Abstract
In the present study, we have observed the development of an inflammatory reaction in the rat hindpaw, following the injection of specific agonists of PAR2 (two PAR2 activating peptides). This inflammation was characterized by oedema and granulocyte infiltration.
Two selective PAR2 activating peptides, SLGRL-NH2 and trans-cinnamoyl-LIGRLO-NH2 induced significant oedema in the rat hindpaw from 1–6 h following subplantar injection. Six hours after the PAR2-activating peptide injection, the paw tissues showed a complete disruption of tissue architecture along with an inflammatory cell infiltrate.
In the inflamed paw, PAR2-immunoreactivity was expressed on endothelial cells as well as on the infiltrating inflammatory cells.
The oedema induced by the injection of the two PAR2 activating peptides was slightly reduced in rats pre-treated with compound 48/80, but was not modified by pre-treatment of rats with cromolyn, a mast cell stabilizer. Pre-treatment of rats with a cyclo-oxygenase inhibitor (indomethacin) or a nitric oxide synthase inhibitor (L-Nω-nitro-L-arginine methyl ester) had no effect on the oedema induced by the PAR2-activating peptides.
These results demonstrate that the administration of PAR2-activating peptides into the rat paw induced an acute inflammatory response characterized by a persistent oedema (at least 6 h) and granulocyte infiltration. The PAR2-induced inflammatory response occurred through a mechanism largely independent of mast cell activation, and of the production of prostanoids and nitric oxide.
Keywords: Proteinase-activated receptor, PAR2, inflammation, oedema, mast cell, prostaglandin, nitric oxide
Introduction
PAR2, is a member of a family of G protein-coupled receptors that are activated by proteinases (Proteinase-Activated Receptors: PARs) (Rasmussen et al., 1991; Vu et al., 1991; Nystedt et al., 1994; Ishihara et al., 1997; Kahn et al., 1998; Xu et al., 1998). Four members of this family have been cloned, three of which can be activated by thrombin (PAR1, PAR3 and PAR4). The fourth, PAR2, can be activated by trypsin or tryptase (Corvera et al., 1997; Molino et al., 1997). The unique mechanism whereby proteinases activate the PARs involves the proteolytic cleavage and unmasking of an N-terminal receptor sequence, that in turn acts as a tethered ligand, which activates the receptor, itself. Short synthetic peptides based on the proteolytically revealed receptor sequences (PAR-activating peptides: PAR-APs) can, in isolation, selectively activate PAR1 (e.g., the peptide TFLLR-NH2) and PAR2 (e.g. SLIGRL-NH2: SL-NH2). PAR3 and PAR4 are both activated by thrombin, but they do not appear to be activated by the PAR-APs derived from PAR1 and PAR2.
The physiological and pathophysiological importance of PAR2 is largely unknown. Previous studies have shown that PAR2 activation can cause relaxation of rat aorta rings by an endothelium-dependent mechanism mediated by nitric oxide production (Al-Ani et al., 1995; Saifeddine et al., 1996). In the intact rat, the intravenous injection of a PAR2-activating peptide has been observed to produce a marked fall in blood pressure (Hwa et al., 1996; Emilsson et al., 1997). The possible involvement of PAR2 in the inflammatory response has been suggested by the finding that PAR2 mRNA is upregulated in cultured endothelial cells by interleukin-1α and tumour necrosis factor-α (Nystedt et al., 1996). Moreover, mast cell tryptase can cleave and activate PAR2 in vitro in endothelial cells, enterocytes and colonic myocytes (Molino et al., 1997; Corvera et al., 1997; Fox et al., 1997).
The studies summarized in the above paragraph suggested that PAR2 may play an important role in inflammation. A recent study has shown that the injection into the rat paw of the PAR2-AP, SL-NH2, can enhance vascular permeability and cause swelling (Kawabata et al., 1998). This vascular effect was observed at a single time point (15 min) after the peptide was administered. Unfortunately, no other components of an inflammatory response were examined. By using the inflammatory model of rat hindpaw oedema (Winter et al., 1962), we wished to determine if PAR2 activation would result in an inflammatory reaction that included infiltration of granulocytes, and we wished to examine in further detail, the time course and the mechanism of this inflammatory response (i.e., involvement of mediators such as nitric oxide and prostaglandins). Two selective PAR2APs were injected into the paw of rats: the SL-NH2 peptide, which corresponds to the tethered-ligand sequence of murine and rat PAR2, and trans-cinnamoyl-LIGRLO-NH2 (tc-NH2), a peptide which has been found to be highly selective for PAR2 (Vergnolle et al., 1998; Saifeddine et al., 1998) and which is more resistant to metabolic degradation by aminopeptidases, than SL-NH2. A partial reverse sequence peptide of the PAR2AP, LSIGRL-NH2, which is unable to activate PAR2 (Hollenberg et al., 1997), was used as a control agonist for paw injection. In addition, we examined the role of mast cells, nitric oxide and prostanoids in mediating the effects of PAR2 activation.
Methods
Animals
Male, Wistar rats (175–200 g) obtained from Charles River Breeding Farms (Montreal, QC, Canada) were used. Animals had free access to food and water and were housed under constant temperature (22°C) and photoperiod (12-h light–dark cycle). All experimental procedures were approved by the Animal Care Committee of the University of Calgary and were performed in accordance with the guidelines established by the Canadian Council on Animal Care.
Paw oedema
The animals were lightly anaesthetized with halothane (5%). Basal paw volume was recorded using a hydroplethismometer (Ugo Basile, Milan, Italy). A subplantar injection of 0.1 ml of the test substance was then administered (n=6 per group in all experiments). The peptides were initially dissolved as stock solutions (1–5 mM) in 25 mM HEPES (pH 7.4) and were diluted for injection in sterile 0.9% saline to give the desired concentrations. The test substances included the PAR2APs, SL-NH2 and tc-NH2 (100 and 500 μg) and the control peptide LS-NH2 (100 and 500 μg) which is not able to activate PAR2. Paw volume was measured every hour for 6 h after the peptide injection. The increase in paw volume was evaluated as difference between the paw volume at each interval and the basal paw volume.
For the evaluation of the effects of drugs on oedema formation, some groups of rats were pre-treated with indomethacin (5 mg kg−1 given orally) or L-Nω-nitro-L-arginine methyl ester (L-NAME; 25 mg kg−1 in saline, intraperitoneally) 1 h before the subplantar injection of tc-NH2. The control group for indomethacin received the appropriate vehicle (5% NAHCO3) and the control group for L-NAME received intraperitoneally, D-NAME, 25 mg kg−1 in saline. Other groups of rats were treated with compound 48/80 to deplete mast cells, as described by Di Rosa et al. (1971). Briefly, compound 48/80 (0.1% solution in 0.9% sterile saline) was injected intraperitoneally each morning and evening for 4 days prior to the paw oedema experiment. The doses employed were 0.6 mg kg−1 for the first six injections and 1.2 mg kg−1 for the last two injections. The test substances in the paw oedema experiments were administered 5–6 h after the final injection of compound 48/80. The control groups received a saline injection (the vehicle for 48/80). Other groups of rats were treated with sodium cromoglycate (cromolyn), a mast cell stabilizer, at 20 mg kg−1 by intravenous injection 1 h before the subplantar injection of the peptides SL-NH2 and tc-NH2 and again 1 h after the subplantar injection of SL-NH2 and tc-NH2. The control group received a saline injection (the vehicle for cromolyn) at the same time-points.
Materials
All peptides, prepared by solid phase synthesis, were obtained from the peptide synthesis facility of the University of Calgary Faculty of Medicine (director, Dr D. McMaster). The composition and the purity of all peptides were confirmed by HPLC analysis, mass spectral analysis and amino acid analysis. Stock solutions (1–5 mM) prepared in 25 mM HEPES buffer, pH 7.4, were analysed by quantitative amino acid analysis to verify peptide concentration and purity. Indomethacin, L-NAME, compound 48/80 and cromolyn were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.).
Histology
Rats were given a 0.1 ml subplantar injection of either the inactive peptide LS-NH2, one of the PAR2APs (SL-NH2 and tc-NH2), or vehicle (n=3 per group) and were killed 6 h thereafter. The injected paws were removed and fixed by immersion in formalin. The paws were then washed in 50% ethanol in distilled water an embedded in paraffin wax. Sections (5 μm) were mounted on glass slides and stained with haematoxylin and eosin.
Histochemistry
Rats were given a 0.1 ml subplantar injection of either the inactive peptide LS-NH2, the PAR2AP SL-NH2, or vehicle (n=3 per group) and were killed 6 h thereafter. The injected paws were removed and fixed by immersion in Carnoy's fixative (ethanol/chloroform/acitic acid, 6 : 3 : 1). The paws were then washed in 50% ethanol in distilled water and embedded in paraffin wax. Sections (5 μm) were mounted on glass slides and stained with Alcian blue 1% (a mast cell-specific dye) and counterstained with Safranin 1%.
Immunohistochemistry
Rats were given a 0.1 ml subplantar injection of either the control peptide LS-NH2 or the PAR2AP tc-NH2 and were killed 6 h after. The injected paws were removed and fixed by immersion in Zamboni's fixative overnight at 4°C. After fixation, they were washed in a phosphate buffered saline (pH 7.4) and cryostat sections (12 μm) were processed for immunohistochemistry. Briefly, sections were incubated for 48 h at 4°C in a specific anti-PAR2 antibody raised in rabbit (B5, 1 : 1000; Kong et al., 1997). After washing, they were incubated in a donkey anti-rabbit IgG conjugated to CY3 (1 : 100, Jackson ImmunoResearch Laboratories, West Grove, PA, U.S.A.) secondary antibody for 1 h at room temperature. Finally they were washed and mounted in bicarbonate-buffered glycerol (1 : 3, pH 8.6). Controls were treated as above, but incubated in primary antibody containing 10 μg ml−1 of the original polypeptide hapten used to raise the antibody. Sections were viewed under a Zeiss Axioplan fluorescence microscope. Micrographs were made by digital capture of the images using a Sony CCD camera and Northern Exposure (Empix Inc., ON, Canada) software. Montages of the original images were made with Corel Draw.
Statistical analysis
All results are reported as mean±s.e.mean. Comparisons among groups were performed using the two-sided Student's t-test with the Bonferroni correction. With all statistical analyses, an associated probability (P value) of less than 5% was considered significant.
Results
Oedema induced by PAR2 activation
Subplantar injection of the PAR2APs (SL-NH2 and tc-NH2) or of an inactive peptide (LS-NH2) caused oedema in the first 2 h, the magnitude of which was significantly greater than that caused by the vehicle alone. Nonetheless, from the third to the sixth hour after the injection, the oedema elicited by 500 μg (765 nmoles) of the selective PAR2-activating peptide SL-NH2 was significantly greater than that caused by the same dose of the control peptide (LS-NH2) that is unable to activate PAR2 (Figure 1). Injection of either 100 or 500 μg (125 or 615 nmoles) of another selective PAR2AP (tc-NH2), which is more resistant to metabolic degradation by aminopeptidases than SL-NH2, provoked a profound oedema, significantly greater than the response caused by the inactive peptide (LS-NH2), at all time points (Figure 1).
Figure 1.
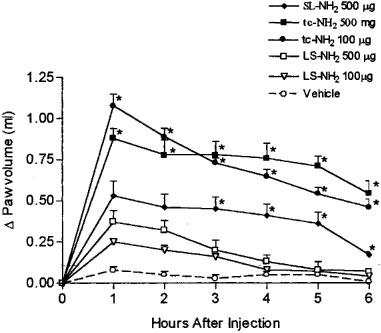
Increase in rat hindpaw volume following administration of PAR2-activating peptides (SL-NH2, 500 μg and tc-NH2, 100 and 500 μg), a control peptide (LS-NH2, 100 and 500 μg) or vehicle. Asterisks denote significant differences (P<0.05) between the control peptide groups and the other groups for the same dose.
Histological alterations associated with PAR2 activation
In rats injected with saline, no sign of tissue damage or cellular infiltration was observed (Figure 2A). The paws of rats injected with the control peptide, LS-NH2, exhibited some disruption of tissue architecture, but only in restricted areas. Moreover, there was a very low degree of cell infiltration (Figure 2B). In contrast, paws injected with either of the PAR2APs (SL-NH2 and tc-NH2) exhibited profound oedema and complete disruption of tissue architecture (Figure 2C and D). Numerous inflammatory cells (mainly granulocytes) were evident in the paws of rats injected with the PAR2APs.
Figure 2.
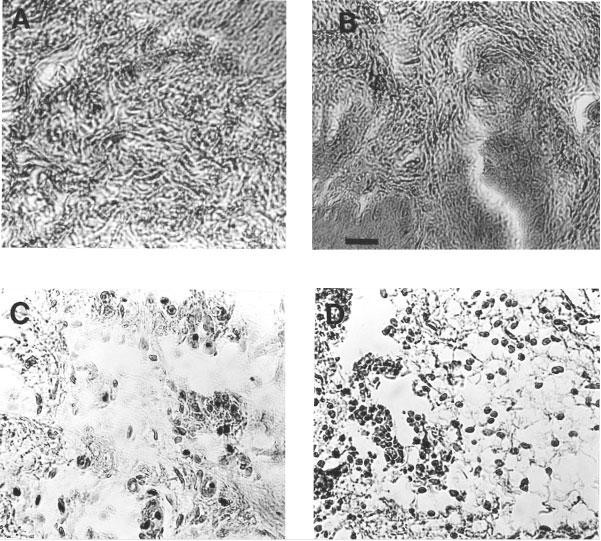
Histological staining of sections of rat hindpaws, 6 h after the injection of vehicle (A), the control peptide (LS-NH2, 100 μg) (B), the two PAR2-activating peptides SL-NH2, 500 μg (C), and tc-NH2, 100 μg (D). Scale bar: 50 μm. The scale bar in B is for A, B, C and D.
Localization of PAR2 receptors
The localization of the PAR2 receptors was assessed by immunohistochemistry. PAR2-immunoreactivity was observed at very low levels in the contralateral (non-injected) paw of rats treated with the PAR2AP or in the injected paw of rats treated with the control peptide (LS-NH2). In the animals injected with the control peptide (LS-NH2), there was faint staining of the endothelium of blood vessels and in a few resident cells in the sub-dermis (Figure 3). Muscle and connective tissue were not labelled. In animals injected 6 h previously with the PAR2-activating peptide, tc-NH2, there was extensive oedema and cellular infiltration. PAR2-immunoreactivity was found on infiltrating cells and was up-regulated on the endothelium of some blood vessels (mostly venules, Figure 3). Incubation of sections with antibody containing the hapten resulted in the complete abolition of staining, suggesting that the labelling observed was specific to PAR2. However, since other protein epitopes might conceivably cross-react with our anti-PAR2 antiserum, we refer to the labelling as PAR2-immunoreactivity.
Figure 3.
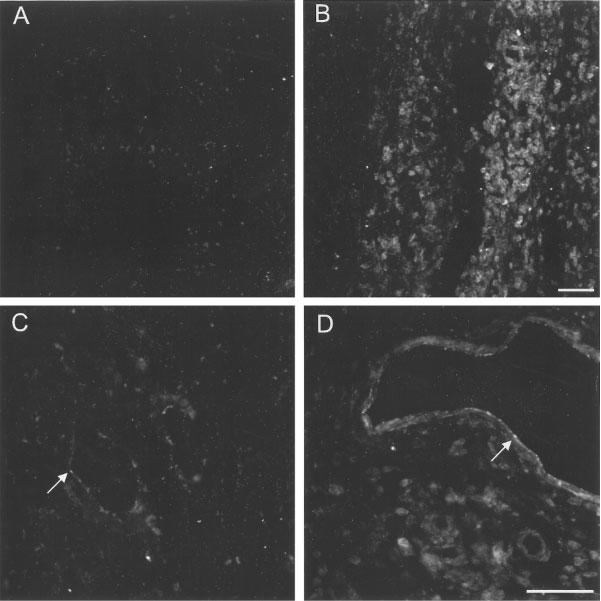
Fluorescence micrographs of PAR2-immunoreactivity in the rat paw from animals injected 6 h earlier with a control peptide (LS-NH2, A and C) and paws injected with a PAR2AP (tc-NH2, B and D). (A) Subdermis from the control paw showing little or no specific labelling. (B) In paws injected with the PAR2AP, there was an extensive infiltration of immunoreactive cells and considerable oedema. (C) On the endothelium of some blood vessels of control tissues (arrow) there was faint PAR2-immunoreactivity. (D) In PAR2AP-treated paws, increased intensity of PAR2-immunoreactivity was found on the endothelium of some blood vessels (mostly venules, arrow). Scale bars: 50 μm. The scale bar in B is for A and B, and the bar in D is for C and D.
Role of mast cells
Administration of the control peptide (LS-NH2) to rats that had been pretreated with compound 48/80 or with cromolyn resulted in a paw oedema response that was not significantly different from that observed in response to saline injection (Figure 4A and B). Pre-treatment with compound 48/80 resulted in a small reduction of the oedema response to the two selective PAR2APs, from the second to the sixth hour after the injection of tc-NH2 and from the fourth to the fifth hour after the injection of the SL-NH2 peptide (Figure 4A). The pre-treatment of animals with doses of cromolyn known to inhibit hypersensitivity reaction in the rat hindpaw (Martel & Klicius, 1997), had no effect on the oedema induced by either tc-NH2 or SL-NH2 (Figure 4B). The histochemical study showed that some mast cells stained positively with Alcian blue in the paws injected with buffer. In the paws injected with the inactive peptide LS-NH2, the Alcian blue staining was diffuse around some mast cells indicating a degranulation. In that tissues, the most part of mast cells failed to take up Alcian blue because of the release of their granule contents. In paws injected with the selective PAR2AP SL-NH2, several mast cells stained positively with Alcian blue among the numerous infiltrated inflammatory cells. A higher amount of Alcian blue-stained mast cells was observed in SL-NH2-injected paws compared to paws injected with buffer.
Figure 4.
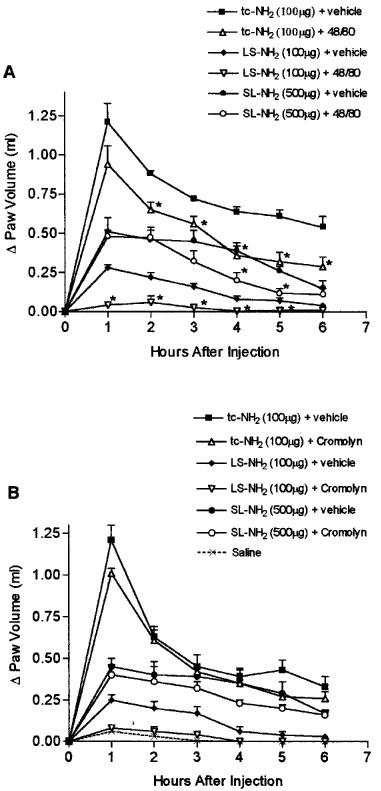
(A) Effects of prior depletion of mast cells (through pre-treatment with compound 48/80) on the increase in hindpaw volume following injection of PAR2-activating peptides (tc-NH2, 100 μg or SL-NH2 500 μg) or a control peptide (LS-NH2, 100 μg). Asterisks denote significant differences (P<0.05) from the corresponding group not treated with compound 48/80. (B) Effects of mast cell stabilization (through pre-treatment with cromolyn) on the increase in hindpaw volume following injection of PAR2-activating peptides (tc-NH2, 100 μg or SL-NH2 500 μg) or a control peptide (LS-NH2, 100 μg). Asterisks denote significant differences (P<0.05) from the corresponding group not treated with cromolyn.
Role of prostanoids
Pre-treatment of the rats with indomethacin at a dose known to inhibit carrageenan-airpouch inflammation (Wallace et al., 1999) resulted in a small but significant decrease in the oedema induced by the PAR2AP, tc-NH2, but this decrease was not apparent until the third through sixth hours after administration of tc-NH2. Indomethacin had no effect on the oedema induced by the control peptide, LS-NH2 (Figure 5).
Figure 5.
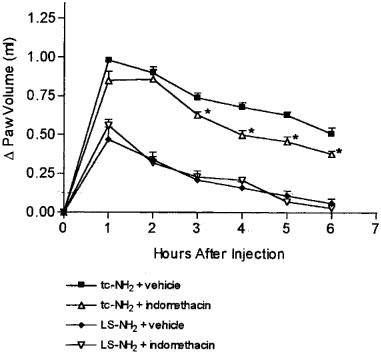
Effects of pre-treatment with the cyclo-oxygenase inhibitor, indomethacin, on the increase in hindpaw volume following injection of PAR2-activating peptide (tc-NH2, 100 μg) or a control peptide (LS-NH2, 100 μg). Asterisks denote significant differences (P<0.05) from the corresponding group not treated with indomethacin.
Role of nitric oxide
Pre-treatment of the rats with 25 mg kg−1 of L-NAME, a dose that has been previously shown to significantly inhibit the carrageenan-induced paw oedema (Honore et al., 1995), did not significantly affect the oedema response to either the PAR2AP, tc-NH2, or the control peptide, LS-NH2 (Figure 6).
Figure 6.
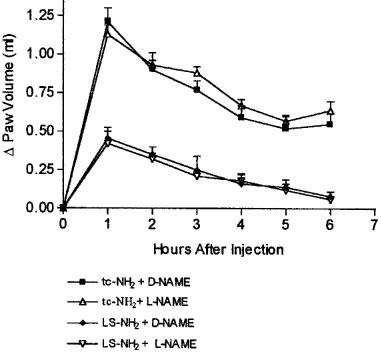
Effects of pre-treatment with the nitric oxide synthase inhibitor, L-NAME, on the increase in hindpaw volume following injection of a PAR2-activating peptide (tc-NH2, 100 μg) or a control peptide (LS-NH2, 100 μg), compared to control treatment with D-NAME. Asterisks denote significant differences (P<0.05) from the corresponding group not treated with L-NAME.
Discussion
In comparison to PAR1, little is known about the physiological and pathophysiological importance of PAR2. Some studies have shown that PAR2 activation leads to effects characteristic of an inflammatory response, such as blood vessel relaxation (Al-Ani et al., 1995; Saifeddine et al., 1996), an increase in vascular permeability (Kawabata et al., 1998) and hypotension (Hwa et al., 1996; Emilsson et al., 1997). However, the ability of PAR2 activation to play a role in other aspects of the inflammatory response, such as leukocyte margination, granulocyte infiltration, cytokine production, prolonged oedema and a disruption of tissue integrity, has yet to be explored in any depth. The study we describe here considerably extends that of Kawabata and colleagues (1998) who demonstrated a rapid (15 min) mast cell-dependent increase in tissue permeability (Evan's blue extravasation) caused by the administration of the PAR2AP, SL-NH2. In our study, we have shown that an acute inflammatory response characterized by persistent oedema (at least 6 h), a dramatic alteration of tissue cyto-architecture and robust granulocyte infiltration was induced by two selective PAR2APs, tc-NH2 and SL-NH2. Moreover, we have shown that the rapid mast cell-dependent increase in vascular permeability observed by Kawabata et al. (1998) may be due only to a minor extent to a PAR2-mediated process, since the PAR2-inactive peptide LS-NH2 can also cause a mast cell-dependent oedema response. Finally, we demonstrated that the acute inflammatory response induced by the potent and selective PAR2AP, tc-NH2, is largely independent of mast cell degranulation and does not involve either prostaglandins or nitric oxide. These findings strongly suggest that PAR2, present in tissue components other than mast cells, can play a pivotal role in the inflammatory process.
In the present study, the magnitude of the oedema response provoked by PAR2 activation after the injection of tc-NH2 was as great as that observed following the injection of 1% (wt vol−1) carrageenan in the well-documented model of rat hindpaw oedema (Winter et al., 1962). Moreover, PAR2 activation in the rat paw resulted not only in an increase in vascular permeability, as reflected by the oedema, but also in the marked infiltration of granulocytes, another hallmark feature of inflammatory reactions. Endothelial cells, which express PAR2 (Al-Ani et al., 1995; Hwa et al., 1996; Mirza et al., 1996; Saifeddine et al., 1996), may represent one of the principal targets for PAR2APs in the paw, in terms of causing increased tissue permeability and oedema. In accord with previous work documenting the presence of PAR2 on neutrophils (Howells et al., 1997), we found that in tissues of rats that had been injected with the PAR2AP, tc-NH2, PAR2 was expressed on infiltrating inflammatory cells as well as on endothelial cells. These results suggest that in addition to its effects on endothelial cells, PAR2 may also have an impact on the inflammatory reaction via its ability to activate cells that migrate into the tissue. In this regard, it is important to note that for at least 6 h after the injection of the PAR2APs a prominent inflammatory reaction was still present, both in terms of oedema and disruption of tissue cyto-architecture.
Surprisingly, we found that a significant oedema response (compared to that observed with saline alone) could be detected during the first 3 h following administration of a control peptide, LS-NH2, that cannot activate PAR2. Comparable results were observed in a previous study we performed with the same model of inflammation but using peptides that selectively activate PAR1 rather than PAR2 (Vergnolle et al., 1999). In that study, the PAR1 control peptide FSLLR-NH2, which cannot activate PAR1, caused an oedema significantly greater than that observed with saline alone, but less than the oedema caused by a PAR1-selective peptide agonist. Like the results we have described for the control PAR1 peptide FSLLR-NH2 (Vergnolle et al., 1999), the response to the control PAR2 peptide LS-NH2 (a partial reverse-sequence peptide of the PAR2 peptide) was completely abolished by pre-treating the animals with compound 48/80 or cromolyn. Moreover, in the present study, the histochemical observations showed that the injection of the LS-NH2 peptide caused mast cell degranulation. Therefore, the effect on the oedema response observed with these control peptides that can not activate PAR1 or PAR2, appeared to be due to mast cell activation via a mechanism that does not involve either PAR1 or PAR2. It is possible that this mast cell-dependent oedema response to control peptides may have been attributable to the aromatic/basic residues in these peptides. Such residues could potentially activate the mast cell in a manner similar to the activation caused by compound 48/80, a formaldehyde Schiff-base conjugate that can also expose mast cells to both aromatic and basic substituents (Mousli et al., 1990). We cannot exclude the possibility that the PAR1 and PAR2 control peptides might also activate receptors other than PAR1 or PAR2 that are located on mast cells to cause a mast cell-dependent oedema.
The mechanism of action of the selective PAR2APs in terms of inducing oedema formation is not clear. As mentioned above, a recent report (Kawabata et al., 1998) described an increase in vascular permeability induced by the intraplantar injection of the SL-NH2 peptide that was eliminated by treating the animals with compound 48/80. Our observations, with respect to oedema formation in rats treated with compound 48/80, suggest a more limited role for the mast cell component than that was suggested by the study of Kawabata et al. (1998) which focused primarily on changes in tissue permeability. Our work showed that in rats pre-treated with compound 48/80, the oedema induced by the injection of the PAR2APs was only slightly reduced from the second to the sixth hour for the tc-NH2 peptide and from the forth to the fifth hour for the SL-NH2 peptide. Even at 6 h after the tc-NH2 injection, the oedema in compound 48/80-treated animals was not abolished and was still greater than in animals injected either with the control peptide or saline. In contrast, the oedema induced by the control PAR2 peptide, LS-NH2, was completely abolished by pre-treatment of the rats with compound 48/80. These differences between the results of Kawabata et al. (1998) and ours may be explained primarily by differences in the time frame of the two studies. In the present study, the oedema responses were monitored for 6 h following the injection of the PAR2AP, while in the study of Kawabata et al. (1998) the vascular permeability was measured at only one time-point: 15 min after peptide injection. At such an early time point after peptide injection (15 min), it may be difficult to distinguish between the events mediated by PAR2 activation and the processes triggered either by LS-NH2 or SL-NH2 in mast cells via a receptor other than PAR2. Another factor to explain the differences between our observations and those reported by Kawabata et al. (1998) may be related to the different peptides and the higher doses we have used. In our study, we have used doses of 100 and 500 μg (125–615 nmoles for the tc-NH2; 155–765 nmoles for the SL-NH2 peptide) of two PAR2APs, tc-NH2 and SL-NH2, and of the control LS-NH2 peptide. The same doses of peptide were used in a previous study that demonstrated the pro-inflammatory effects of a PAR1-activating peptide in the rat hindpaw model (Cirino et al., 1996). Because of the non-PAR2-mediated effect of the control peptide (LS-NH2) on the oedema response, we were unable to detect with confidence, differences between the control peptide and the PAR2-activating peptide (SL-NH2) at doses below 100 μg (155 nmoles; data not shown). In the study of Kawabata et al. (1998), the highest dose of SL-NH2 they used was 65 μg (100 nmoles), and a mixture of amino acids rather than an intact scrambled sequence peptide was used as a ‘control'. Thus, the mast cell-dependent effects of low doses of SL-NH2 (⩽65 μg or 100 nmoles) on vascular permeability that were described by Kawabata et al. (1998) may have been due not only to the activation of PAR2 on mast cells, but also to the activation of another mast cell receptor. In our study, the use of higher doses of the SL-NH2 peptide (500 μg; 765 nmoles) or the use of the more potent tc-NH2 peptide, which is more resistant to aminopeptidase degradation than SL-NH2, allowed us to distinguish the effect of PAR2 activation from the mast cell-dependent effect induced most likely via a receptor other than PAR2 or by a non-receptor mediated mechanism (Mousli et al., 1990). Apart from the compound 48/80 treatment, we have also treated two groups of rats with cromolyn, a mast cell stabilizer, and observed that the oedema induced by the injection of each of the PAR2APs was not modified (Figure 4B). The experiments performed with 48/80- and cromolyn-treated animals clearly showed that the oedema induced by the injection of PAR2APs is largely unrelated to mast cell activation. Nonetheless, histochemical examination indicated that among the numerous infiltrated inflammatory cells observed in paws 6 h after the injection of the PAR2AP SL-NH2, several were identified as mast cells. These results indicate that even if the PAR2APs-induced oedema is largely independent of mass cell activation, mast cells may participate in a later phase of the inflammatory reaction initiated by PAR2 activation. PAR2 activation has been shown to result in the release of arachidonic acid and the generation of prostaglandin E2 and F1α (Kong et al., 1997). These mediators could contribute to the inflammatory response. However, the oedema caused by the injection of the PAR2AP, tc-NH2, was inhibited only to a small extent by indomethacin, suggesting that the pro-inflammatory effect was mostly independent of prostanoids. PAR2 activation can also result in the liberation of nitric oxide (Al-Ani et al., 1995; Hwa et al., 1996; Saifeddine et al., 1996). However, we found that pre-treatment with a nitric oxide synthase inhibitor (L-NAME) failed to modify the oedema response to a selective PAR2AP (tc-NH2).
PAR2 can be activated by trypsin in certain tissues, such as enterocytes (Kong et al., 1997). However, other tissues that express PAR2 are not exposed to trypsin. Other proteinases might therefore be responsible for PAR2 activation in vivo, particularly in the case of inflammatory reactions. Mast cells are involved in many inflammatory reactions as effector cells that initiate the inflammatory response by releasing a variety of pro-inflammatory mediators. Among the mediators released during mast cell degranulation, proteinases represent the major protein constituents. Tryptase, which is one of the proteinases released by activated human mast cells, is able to cleave and activate PAR2 (Corvera et al., 1997; Fox et al., 1997; Molino et al., 1997). Thus, tryptase or other mast cell proteinases represent good candidates for the activation of PAR2 in vivo during inflammatory processes. PAR2 activation by mast cell proteinases may play an important role in inflammatory states characterized by mast cell infiltration and degranulation. To date, the use of peptides that selectively activate PAR2 has provided considerable insights concerning the possible consequences of activating PAR2 in vivo. Such receptor-activating peptides yield information that is more readily interpreted than information obtained by administering proteolytic enzymes, which can cause many effects in addition to the activation of PAR2. Unfortunately, potent and selective PAR2 antagonists are not yet available either to verify the target (presumably PAR2) of the PAR2APs that we have used or to assess the precise contribution of PAR2 itself in the inflammatory response of tissues to proteinases.
In summary, our data obtained with the PAR2APs suggest that PAR2 activation can cause a profound inflammatory response, characterized by oedema and granulocyte infiltration. The mechanism of the early inflammatory reaction induced by PAR2 activation is largely independent of mast cell activation and of the production of prostaglandins and nitric oxide.
Acknowledgments
We are grateful to Dr Denis McMaster at the University of Calgary Peptide Synthesis Facility for the efficient provision of synthetic peptides. This work was supported by grants to Drs Hollenberg, Sharkey and Wallace from the Medical Research Council of Canada (MRC). Dr Vergnolle is supported by a fellowship from the Canadian Association of Gastroenterology/Abbott Laboratories/MRC. Dr Wallace is an MRC Senior Scientist and an Alberta Heritage Foundation for Medical Research (AHFMR) Senior Scientist. Dr Sharkey is an AHFMR Senior Scholar. The authors are grateful to Webb McKnight and Kevin Chapman for their assistance in performing these studies.
Abbreviations
- L-NAME
Nω-nitro-L-arginine methyl ester
- LS-NH2
LSIGRL-NH2
- PAR1
proteinase-activated receptor-1
- PAR2
proteinase-activated receptor-2
- PAR3
proteinase-activated receptor-3
- PAR4
proteinase-activated receptor-4
- PAR-APs
PAR-activating peptides
- PAR2APs
proteinase-activated receptor-2-activating peptides
- PARs
proteolytically-activated-receptors
- SL-NH2
SLIGRL-NH2
- tc-NH2
trans-cinnamoyl-LIGRLO-NH2
References
- AL-ANI B., SAIFEDDINE M., HOLLENBERG M.D. Detection of functional receptors for the proteinase-activated receptor-2-activating polypeptides, SLIGRL-NH2 in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- CIRINO G., CICALA C., BUCCI M.R., SORRENTINO L., MARAGANORE J.M., STONE S.R. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGUE K., BOHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates colonic myocytes through proteinase-activated receptor-2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI ROSA M., GIROUD J.P., WILLOUGHBY D.A. Studies of the mediators of the acute inflammatory response induced in rats at different sites by carrageenan and turpentine. J. Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- EMILSSON K., WAHLESTEDT C., SUN M.K., NYSTEDT S., OWMAN C., SUNDELIN J. Vascular effects of proteinase-activated receptor 2 agonist peptide. J. Vasc. Res. 1997;34:267–272. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- FOX M.T., HARRIOTT P., WALKER B., STONE S.R. Identification of potential activators of proteinase-activated receptor-2. FEBS Lett. 1997;417:267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., KAWABATA A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- HONORE P., CHAPMAN V., BURITOVA J., BESSON J.M. Reduction of carrageenin oedema and the associated c-Fos expression in the rat lumbar spinal cord by nitric oxide synthase inhibitor. Br. J. Pharmacol. 1995;114:77–74. doi: 10.1111/j.1476-5381.1995.tb14908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELLS G.L., MACEY M., CHINNI C., HOU L., FOX M.T., HARRIOTT P., STONE S. Proteinase-activated receptor-2: expression by human neutrophils. J. Cell. Sci. 1997;110:881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHINTALA M., ZHANG R., CHATTERJEE M., SYBERTZ E. Evidence for the presence of proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ. Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Proteinase-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., JR, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., MINAMI T., KATAOKA K., TANEDA M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br. J. Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG W., MCCONALOGUE K., KHITIN L.M., HOLLENBERG M.D., PAYAN D.G., BOHM S.K., BUNNETT N.W. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Acad. Natl. Sci. U.S.A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTEL R.R., KLICIUS J. Reagin-mediated hypersensitivity reaction in the rat hindpaw and its inhibition by antianaphylactic drugs. Int. Arch. Allergy Appl. Immunol. 1994;54:205–209. doi: 10.1159/000231828. [DOI] [PubMed] [Google Scholar]
- MIRZA H., YATSULA V., BAHOU W.F. The proteinase activated receptor-2 (PAR2) mediates mitogenic responses in human vascular endothelial cells. J. Clin. Invest. 1996;97:1705–1714. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECHTER J.A., WOOLKALIS M., BRASS L.F. Interaction of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BUEB J.L., BRONNER C., ROUOT B., LANDRY Y. G-protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol. Sci. 1990;11:358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., RAMAKRISHNAN V., SUNDELIN J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. J. Biol. Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN U.B., VOURET-CRAVIARI V., JALLAT S., SCHLESINGER Y., PAGES G., PAVIRANI A., LECOCQ J.P., POUYSSEGUR J., VAN OBBERGHEN-SCHILLING E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides and in gastric and vascular tissues. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., ROY S.S., AL-ANI B., TRIGGLE C.R., HOLLENBERG M.D. Endothelium-dependent contractile actions of proteinase-activated receptor-2-activating peptides in human umbilical vein: release of a contracting factor via a novel receptor. Br. J. Pharmacol. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR-1) Br. J. Pharmacol. 1999;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor-2-activating peptides: identification of a receptor that regulates intestinal transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., CHAPMAN K., MCKNIGHT W. Limited anti-inflammatory efficacy of cyclooxygenase-2 inhibition in carrageenan-airpouch inflammation. Br. J. Pharmacol. 1999;126:1200–1205. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER C.A., RISLEY E.A., NUSS G.W. Carrageenan-induced oedema in the hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- XU W., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


