Abstract
We assessed whether pharmacological inhibition of CuZn-superoxide dismutase (SOD) mimics the molecular mechanism of either in vitro or in vivo nitrovasodilator tolerance.
In endothelium-intact aortic rings from in vivo tolerant rabbits the GTN- and acetylcholine (ACh)-induced maximal relaxation was attenuated by 36 and 23%, respectively. In vitro treatment of control rings with GTN (1 h 10 μM) similarly attenuated the vasorelaxant response to GTN, but not to ACh.
Formation of superoxide radicals (•O2−) in endothelium-intact rings (lucigenin-chemiluminescence) increased 2.5 fold in in vivo tolerance, but significantly decreased in in vitro tolerance. The membrane associated NADH oxidase activity was increased 2.5 fold in homogenates of in vivo tolerant aortae, but was not changed in in vitro tolerant aorta.
Conversely, SOD activity and protein expression was halved in in vivo tolerance, but SOD activity was not altered by in vitro tolerance. The •O2− scavenger tiron (10 mM) effectively restored the vasorelaxant response to GTN in in vivo tolerant aortic rings, but not the reduced response to GTN in in vitro tolerant rings.
Pretreatment (1 h) of vessels with diethyldithiocarbamate (DETC; 10 mM) attenuated vasorelaxant responses to GTN and ACh, increased vascular •O2− production, and inhibited SOD activity in vessel homogenates to a similar degree as observed in in vivo tolerance. DETC-treatment of in vivo-tolerant vessels induced an additional increase in •O2− production.
Increased •O2− production in in vivo nitrate tolerant aorta is associated with activation of vascular NADH oxidase and inactivation of CuZnSOD. Therefore, in vivo tolerance can be mimicked by in vitro inhibition of CuZnSOD, but not by in vitro exposure to GTN, which does not affect vascular •O2− production, NADH oxidase and CuZnSOD.
Keywords: Glycerol trinitrate, nitrate tolerance, superoxide anion radical, CuZn-superoxide dismutase, NADH oxidase, diethyldithiocarbamate, tiron, rabbit aorta
Introduction
The vasodilator capacity of organic nitrates is rapidly attenuated due to the development of nitrate tolerance (Münzel et al., 1996a; Roth et al., 1987). The mechanisms underlying this phenomenon are likely to be multifactorial and involve both neurohormonal adjustments and changes in intracellular glyceryl trinitrate (GTN) metabolism (Packer et al., 1987; Elkayam et al., 1992). Recently we demonstrated that prolonged treatment of rabbits with GTN increases vascular •O2− production (Münzel et al., 1995b) and that diphenylene iodonium, an inhibitor of flavin-dependent enzymes, corrected •O2− production in these vessels (Münzel et al., 1995b). In these studies, we excluded a significant contribution of xanthine oxidase, NO-synthase, and mitochondrial NADH-dehydrogenases as sources of •O2−. In additional studies, we found that the activity of a membrane bound NADH-driven oxidase was increased in vessels from nitrate tolerant animals (Münzel et al., 1995a,1995b). This finding was somewhat unexpected, since it has been reported that the acute administration of NO or nitrovasodilators inhibits the activity of the NADPH oxidase in neutrophils (Clancy et al., 1992). These findings would suggest that either the vascular oxidase systems are very different from the neutrophil oxidase, or that the acute in vitro administration of NO affects the vascular system differently than prolonged in vivo administration.
A related issue is the method of inducing nitrate tolerance. Often, tolerance is induced by short-term exposure of isolated blood vessels in vitro to high concentrations of GTN (Needleman, 1970; Needleman et al., 1973; Needleman & Johnson, 1973). In general, this approach may be questioned since in vitro tolerance lacks the physiological counterregulatory mechanisms such as an activation of the renin-angiotensin system.
In order to reveal potential differences in the molecular mechanisms underlying in vivo and in vitro nitrate tolerance we examined relaxant responses to GTN and ACh, vascular •O2− production, NADH oxidase and SOD activity of isolated rabbit aortic rings, either from in vivo GTN-tolerant rabbits, or after acute exposure to GTN in vitro. Furthermore, we assessed whether acute inhibition of CuZnSOD by diethyldithiocarbamate (DETC)-treatment of isolated aortic rings in vitro could mimic the effects of in vivo nitrate tolerance with respect to changes in nitrovasodilator-responsiveness and •O2− formation.
Methods
Animal model, in vivo nitrate tolerance
New Zealand White rabbits of either sex, weighing 3–4 kg were studied. A region either on the dorsal aspect of the thorax or between the scapulae was shaved and a GTN patch was applied to the skin. This treatment period was started between 0800 h and 1000 h and the GTN patch was changed each morning of the ensuing days. On the morning of the third day following initiation of GTN treatment, the animals were given an intravenous injection of 100 U of heparin and sufficient pentobarbital to produce death. The chest was then rapidly opened and the descending thoracic aorta removed. Rabbits of a similar size and sex distribution served as controls.
Vessel preparation and organ chamber experiments
The aorta was placed in chilled Krebs buffer and cleaned of excessive adventitial tissue. Eight 5 mm ring segments of thoracic aorta were suspended in individual organ chambers (25 ml) filled with carbogen-equilibrated Krebs buffer of following composition (mM): NaCl, 118.3; KCl, 4.69; CaCl2, 1.87; MgSO4, 1.20 K2HPO4, 1.03; NaHCO3 25.0; and glucose 11.1; pH: 7.40. During the following hour the resting tension was increased to optimize contractions to KCl (80 mM) as described (Münzel et al., 1996b). This optimum occurred at 5 g resting tension for both, GTN tolerant and control aortic rings. Control and tolerant rings were then preconstricted with phenylephrine (0.1–0.3 μM) to achieve 30–50% of maximal tone. When the tone had reached a stable plateau GTN and ACh were applied to the organ baths in cumulative concentrations (1 nM–3 μM in semi-logarithmic concentration steps) and relaxant responses were continuously recorded.
To induce in vitro tolerance aortic rings were incubated in carbogenated Krebs buffer with 10 μM GTN for 1 h or with 10 mM DETC for 30 min.
Measurement of •O2− production in endothelium-intact vessel segments
Superoxide production in endothelium-intact aortic segments from control and GTN treated animals was measured using lucigenin chemiluminescence. The details of this method have been reported previously (Münzel et al., 1995b; 1996b). Briefly, after preparation the segments were equilibrated for 30 min at 37°C in modified Krebs buffer pH 7.4 containing 20 mM N - [2 - hydroxyethyl] piperazine - N′ - [2 - ethanesulphonic acid] (HEPES). Scintillation vials containing 1.5 ml Krebs/HEPES buffer with 500 μl lucigenin (250 μM) were placed into a scintillation counter switched to the out of coincidence mode. After 15 min, background counts were recorded and a vascular segment then added to the vial. Scintillation counts were recorded after 15 min and the respective background subtracted. The vessels were then dried for 24 h at 90°C, allowed to cool and weighed. In a separate set of experiments the effects of in vitro incubation with either GTN (10 μM, 1 h) or DETC (10 mM, 30 min) on •O2− production were determined.
Measurement of •O2− production in vessel homogenates
All vessels were homogenized on ice with a motor-driven glass/glass tissue homogenizer for 2 min in phosphate buffered saline. The homogenate was then centrifuged at 750×g for 1 min. The pellet was discarded and the supernatant stored on ice until use. The protein content was measured in an aliquot of the homogenate by the method of Bradford (1976).
NADH oxidase activity was measured by chemiluminescence in a scintillation vial containing Krebs/HEPES buffer, 250 μM lucigenin and 100 μM NADH as the substrate. No oxidase activity could be measured in the absence of NADH. The reaction was always started by addition of 25 μl homogenate (25–50 μg protein). Lucigenin chemiluminescence was measured after addition of NADH for 9 min. The area under the curve of chemiluminescence vs time was integrated and converted to nmol •O2− using a xanthine/xanthine oxidase standard as previously described (Münzel et al., 1996b).
In further experiments NADH-driven •O2− production was measured with membranes of aortas from control rabbits and rabbits treated with GTN for 3 days, as well as from aortic rings incubated with GTN (10 μM) or DETC (10 mM). This was accomplished by centrifuging the supernatant from the 750×g spin for 30 min at 50,000×g. The pellet was resuspended in 200 μl of Krebs/HEPES buffer.
SOD activity assay
To assess the SOD activity of control and tolerant aortic rings these were homogenized (10% w v−1) in KH2PO4 (50 mM), EDTA, pH 6.5 (0.05 mM). The homogenates were cleaned of debris by centrifugation at 10,000×g for 20 min. Thereafter, SOD activity was assessed in the supernatants by measuring the rate of SOD-sensitive autooxidation of 6-hydroxydopamine (6-HDOPA). In contrast to the cytochrome C and the nitroblue tetrazolium assay, this method is insensitive to interference by DETC (A Mülsch, unpublished). Autooxidation of 6-HDOPA is catalyzed by •O2−, yields a red adrenochrome (λmax 490 nm), and is inhibited by SOD in a concentration-dependent fashion. The aortic extracts (10–100 μg protein in 0.9 ml homogenization buffer) were incubated at room temperature in plastic cuvettes. The reactions were started by addition of 6-HDOPA (final concentration 0.2 mM; 0.1 ml 2 mM stock solution dissolved in N2-gassed bidest). The increase in absorbance at 490 nm was continuously monitored for 3 min in a double-beam spectrophotometer. In the absence of tissue extracts the absorbance increased by 0.10±0.03 units min−1 due to spontaneous oxidation of 6-HDOPA. This rate was decreased by assessing the rate of absorbance changes in the presence of known amounts of pure CuZnSOD (bovine erythrocytes, Sigma). An apparently linear concentration/rate-relationship was observed from 0.1–0.5 U SOD.
Western blot analysis
Rat aortic tissue was homogenized in ice cold homogenization buffer containing (mM) NaCl 99.01, KCl 4.69, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, K2HPO4 1.03, Na-HEPES 20, D-glucose 11.1, aprotinin 0.0015, leupeptin 0.0107, pepstatin 0.0102 and phenylmethylsulphonyl fluoride (PMSF) 0.0028 using a glass/glass homogenizer. The homogenate was centrifuged with a low speed spin at 3000×g for 5 min to remove insoluble material. A total of 15μg protein was subjected to a SDS–PAGE and transferred to nitrocellulose membranes (Bio-Rad). Immunoblotting was performed with a polyclonal sheep antibody to human erythrocyte CuZnSOD for 2 h at room temperature (1 : 3000 dilution, Transduction Laboratories, Lexington, KY, U.S.A.). Immunodetection was accomplished with an anti sheep secondary antibody (1 : 2000 dilution, Upstate Biotechnology, Lake Placid, NY, U.S.A.) and the enhanced chemiluminescence kit (Amersham). Quantification of the 16 kDa CuZnSOD subunits was performed by densitometry. The signals were integrated and the results expressed as per cent of controls. In separate experiments an ultracentrifugation step was included to separate cytosolic and membrane fractions (100,000×g for 1 h).
Materials
GTN was supplied by Pohl Boskamp, Hohenlockstedt, Germany. All other chemicals were purchased from Sigma (Deisenhofen, Germany).
Statistical analysis
Results are expressed as mean±s.e.mean. The EC50 value for each experiment was obtained by logit transformation. To compare NADH-driven •O2− production in normal and nitrate tolerant vessels, one way ANOVA was employed. Comparisons of vascular responses were performed using multivariate analysis of variance with vessel treatment (control vs tiron treated) as independent variables and per cent relaxation and EC50 as dependent variables. A Scheffe's post hoc test was used to examine differences between groups when significance was indicated. P values <0.05 were considered significant.
Results
Effects of in vivo and in vitro glyceryl trinitrate treatment or in vitro treatment with DETC on GTN- and ACh-induced vasorelaxation
In control rabbits, GTN dose dependently relaxed rabbit aorta with a maximal relaxation of 94±2%. Both in vitro tolerance caused by 1 h incubation of aortic rings from control rabbits with GTN (10 μM) or in vivo tolerance with GTN for 3 days significantly attenuated relaxations to GTN to a similar extent (in vivo tol: 57±3%, in vitro tol. 56±3%; Figure 1A). As reported previously, in vivo tolerance, but not in vitro tolerance was associated with cross tolerance to endogenous nitric oxide released by ACh (Figure 1B). Incubation of intact aortic rings with DETC reduced maximal relaxation in response to GTN and to the endothelium dependent vasodilator ACh (Figure 1, Table 1).
Figure 1.
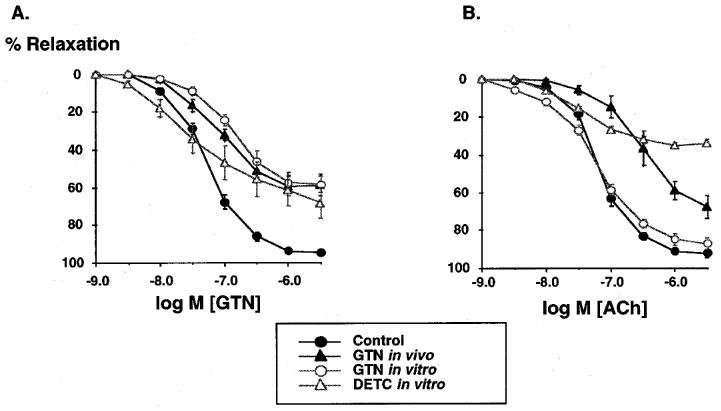
Effects of in vivo treatment with glyceryl trinitrate (GTN) or in vitro treatment with GTN or DETC on the relaxations to GTN (1 nM–30 μM, A) and to acetylcholine (ACh, 1 nM–30 μM, B) in rabbit aortic ring segments. Segments were preconstricted with phenylephrine (0.1 μM) and relaxations to cumulative concentrations of GTN and ACh were examined. In vivo treatment with GTN and in vitro incubation with GTN and DETC markedly attenuated the dose response relationship to GTN. In contrast, ACh was attenuated in response to in vivo GTN treatment or incubation with DETC, but remained unchanged following GTN incubation. Data are mean±s.e.mean from 6–10 independent experiments.
Table 1.
Effects of in vivo treatment with GTN or in vitro treatment with GTN or DETC on potency and maximal relaxations to GTN and ACh

Effects of in vivo or in vitro GTN or in vitro DETC treatment on •O2− production
In control aortic segments with intact endothelium lucigenin-enhanced chemiluminescence values averaged 820±102 counts mg−1 min−1 (n=8). Incubation of aortic rings with 10 μM GTN had no significant effect vascular •O2− production (780 ±125 counts mg−1 min−1). In vivo treatment with GTN caused a marked increase in vascular •O2− production to 2262±406 counts mg−1 min−1. Incubation of aortic rings with DETC increased lucigenin-enhanced chemiluminescence to a similar degree (2403±358 counts mg−1 min−1, P<0.05). Incubation of in vivo tolerant tissue with DETC caused a further significant increase to 3370±421 counts mg−1 min−1 (n=4).
Effects of in vivo or in vitro treatment with GTN or in vitro DETC treatment on the activity of vascular NADH oxidase
In vessel homogenates from animals treated with GTN for 3 days (in vivo tolerance) the NADH-dependent •O2− formation was increased approximately 2 fold compared to controls (7.2±0.2 vs 3.3±0.2 nmol mg−1 min−1; P<0.05; n=8). In contrast, NADH oxidase activity was slightly but significantly inhibited in homogenates prepared from aortic tissue incubated with high doses of GTN in vitro (to 2.2±0.3 nmol mg−1 min−1; P<0.05; n=8). Incubation of control aortic rings with DETC increased the NADH oxidase-induced chemiluminescence signal to 8.0±0.88 nmol mg−1 min−1 (P<0.05 vs control; n=8).
Studies on the subcellular distribution of NADH oxidase activity in vessel homogenates demonstrated that >90% of the enzyme activity resided in the particulate fraction in both control and tolerant vessels. In parallel with the findings in homogenates the NADH-dependent •O2− formation was increased about 3 fold in aortic membranes from in vivo GTN tolerant rabbits compared to controls (83.5±7.6 vs 28.8±3.11 nmol mg−1 min−1; P<0.05; n=4). Similarly, NADH oxidase activity was significantly increased in membranes from DETC pretreated tissue (62.6±5.5 nmol mg−1 min−1; P<0.05; n=4), but remained unchanged in membranes from GTN-incubated blood vessels (32.2±7 nmol mg−1 min−1; P<0.05; n=4).
Effects of the radical scavenger tiron on in vivo and in vitro tolerance
Incubation with tiron (10 mM) for 10 min had no effect on phenylephrine-induced tone (control: 8.3±1.1 g; tiron: 7.7±1.6 g; n=5). Tiron did not affect relaxations caused by GTN in control vessels and vessels made nitrate tolerant by in vitro treatment with GTN (Table 1, Figure 2). In striking contrast, tiron significantly improved GTN-evoked relaxations in vessels from rabbits treated for 3 days with GTN (tolerant: 58.8±5.9% vs tolerant+tiron: 89±1.8%; Figure 2, Table 1).
Figure 2.

Effects of the radical scavenger tiron on the GTN dose response-relationship of in vivo and in vitro tolerant rabbit aorta. Aortic rings were incubated for 15 min at 37°C in a Krebs/HEPES buffer containing tiron (10 mM). Thereafter the relaxant responses to cumulative concentrations of GTN were assessed. Tiron markedly improved the GTN responses of in vivo tolerant vessels, but failed to restore GTN responses in in vitro tolerant tissue. Data are presented as mean±s.e.mean from five separate experiments.
Effects of in vivo or in vitro GTN treatment or in vitro DETC treatment on vascular SOD activity
The vascular SOD activity as assessed by inhibition of 6-HDOPA autooxidation in tissue homogenates prepared from endothelium intact control aortic rings averaged 5.8±0.4 U per mg protein (Figure 3). Removal of the endothelium slightly but significantly decreased SOD activity, whereas SOD activity was markedly decreased in homogenates from in vivo tolerant aortas, and removal of the endothelium caused no further significant change (Figure 3). Incubation of control aortic rings with 10 mM DETC for 30 min reduced SOD activity significantly by more than 50%, while in vitro incubation with 10 μM GTN for 1 h did not affect vascular SOD activity (Figure 3).
Figure 3.
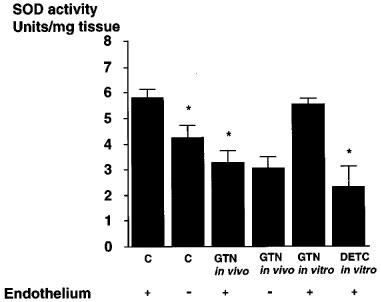
Effects of in vivo treatment of rabbits with glyceryl trinitrate (GTN) or in vitro treatment of isolated aortic segments with GTN or DETC on vascular superoxide dismutase (SOD) activity. In vivo treatment with GTN as well as incubation with DETC significantly decreased SOD activity. In contrast, SOD activity remained unchanged in in vitro incubations with GTN. Data are presented as mean±s.e.mean from 5–7 separate experiments. *P<0.05 vs control endothelium.
Effects of in vivo and in vitro GTN treatment on the expression of vascular CuZnSOD
As depicted in Figure 4 in vivo treatment with GTN markedly reduced the expression of vascular CuZnSOD by 60±20% (P<0.05; n=4), while the protein expression did not change following incubation of aortas with NTG in vitro. When tissue homogenates were further separated by ultracentrifugation and probed for CuZnSOD protein abundance by Western blot the protein localized entirely to the cytosolic fraction (Figure 4). In contrast, in vitro incubation of rabbit aortic tissue for 1 h with 10 μM GTN did not modify SOD expression (control 104±14 vs in vitro: 98±18%).
Figure 4.
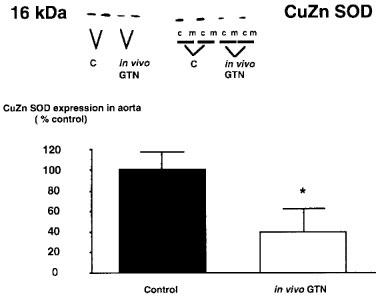
Representative Western blot illustrating the effect of in vivo treatment with glyceryl trinitrate (GTN) on the expression of the CuZn superoxide dismutase (SOD) in whole vessel homogenates (left hand panel) and in the cytosolic (c) or membrane (m) fractions (right hand panel). Densitometric analysis of protein expression in the homogenate is shown below. Densitometric data are presented as mean±s.e.mean from five separate experiments. *P<0.05 vs control. In vivo treatment with GTN for 3 days caused a significant decrease in protein expression of the CuZn superoxide dismutase.
Discussion
The present study shows that in vivo treatment with GTN as well as short-term exposure of aortic tissue to high concentrations of GTN results in a marked attenuation of GTN's vasodilator activity. In contrast, however, to in vitro tolerance, only in vivo tolerance was associated with a significant cross tolerance to the endothelium-dependent vasodilator ACh, increased vascular superoxide production and a decrease in vascular SOD activity and expression. Accordingly, only the relaxant responses to GTN of aortic rings obtained from animals treated in vivo with GTN were improved by the radical scavenger tiron. We have previously shown that tiron also improves cross-tolerance to ACh (Münzel et al., 1995b). In vivo tolerance could be mimicked by incubation of rings with DETC, an inhibitor of CuZnSOD, resulting in tolerance to GTN and cross tolerance to ACh, in increases in •O2− production and increased NADH-oxidase activity. These data suggest that the mechanisms underlying in vivo and in vitro tolerance differ substantially and therefore raise doubt whether in vitro treatment of blood vessels with high doses of GTN is a suitable model to study mechanisms underlying in vivo tolerance.
In vivo vs in vitro tolerance: different degree of cross tolerance
With the present studies we can demonstrate that in agreement with previous observations in vivo and in vitro tolerance is associated with a marked attenuation of GTN's vasodilator effects while the degree of cross tolerance to endothelium-dependent and -independent vasodilators differs considerably. Needleman & Johnson (1973) and others (Keith et al., 1982) demonstrated in their in vitro tolerance models a strong attenuation of vasodilator responses to GTN but no cross tolerance to the relaxant effects of sodium nitroprusside or nitric oxide itself. In addition, the cyclic GMP response to sodium nitroprusside was unaffected by in vitro GTN-pretreatment. In contrast, Axelsson & Anderson (1983) demonstrated that the activation of soluble guanylyl cyclase by sodium nitroprusside was markedly inhibited in homogenates of thoracic aortae from in vivo GTN-treated animals. Similarly, we and others have shown that in vivo treatment with GTN induced tolerance to GTN with a considerable degree of cross tolerance to sodium nitroprusside, to the sydnonimine SIN-1 (both are NO donors) and to endothelium-dependent vasodilators such as ACh and calcium ionophore (Molina et al., 1987; Münzel et al., 1995a,1995b). Since in vivo tolerance was associated with a blunted cyclic GMP increase in response to acute GTN challenges it was concluded that the target enzyme of GTN, the guanylyl cyclase, was desensitized as a consequence of the chronic stimulation with GTN (Molina et al., 1987; Münzel et al., 1995a,1995b). More recently, however, we found that removal of the endothelium and incubation with SOD markedly improved GTN-elicited relaxations in tolerant tissue suggesting that increased endothelial •O2− formation rather than smooth muscle guanylyl cyclase desensitization is the decisive determinant of in vivo nitrate tolerance (Münzel et al., 1995a,1995b).
In vivo vs in vitro tolerance: different effects on vascular superoxide production
In keeping with previous observations we found that in vivo tolerance was associated with a significant increase in vascular •O2− compared to aortic tissue from control animals (Münzel et al., 1995a,1995b). In contrast, incubation of aortic rings from control animals with a high GTN concentration for 1 h did not modify the lucigenin enhanced chemiluminescence signal.
As a potential enzymatic •O2− source in nitrate tolerance we recently demonstrated a significant increase in the activity of the membrane associated, NADH dependent oxidase (Münzel et al., 1995a,1995b). The present data support these observations. In in vivo GTN-treated animals NADH oxidase activity of aortic tissue was about 2–3 fold higher when compared to that of control animals. The increase in NADH-oxidase activity in GTN treated animals is somewhat surprising since NO (in μM concentrations) has been demonstrated to inhibit significantly the NAD(P)H oxidase activity of neutrophils (Clancy et al., 1992) in vitro by preventing the assembly of the activated multiprotein complex. One explanation for this apparent paradox might be that in vivo treatment with GTN is associated with a strong activation of neurohormonal counterregulatory mechanisms such as the renin-angiotensin-aldosterone system (Münzel et al., 1996a). Increased circulating levels of angiotensin II have been shown to increase the activity and expression of the vascular NAD(P)H oxidase (Fukui et al., 1997; Rajagopalan et al., 1996). Increased NAD(P)H oxidase activity in angiotensin II infused rats has also been shown to cause a tolerance like attenuation of the GTN response and cross tolerance to endothelium-dependent vasodilators (Rajagopalan et al., 1996). The hypothesis that the neurohormonal environment contributes at least in part to in vivo tolerance is supported by preliminary data demonstrating inhibition of tolerance and increased •.O2− formation by concomitant treatment with the angiotensin receptor blocker (AT-1) losartan (Kurz et al., 1999). These findings further strengthen the concept that mechanisms underlying in vivo tolerance may completely differ from the in vitro tolerance mechanisms.
The concept that increased vascular •O2− production contributes at least in part to tolerance of in vivo treated animals is further substantiated by the differential effects of the radical scavenger tiron. In aortic rings from animals treated with GTN in vivo, tiron markedly improved vascular relaxations induced by GTN, while the dose response relationship to GTN was not altered by tiron under control and in vitro tolerance conditions. These data are in line with previous observations demonstrating that liposomal SOD was able to improve GTN- and ACh-induced vasorelaxations in the setting of in vivo tolerance (Münzel et al., 1995a,1995b), indicating that increased vascular •O2− production may be a major factor in limiting the GTN vasodilator effects in in vivo nitrate tolerance.
In vivo vs in vitro tolerance: different effects on vascular SOD activity
In addition to differences in vascular •O2− production we found that in vivo GTN-treated animals exhibited a significant decrease in vascular SOD activity while the SOD activity of animals treated in vitro with GTN was not affected at all. Likewise we can now demonstrate that changes in SOD activity were paralleled by corresponding changes in the expression of the cytosolic CuZnSOD. More recently, we have shown that the activity and expression of the CuZnSOD in endothelial cells is strongly dependent on shear stress (Inoue et al., 1996). GTN preferentially dilates large conductance vessels such as the coronary arteries and the aorta (Fam & McGregor, 1968) while having minimal or no effect on the tone of arterioles with a diameter of less than 100 μm (Sellke et al., 1990; 1991). Therefore, by applying GTN in vivo, shear stress on endothelial cells of large arteries is dramatically reduced. Interestingly, removal of the endothelium in control vessels significantly decreased whole vessel SOD activity, a phenomenon which was not observed in in vivo tolerant tissue. It is therefore tempting to speculate that the observed reduction of SOD activity in the vascular wall of GTN treated animals is mediated at least in part through GTN-induced reduction in physico-chemical forces exerted by the streaming blood on endothelial cells.
Based on these observations we incubated aortic tissue from control animals with the inhibitor of CuZnSOD, DETC. Interestingly, with this in vitro approach we could completely mimic the effects of chronic in vivo GTN treatment. DETC-incubation decreased SOD activity in rabbit aorta, increased vascular •O2− production, increased NADH-oxidase induced lucigenin chemiluminescence, caused a tolerance-like attenuation of the GTN-dose response relationship and cross tolerance to the endothelium dependent vasodilator ACh.
The critical question which must be addressed is whether the loss of responsiveness to GTN and ACh in in vivo tolerant vessels is due to increased production in •O2− or decreased activity of SOD. To address this issue we incubated in vivo GTN tolerant tissue with DETC and found that levels of •O2− are still strikingly higher as compared to control vessels incubated with DETC. This observation suggests that the attenuation of vascular relaxations in in vivo tolerant vessels are in part due to reductions in SOD activity, but also likely secondary to an activation of an •O2− producing enzyme such as the NADH oxidase (Münzel et al., 1995a; 1996b).
The present paper has important implications. In vivo treatment with GTN significantly reduced vascular SOD activity by reducing the expression of the protein. We therefore identified an additional mechanism by which long-lasting GTN-treatment may increase oxidative stress within the vasculature. Although in vitro tolerance was associated with a comparable degree of tolerance to GTN's vasodilator action, high concentrations of GTN did not modify vascular •O2− production. In contrast, in vitro tolerance could be mimicked by incubating aortic tissue with the inhibitor of CuZnSOD, DETC, suggesting that this approach could be used in order to study molecular mechanisms underlying in vivo tolerance.
Acknowledgments
The study was supported by a DFG grant Mu 1079/2-1 and in part by the GIF (I-504-178.13/96).
Abbreviations
- ACh
acetylcholine
- DETC
diethyldithiocarbamate
- GTN
glyceryl trinitrate
- SOD
superoxide dismutase
- 6-HDOPA
6-hydroxydopamine
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]
- PMSF
phenylmethylsulphonyl fluoride
References
- AXELSSON K.L., ANDERSSON R.G. Tolerance towards nitroglycerin, induced in vivo, is correlated to a reduced cGMP response and an alteration in cGMP turnover. Eur. J. Pharmacol. 1983;88:71–79. doi: 10.1016/0014-2999(83)90393-x. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLANCY R.M., LESZCZYNSKA-PIZIAK J., ABRAMSON S.B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELKAYAM U., MEHRA A., SHOTAN A., OSTRZEGA E. Nitrate resistance and tolerance: potential limitations in the treatment of congestive heart failure. Am. J. Cardiol. 1992;70:98B–104B. doi: 10.1016/0002-9149(92)90601-t. [DOI] [PubMed] [Google Scholar]
- FAM W.M., MCGREGOR M. Effect of nitroglycerin and dipyridamole on regional coronary resistance. Circ. Res. 1968;22:649–659. doi: 10.1161/01.res.22.5.649. [DOI] [PubMed] [Google Scholar]
- FUKUI T., ISHIZAKA N., RAJAGOPALAN S., LAURSEN J.B., CAPERS Q., IV, TAYLER W.R., HARRISON D.G., DE LEON H., WILCOX J.N., GRIENDLING K.K. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- INOUE N., RAMASAMY S., FUKAI T., NEREM R.M., HARRISON D.G. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ. Res. 1996;79:32–37. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- KEITH R.A., BURKMAN A.M., SOKOLOSKI T.D., FERTEL R.H. Vascular tolerance to nitroglycerin and cyclic GMP generation in rat aortic smooth muscle. J. Pharmacol. Exp. Ther. 1982;221:525–531. [PubMed] [Google Scholar]
- KURZ S., HINK U., NICKENIG G., BORTHAYRE A.B., HARRISON D.G., MUNZEL T. A role for angiotensin II in nitrate tolerance: chronic AT-1 receptor blockade prevents development of tolerance and cross tolerance. Circulation. 1999. [DOI] [PubMed]
- MOLINA C.R., ANDRESEN J.W., RAPOPORT R.M., WALDMAN S., MURAD F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J. Cardiovasc. Pharmacol. 1987;10:371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- MÜNZEL T., HEITZER T., KURZ S., HARRISON D.G., LUHMAN C., PAPE L., OLSCHEWSKI M., JUST H. Dissociation of coronary vascular tolerance and neurohormonal adjustments during long-term nitroglycerin therapy in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 1996a;27:297–303. doi: 10.1016/0735-1097(95)00475-0. [DOI] [PubMed] [Google Scholar]
- MÜNZEL T., KURZ S., RAJAGOPALAN S., TARPEY M., FREEMAN B., HARRISON D.G.Identification of the membrane bound NADH oxidase as the major source of superoxide anion in nitrate tolerance Endothelium 1995a3suppl.s14(abstract) [Google Scholar]
- MÜNZEL T., KURZ S., RAJAGOPALAN S., THOENES M., BERRINGTON W.R., THOMPSON J.A., FREEMAN B.A., HARRISON D.G. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase: a new action for an old drug. J. Clin. Invest. 1996b;98 suppl.:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜNZEL T., SAYEGH H., FREEMAN B.A., TARPEY M.M., HARRISON D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Invest. 1995b;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEEDLEMAN P. Tolerance to the vascular effects of glyceryl trinitrate. J. Pharmacol. Exper. Therap. 1970;171:98–102. [PubMed] [Google Scholar]
- NEEDLEMAN P., JAKSCHIK B., JOHNSON E., JR Sulfhydryl requirement for relaxation of vascular smooth muscle. J. Pharmacol. Exper. Therap. 1973;187:324–331. [PubMed] [Google Scholar]
- NEEDLEMAN P., JOHNSON E.M.J. Mechanism of tolerance development to organic nitrates. J. Pharmacol. Exper. Therap. 1973;184:709–715. [PubMed] [Google Scholar]
- PACKER M., LEE W., KESSLER P.D., GOTTLIEB S.S., MEDINA N., YUSHAK M. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N. Engl. J. Med. 1987;317:799–804. doi: 10.1056/NEJM198709243171304. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN S., KURZ S., MÜNZEL T., TARPEY M., FREEMAN B.A., GRIENDLING K.K., HARRISON D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH A., KULICK D., FREIDENBERGER L., HONG R., RAHIMTOOLA S.H., ELKAYAM U. Early tolerance to hemodynamic effects of high dose transdermal nitroglycerin in responders with severe chronic heart failure. J. Am. Coll. Cardiol. 1987;9:858–864. doi: 10.1016/s0735-1097(87)80242-5. [DOI] [PubMed] [Google Scholar]
- SELLKE F.W., MYERS P.R., BATES J.N., HARRISON D.G. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am. J. Physiol. 1990;258:H515–H520. doi: 10.1152/ajpheart.1990.258.2.H515. [DOI] [PubMed] [Google Scholar]
- SELLKE F.W., TOMANEK R.J., HARRISON D.G. L-cysteine selectively potentiates nitroglycerin-induced dilation of small coronary microvessels. J. Pharmacol. Exp. Ther. 1991;258:365–369. [PubMed] [Google Scholar]


