–45. doi: 10.1007/BF00169062. [DOI] [PubMed] [Google Scholar]
- CHAMBERS P., NEAL D.E., GILLESPIE J.I. Ca2+ signalling in cultured smooth muscle cells from human bladder. Exp.Physiol. 1996;81:553–564. doi: 10.1113/expphysiol.1996.sp003958. [DOI] [PubMed] [Google Scholar]
- CHAMBERS P., NEAL D.E., GILLESPIE J.I. Ryanodine receptors in human bladder smooth muscle. Exp. Physiol. 1999;84:41–46. doi: 10.1111/j.1469-445x.1999.tb00070.x. [DOI] [PubMed] [Google Scholar]
- DAMASER M.S., KIM K-B., LONGHURST P.A., WEIN A.J., LEVIN R.M. Calcium regulation of urinary bladder function. J. Urol. 1997;157:732–738. [PubMed] [Google Scholar]
- FOVAEUS M., ANDERSSON K-E., BATRA S., MORGAN E., SJÖGREN C. Effects of calcium, calcium channels blockers, and Bay K8644 on contractions induced by muscarinic receptor stimulation of isolated bladder muscle from rabbit and man. J. Urol. 1987;137:798–803. doi: 10.1016/s0022-5347(17)44214-5. [DOI] [PubMed] [Google Scholar]
- FRY C. H. &, WU C. Initiation of contraction in detrusor smooth muscle. Scand. J. Urol. Nephrol. 1997;184 31 (Suppl):7–14. [PubMed] [Google Scholar]
- HASAN S.T., NEAL D.E. Diferrent actions of carbachol, histamine, bombesin and neuropeptide Y on isolated human detrusor muscle. J. Physiol. 1995;483P:117P. [Google Scholar]
- HIROSE K., KADOWAKI S., IINO M. Allosteric regulation by cytoplasmic Ca2+ and IP3 of the gating of IP3 receptors in permeabilized guinea-pig vascular smooth muscle cells. J. Physiol. 1998;506:407–414. doi: 10.1111/j.1469-7793.1998.407bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IACOVOU J.W., HILL S.J., BIRMINGHAM A.T. Agonist-induced contraction and accumulation of Inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J. Urol. 1990;144:775–779. doi: 10.1016/s0022-5347(17)39590-3. [DOI] [PubMed] [Google Scholar]
- IINO M., ENDO M. Calcium-dependent immediate feedback control of Inositol 1,4,5-trisphosphate-induced Ca2+ release. Nature. 1992;360:76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- INOUE R., BRADING A.F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br. J. Pharmacol. 1990;100:619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., BRADING A.F. Human, pig and guinea-pig bladder smooth muscle cells generate similar inward currents in response to purInoceptor activation. Br. J. Pharmacol. 1991;103:1840–1841. doi: 10.1111/j.1476-5381.1991.tb12338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN R.M., HYPOLITE J., LONGHURST P.A., WEIN A.J. Comparison of the contractile and metabolic effects of muscarinic stimulation with those of KCl. Pharmacol. 1991;42:142–150. doi: 10.1159/000138791. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., PATACCHINI R., TURINI D., BARBANTI G., GIACHETTI A., MELI A. Multiple sources of calcium for contraction of the human urinary bladder muscle. Br. J. Pharmacol. 1989;98:1021–1031. doi: 10.1111/j.1476-5381.1989.tb14634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKKELSON E. O., THASTRUP O., BRØGGER CHRISTENSEN. E. Effects of Thapsigargin in isolated rat aorta. Pharmacol. Toxicol. 1988;62:7–11. doi: 10.1111/j.1600-0773.1988.tb01835.x. [DOI] [PubMed] [Google Scholar]
- MISSIAEN L., DE SMEDT H. , PARYS J.B., CASTEELS R. Co-activation of Inositol triphophate-induced Ca2+ release by cytosolic Ca2+ is loading dependent. J. Biol. Chem. 1994;269:7238–7242. [PubMed] [Google Scholar]
- MOSTWIN J.L. Receptor operated intracellular calcium stores in the smooth muscle of the guinea pig bladder. J. Urol. 1985;133:900–905. doi: 10.1016/s0022-5347(17)49277-9. [DOI] [PubMed] [Google Scholar]
- NEUSSER M., GOLINSKI P., ZHU Z., TEPEL M., ZIDEK W. Effects of protein kinase C activation on intracellular Ca2+ distribution in vascular smooth muscle cells of spontaneously hypertensive rats. J. Vasc. Res. 1993;30:116–120. doi: 10.1159/000158983. [DOI] [PubMed] [Google Scholar]
- SAITO M., HYPOLITE J.A, , WEIN A.J., LEVIN R.M. Effect of partial outflow obstruction on rat detrusor contractility and intracellular free calcium concentration. Neurourol. Urodyn. 1994;13:297–305. doi: 10.1002/1520-6777(1994)13:3<297::aid-nau1930130311>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- SHMIGOL A., KOSTYUK P., VERKHRATSKY A. Dual action of thapsigargin on calcium mobilization in sensory neurons: Inhibition of Ca2+ uptake by caffeine-sensitive pools and blockade of plasmalemmal Ca2+ channels. Neuroscience. 1995;65:1109–1118. doi: 10.1016/0306-4522(94)00553-h. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- THASTRUP O., DAWSON A.P., SCHARFF O., FODER B., CULLEN P.J., DROBAK B.K., BJERRUM P.J., CHRISTENSEN S.B., HANLEY M.R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1994;43:187–193. doi: 10.1007/BF01986687. [DOI] [PubMed] [Google Scholar]
- YOSHINO M., YABU H. Muscarinic suppression of Ca2+ current in smooth muscle cells of the guinea-pig urinary bladder. Exp. Physiol. 1995;80:575–587. doi: 10.1113/expphysiol.1995.sp003868. [DOI] [PubMed] [Google Scholar]


