Abstract
Microbial stimuli such as bacterial lipopolysaccharide (LPS) or glycosylphosphatidylinositol-mucins derived from Trypanosoma cruzi trypomastigotes (tGPI-mucins) are effective stimulators of the synthesis of cytokines by macrophages. Here, we evaluated the ability of cyclic AMP mimetic or elevating agents to modulate TNF-α and IL-12 synthesis by murine inflammatory macrophages.
Cholera Toxin (ChTx) inhibited tGPI-mucins (2.5 nM) or LPS (100 ng ml−1) induced TNF-α and IL-12(p40) synthesis in a concentration-dependent manner. Similarly, the cyclic AMP mimetics, 8-bromo cyclic AMP or dibutyryl cyclic AMP, or prostaglandin (PG) E2 inhibited the synthesis of both cytokines by macrophages exposed to microbial stimuli.
The protein kinase A inhibitor H-89 partially reversed the inhibitory effects of dibutyryl cyclic AMP and PGE2 on both IL-12(p40) and TNF-α synthesis.
Pretreatment of macrophages with dibutyryl cyclic AMP or ChTx augmented the synthesis of IL-10 triggered by microbial products. Elevation of cyclic AMP inhibited the synthesis of TNF-α, but not IL-12(p40), by inflammatory macrophages from IL-10 knockout mice.
Kinetic studies showed that synthesis of both TNF-α and IL-10 peaked at 8 h and IL-12 at 24 h after stimulation with microbial stimuli.
Together, our findings favour the hypothesis that the cyclic AMP inhibitory activity on IL-12(p40) but not on TNF-α synthesis is dependent on de novo protein synthesis, most likely involving IL-10, by macrophages stimulated with microbial products. Accordingly, dibutyryl cyclic AMP inhibited IL-12(p40) synthesis only when added before or at the same time of the stimuli. In contrast, the effect of this cyclic AMP analogue on TNF-α synthesis was protracted and observed even 2 h after the addition of the stimuli.
Keywords: Cyclic AMP, glycosylphosphatidylinositol-mucins, IL-12, IL-10, lipopolysaccharide, macrophage, microbial stimuli, prostaglandins, TNF-α, Trypanosoma cruzi
Introduction
Interleukin-12 (IL-12) is a heterodimeric cytokine which has two protein chains encoded by unrelated genes (Kobayashi et al., 1989). The light chain of 35 kDa (p35) is synthesized constitutively by a broad range of cells, whereas the 40 kDa (p40) unit is tightly regulated and secreted mainly by macrophages and dendritic cells upon microbial stimulation (Trinchieri, 1995). A broad list of bacteria, viruses and intracellular parasites has been described as inducers of the IL-12 heterodimer by macrophages both in vitro and in vivo (Biron & Gazzinelli, 1995; Trinchieri, 1995; Trinchieri & Scott, 1995). Once released by professional phagocytic cells exposed to microbial products, IL-12 induces IFN-γ synthesis by natural killer (NK) cells as well as drives the differentiation of naive Th precursor cells into Th1 lymphocytes. In this way, IL-12 is thought to play a primordial role on establishment of protective immunity against intracellular parasites (Biron & Gazzinelli, 1995; Scott, 1993; Trinchieri, 1995; Trinchieri & Scott, 1995). In agreement with this suggestion, animals treated with neutralizing doses of mAb against IL-12(p40) or lacking either IL-12(p40) or IL-12(p35) genes are highly susceptible to various intracellular pathogens (Biron & Gazzinelli, 1995; Trinchieri & Scott, 1995).
The synthesis of the IL-12(p40) chain is also regulated by a broad list of cytokines (D'Andrea et al., 1993; 1995; Flesch et al., 1995; Gazzinelli et al., 1993; 1994; Kubin et al., 1994; Ma et al., 1996; Murphy et al., 1995; Skeen et al., 1996). In fact, it is widely accepted that for optimal production of IL-12 by macrophages stimulated with microbial products, co-stimulation with IFN-γ is required (Gazzinelli et al., 1993; Ma et al., 1996; Murphy et al., 1995). Other cytokines such as GM-CSF, IL-1 and TNF-α (D'Andrea et al., 1993; Kubin et al., 1994), although not essential for maximal IL-12 synthesis, have also been shown to potentiate the synthesis and/or enhance IL-12 activity. Interestingly, IL-4 and IL-13 may play either negative or positive regulatory activity on IL-12 synthesis, depending on whether they are added to macrophage cultures before or after microbial stimuli, respectively (D'Andrea et al., 1995). In contrast, IL-10 is a major negative regulator of IL-12 synthesis both in vitro and in vivo (D'Andrea et al., 1993; Gazzinelli et al., 1996; Hunter et al., 1997).
We have recently shown that macrophages activated with glycosylphosphatidylinositol (GPI)-anchored mucin-like glycoproteins derived from Trypanosoma cruzi trypomastigotes (tGPI-mucins) have the ability to secrete several cytokines, including IL-12 and TNF-α (Camargo et al., 1997a, 1997b). Similarly to bacterial lipopolysaccharide (LPS), tGPI-mucins also activate macrophages to express IL-10. The precise role of tGPI-mucins in the pathophysiology of experimental and human infection by T. cruzi is not known, but tGPI-mucins induce several cytokines (such as IL-12, IL-10 and TNF-α) which are thought to be involved in protection and pathology in experimental Chagas' disease (Brener & Gazzinelli, 1997). Thus, knowledge of the mechanisms underlying and controlling macrophage activation by tGPI-mucins may help to understand the interaction of T. cruzi and its host.
Recent evidence suggests that the intracellular levels of cyclic AMP play an important role in re-directing cytokine synthesis by LPS-activated macrophages (Benbernou et al., 1997; Eigler et al., 1998; Meisel et al., 1996; van der Pouw Kraan et al., 1995). Thus, PGE2, a potent and physiological inducer of cyclic AMP synthesis, as well as cyclic AMP derivatives have been shown to inhibit both IL-12 and TNF-α synthesis by macrophages exposed to LPS (Eigler et al., 1998; Kambayashi et al., 1995a, 1995b; Panina-Bordignon et al., 1997; van der Pouw Kraan et al., 1995). Although controversial, it is suggested that the release of IL-10 by macrophages upon activation with LPS may account, at least in part, for the inhibitory effects of cyclic AMP on TNF-α production both in vitro and in vivo (Eigler et al., 1998; Kambayashi et al., 1995a, 1995b; Seldon et al., 1998; Strassmann et al., 1994; van der Pouw Kraan et al., 1995). Much less is known about the ability of the cyclic AMP-induced IL-10 in modulating the release of IL-12.
In the present work we have assessed the effects of several cyclic AMP-elevating agents and cyclic AMP mimetic drugs on modulating both IL-12 and TNF-α production by macrophages activated with tGPI-mucins. Moreover, we addressed whether endogenous IL-10 played a role on the cyclic AMP-mediated inhibition of cytokine release. For comparison we studied the effects of these strategies on macrophages stimulated with LPS. Our results clearly indicate a role for cyclic AMP in modulating the synthesis of pro-inflammatory cytokines. We also provide strong evidence that cyclic AMP modulates tGPI-mucin-induced IL-12 production by macrophages indirectly, following the release of IL-10. In contrast, the effects of cyclic AMP regulation of TNF-α production are probably direct, and largely independent of IL-10 production.
Methods
Animals
Male C3H/HeJ, C57BL/6 and IL-10 knockout (KO) mice in the C57BL/6 genetic background, 6–7 week old, were obtained from the animal house of FIOCRUZ (Rio de Janeiro, RJ, Brazil) and from the Division of Cancer Treatment, National Cancer Institute (Frederick, MD, U.S.A.) and used as source of inflammatory macrophages.
Reagents
ChTx, PGE2 and H89 were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.); SQ-22536, dibutyryl cyclic AMP (DB) and 8-bromo cyclic AMP were obtained from Calbiochem (La Jolla, CA, U.S.A.) and used according with the recommended specification. IFN-γ and LPS were purchased from Genzyme Corp. (Cambridge, MA, U.S.A.) and Sigma Chemical Co., respectively.
T. cruzi GPI-anchored mucins
tGPI-mucins were isolated from T. cruzi trypomastigotes as previously described (Almeida et al., 1994a; Camargo et al., 1997a) using sequential butanol:water partition and hydrophobic-interaction chromatography in an octyl-Sepharose column (Pharmacia Biotech, Uppsala, Sweden) eluted with a propan-1-ol gradient (5–60%). In addition, we isolated the active moiety of the tGPI-mucins consisting of the GPI anchors (tGPI). A preparation of highly purified tGPI showed an activity identical with that of tGPI-mucins on a molar basis. The tGPI anchor showed no detectable contamination with any known lipopetide from Mycoplasma sp. as indicated by matrix-assisted laser desorption ionization spectrometry (MALDI) as well as by aminoacid sequence analysis, using an authentic synthetic lipopeptide as a standard (MALP-2; a kind gift from Peter Mühlradt, Germany; Mühlradt et al., 1997) (data not shown).
Macrophage culture
Mice were inoculated intraperitoneally with 2 ml of 3% thioglycollate and, 4 days later, the elicited peritoneal exudate cells were harvested in cold serum-free DMEM. The medium used in the macrophage cultures (MacMed) consisted of DMEM (Gibco, Grand Island, NY, U.S.A.) supplemented with 40 μg ml−1 gentamicin and 5% heat-inactivated foetal calf serum (FCS). Macrophages were resuspended in MacMed at 2×106 ml−1, and 100 μl aliquots dispensed into wells of a 96-well plate. Cells were allowed to adhere at 37°C and 5% CO2 for 3 h, and were then washed once with serum-free DMEM and 100 μl of MacMed added to each well. When needed, the macrophages were primed with 50 U ml−1 IFN-γ. The plates were incubated overnight at 37°C and 5% CO2. Different macrophage stimulating perparations were added to the macrophage cultures in a final volume of 200 μl well−1. Aliquots of the supernatant (50 and 100 μl) were collected after 24 and 48 h of culture for TNF-α/IL-10 and IL-12(p40)/IL-12(p70) measurements, respectively (Camargo et al., 1997a, 1997b).
Determination of cytokine levels on supernatants of unstimulated and stimulated macrophages
TNF-α and IL-12(p70) were quantified employing ELISA kits (Genzyme, Duoset kit). IL-10 and IL-12(p40) were assayed by sandwich ELISA, using Maxsorp plates (Nunc, Denmark) as previously described (Gazzinelli et al., 1994; Jankovic et al., 1997). Capture (26571E) and detection (biotinylated 26572E) antibody (Pharmingen), and the enzyme reagent (streptavidin-horseradish peroxidase conjugated, 26167E), were used for quantifying IL-10. C17.1.5 (1510) rat mAb (5 μl ml−1) and biotinylated C15.6 (676) rat mAb (1 : 750) were used for capture and detection of bound IL-12(p40), respectively. Recombinant murine IL-10 and IL-12 were used as standards for the respective cytokine measurements. For TNF-α, IL-12(40) and IL-12(70) sandwich ELISAs were developed with 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid) (Sigma) and read in a ELISA reader (SpectraMax Plus, Molecular Devices, U.S.A.) at 405 nm. For IL-10 ELISAs the reaction was developed with an ECL-PLUS chemiluminescent substrate, as previously described (Almeida et al., 1994b). Briefly, plates (Fluoronunc Maxisorp - Nunc) were developed for 20 min using ECL-PLUS substrate (Amersham, Life Science, RPN-2132) and exposed for 10–20 min to Hyperfilm SL (Amersham, Life Science, RPN-3103H) placed at the top of ELISA plates. Developed films were attached to the top of unused plates and introduced in an ELISA reader (SpectraMax Plus, Molecular Devices) at 405 nm.
Inhibition of cytokine synthesis
Different signalling inhibitors were added to macrophage cultures in titration assays and incubated for 30 min, at 37°C and 5% CO2. The cultures were incubated with tGPI-mucins or LPS at the concentrations indicated in presence or absence of IFN-γ. The cultures were maintained at 37°C and 5% CO2, and 50 and 100 μl of supernatant were collected after 24 and 48 h, respectively, for TNF-α and IL-12 measurements. By the end of 48 h of incubation, cells were washed in pre-warmed PBS and assayed for viability.
Cell viability assay
Cell viability was determined as previously described (Miller, 1994). Briefly, cells were incubated with 100 μl well−1 of supplemented medium containing 0.5 mg ml−1 of MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), overnight at 37°C and 5% CO2. Cells were then washed and treated with 100 μl well−1 of 10% SDS in dimethylformamide:H2O (1 : 1). Absorbency was read at 570 nm. Cell viability was calculated as a relative index of control cells (100% of viable cells).
IC50 calculation
A dose-response curve was obtained by adding increasing concentrations of cholera toxin, cyclic AMP derivatives or PGE2, 30 min before stimulation with microbial products, and evaluation of cytokine production after indicated times. Concentrations of effector compounds which rendered 50% inhibition of the maximum response were designated IC50.
Statistical analysis
Differences between groups were determined using analysis of variance and P values assigned using Student-Newman-Keuls post hoc test. Results were considered significant when P<0.05.
Results
Cyclic AMP is a negative regulator of IL-12 synthesis by inflammatory macrophage
Recent evidence suggests that the increase in intracellular levels of cyclic AMP is accompanied by a significant decrease of cytokine production by LPS-activated macrophages (Eigler et al., 1998; Meisel et al., 1996; van der Pouw Kraan et al., 1995). We carried out preliminary studies with cholera toxin (ChTx), an effective stimulator of adenylate cyclase, in murine macrophages activated by LPS. Pretreatment with ng ml−1 concentrations of ChTx completely inhibited the production of both TNF-α and IL-12 from LPS-stimulated macrophages (Table 1). Similarly, ChTx totally inhibited the production of IL-12 and TNF-α by macrophages stimulated with tGPI-mucins in the presence or absence of IFN-γ (Figure 1 and Table 1). We also tested two cyclic AMP derivatives (DB and 8-bromo-cyclic AMP), that mimic the action of cyclic AMP, and PGE2 in the regulation of IL-12 and TNF-α production by macrophages activated by microbial products. Both cyclic AMP derivatives effectively inhibited TNF-α and IL-12 synthesis induced by LPS or tGPI-mucins (Table 1 and Figure 1). PGE2 also significantly blocked the production of cytokines by either LPS or tGPI-mucins but it was slightly less effective than the other modulators at the concentrations used (Table 1 and Figure 1). Overall, the inhibitory effects of cyclic AMP-based strategies were equally effective but more pronounced against cytokine synthesis induced by tGPI-mucins than by LPS (Table 1). In contrast to these inhibitory effects, blockade of endogenous cyclic AMP synthesis with SQ-22536 and of PKA with H89, had little effect on IL-12 or TNF-α production induced by either tGPI-mucins or LPS (Table 1). Overall the results domonstrate that cyclic AMP is an effective modulator of both IL-12 and TNF-α production by macrophages stimulated with LPS or tGPI-mucins.
Table 1.
Inhibitory concentrations (IC50) of different inhibitors or activators of adenylate cyclase and/or Protein Kinase A on IL-12(p40) or TNF-α synthesis by macrophages stimulated with tGPI mucins or LPS*

Figure 1.

Inhibitory activity of cholera toxin, cyclic AMP derivatives and prostaglandin E2 in the synthesis of TNF-α and IL-12(p40) by macrophages exposed to tGPI-mucins. Inflammatory macrophages were pre-treated for 30 min with range of concentrations of ChTx (left panel), DB (central panel) or PGE2 (right panel) before stimulation with tGPI-mucins (2.5 nM) in the presence of 50 U ml−1 of IFN-γ. TNF-α and IL-12(p40) were assayed in supernatants collected 24 and 48 h, respectively, after macrophage stimulation. Control cytokine levels in absence of inhibitors are shown by dashed (IL-12) and continuous (TNF-α) lines. Values are means±s.d. of triplicates. Similar results were obtained in three different experiments.
The inhibitory effect of cyclic AMP on both IL-12(p40) and TNF-α synthesis involves PKA activation
Next, we examined the role of protein kinase A (PKA) in mediating the modulatory effects of cyclic AMP in macrophages. IFN-γ-primed macrophages were incubated in presence of the PKA inhibitor H89 (at 1 μM) for 30 min before addition of PGE2 or DB. The macrophages were then cultured in the presence or absence of LPS or tGPI-mucins and cytokines measured in the macrophage culture supernatants by ELISA. In these experiments, PGE2 and DB caused a reduction of 50% or more of IL-12 and TNF-α production that was partially reversed by the presence of H89 (Figure 2). H89 alone had no significant effect on the IL-12 synthesis but caused a slight increase on TNF-α production. These results suggest that down-regulation of IL-12 and TNF-α synthesis caused by components that promote enhancement in the intracellular levels of cyclic AMP is, at least in part, mediated by PKA activation.
Figure 2.

Protein Kinase A involvement in the inhibitory effect of DB and prostaglandin E2 on the synthesis of TNF-α and IL-12(p40) by activated macrophages. Inflammatory macrophages were pre-treated with 1 μM H89 in the presence or absence of 10 μM PGE2 or 0.3 mM DB, for 30 min before stimulation with tGPI-mucins (2.5 nM) or LPS (100 ng ml−1) in the presence of 50 U ml−1 of IFN-γ. TNF-α and IL-12(p40) were assayed in supernatants collected 24 and 48 h, respectively, after macrophage stimulation. Bars indicated by the same letters represent values that are statistically different (P<0.05). Values are means±s.d. of triplicates and are representative of two experiments.
IL-10 is produced upon macrophage stimulation by tGPI-mucins and LPS in the presence of cyclic AMP
Since increased intracellular levels of cyclic AMP have been shown to augment the synthesis of IL-10 in both T lymphocytes (Benbernou et al., 1997) and macrophages (Eigler et al., 1998; Meisel et al., 1996; van der Pouw Kraan et al., 1995) and since IL-10 modulates the synthesis of pro-inflammatory cytokines by macrophages, we investigated whether a similar phenomenon was involved in our system. Increasing concentrations of DB (Figure 3, left panel) or ChTx (Figure 3, right panel) added to IFN-γ-primed macrophages 30 min before stimulation with tGPI-mucins or LPS augmented the synthesis of IL-10 in a dose-dependent manner. Note that both DB and ChTx were considerably less potent at inducing IL-10 production than inhibiting IL 12 and/or TNF-α production (compare Figures 1 and 3). The latter results may, however, be explained by the lower sensitivity of our IL-10 ELISA.
Figure 3.

Cholera Toxin and DB enhance the synthesis of IL-10 by stimulated inflammatory macrophages. Inflammatory macrophages were stimulated with tGPI-mucins (2.5 nM) or LPS (100 ng ml−1) in the absence or presence of increasing concentrations of ChTx or DB, as indicated. IL-10 was measured in the supernatants collected at 24 h post-stimulation. Values are means±s.d. of triplicates. Similar results were obtained in two different experiments.
Figure 4 demonstrates the effects of IL-10 on the synthesis of IL-12(p40) and TNF-α by IFN-γ primed macrophages stimulated with either LPS or tGPI-mucins. We observed that IL-10 inhibited both TNF-α and IL-12(p40) production in a dose dependent way, in either LPS or tGPI-mucin-stimulated macrophages (Figure 4).
Figure 4.

Inhibitory effect of IL-10 on the synthesis of TNF-α and IL-12(p40) by macrophages exposed to tGPI-mucins. IFN-γ-primed inflammatory macrophages were incubated for 30 min without (columns) or with (lines) different concentrations of rIL-10 before stimulation with LPS (100 ng ml−1) or tGPI-mucins (2.5 nM). TNF-α and IL-12(p40) were measured on culture supernatants at 24 and 48 h, respectively, after stimulation with microbial products. Values are means±s.d. of triplicates and are representative of two different experiments. Standard deviations are too small and they don't appear in this figure.
Endogenous IL-10 is necessary for the inhibitory effect of cyclic AMP on IL-12(p40) but not on TNF-α synthesis by stimulated macrophages
The inhibitory activity of endogenous IL-10 on IL-12 and TNF-α synthesis was further investigated using IL-10 KO mice (Tables 2 and 3). We observed that, although ChTx. PGE2, 8-bromo-cyclic AMP and DB caused a major reduction of IL-12(p40) synthesis in wild type animals, these same compounds did not display any inhibitory activity on IL-12 synthesis in macrophages from IL-10−/− KO mice exposed to either LPS or tGPI-mucins in the presence or absence of IFN-γ (Table 2). These results strongly suggest that the inhibitory activity on IL-12 production by compounds that cause an increase or mimic intracellular levels of cyclic AMP is in fact mediated by IL-10. Interestingly, ChTx, PGE2, 8-bromo cyclic AMP and DB inhibited TNF-α synthesis induced by tGPI-mucins or LPS even in macrophages from IL-10−/− KO mice (Table 3). This indicates that the inhibitory activity of cyclic AMP on cytokine synthesis may involve more than one pathway, which may or may not be mediated by endogenous IL-10.
Table 2.
IL-12(p40) synthesis by inflammatory macrophages from wild type or IL-10−/− KO mice treated with IFN-γ, and with either tGPI-mucins or LPS in the presence of cyclic AMP derivatives, cholera toxin or PGE2*

Table 3.
TNF-α synthesis by inflammatory macrophages from wild type or IL-10−/− KO mice treated with IFN-γ and with either tGPI-mucins of LPS in the presence or absence of cyclic AMP derivatives, cholera toxin or PGE2*

The kinetics of TNF-α and IL-12(p40) synthesis and inhibition by IL-10 and DB
Inasmuch as IL-10 is produced in response to high intracellular levels of cyclic AMP and it was capable of blocking the production of both IL-12 and TNF-α by macrophages, the contrasting results in macrophages from IL-10−/− KO mice were at first unexpected. The next experiments were designed to investigate whether a kinetic difference in IL-12 and TNF-α production could explain the endogenous IL-10 dependency of the inhibitory activity of cyclic AMP. Macrophages primed or not with IFN-γ were exposed to either tGPI-mucins or LPS and supernatants collected at various times. Our results show that TNF-α synthesis was already detectable at 2 h and peaked between 4–8 h after stimulation of macrophages (Figure 5). In contrast, synthesis of IL-12(p40) was delayed and only measurable after 8 h (peaking at 24 h or later) post-stimulation with either LPS or tGPI-mucins (Figure 5). Identical kinetics were observed for IL-12(p40) and IL-12(p70) synthesis upon microbial stimuli in the presence or absence of DB (Figure 5).
Figure 5.
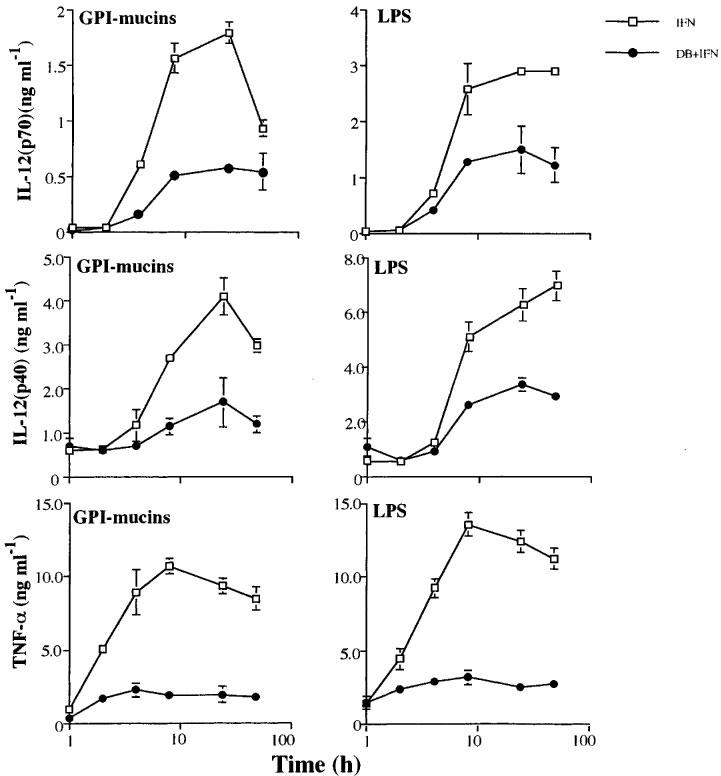
Kinetics of TNF-α, IL-12(p40) and IL-12(p70) synthesis by macrophages exposed to microbial stimuli. IFN-γ-primed or unprimed macrophages were stimulated with tGPI-mucins (2.5 nM) and LPS (100 ng ml−1). IL-12 and TNF-α levels were determined at different time after stimulation of macrophage. Values are means±s.d. of triplicates. Similar results were obtained in two different experiments.
Next, we studied the kinetics of the inhibitory effects of IL-10 on TNF-α and IL-12(p40) synthesis by activated macrophages. The cells were stimulated with either LPS or tGPI-mucins and IL-10 was added to the cells in culture at different times after the initial stimulation. Our results show that the inhibitory effect of IL-10 was immediate and protracted for both TNF-α and IL-12(p40) synthesis in stimulated macrophages (Figure 6). IL-10 inhibited the synthesis of either cytokines even when added at 2 or in some case 4 h after stimulation with tGPI-mucins or LPS. Interestingly, kinetic experiments showed that IL-10 synthesis by macrophages stimulated with either stimuli in the presence of DB peaked at 8 h post-stimulation, before IL-12(p40) and IL-12(p70) reach maximal levels in the culture supernatants, but after most TNF-α being produced (Figure 7). Therefore, our results indicate that upon microbial stimuli, IL-10 is produced and may play a role on macrophage deactivation, only after most of the TNF-α, but not IL-12(p40) and IL-12(p70), has been produced. In fact, published data suggest that the synthesis of IL-10 by LPS-stimulated macrophages occur after generation of TNF-α (Eigler et al., 1998).
Figure 6.

Time-dependence of the inhibitory effect of IL-10. IFN-γ-primed macrophages were stimulated with tGPI-mucins and LPS in the presence or absence of IL-10 (1.25 ng ml−1) added at the indicated time points. IL-12 and TNF-α levels were determined after a 24 h culture. Values are means±s.d. of triplicates. Similar results were obtained in two different experiments.
Figure 7.
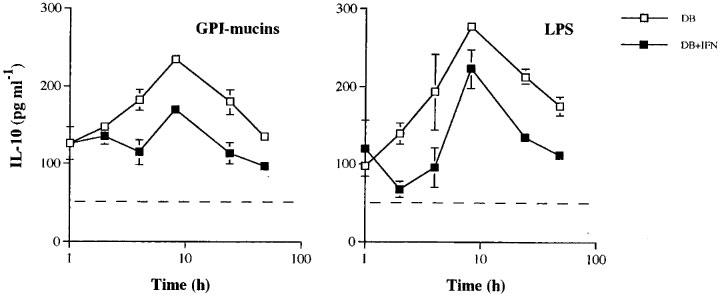
Kinetics of IL-10 synthesis by macrophages exposed to microbial stimuli in the presence of DB. IFN-γ-primed or unprimed macrophages were stimulated with tGPI-mucins (2.5 nM) and LPS (100 ng ml−1) in the presence of 0.3 mM of DB. IL-10 levels were determined at different time after stimulation of macrophage. Horizontal dashed lines indicate the levels of IL-10 in culture supernatants from unstimulated macrophages. Values are means±s.d. of triplicates. Similar results were obtained in two different experiments.
Consistent with the hypothesis that DB inhibitory effect on IL-12, but not TNF-α, production is dependent on synthesis of endogenous IL-10, DB was only inhibitory for IL-12(p40) synthesis if added prior to or at the same time of macrophage stimulation (Figure 8). In contrast, DB had a much more protracted action on TNF-α synthesis, and was inhibitory even when added 2 h after macrophage stimulation with either LPS or tGPI-mucins (Figure 8). The delayed DB inhibitory effect on IL-12(p40), as compared to TNF-α synthesis, is even more dramatic if we consider that most TNF-α is produced in the first 8 h, whereas most IL-12(p40) is produced from 8–24 h post macrophage stimulation with microbial products.
Figure 8.

Time-dependence of the inhibitory effect of DB. IFN-γ-primed macrophages were stimulated with tGPI-mucins (2.5 nM) and LPS (100 ng ml−1) in the presence or absence of DB (0.3 mM) added at the indicated time points. IL-12 and TNF-α levels were determined after a 24 h culture. Values are means±s.d. of triplicates. Similar results were obtained in two different experiments.
Discussion
IL-12 is a key cytokine responsible for initiation of IFN-γ synthesis and establishment of cell mediated immunity (CMI), thus being an important determinant of resistance against a large number of pathogens (Biron & Gazzinelli, 1995; Scott, 1993; Trinchieri, 1995; Trinchieri & Scott, 1995). Consistent with this, the IL-12 heterodimer has been successfully used as vaccine adjuvant for selective induction of strong Th1 lymphocytes and resistance against some intra and extracellular pathogens (Afonso et al., 1994; Miller et al., 1995; Schijns et al., 1995; Wynn et al., 1995). Conversely, uncontrolled synthesis of this cytokine as in experimental infections with parasitic protozoa (Gazzinelli et al., 1996; Hunter et al., 1997), challenge with bacterial endotoxin (Heinzel et al., 1994; Ozmen et al., 1994; Wysoka et al., 1995), or certain autoimmune diseases (Germann et al., 1995; Leonard et al., 1995), results in detrimental effects due to cytokine shock and/or excessive activation of the cellular compartment of the immune system. Moreover, in Chagas' disease, the IL-12-driven CMI may contribute to both resistance (Aliberti et al., 1996; Hunter et al., 1996) and damage to vital organs such as the heart (Hunter et al., 1997). Therefore, a better understanding of the biochemical signalling pathway(s) involved in activation and expression of IL-12 genes may lead to the development of new reagents and/or strategies that can be used either to induce desired, or inhibit detrimental, immune responses mediated by IFN-γ and CMI.
Recently, we have purified tGPI-mucins from T. cruzi trypomastigotes and determined that these components induce macrophage activation (Camargo et al., 1997a, 1997b). In the presence of IFN-γ, macrophages activated with tGPI-mucins produced significant amounts of nitric oxide (NO) and the pro-inflammatory cytokines IL-12 and TNF-α activation (Camargo et al., 1997a, 1997b). The precise role of tGPI-mucins in the pathophysiology of Chagas' disease is poorly understood, but the ability of these glycoconjugates to induce macrophages to secrete NO and cytokines such as IL-12 may underlie a fundamental host–parasite interaction (Aliberti et al., 1996; Hunter et al., 1996; 1997), akin to the importance of LPS in bacterial diseases.
In the present study we investigated the role of cyclic AMP-based strategies in modulating IL-12 and TNF-α synthesis in macrophages stimulated by tGPI-mucins and LPS. Because IL-12(p40) has been shown to be tightly regulated in cells from macrophage lineage (Ma et al., 1996; Murphy et al., 1995), whereas IL-12(p35) is expressed constitutively by different types of cell (Trinchieri, 1995), we focused on the expression of IL-12(p40) rather than on that of the IL-12 heterodimer. Moreover, we assessed whether the endogenous production of IL-10 was important for the inhibitory activity of cyclic AMP. Our results show that in IFN-γ primed macrophages the synthesis of TNF-α and IL-12 is favoured over that of IL-10. In fact, significant amounts of both TNF-α and IL-12 but not of IL-10 were detected upon activation with tGPI-mucins (or LPS). In conditions, however, which favour the induction of (or mimic) high levels of intracellular cyclic AMP, the synthesis of IL-10 is enhanced, whereas that of TNF-α, IL-12(p40) and IL-12(p70) heterodimer is mostly abolished. These results are in agreement with early studies performed elsewhere, which show that PGE2, via its ability to raise intracellular cyclic AMP, is a potent regulator of IL-12 and TNF-α synthesis (van der Pouw Kraan et al., 1995). Moreover, we show that the effects of cyclic AMP-based strategies were at least in part mediated by PKA, inasmuch as the PKA inhibitor H89 reversed the modulatory effects of elevated cyclic AMP. Thus, it is tempting to speculate that in situations where the cyclic AMP levels are enhanced in antigen presenting cells (APCs), T cell differentiation will be favoured towards the Th2 phenotype (Panina-Bordignon et al., 1997). Conversely, priming with IFN-γ and low intracellular levels of cyclic AMP will produce high levels of IL-12 in APCs, which will favour the development of Th1 lymphocytes (Hsieh et al., 1993; Seder et al., 1993).
IL-10 has been shown to be a potent modulator of IL-12 and TNF-α expression (D'Andrea et al., 1993; Fiorentino et al., 1991; Gazzinelli et al., 1996). In addition synthesis of IL-10 stimulated by LPS appears to be potentiated by cyclic AMP (Eigler et al., 1998; van der Pouw Kraan et al., 1995). Therefore, we investigated whether tGPI-mucins induced production of IL-10 protein in the presence of cyclic AMP and if the inhibitory effect of cyclic AMP on TNF-α and IL-12 synthesis occurred via IL-10. Our results clearly demonstrate that tGPI-mucins (and LPS) induce considerable amounts of IL-10 when activation occurs in the presence of elevated intracellular levels of cyclic AMP. Moreover, our results demonstrate that IL-10 is clearly capable of inhibiting the synthesis of both IL-12 and TNF-α induced by tGPI-mucins (or LPS) even when added up to 2 h after stimulation. However, cyclic AMP was still inhibitory for TNF-α, but not IL-12(p40), production by inflammatory macrophages lacking functional genes for IL-10. Thus, we suggest that, although IL-10 is capable of modulating both IL-12(p40) and TNF-α production, the regulatory activity of cyclic AMP on IL-12 but not on TNF-α synthesis is dependent on endogenous IL-10 activity.
The lack of inhibition of IL-12 production, but not of TNF-α, by cyclic AMP in macrophages from IL-10 knockout mice in response to tGPI mucins or LPS stimulation was intriguing. To examine this issue in more detail we performed kinetic studies to compare the onset of secretion of these cytokines in response to both stimulants in the presence and absence of cyclic AMP-elevating agents. Our results showed that whereas TNF-α production was first detected at 2 h and peaked around 4–8 h, IL-12 production began and peaked at much later time point. In addition, there is evidence showing that the production of IL-10 by activated macrophages has a late onset (Eigler et al., 1998) similarly to that of IL-12, therefore later than the onset of TNF-α synthesis.
Together, our findings favour the hypothesis that the cyclic AMP inhibitory activity on IL-12(p40) or IL-12(p70) but not on TNF-α synthesis is dependent on de novo protein synthesis, most likely IL-10 synthesis, by macrophages stimulated with microbial products. In contrast, the IL-10 inhibitory effect on both IL-12(p40) and TNF-α expression was immediate and most likely independent of de novo protein synthesis. Thus, we can speculate that increased levels of cyclic AMP, which are normally transient, may be directly able to control genes expressed early (e.g. TNF-α) but not later after macrophage stimulation with microbial products. However, by inducing IL-10 gene expression, cyclic AMP may trigger anti-inflammatory activity (e.g. blockade of IL-12 production), which will persist even after the levels of cyclic AMP have decreased to normal.
Acknowledgments
This work was supported in part by the WHO-TDR (Id Nos: 970506 and 970728), CNPq (522.056/95-4), PADCT (02-SB10 01/97 04/03-35) and the Wellcome Trust. R.T. Gazzinelli, M.M. Teixeira and L.R. Travassos are research fellows of CNPq. DOP and MMC are post-graduate students with scholarships from CNPq. ICA is a post-doctoral fellow with fellowship (no. 96/04260-0) from FAPESP. MAJF is a Howard Hughes Medical Institute international scholar. The IL-10−/− knockout mice were kindly provided by Dr Werner Müller of the Institute of Genetics, University of Köln, D50923 Köln, Germany. We are grateful to Luiz S. Silva for the excellent technical assistance.
Abbreviations
- APCs
antigen presenting cells
- cyclic AMP
cyclic adenosine monophosphate
- ChTx
cholera toxin
- DB
dibutyryl cyclic AMP
- GPI
glycosylphosphatidylinositol
- IL-12(p40)
40 kDa peptide of IL-12
- IL-12(p35)
35 kDa peptide of IL-12
- IL-12(p70)
IL-12(p40)/IL-12(p35) heterodimer
- LPS
Escherichia coli lipopolysaccharide
- MacMed
DMEM supplemented with 40 μg ml−1 gentamicin and 5% heat-inactivated foetal calf serum
- PGE2
prostaglandin E2
- PKA
Protein Kinase A
- tGPI-mucins
GPI-anchored mucin-like glycoproteins from Trypanosoma cruzi trypomastigotes
References
- AFONSO L.C.C., SCHARTON T.M., VIEIRA L.Q., WYSOCKA M., TRINCHIERI G., SCOTT P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- ALIBERTI J.C.S., CARDOSO M.A.G., MARTINS G.A., GAZZINELLI R.T., VIEIRA L.Q., SILVA J.S. IL-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALMEIDA I.C., FERGUSON M.A.J., SCHENKMAN S., TRAVASSOS L.R. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 1994a;304:793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALMEIDA I.C., RODRIGUES E.G., TRAVASSOS L.R. Chemiluminescent immunoassays: discrimination between the reactivities of natural and human patient antibodies with antigens from eukaryotic pathogens, Trypanoama cruzi and Paracoccidioides brasiliensis. J. Clin. Lab. Analysis. 1994b;8:424–431. doi: 10.1002/jcla.1860080614. [DOI] [PubMed] [Google Scholar]
- BENBERNOU N., ESNAULT S., SHIN H.C.K., FEKKAR H., GUENOUNOU M. Differential regulation of IFN-γ, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunoiogy. 1997;91:361–368. doi: 10.1046/j.1365-2567.1997.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRON C.A., GAZZINELLI R.T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr. Opin. Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- BRENER Z., GAZZINELLI R.T. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- CAMARGO M.M., ALMEIDA I.C., PEREIRA M.E., FERGUSON M.A., TRAVASSOS L.R., GAZZINELLI R.T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J. Immunol. 1997a;158:5890–5901. [PubMed] [Google Scholar]
- CAMARGO M.M., ANDRADE A.C., ALMEIDA I.C., TRAVASSOS L.R., GAZZINELLI R.T. Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania sp. parasite membranes trigger nitric oxide synthesis as well as microbicidal activity by IFN-γ-primed macrophages. J. Immunol. 1997b;159:6131–6139. [PubMed] [Google Scholar]
- D'ANDREA A., ASTE-AMEZAGA M., VALIANTE N., MA X., KUBIN M., TRINCHIERI G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon-γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA A., MA X., ASTE-AMEZAGA M., PAGANIN C., TRINCHIERI G. Stimulatory and inhibitory effects of IL-4 and IL-13 on production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and TNF-α production. J. Exp. Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIGLER A., SIEGMUND B., EMMERICH U., BAUMMANN K.H., HARTMANN G., ENDRES S. Anti-inflammatory activities of cyclic AMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF-α production. J. Leuk. Biol. 1998;63:101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- FIORENTINO D.F., ZLOTNICK T., MOSMANN T., HOWARD M., O'GARRA A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- FLESCH I.E.A., HESS J.H., HUANG S., AGUET M., ROTHE J., BLUETHMANN H., KAUFMANN S.H.E. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon-γ and tumor necrosis factor-α. J. Exp. Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZZINELLI R.T., HIENY S., WYNN T., WOLF S., SHER A. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-deficient hosts. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZZINELLI R.T., WYSOCKA M., HAYASHI S., DENKERS E.Y., HIENY S., CASPAR P., TRINCHIERI G., SHER A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- GAZZINELLI R.T., WYSOCKA M., HIENY S., SCHARTON-KERSTEN T., CHEEVER A., KÜHN R., MÜLLER W., TRINCHIERI G., SHER A. In absence of endogenous IL-10 mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, TNF-α. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- GERMANN T., SZELIGA J., HESS H., STORKEL S., PODLASKI F.J., GATELY M.K., SCHMITT E., RUDE E. Administration of interleukin 12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4823–4827. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINZEL F.P., RERKO R.M., LING P., HAKIMI J., SCHOENHAUT D.S. Interleukin-12 is produced in vivo during endotoxemia and stimulates synthesis of interferon-γ. Infect. Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIEH C.S., MACATONIA S.E., TRIPP C.S., WOLF S., O'GARRA A., MURPHY K.M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- HUNTER C.A., ELLIS-NEYES L.A., SLIFER T., KANALY S., GRUNIG G., FORT M., RENNICK D., ARAUJO F.G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- HUNTER C.A., SLIFER T., ARAUJO F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor-α and interferon-γ. Infect. Immun. 1996;64:2381–2386. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANKOVIC D., CASPAR P., ZWEIG M., GARCIA-MOLL M., SHOWALTER S.D., VOGEL F.R., SHER A. Adsortion to aluminum hidroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp 120. J. Immunol. 1997;159:2409–2417. [PubMed] [Google Scholar]
- KAMBAYASHI T., ALEXANDER H.R., FONG M., STRASSMANN G. Potential involvement of IL-10 in suppressing tumor associated macrophages. Colon-26-derived Prostaglandin E2 inhibits TNF-α release via a mechanism involving IL-10. J. Immunol. 1995a;154:3383–3390. [PubMed] [Google Scholar]
- KAMBAYASHI T., JACOB C.O., ZHOU D., MAZUREK N., FONG M., STRASSMANN G. Cyclic nucleotide phosphodiesterase Type IV participates in the regulation of IL-10 and in subsequent inhibition of TNF-α and IL-6 release by endotoxin-stimulated macrophages. J. Immunol. 1995b;155:4909–4916. [PubMed] [Google Scholar]
- KOBAYASHI M., FITZ L., RYAN M., HEWIK R.M., CLARK S.C., CHAN S., LOUDON R., SHERMAN F., PERUSSIA B., TRINCHIERI G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBIN M., CHOW M.J., TRINCHIERI G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor-α, and IL-1β, production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- LEONARD J.P., WALDBURGER K.E., GOLDMAN S.J. Prevention of experimental autoimmune encephalomyelitis by antibodies against IL-12. J. Exp. Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA X., CHOW J.M., GRI G., CARRA G., GEROSA F., WOLF S.F., DZIALO R., TRINCHIERI G. The interleukin 12 p40 promoter is primed by interferon-γ in monocytic cells. J. Exp. Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISEL C., VOGT K., PLATZER C., RANDOW F., LIEBENTHAL C., VOLK H.D. Differential regulation of monocytic tumor necrosis factor-α and interleukin-10 expression. Eur. J. Immunol. 1996;26:1580–1586. doi: 10.1002/eji.1830260726. [DOI] [PubMed] [Google Scholar]
- MILLER M.A., SKEEN M.J., ZIEGLER H.K. Nonviable bacterial antigens administered with IL-12 generate antigen specific T cell responses and protective immunity against Listeria monocytogenis. J. Immunol. 1995;155:4817–4828. [PubMed] [Google Scholar]
- MILLER R.A. Quantification of functional T cells by limiting dilution. Curr. Protocols Immunol. 1994;1:3.15.5. doi: 10.1002/0471142735.im0315s35. [DOI] [PubMed] [Google Scholar]
- MÜHLRADT P.F., KIEB M., MEYER H., SÜBMUTH R., JUNG G. Isolation, structure and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY T., CLEVELAND M., KULEZKA P., MAGRAM J., MURPHY K. Regulation of interleukin 12 p40 expression through an NF-kB half-site. Mol. Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZMEN L., PERICIN M., HAKIMI J., CHIZZONITE R.A., WYZOCKA M., TRINCHIERI G., GATELY M., GAROTTA G. Interleukin-12, interferon-γ and tumour necrosis factor-α are key cytokines of the generalized Swartzman reaction. J. Exp. Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANINA-BORDIGNON P., MAZZEO D., LUCIA P.D., D'AMBROSIO D., LANG R., FABBRI L., SELF C., SINIGAGLIA F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIJNS V.E.C.J., HAAGMANS B.L., HORZINEK M.C. IL-12 stimulates an anti-viral type 1 cytokine response but lacks adjuvant activity in IFN-γ receptor deficient mice. J. Immunol. 1995;155:2525–2532. [PubMed] [Google Scholar]
- SCOTT P. IL-12: initiation cytokine for cell mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- SEDER R.A., GAZZINELLI R.T., SHER A., PAUL W.E. IL-12 acts directly on CD4+ T cells to enhance priming for IFN-γ production and dimminishes IL-4 inhibition of such priming. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10188–10193. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELDON P.M., BARNES P.J., GIEMBYCZ M.A. Interleukin-10 does not mediate inhibitory effect of PDE-4 inhibitors and other cyclic AMP-elevating drugs on lipopolysaccharide-induced tumor necrosis factor-α generation from human peripheral blood monocytes. Cell. Biochem. Biophys. 1998;29:179–201. doi: 10.1007/BF02737835. [DOI] [PubMed] [Google Scholar]
- SKEEN M.J., MILLER M.A., SHINNICK T.M., ZIEGLER H.K. Regulation of murine IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J. Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- STRASSMANN G., PATIL-KOOTA V., FINKELMAN F., FONG M., KAMBAYASHI T. Evidence for the involvement of Interleukin-10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRINCHIERI G. Interleukin-12: a pro-inflammatory cytokine with immuno-regulatory functions that bridge innate resistance and antigen-specitic adaptative immunity. Ann. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- TRINCHIERI G., SCOTT P. 62nd Forum in Immunology: immunoregulation by interleukin-12. Res. Immunol. 1995;146:419–422. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- VAN DER POUW KRAAN T.C., BOEIJE L.C., SMEENK R.J., WIJDENES J., AARDEN L.A. Prostaglandin E-2 is a potent inhibitor of human interleukin-12 production. J. Exp. Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYNN T.A., JANKOVIC D., HIENY S., CHEEVER A.W., SHER A. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J. Immunol. 1995;154:4701–4709. [PubMed] [Google Scholar]
- WYSOKA M., KUBIN M., VIEIRA L.Q., OZMEN L., GAROTTA G., SCOTT P., TRINCHIERI G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]


