Abstract
PMC, a potent α-tocopherol derivative, dose-dependently (5–25 μM) inhibited the ATP-release reaction and platelet aggregation in washed human platelets stimulated by agonists (collagen and ADP).
PMC also dose-dependently inhibited the intracellular Ca2+ mobilization, whereas it did not inhibit phosphoinositide breakdown in human platelets stimulated by collagen.
PMC (10 and 25 μM) significantly inhibited collagen-stimulated thromboxane A2 (TxA2) formation in human platelets. On the other hand, PMC (25 and 100 μM) did not increase the formation of cyclic AMP or cyclic GMP in platelets. Moreover, PMC (25, 100, and 200 μM) did not affect the thromboxane synthetase activity of aspirin-treated platelet microsomes.
PMC (10 and 25 μM) markedly inhibited the exogenous arachidonic acid (100 μM)-induced prostaglandin E2 (PGE2) formation in the presence of imidazole (600 μM) in washed human platelets, indicating that PMC inhibits cyclo-oxygenase activity.
We conclude that PMC may exert its anti-platelet aggregation activity by inhibiting cyclo-oxygenase activity, which leads to reduced prostaglandin formation; this, in turn, is followed by a reduction of TxA2 formation, and finally inhibition of [Ca2+]i mobilization and ATP-release.
Keywords: PMC, α-tocopherol, platelet aggregation, cyclo-oxygenase
Introduction
α-Tocopherol (vitamin E) is well known for its antioxidant properties in biological membranes, where it acts to prevent the peroxidation of membrane lipids (Burton et al., 1986). It is also known to effectively inhibit the activation of cytokine-induced nuclear factor-κB (NF-κB) (Suzuki & Packer, 1993). NF-κB is believed to play an important role in the activation of the human immunodeficiency virus (HIV), and inducible nitric oxide synthase (iNOS) which may play a key role in septic shock (Kilbourn & Griffith, 1992). Therefore, antioxi-dants such as α-tocopherol may have a possible therapeutic use for preventing HIV activation and septic shock.
α-Tocopherol is also known to inhibit platelet aggregation in vitro (Steiner & Anastasi, 1976; Agradi et al., 1981), an effect that was initially attributed to the inhibition of lipid peroxidation (Steiner & Anastasi, 1976). α-Tocopherol has been tested in human plasma, where a concentration of about 1 mM inhibits platelet aggregation (Steiner & Anastasi, 1976). These findings are of scientific interest since they suggest a possible role of α-tocopherol in the modulation of platelet function; however, they are of no clinical value because a concentration of 1 mM α-tocopherol cannot be achieved in human blood by α-tocopherol supplementation (Vatassery et al., 1983). Although its activity was found to be rather weak, interest in α-tocopherol has continued as it represents one of the few natural substances with platelet aggregation inhibiting activity.
The mechanism of action of α-tocopherol has proved difficult to define, owing to its highly lipophilic nature. Therefore, finding a more hydrophilic analogue is important for exploring the mechanisms of α-tocopherol's anti-platelet aggregation activity. Of the α-tocopherol analogues studied, PMC (2, 2, 5, 7, 8, -pentamethyl-6-hydroxychromane), in which the phytyl chain is replaced by a methyl group (Figure 1), is the most potent derivative of α-tocopherols in antioxidation (Suzuki & Packer, 1993). PMC is more hydrophilic than other α-tocopherol derivatives, and has potent radical scavenging activity (Suzuki & Packer, 1993). Although the effect of α-tocopherol on aggregation of human platelets has been studied, the molecular mechanisms underlying the α-tocopherol signalling pathway remain obscure. We therefore systematically examined the influence of PMC on washed human platelets, and utilized the findings to characterize the mechanisms of action of this α-tocopherol derivative.
Figure 1.

Chemical structures of α-tocopherol and PMC.
Methods
Preparation of human platelet suspensions
Human platelet suspensions were prepared as previously described (Huang et al., 1991). Blood was collected from healthy human volunteers who had not taken any medicine during the preceding 2 weeks, and was mixed with acid/citrate/glucose (9:1, v v−1). After centrifugation at 120×g for 10 min at room temperature, the supernatant (platelet-rich plasma; PRP) was supplemented with PGE1 (0.5 μM) and heparin (6.4 IU ml−1), and then incubated for 10 min at 37°C and centrifuged at 500×g for 10 min. The platelet pellets were suspended in 5 ml of Tyrode's solution, pH 7.3 [containing (mM) NaCl 11.9, KCl 2.7, MgCl2 2.1, NaH2PO4 0.4, NaHCO3 11.9, and glucose 11.1]. Apyrase (1.0 U ml−1), PGE1 (0.5 μM), and heparin (6.4 IU ml−1) were then added, and the mixture was incubated for 10 min at 37°C. After centrifugation of the suspensions at 500×g for 10 min, the washing procedure was repeated. The washed platelets were finally suspended in Tyrode's solution containing bovine serum albumin (BSA) (3.5 mg ml−1) and adjusted to a concentration of 4.5×108 platelets ml−1. The final concentration of Ca2+ in the Tyrode's solution was 1 mM.
Platelet aggregation
The turbidimetric method (Born & Cross, 1963) was applied to measure platelet aggregation, using a Lumi-Aggregometer (Payton, Canada). Platelet suspensions (0.4 ml) were pre-warmed at 37°C for 2 min (stirring at 1200 r.p.m.) in a silicone-treated glass cuvette. PMC, α-tocopherol or vehicle solvent (0.4% DMSO) was added 3 min before the addition of platelet-aggregation inducers. The reaction was allowed to proceed for at least 6 min and the extent of aggregation was expressed as the percentage of the control value (in the absence of PMC). The degree of aggregation was expressed in light-transmission units. While measuring ATP release, 20 μl of luciferin/luciferase mixture was added 1 min before the addition of agonists and ATP release was compared with that of control. For PMC and α-tocopherol, the inhibitory concentrations IC50 was determined as that concentration required to reduce, by a half, the maximum extent of the change in light transmission achieved on stirring the aggregating agent with platelet suspensions preincubated with the solvent control alone.
Analysis of platelet surface GP IIb/IIIa complex by flow cytometry
Triflavin, a specific fibrinogen receptor (GP IIb/IIIa complex) antagonist, was prepared as previously described (Sheu et al., 1992). FITC-conjugated triflavin was also prepared as previously described (Sheu et al., 1996). The final concentration of FITC-conjugated triflavin was adjusted to 1 mg ml−1. Human platelet suspensions were prepared as described above. Aliquots of platelet suspensions (4.5×108 ml−1) were pre-incubated with PMC (25 and 200 μM) or vehicle solution (0.4% DMSO) for 3 min, followed by the addition of 2 μl of FITC-triflavin. The suspensions were then incubated for another 5 min, and the volume was adjusted to 1 ml tube−1 with Tyrode's solution. The suspensions were then assayed for fluorescein-labeled platelets with a flow cytometer (Becton Dickinson, FACScan Sys.). Data were collected from 50,000 platelets per experimental group. All experiments were repeated at least five times to ensure reproducibility.
Labelling of membrane phospholipids and measurement of the production of [3H]-inositol phosphates
Citrated human PRP was centrifuged at 500×g for 10 min at room temperature, and the platelet pellets were then suspended in 1 ml of a Ca2+-free and BSA-free Tyrode's solution containing [3H]-inositol (75 μCi ml−1). Platelet pellets were incubated at 37°C for 2 h, followed by centrifugation. Platelets were finally resuspended in Ca2+-free Tyrode's solution, and the platelet count was adjusted to 5×108 platelets ml−1. One-ml aliquots of platelet suspensions were pre-warmed at 37°C with 5 mM LiCl in a 3.5 ml cuvette. PMC (5 and 25 μM) or vehicle solution (0.4% DMSO) was pre-incubated with loaded platelets at room temperature for 3 min, and collagen (10 μg ml−1) was then added to trigger aggregation. Six minutes later, the reaction was stopped by adding ice-cold trichloroacetic acid (TCA, 10%, w v−1) and the samples were centrifuged at 1000×g for 4 min. One-ml aliquots of each of the supernatants were transferred to test tubes. TCA was removed by extraction with 10 ml of ethyl ether three times. The mixture was then incubated over water at 80°C to remove the residual ethyl ether. The inositol phosphates were separated in a Dowex-1 anion exchange column (50%, w v−1, 1 ml) as described by Neylon & Summers (1987). Only [3H]-inositol monophosphate (IP) was measured as an index of the total inositol phosphate formation, because the levels of inositol bisphosphate (IP2) and inositol trisphosphate (IP3) were very low.
Measurement of platelet [Ca2+]i mobilization by fura 2-AM fluorescence
Citrated whole blood was centrifuged at 120×g for 10 min. The supernatant was protected from light and incubated with Fura 2-AM (5 μM) at 37°C for 1 h. Human platelet suspensions were then prepared as described above. Finally, the external Ca2+ concentration of the platelet suspensions was adjusted to 1 mM. The [Ca2+]i rise was measured using a fluorescence spectrophotometer (CAF 110, Jasco, Japan) at excitation and emission wavelengths of 340 and 500 nm, respectively. [Ca2+]i was calculated from the fluorescence, using 224 nM as the Ca2+-Fura 2 dissociation constant (Grynkiewicz et al., 1985).
Measurement of TxB2 formation
Washed human platelet suspensions (0.4 ml, 4.5×108 ml−1) were preincubated for 3 min in the presence of PMC (10 and 25 μM) or vehicle solution (0.4% DMSO) before the addition of collagen (5 μg ml−1). Six minutes after the addition of collagen, EDTA (2 mM) and indomethacin (50 μM) were added to the reaction suspensions. The vials were then centrifuged in an Eppendorf centrifuge (Model 5414) for 3 min at 14,000 r.p.m. The TxB2 levels of the supernatants were measured using an EIA (enzyme immunoassay) kit (Cayman, U.S.A.) according to the manufacturer's instructions.
Assay for thromboxane synthetase activity
The thromboxane synthetase activity was determined using a kit of thromboxane synthetase inhibitor according to the manufacturer's instructions (Biomol Res. Lab., PA, U.S.A.). In brief, 0.1-ml aliquots of aspirin-treated platelet microsomes were placed in tubes, followed in some cases by the addition of 10 μl imidazole (1 mM) or PMC (25, 100, and 200 μM). The mixtures were then incubated at 25°C with shaking for 3 min. Then, 2 μl of PGH2 solution were added to the tubes, and the tubes were vortexed and placed in a 25°C water bath for 3 min. Next, 10 μl FeCl2 solution was added, followed by incubation at room temperature for 15 min, the tubes were then returned to the ice bath. Finally, the tubes were centrifuged at 3000×g at 4°C for 10 min, and the TxB2 levels in the supernatants were measured using an EIA kit according to the manufacturer's instructions.
Measurement of PGE2 formation
PMC (10 and 25 μM) or vehicle solution (0.4% DMSO) was preincubated for 3 min in the presence of imidazole (600 μM) in washed human platelets (0.4 ml, 4.5×108 ml−1). Six minutes after the addition of arachidonic acid (100 μM), EDTA (10 mM) and indomethacin (500 μM) were added to the reaction suspensions. The vials were then centrifuged and the PGE2 levels of the supernatants were measured using an EIA kit (Cayman, U.S.A.) according to the manufacturer's instructions.
Estimation of platelet cyclic AMP and cyclic GMP
The method of Karniguian et al. (1982) was followed. Platelet suspensions were first prewarmed at 37°C for 1 min. Then, either PGE1 (10 μM), nitroglycerin (10 μM), or PMC (25 and 100 μM) was added, and the suspensions were incubated for 3 min. The incubation was stopped by adding 10 mM EDTA and immediately boiling for 5 min. The reaction mixtures were cooled to 4°C, and the precipitated protein was collected as sediment after centrifugation in an Eppendorf centrifuge. The supernatant (400 μl) was freeze-dried and the residue was dissolved in 100 ml of distilled water. To determine the cyclic AMP and cyclic GMP concentrations, 50 μl of the reconstituted supernatant was acetylated and used for EIA, as described by the manufacturer (Cayman, U.S.A.).
Materials
Collagen (Type I, bovine achilles tendon), adenosine 5′-diphosphate (ADP), sodium citrate, luciferin-luciferase, Dowex-1 (100–200 mesh; X8, chloride form), indomethacin, prostaglandin E1 (PGE1), nitroglycerin, apyrase, heparin, arachidonic acid, and myoinositol were purchased from Sigma Chem. Co. (St. Louis, MO, U.S.A.). Fura 2-AM and fluorescein iso-thiocyanate (FITC) were purchased from Molecular Probe Inc. (Eugene, OR, U.S.A.). Trimeresurus flavoviridis venom was purchased from Latoxan (Rosans, France). Myo-2-[3H]-inositol was purchased from Amersham (Buckinghamshire, HP, U.K.). Cyclic AMP, cyclic GMP, thromboxane B2 (TxB2), and PGE2 (EIA) kits were purchased from Cayman Co. (Ann Arbor, MI, U.S.A.). PMC, obtained from Wako Pure Chemical Co. (Osaka, Japan), was dissolved in dimethyl sulphoxide (DMSO) and stored at −4°C until use.
Statistical analysis
The experimental results are expressed as the means±s.e.mean and are accompanied by the number of observations. Data were assessed with analysis of variance (ANOVA). If this analysis indicated significant differences among the group means, then each group was compared by the Newman-Keuls method. A P value less than 0.05 was considered statistically significant. The IC50 values were obtained by regression analysis.
Results
Effect of PMC on platelet aggregation in human platelet suspensions
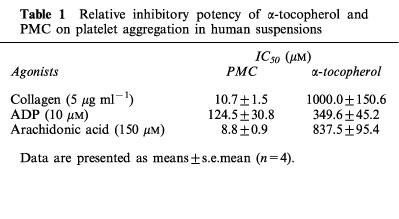
PMC (5–25 μM) dose-dependently inhibited collagen (5 μg ml−1)-induced ATP-release and platelet aggregation in human platelet suspensions (Figures 2 and 3). It also inhibited fibrinogen-induced aggregation of ADP (10 μM)-stimulated platelets (Figure 3). Furthermore, PMC (25 μM) also inhibited arachidonic acid (150 μM)-induced platelet aggregation by about 85% (data not shown). At a dose of 10 μM, PMC was less potent at inhibiting ADP (10 μM)-induced platelet aggregation than collagen (5 μg ml−1). α-Tocopherol (100–500 μM) also dose-dependently inhibited ADP (10 μM) and collagen (5 μg ml−1)-induced platelet aggregation, but less efficiently than PMC. The IC50 values of PMC and α-tocopherol for collagen-, ADP- and arachidonic acid-induced platelet aggregation were shown in Table 1. On a molar basis, PMC was about 90 fold more potent than α-tocopherol at inhibiting collagen-induced platelet aggregation (Table 1).
Figure 2.

Typical patterns of the inhibitory effect of PMC on collagen-induced platelet aggregation and ATP release from washed human platelet suspensions. Platelets (4.5×108 ml−1) were preincubated with PMC (5 and 25 μM) at 37°C for 3 min, then collagen (5 μg ml−1) was added to trigger aggregation (upward tracing) and ATP release (downward tracing). Luciferin/luciferase (10 μl) was added 1 min before the collagen for measurement of ATP release.
Figure 3.

Dose-inhibition curves of PMC and α-tocopherol in collagen and ADP-induced platelet aggregation. Human platelet suspensions were preincubated with various concentrations of PMC (5, 10, and 25 μM) or α-tocopherol (100, 200, and 500 μM) at 37°C for 3 min, followed by the addition of collagen (5 μg ml−1) or ADP (10 μM) to trigger platelet aggregation. Data are presented as percentage of the control (in the absence of PMC or α-tocopherol) (means±s.e.mean, n=4).
Table 1.
Relative inhibitory potency of α-tocopherol and PMC on platelet aggregation in human suspensions
Effect of PMC on collagen-induced GP IIb/IIIa complex exposure in human platelets
Triflavin is a specific fibrinogen receptor (GP IIb/IIIa complex) antagonist, purified from Trimeresurus flavoviridis snake venom (Sheu et al., 1992). Because the binding of fibrinogen to the GP IIb/IIIa complex is the final common pathway for agonist-induced platelet aggregation (Sheu et al., 1992), we decided to evaluate whether or not PMC inhibits agonist-induced platelet aggregation by binding directly to the platelet GP IIb/IIIa complex. In this study, FITC-triflavin (2 μg ml−1) bound directly to collagen (5 μg ml−1)-activated platelets (27.4±2.8, n=4). The intensity of the fluorescence was markedly reduced in the presence of 10 mM EDTA (negative control, 2.4±0.8, n=4). PMC (25 and 200 μM) did not significantly inhibit FITC-triflavin binding to the GP IIb/IIIa complex in platelet suspensions (28.6±1.4 and 30.6±4.3, respectively, n=4), indicating that the mechanism of the inhibitory effect of PMC on platelet aggregation does not involve binding to the GP IIb/IIIa complex.
Effect of PMC on phosphoinositide breakdown in human platelet suspensions
Phosphoinositide breakdown occurs in platelets activated by many different agonists (Broekman et al., 1980). In this study, we found collagen (10 μg ml−1) induced rapid formation of radioactive IP, IP2, and IP3 in human platelets loaded with [3H]-inositol, although we measured only [3H]-IP as an index of the total inositol phosphate formation. In this study, the addition of collagen resulted in a rise of IP formation of about 1.4 fold compared with that in resting platelets (9650±260 c.p.m. versus 13,120±560 c.p.m., n=4). The addition of PMC (5 and 25 μM) did not significantly decrease IP formation in collagen-stimulated human platelets (11,640±1290 c.p.m. versus 12,210±680 c.p.m., respectively, n=4), even at a high concentration (200 μM) (data not shown). These results indicate that the mechanisms of the inhibitory effect of PMC may not involve inhibition of phospholipase C.
Effect of PMC on [Ca2+]i mobilization
Free cytoplasmic Ca2+ concentrations in human platelets were measured by the Fura 2-AM loading method. As shown in Figure 4, collagen (5 μg ml−1) evoked an increase in [Ca2+]i from 17.8±4.2 to 193.5±13.2 (nM). This collagen-evoked increase in [Ca2+]i was markedly inhibited in the presence of PMC (10 μM, 75.6±8.6 nM; 25 μM, 19.2±1.7 nM; n=4), suggesting that PMC exerts an inhibitory effect on collagen-stimulated [Ca2+]i mobilization in human platelets.
Figure 4.

Effect of PMC on collagen-induced intracellular Ca2+ mobilization of Fura 2-AM-loaded human platelets. Platelet suspensions were incubated with Fura 2-AM (5 μM) at 37°C for 30 min, followed by the addition of collagen (5 μg ml−1) in the absence or presence of PMC (10 and 25 μM), which was added 3 min prior to the addition of collagen. The profiles are representative examples of four similar experiments.
Effect of PMC on TxB2 formation
As shown in Table 2, resting platelets produced relatively little TxB2 compared with collagen-activated platelets. PGE1 (5 μM) inhibited TxB2 formation in collagen-activated platelets by 82% (data not shown). Furthermore, results obtained using various concentrations of PMC indicated that PMC (10 and 25 μM) significantly inhibited TxB2 formation in platelets stimulated by collagen (5 μg ml−1) (Table 2). These results suggest that PMC may also exert an inhibitory effect on TxA2 formation.
Table 2.
Effect of PMC on collagen (5 μM ml−1)-induced thromboxane B2 formation in washed human platelets

Effect of PMC on cyclic AMP and cyclic GMP levels in human platelets
The levels of cyclic AMP and cyclic GMP in unstimulated platelets were low (26.8±5.6 and 4.2±1.3 pmol 10−9 platelets, respectively, n=4). Addition of PGE1 (10 μM) and nitro-glycerin (10 μM) increased the cyclic AMP and cyclic GMP levels to 201.9±62.3 and 20.3±7.1 pmol 10−9 platelets, respectively (n=4). However, addition of PMC at concentrations of up to 200 μM resulted in no significant increase in platelet cyclic AMP or cyclic GMP levels (data not shown).
Effect of PMC on thromboxane synthetase activity
We evaluated further whether or not PMC inhibits TxB2 formation through the inhibition of thromboxane synthetase activity. In this study, the production of TxB2 in unstimulated aspirin-treated platelet microsomes was about 1227.8±24.3 ng ml−1 (n=4). PMC (25, 100, and 200 μM) did not significantly decrease the production of TxB2 in aspirin-treated platelet microsomes (1211.0±28.5, 1206.9±39.1, and 1232.8±60.7 ng ml−1, respectively, n=4), indicating that the inhibitory effect of PMC on TxB2 formation was not through the inhibition of thromboxane synthetase activity. On the other hand, imidazole, a thromboxane synthetase inhibitor (positive control) (Needleman et al., 1977), markedly inhibited TxB2 formation in aspirin-treated platelet microsomes (932.4±25.1 ng ml−1, P<0.001, n= 4).
Effect of PMC on PGE2 formation
As shown in Table 3, exogenous arachidonic acid (100 μM) induced relatively high levels of PGE2 formation in washed human platelets in the presence of imidazole (600 μM). Furthermore, results obtained using various concentrations of PMC indicated that PMC (10 and 25 μM) significantly inhibited PGE2 formation in platelets stimulated by arachidonic acid (Table 3). These results suggest that PMC inhibits eicosanoid synthesis by preferential inhibition of cyclo-oxygenase activity.
Table 3.
Effect of PMC on arachidonic acid (100 μM)-induced prostaglandin E2 formation in washed human platelets

Discussion
The principal objective of this study was to study the detailed mechanisms involved in the inhibition of platelet aggregation by PMC, the most potent derivative of the α-tocopherols. Inhibition of platelet aggregation by α-tocopherol was initially attributed to the inhibition of lipid peroxidation (Steiner & Anastasi, 1976). However, observations that the oxidized form of α-tocopherol (which is devoid of antioxidant activity) also inhibits platelet aggregation cast doubt on this hypothesis (Cox et al., 1980). Subsequently, numerous reports have appeared concerning the action of α-tocopherol upon platelet aggregation. For example, Steiner & Mower (1982) reported that α-tocopherol at higher concentrations (0.25–1.0 mM) inhibits cyclo-oxygenase and cyclic AMP-specific phosphodiesterase. Kakishita et al. (1990) reported that at lower concentrations (0.02–0.4 mM), α-tocopherol inhibits platelet-activating factor (PAF) release and synthesis. Recently, Freedman et al. (1996) reported that α-tocopherol inhibits human platelet aggregation through a protein kinase C-dependent mechanism. Thus, α-tocopherol's effect on human platelets remains unclear and controversial. The difficulties in clearly defining the mechanisms of the antiplatelet activity of α-tocopherol may be due, at least partly, to its highly lipophilic nature.
Because PMC is the most effective α-tocopherol analogue, investigations of the mechanism of antiplatelet activity focused only on PMC in this study. The results demonstrated that PMC exerts an inhibitory activity upon human platelet aggregation, which has not been described previously. PMC was about 90 fold more potent than α-tocopherol at inhibiting collagen-induced platelet aggregation (Figure 3 and Table 1). Our results clearly show that PMC, which possesses a methyl group instead of phytyl side chain (Figure 1), exerts a stronger inhibitory effect than α-tocopherol.
In this study, agonist-induced platelet aggregation and ATP-release both appeared to be inhibited in the presence of PMC. Therefore, PMC may affect Ca2+ release from intracellular Ca2+-storage sites (i.e., dense tubular systems or dense bodies) (Figure 4). This is in accord with the concept that intracellular Ca2+ release is responsible for the ATP-release (Charo et al., 1976).
PMC significantly inhibited platelet aggregation stimulated by collagen, ADP, and arachidonic acid, despite the differences in their mechanisms. This implies that PMC may block a common step shared by these inducers. Our results also indicate that the site of action of PMC is not at the receptor level of individual agonists. Triflavin acts by binding to the GP IIb/IIIa complex on the platelet surface membrane, resulting in interference with the interaction of fibrinogen with its specific receptor (Sheu et al., 1992). In this study, we found that PMC did not significantly inhibit the binding of FITC-triflavin to the GP IIb/IIIa complex, indicating that the antiplatelet effect of PMC may not be directly due to interference with the binding of fibrinogen to its specific receptor on the platelet membrane surface.
The importance of cyclic AMP and cyclic GMP in modulating platelet reactivity is well established (Karniguian et al., 1982; McDonald & Murad, 1996). In addition to inhibiting most platelet responses, elevated levels of cyclic AMP or/and cyclic GMP decrease intracellular Ca2+ concentrations by promoting the uptake of Ca2+ into dense tubular systems (Zavoico & Feinstein, 1984; McDonald & Murad, 1996). Our data suggest that PMC does not exert its antiplatelet activity by increasing the levels of cyclic AMP and cyclic GMP. Furthermore, stimulation of platelets by agonists (i.e., collagen) results in phospholipase C-catalyzed hydrolysis of the minor plasma membrane phospholipid phosphatidylinositol 4,5-bisphosphate, with concomitant formation of inositol 1,4,5-trisphosphate and diacylglycerol (Berridge, 1983. There is strong evidence that inositol 1,4,5-trisphosphate induces the release of Ca2+ from intracellular stores (Berridge, 1983). Diacylglycerol activates protein kinase C, inducing protein phosphorylation and a release reaction. In this study, phosphoinositide breakdown of collagen-activated platelets was not inhibited by PMC, suggesting that inhibition of platelet aggregation by PMC is not related to inhibition of phospholipase C activity.
TxA2 is an important mediator of release reaction and aggregation of platelets (Hornby, 1982). It has also been proposed to act as an intracellular ionophore, releasing Ca2+ directly (Gerrard et al., 1981). Collagen-induced formation of TxB2, a stable metabolite of TxA2, was markedly inhibited by PMC (10 and 25 μM) (Table 2). Thus, it seems likely that reduction of TxB2 formation plays an important role in the mediation of the inhibitory effect of PMC on platelet aggregation.
PMC did not significantly affect the thromboxane synthetase activity in aspirin-treated platelet microsomes, indicating that the inhibition of TxB2 formation by PMC is not due to the inhibition of thromboxane synthetase in human platelets. On the other hand, PMC is capable of inhibiting arachidonic acid-induced synthesis of PGE2 (Table 3), indicating that PMC can significantly affect the cyclo-oxygenase activity in human platelets. In contrast, α-tocopherol (1 mM) did not inhibit PGE2 synthesis in the same reaction (data not shown). This may be due to poor uptake into platelets or the inability of α-tocopherol to suppress cyclo-oxygenase activity. We also found that PMC (100 μM) did not significantly affect the phospholipase A2 activity in [3H]-arachidonic acid-labeled resting platelets (unpublished data). These results indicate that inhibition of TxA2 formation in human platelets by PMC, may be through a direct effect on the cyclo-oxygenase activity in the platelet membrane.
In conclusion, platelet aggregation plays a pathophysio-logical role in a variety of thromboembolic disorders, including myocardial infarction, atherosclerosis and stroke. Therefore, prevention of platelet aggregation by drugs should provide effective prophylactic and/or therapeutic means of treating such diseases. Our findings suggest that PMC inhibits agonist-induced platelet aggregation in human platelets, probably via the initial inhibition of cyclo-oxygenase activity, which leads to inhibition of TxA2 formation and [Ca2+]i mobilization. These findings suggest that PMC may be an effective tool in treating thromboembolic-related disorders.
Acknowledgments
This work was supported by a grant from the National Science Council of Taiwan (NSC86-2314-B-038-015-M36).
Abbreviations
- BSA
bovine serum albumin
- [Ca2+]i
intracellular calcium
- EIA
enzyme immunoassay
- FITC
fluorescein iso-thiocyanate
- HIV
human immunodeficiency
- iNOS
inducible nitric oxide synthase
- IP
inosito monophosphate
- IP2
inositol bisphosphate
- IP3
inositol trisphosphate
- NF-κB
nuclear factor-κB
- PGE1
prostaglandin E1
- PRP
platelet-rich plasma
- TxA2
thromboxane A2
References
- AGRADI E., PETRONI A., SOCINI A., GALLI C. In vitro effects of synthetic antioxidants and vitamin E on arachidonic acid metabolism and thromboxane formation in human platelets and on platelet aggregation. Prostaglandins. 1981;22:255–266. doi: 10.1016/0090-6980(81)90040-x. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem. J. 1983;212:249–258. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G.V.R., CROSS M.J. The aggregation of blood platelets. J. Physiol. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROEKMAN M.J., WARD J.W., MARCUS A.J. Phospholipid metabolism in phosphatidyl inositol, phosphatic acid and lysophospholipids. J. Clin. Invest. 1980;66:275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON G.W., CHENG S.C., WEBB A., INGOLD K.U. Vitamin E in young and old human red blood cells. Biochim. Biophys. Acta. 1986;860:84–90. doi: 10.1016/0005-2736(86)90501-8. [DOI] [PubMed] [Google Scholar]
- CHARO I.F., FEINMAN R.D., DETWILER T.C. Inhibition of platelet secretion by an antagonist of intracellular calcium. Biochem. Biophys. Res. Commun. 1976;72:1462–1467. doi: 10.1016/s0006-291x(76)80178-7. [DOI] [PubMed] [Google Scholar]
- COX A.C., RAO G.H., GERRARD J.M., WHITE J.G. The influence of vitamin E quinone on platelet structure, function, and biochemistry. Blood. 1980;55:907–914. [PubMed] [Google Scholar]
- FREEDMAN J.E., FARHAT J.H, , LOSCALZO J., KEANEY J.F. Alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation. 1996;94:2434–2440. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- GERRARD J.M., PETERSON D.A., WHITE J.G.Calcium mobilization Platelets in Biology and Pathology 1981Amsterdam: Elsevier; 406–436.ed. Gordon, J.L. pp [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ induces with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HORNBY E.J. Evidence that prostaglandin endoperoxides can induce platelet aggregation in the absence of thromboxane A2 production. Biochem. Pharmacol. 1982;31:1158–1160. doi: 10.1016/0006-2952(82)90359-8. [DOI] [PubMed] [Google Scholar]
- HUANG T.F., SHEU J.R., TENG C.M. A potent antiplatelet peptide, triflavin, from Trimeresurus flavoviridis snake venom. Biochem. J. 1991;277:351–357. doi: 10.1042/bj2770351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKISHITA E., SUEHIRO A., OURA Y., NAGAI K. Inhibitory effect of vitamin E (alpha-tocopherol) on spontaneous platelet aggregation in whole blood. Thromb. Res. 1990;60:489–499. doi: 10.1016/0049-3848(90)90233-3. [DOI] [PubMed] [Google Scholar]
- KARNIGUIAN A., LEGRAND Y.J., CAEN J.P. Prostaglandins: specific inhibition of platelet adhesion to collagen and relationship with cyclic AMP level. Prostaglandins. 1982;23:437–457. doi: 10.1016/0090-6980(82)90108-3. [DOI] [PubMed] [Google Scholar]
- KILBOURN R.G., GRIFFITH O.W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J. Natl. Cancer Invest. 1992;84:827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- MCDONALD L.J., MURAD F. Nitric oxide and cyclic GMP signaling. Proc. Soc. Exp. Biol. Med. 1996;211:1–6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- NEEDLEMAN P., RAZ A., FERRENDELLI J.A., MINKES M. Application of imidazole as a selective inhibitor of thromboxane synthetase in human platelets. Proc. Natl. Acad. Sci. U.S.A. 1977;74:1716–1720. doi: 10.1073/pnas.74.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEYLON C.B., SUMMERS R.J. Stimulation of α1-adrenoceptor in rat kidney mediates increased inositol phospholipid hydrolysis. Br. J. Pharmacol. 1987;91:367–376. doi: 10.1111/j.1476-5381.1987.tb10291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEU J.R., LIN C.H., PENG C.H., HUANG T.F. Triflavin, an Arg-Gly-Asp-containing peptide, inhibits the adhesion of tumor cells to matrix protein via binding to multiple integrin receptors expressed on human hepatoma cells. Proc. Soc. Exp. Biol. Med. 1996;213:71–79. doi: 10.3181/00379727-213-44038. [DOI] [PubMed] [Google Scholar]
- SHEU J.R., TENG C.M., HUANG T.F. Triflavin, an RGD-containing antiplatelet peptide, binds to GP IIIa of ADP-stimulated platelets. Biochem. Biophys. Res. Commun. 1992;189:1236–1242. doi: 10.1016/0006-291x(92)92337-w. [DOI] [PubMed] [Google Scholar]
- STEINER M., ANASTASI J. Vitamin E: an inhibitor of the platelet release reaction. J. Clin. Invest. 1976;57:732–737. doi: 10.1172/JCI108331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINER M., MOWER R. Mechanism of action of vitamin E on platelet function. Ann. N.Y. Acad. Sci. 1982;393:289–299. doi: 10.1111/j.1749-6632.1982.tb31269.x. [DOI] [PubMed] [Google Scholar]
- SUZUKI Y.J., PACKER L. Inhibition of NF-kappa B activation by vitamin E derivatives. Biochem. Biophys. Res. Commun. 1993;193:277–283. doi: 10.1006/bbrc.1993.1620. [DOI] [PubMed] [Google Scholar]
- VATASSERY G.T., MORLEY J.E., KUSKOWSKI M.A. Vitamin E in plasma and platelets of human diabetic patients and control subjects. Am. J. Clin. Neut. 1983;37:641–644. doi: 10.1093/ajcn/37.4.641. [DOI] [PubMed] [Google Scholar]
- ZAVOICO G.B., FEINSTEIN M.B. Cytoplasmic Ca2+ in platelets is controlled by cyclic AMP: antagonism between stimulators and inhibitors of adenylate cyclase. Biochem. Biophys. Res. Commun. 1984;120:579–585. doi: 10.1016/0006-291x(84)91294-4. [DOI] [PubMed] [Google Scholar]


