Abstract
Experiments have been conducted to investigate the actions of endothelins on the guinea-pig prostate gland.
Saturation experiments with [125I]-endothelin-1 (2–800 pM) in guinea-pig prostatic homogenates indicated the presence of high affinity binding sites with an equilibrium dissociation constant (KD) of 230±50 pM, a maximum number of binding sites (Bmax) of 52±16 fmol mg−1 protein or 269±61 fmol g−1 tissue and a Hill coefficient (nH) of 1.01±0.03 (n=3). Competition experiments revealed that binding of [125I]-endothelin-1 (20 pM) was inhibited with the following order of potency: endothelin-1 >>BQ-788 (N-cis-2,6-dimethylpiperidinocarbonyl-L-γ-methyl-Leu-D-Trp[1-CO2CH3-D-Nle-ONa])>BQ-123 (cyclo-D-Asp-L-Pro-D-Val-Leu-D-Trp) ⩾sarafotoxin S6c.
At concentrations with negligible influence on smooth muscle tone, endothelin-1, endothelin-2 and sarafotoxin S6b (1 nM–0.1 μM) produced concentration-dependent potentiation of the contractions evoked by electrical field stimulation with trains of 20 pulses at 10 Hz every 50 s, 0.5 ms pulse width and a dial setting of 60 V. In contrast, the endothelin ETB receptor-preferring agonist endothelin-3 (1 nM–1 μM) was much less potent, and the endothelin ETB receptor-selective agonists sarafotoxin S6c and BQ-3020 (Ac-[Ala11,15]-endothelin-1 (6-21)), up to 1 μM, were without effect.
The endothelin ETA receptor antagonist BQ-123 (1 μM) markedly inhibited the potentiation induced by endothelin-1, endothelin-2 and sarafotoxin S6b while the endothelin ETB receptor antagonist BQ-788 (1 μM) was less effective.
While our binding data indicates the presence of ETA and ETB binding sites in the guinea-pig prostate, the endothelin-induced facilitation of neurotransmission to the prostatic smooth muscle is mediated largely via activation of endothelin receptors of the ETA subtype.
Keywords: Endothelin ETA and ETB receptors; endothelin-1, -2 and -3; sarafotoxins S6b and S6c; BQ-123; BQ-788; BQ-3020; smooth muscle contraction; field stimulation; guinea-pig prostate
Introduction
Since the discovery of endothelin in the porcine vascular endothelial cell cultures in 1988 by Yanagisawa et al. (1988), the presence of this peptide and its receptors has been demonstrated in a variety of tissues and organs (see reviews by Huggins et al., 1993; Rubanyi & Polokoff, 1994; Sokolovsky, 1995). The existence of three genes encoding a family of structurally similar peptides, endothelin-1, -2 and -3, has been described (Inoue et al., 1989). Endothelins have been reported to have roles not only in smooth muscle contraction of vascular and non-vascular tissues, but also in promoting cell growth (see reviews by Battistini et al., 1993; Hay, 1995) and in the modulation of neuroeffector transmission in the airways (Takimoto et al., 1993; Henry & Goldie, 1995; Fernandes et al., 1996) and in the urogenital tract (urinary bladder: Saenz de Tejada et al., 1992; Donoso et al., 1994; vas deferens: Wiklund et al., 1989; Donoso et al., 1992; Lau et al., 1995; Maas et al., 1995).
There is now a growing body of evidence that indicates the presence of endothelins in the prostate. Endothelin precursors and the endothelin converting enzyme (ECE-1) have been reported to be expressed in the human prostate (Rossi et al., 1995; Prayer-Galetti et al., 1997; Walden et al., 1998). To date, two major endothelin receptor subtypes have been identified (Masaki et al., 1994), and both endothelin ETA and ETB receptors are detected in human cultured prostatic smooth muscle cells (Saita et al., 1997), but the ETA receptors are predominantly expressed in prostatic membrane homogenates (Le Brun et al., 1996) and slide-mounted prostatic sections (Kobayashi et al., 1994a; Imajo et al., 1997). Quantitative receptor autoradiographic studies by Kobayashi et al. (1994b), have, however, shown a preferential localization of endothelin ETA and ETB receptors in stroma and epithelium, respectively.
Endothelin-1 immunoreactivity is prominent in the glandular epithelium (Langenstroer et al., 1993). It is possible that endothelins play a physiological role in modulation of secretion in the gland, or a physiological or pathophysiological role to attenuate apoptosis (Wu-Wong et al., 1997a) or to exert mitogenic effects in human prostatic smooth muscle cells (Saita et al., 1998). In addition, endothelin-1 potently contracts the human prostate in vitro. These contractions are shown to be mediated by both endothelin receptor subtypes (Kobayashi et al., 1994a) although it has also been proposed that in prostatic stromal cells, contraction is mediated by ETB receptors (Webb et al., 1995). Endothelins may thus play an important role in the regulation of the contractile function of smooth muscle cells associated with prostatic tension and secretory processes. Recent reports of upregulated endothelin binding sites in the benign hyperplastic prostate (Kondo et al., 1995; Moriyama et al., 1996) and of increased levels of endothelin-1 with decreased expression of endothelin ETB binding sites in prostate cancer (Nelson et al., 1996) suggest a possible involvement of endothelins in prostatic disorders. Prayer-Galetti et al. (1997) have proposed that zonal-specific distribution of endothelin receptors in the human prostate may be of relevance in the pathogenesis of benign prostatic hyperplasia and prostate cancer.
The main aim of the present study was to examine the actions of endothelins and to characterize endothelin receptor subtype/s in the prostate, using the guinea-pig as an experimental model. The guinea-pig prostate has a substantial stromal component, which comprises predominantly smooth muscle cells (Ricciardelli et al., 1989). With age, the guinea-pig prostate develops a stromal hyperplasia which is histologically indistinguishable from that observed in ageing men (Horsfall et al., 1994). The influences of endothelins and related sarafotoxins on prostatic smooth muscle contractility and on neurotransmission in the guinea-pig isolated prostate were examined. Pharmacological tools including the endothelin ETB receptor agonists sarafotoxin S6c (Williams et al., 1991) and BQ-3020 (Ihara et al., 1992b), and the endothelin ETA and ETB receptor-selective antagonists, BQ-123 (Ihara et al., 1992a) and BQ-788 (Ishikawa et al., 1994), respectively, have been employed in the characterization of endothelin receptor subtype/s in the guinea-pig prostate gland.
Preliminary accounts of some of the results of this study have been communicated to the Australian Neuroscience Society (Pennefather et al., 1998; Lau et al., 1999).
Methods
Animals
Adult male Dunkin-Hartley and Monash strain guinea-pigs (450–680 g) were housed in open runs at 22°C with a 12 h : 12 h light : dark cycle. Rodent chow, fruits, vegetables and water were provided ad libitum. Prior approval for animal experimentation was obtained from the Monash University Standing Committee on Ethics in Animal Experimentation (SCEAE Approval No. 96/069). Guinea-pigs were killed by cervical dislocation followed by exsanguination. The prostates were rapidly dissected out and trimmed of connective tissue and fat.
Radioligand binding studies
The [125I]-endothelin-1 binding assay was conducted as described previously by Williams et al. (1991). Immediately after removal from the guinea-pig, the whole prostate was placed in ice-cold Tris buffer containing Tris HCl (50 mM), sucrose (250 mM) and the protease inhibitors pepstatin A (7 μg ml−1) and leupeptin (0.5 μg ml−1) at pH 7.4. The prostate was blotted dry, weighed (mean weight=0.46± 0.02 g, n=18), finely minced with scissors and homogenized twice for 10 s in 10 ml of ice-cold buffer with a 2 min interval on ice using a Polytron homogenizer (setting 5). The homogenates were centrifuged at 47,766×g at 0–4°C for 10 min. The supernatant was discarded, the pellets homogenized and centrifuged as before for a further 30 min. The resultant pellets, containing membranes, were finally suspended in 7 ml of Tris buffer.
[125I]-endothelin-1 binding was conducted in assay buffer containing potassium phosphate (KH2PO4 50 mM; pH 7.5) with 0.1% bovine serum albumin (BSA). For the binding assay, 100 μl of the membrane suspension was added to tubes containing a final volume of 250 μl (approximately 60 μg of protein as determined by the method of Lowry et al. (1951)). Saturation experiments with [125I]-endothelin-1 were performed at eight concentrations in duplicate ranging from 2–800 pM. In competition experiments, at least twelve concentrations in triplicate of unlabelled endothelin-1 (10 pM–200 nM), sarafotoxin S6c (50 nM–5 μM), BQ-123 (1 nM–20 μM) and BQ-788 (0.5 nM–20 μM) were used with [125I]-endothelin-1 (20 pM). Non-specific binding was determined in the presence of unlabelled endothelin-1 (200 nM). Following a 60 min incubation at 37°C, the binding reaction was terminated by rapid filtration through Whatman GF/B glass fibre filters using a Brandel M-48 cell harvester. The filters had been presoaked overnight in 2% BSA to reduce non-specific binding to filters. After washing twice with 5 ml of ice-cold buffer and partial drying under vacuum, tissue-bound radioactivity was determined by a United Technologies Packard gamma counter with an efficiency of 100%.
Functional studies
Preparations of the guinea-pig isolated prostate were mounted vertically in 5-ml siliconised (Coatasil, Searle) organ baths containing Krebs-Henseleit solution (pH 7.4, maintained at 37°C and bubbled with 5% CO2 in O2) of the following composition (mM): NaCl 118.1, KCl 4.87, KH2PO4 1.2, NaHCO3 25.0, glucose 11.7, MgSO4.7H2O 0.5, CaCl2.2H2O 2.5. Tissues were allowed to equilibrate for 30 min under a rest-ing force of 0.5 g, with bath medium changes every 5–10 min. Nerve terminals within the prostatic smooth muscle preparations of the guinea-pig were then electrically field stimulated with trains of 20 pulses at 10 Hz every 50 s with a dial setting of 60 V and 0.5 ms pulse width via two parallel electrodes incorporated into the tissue holder, connected to a Grass S88 stimulator. Isometric contractions of the prostatic smooth muscle were recorded with Grass FT03C force-displacement transducers connected to a MacLab data acquisition system (Chart 3.3) interfaced with a Macintosh LC575 computer.
After the field stimulated preparations of the guinea-pig prostate had been allowed to equilibrate for 30 min and when the stimulation-induced contractions were stable, the effects of endothelin receptor agonists including endothelin-1, endothelin-2 (1 nM–0.1 μM), endothelin-3 (1 nM–1 μM), sarafotoxin S6b (1 nM–0.1 μM) and sarafotoxin S6c and BQ-3020 (1 nM–1 μM) on prostatic smooth muscle tone and on field stimulation-induced contractions were assessed. Log concentration-response curves to the endothelin receptor agonists were constructed cumulatively using half log unit increments of agonist concentration. Increasing concentration of agonist was added when responses to the previous concentration reached a plateau; this usually occurred within 5 min for lower concentrations, or after agonist exposure time of 10 min.
To investigate the endothelin receptor subtype/s involved in mediating the agonist-induced influence on neurotransmission to the smooth muscle of the prostate from the guinea-pig, log concentration-response curves to the endothelin receptor agonists were constructed in the absence and presence of the subtype-selective endothelin receptor antagonists BQ-123 (ETA: 1 μM) or BQ-788 (ETB: 1 μM), with antagonist incubation period of 30 min. Control experiments were also conducted in parallel in order to determine whether agonist concentration-response curves, at 60–80 min intervals, were reproducible over the experimental period and to correct for any tissue sensitivity changes due to time and/or vehicle. The vehicle was 0.01% dimethyl sulphoxide (DMSO). The effects of the endothelin receptor antagonists, alone and in combination, on the magnitude of field stimulation-induced contractions were examined after 15–30 min exposure of the prostatic preparations to BQ-123 (1 μM) or BQ-788 (1 μM).
In a separate series of experiments, the postjunctional action of endothelin-1 was determined by generating discrete concentration-contractile response curves to noradrenaline (0.1 μM–1 mM), noradrenaline exposure time of 60 s with a 10 min dose-cycle, before and 10 min after incubating prostatic preparations with endothelin-1 (10 nM).
Measurement and analysis of data
Binding data were analysed using software programs EBDA and LIGAND (McPherson, 1985).
In functional studies, the effects of endothelin receptor agonists on the magnitude of contractions evoked by trains of stimuli were expressed as percentage increases in field stimulation-induced responses. Mean peak force developed (in g) of four field stimulation-induced contractions just prior to and 5–10 min after exposure to each concentration of agonists were obtained in the absence and presence of antagonists. Mean log concentration-response curves to agonists were constructed by pooling data from individual curves. Agonist concentration required to produce 50% of the increase in stimulation-induced response induced by maximal concentration (0.1 μM) of agonist examined, EC50 (0.1 μM) with 95% confidence limits (C.L.) and degrees of freedom (d.f.), was derived from non-linear regression analyses using the GraphPad PRISM software program. The EC50 (0.1 μM) values were then converted to the negative logarithm and expressed as pEC50 (0.1 μM).
Data are presented as mean values with vertical bars representing standard error of the mean (s.e.mean); n represents the number of experimental animals. Statistical evaluation of data was performed with the GraphPad PRISM software program using Student's paired t-tests and one- or two-way analyses of variance (ANOVA), where appropriate. In all cases, values of P<0.05 were considered statistically significant.
Drugs
Endothelin-1, endothelin-2, endothelin-3, BQ-3020 (Ac-[Ala11,15]-endothelin-1 (6-21)), sarafotoxin S6b, sarafotoxin S6c, BQ-123 (cyclo-D-Asp-L-Pro-D-Val-Leu-D-Trp and BQ-788 (N-cis-2,6-dimethylpiperidinocarbonyl-L-γ-methyl-Leu-D-Trp[1-CO2CH3-D-Nle-ONa] were obtained from the American Peptide Company. [125I]-endothelin-1, at specific activity of 2000 Ci mmol−1, was prepared at the Baker Institute (Australia). Pepstatin A and leupeptin were from Auspep. (−)-Arterenol (noradrenaline) bitartrate was from Sigma. Bovine serum albumin (BSA) was from Commonwealth Serum Laboratory Ltd. (Australia). Stock concentrations of endothelins (0.1 mM), BQ-3020 (0.1 mM) and BQ-788 (1 mM) were prepared in 5–10% dimethyl sulphoxide (DMSO). BQ-123, sarafotoxins and leupeptin were dissolved in distilled water and noradrenaline was dissolved and diluted in a catecholamine diluent (mM: NaCl 154.0, NaH2PO4 1.2 and ascorbic acid 0.2). Pepstatin A was first dissolved in equivalent volumes of glacial acetic acid and ethanol and then made up to a desired concentration of 1 mg ml−1 in distilled water. Subsequent dilutions of peptides were made in KH2PO4 buffer with 0.1% BSA and Krebs-Henseleit solution in binding and functional experiments, respectively.
Results
Radioligand binding studies
Saturation experiments
Specific binding of [125I]-endothelin-1 (2–800 pM) to the guinea-pig prostatic homogenates was saturable and of high affinity with a KD of 230±50 pM and a Bmax of 52±16 fmol mg−1 protein or 269±61 fmol g−1 tissue. The Hill coefficient (nH) was 1.01±0.03 (n=3), indicating the presence of a homogeneous population of binding sites. Typical saturation and Scatchard plots are shown in Figure 1. Non-specific binding, determined in the presence of unlabelled endothelin-1 (200 nM), represented 20–50% of the total binding of [125I]-endothelin-1, at the concentration range employed (2–800 pM) (Figure 1).
Figure 1.
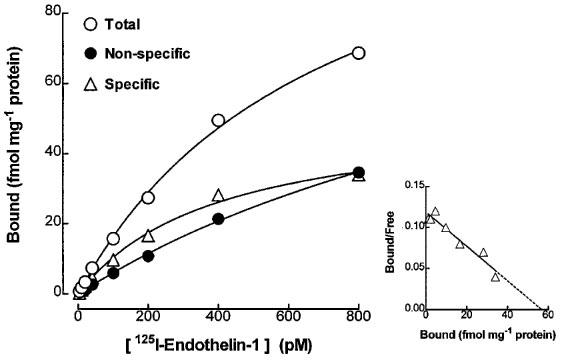
Saturation plot showing the specific binding of [125I]-endothelin-1, determined as the difference between total and non-specific (in the presence of 200 nM endothelin-1) binding in the guinea-pig prostatic homogenates. Each data point represents the mean of duplicate samples in a single experiment. Corresponding Scatchard transformation of specific [125I]-endothelin-1 is shown in the inset. These figures are representative of three similar experiments.
Competition experiments
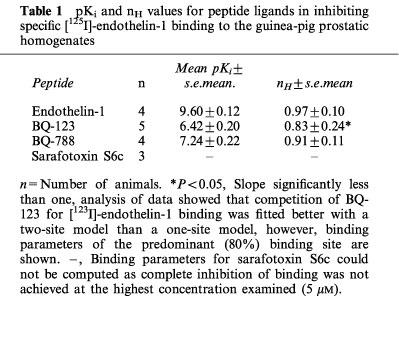
In competition studies the specific [125I]-endothelin-1 (20 pM) binding to the guinea-pig prostatic homogenates was inhibited by endothelin-1, sarafotoxin S6c, BQ-123 and BQ-788 (Figure 2). Individual competition curves for endothelin-1 and BQ-788 were consistently better fitted by a one-site model whilst curves for BQ-123 were better fitted by a two-site model. Sarafotoxin S6c, at the highest concentration used (5 μM), caused approximately 70% inhibition of the specific [125I]-endothelin-1 binding to the guinea-pig prostatic homogenates. The rank order of potency was endothelin-1 >>BQ-788>BQ-123⩾sarafotoxin S6c with respective mean values of the negative logarithm of dissociation constants (pKi) and slopes of the Hill plot (nH) from estimates of individual experiments shown in Table 1.
Figure 2.
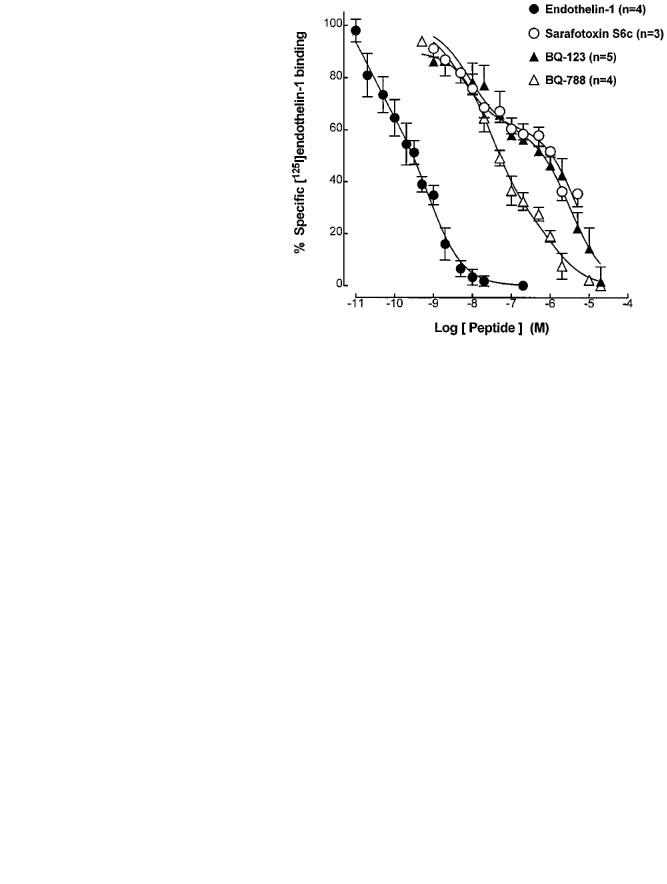
Competition of specific [125I]-endothelin-1 binding to the guinea-pig prostatic homogenates by unlabelled endothelin-1 (n=4), sarafotoxin S6c (n=3), BQ-123 (n=5) and BQ-788 (n=4). Non-specific binding was determined in the presence of endothelin-1 (200 nM). Mean values±s.e.mean of 3–5 separate experiments performed in triplicates are shown.
Table 1.
pKi and nH values for peptide ligands in inhibiting specific [125I]-endothelin-1 binding to the guinea-pig prostatic homogenates
Functional studies
Electrical field stimulation, delivered as trains of 20 pulses at 10 Hz every 50 s with 0.5 ms pulse width and a dial setting of 60 V, of nerve terminals within the prostate evoked regular and reproducible contractions (0.12±0.2 g; n=12) of the prostatic smooth muscle preparations from the guinea-pig. It has previously been shown that neurotransmission to the guinea-pig prostatic smooth muscle is predominantly sympathetic and noradrenergic in nature (Ohkawa, 1983; Lau & Pennefather, 1995; Najbar-Kaszkiel et al., 1997; Lau et al., 1998).
Cumulative addition of endothelin-1, endothelin-2 and sarafotoxin S6b (1 nM–0.1 μM) to the guinea-pig prostatic smooth muscle preparations produced concentration-dependent potentiation of the field stimulation-induced contractions, but had negligible effect on smooth muscle tone. Endothelin-3 (1 nM–1 μM) was similarly without effect on smooth muscle tone and much less potent in producing facilitation. Figure 3 shows a representative trace illustrating the enhancing effects of endothelin-1 on field stimulation-induced contractions of the guinea-pig prostatic smooth muscle. Mean responses to endothelin-1, endothelin-2 and sarafotoxin S6b at the maximal concentration (0.1 μM) of agonist examined were not significantly (P>0.05, one-way ANOVA) different (Table 2 and Figure 4). The three agonists were equipotent in eliciting enhancement of stimulation-induced contractions (Table 2 and Figure 4). In sharp contrast, the endothelin ETB receptor agonists sarafotoxin S6c and BQ-3020 (1 nM–1 μM) were without effect on the field stimulation-induced contractions of the guinea-pig prostatic preparations (Figure 4).
Figure 3.
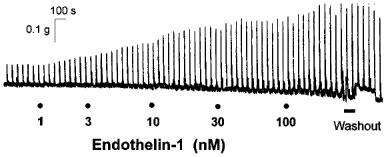
Representative trace showing the enhancing effect of endothelin-1 (1 nM–0.1 μM) on contractions evoked by trains of stimuli (0.5 ms pulse width, a dial setting of 60 V, 20 pulses at 10 Hz every 50 s) of the guinea-pig prostatic preparation.
Table 2.
Potencies of agonists in facilitating neurotransmission to the guinea-pig prostatic smooth muscle
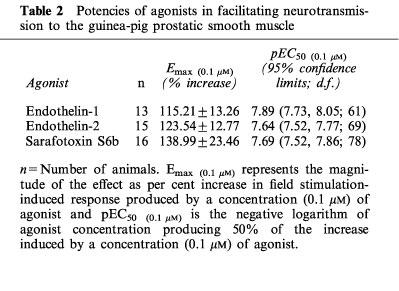
Figure 4.
Mean log concentration-response curves for the facilitatory effects of endothelin-1 (n=13), endothelin-2 (n=15), endothelin-3 (n=4), sarafotoxin S6b (n=15), sarafotoxin S6c (n=3) and BQ-3020 (n=3) on the field stimulation-induced contractions in guinea-pig prostatic preparations.
In time and/or vehicle control experiments, neither the magnitude of field stimulation-induced contractions (P>0.05, Student's paired t-tests) (Table 3) nor the log concentration-response curves to the endothelin receptor agonists (P>0.05, two-way ANOVA) were affected in the presence of 0.01% DMSO. Exposure of the prostatic preparations to BQ-123 (1 μM) or BQ-788 (1 μM) alone and in combination had no significant (P>0.05, Student's paired t-tests) effect on the magnitude of field stimulation-induced contractions (Table 3). The agonist-induced enhancements were, however, markedly attenuated in the presence of BQ-123 (1 μM) (Figure 5A). BQ-788 (1 μM) caused a slight but significant (P<0.05, Student's paired t-tests) inhibition of the enhancing effects of endothelin-1 (30 and 100 nM) and sarafotoxin S6b (10 and 30 nM), but not those of endothelin-2, in the field stimulated preparations of the guinea-pig prostate (Figure 5B).
Table 3.
The effects of vehicle (0.01% DMSO), BQ-123 and BQ-788 on the field stimulation-induced contractions in guinea-pig prostatic preparations
Figure 5.
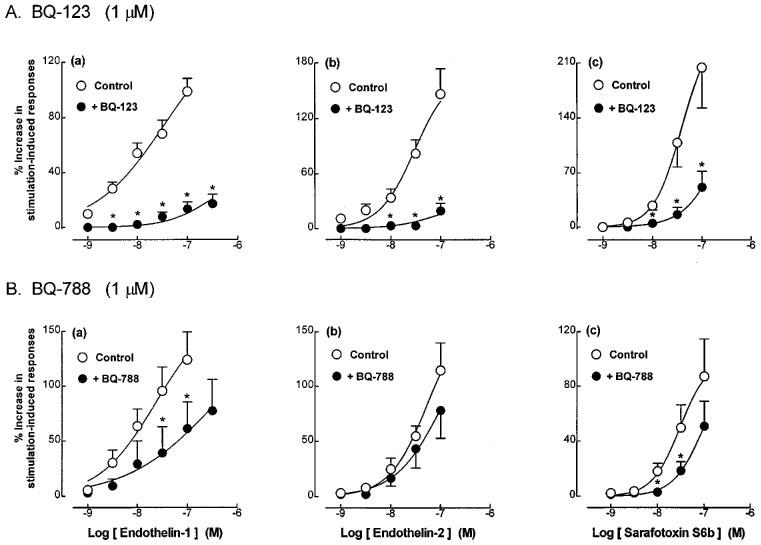
The effects of (A) BQ-123 (1 μM) and (B) BQ-788 (1 μM) on the mean log concentration-response curves to (a) endothelin-1 (n=4–5), (b) endothelin-2 (n=4–5) and (c) sarafotoxin S6b (n=5–8) in field stimulated preparations of the guinea-pig prostate. *P<0.05, 2-way ANOVA and Student's paired t-tests.
Application of exogenous noradrenaline (0.1 μM–1 mM) produced concentration-dependent contractions of the guinea-pig prostatic smooth muscle preparations; these were not modified by the presence of endothelin-1 (10 nM) (Figure 6).
Figure 6.
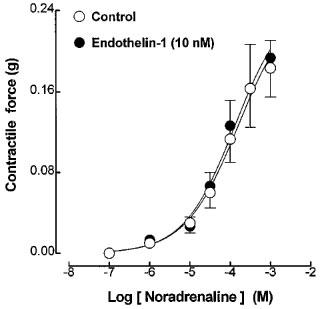
The effects of endothelin-1 (10 nM) on the mean log concentration-contractile response curves to noradrenaline in guinea-pig prostatic preparations (n=3).
Discussion
Radioligand binding and functional experiments have been conducted to characterize the endothelin receptor subtype/s present and their role in smooth muscle contraction in the guinea-pig prostate gland. The major findings from this study are (1) that guinea-pig prostatic homogenates contain both endothelin ETA and ETB binding sites and (2) that endothelin-induced facilitation of neuromuscular transmission to the smooth muscle of the prostate from the guinea-pig is mediated predominantly through activation of the endothelin ETA receptors.
Saturation binding experiments in the guinea-pig prostatic homogenates revealed that [125I]-endothelin-1 binding involved an apparently single class of saturable and high affinity binding sites. The mean KD value of 230±50 pM obtained is well within the range of KD values previously reported in the human prostate (70 pM: Saita et al., 1997; 550 pM: Imajo et al., 1997; 720 pM: Kobayashi et al., 1994a).
In competition binding studies, the rank order of affinity of endothelin-1>>BQ-788>BQ-123⩾sarafotoxin S6c in inhibiting [125I]-endothelin-1 binding to the guinea-pig prostatic homogenates indicated the presence of both endothelin ETA and ETB binding sites. Endothelin-1 is known to bind to ETA and ETB receptors with equally high affinity. Reported pKi values ranged from 9.24–10.64 at cloned human ETA receptors transfected into Chinese hamster ovary cells, and from 9.92–10.97 at ETB receptors in similar cells (Williams et al., 1993; Latifpour et al., 1995). The pKi value (9.60) obtained for endothelin-1 in the present study approximates these ranges. Additionally, similar affinity constants were obtained for the radiolabelled (230 pM) and unlabelled (250 pM) endothelin-1 in saturation and competition experiments, respectively.
Analysis of binding data indicated that specific [125I]-endothelin-1 binding to the guinea-pig prostatic homogenates was inhibited by the endothelin ETA receptor-selective antagonist BQ-123 in a biphasic manner; the predominant proportion (80%) of binding yielding a pKi value of 6.42. The endothelin ETB receptor-selective antagonist BQ-788 competed for [125I]-endothelin-1 binding with a pKi value of 7.24. These estimates are somewhat lower than those reported at corresponding cloned ETA and ETB receptor systems. Values ranging from 7.1–7.9 have been reported for BQ-123, and from 8.7–9.2 for BQ-788 at cloned ETA and ETB receptors, respectively (Williams et al., 1993; Buchan et al., 1994; Ishikawa et al., 1994; Latifpour et al., 1995). In the human prostate pKi values of 8.26 (Kobayashi et al., 1994a), 8.89 (Imajo et al., 1997) and 9.29 (Saita et al., 1997) were reported for BQ-123 and values of 8.77 (Saita et al., 1997) and 9.15 (Walden et al., 1998) for BQ-788. The reasons for the lower values we have obtained remain uncertain. It may be that these antagonists are binding to more than one receptor subtype in the guinea-pig prostate gland, but, as outlined by De Lean et al. (1982) further resolution of the binding profile would require 15–18 data points to validate this. Furthermore, differences in binding profiles may be influenced by the use of different preparations (intact cells vs cell membranes, Hara et al., 1998) and incubation conditions (temperature and time). The present study employed conditions similar to those described by Williams et al. (1991). Bovine serum albumin is included in the binding buffer in this study and that by Williams et al. (1991); serum albumin has been proposed to decrease the potency of antagonists in inhibiting endothelin receptor binding (Wu-Wong et al., 1997b).
The endothelin ETB receptor agonist sarafotoxin S6c, at the highest concentration tested, caused approximately 70% inhibition. Taken together with the data for the endothelin antagonists, its low potency suggests that either low affinity ETB as well as ETA sites are present in the guinea-pig prostate. Additionally, atypical ETB receptors or subtypes of endothelin ETB receptors may be present in this tissue as proposed for other tissues including the human bronchus (Hay et al., 1998; see Bax & Saxena, 1994 for a review).
The results from our binding experiments indicated that the prostate gland of guinea-pig, as that in man, contains both endothelin ETA and ETB receptors. The guinea-pig prostate, like the human prostate, is composed primarily of glandular and stromal components. Although differential distribution and proportion of endothelin receptor subtypes between smooth muscle cells and other components in the guinea-pig prostatic homogenates may exist, the prostatic stroma from the guinea-pig has been shown to comprise predominantly (95%) smooth muscle cells (Ricciardelli et al., 1989; Horsfall et al., 1994). In the human prostate, ETA receptors were originally shown to be mainly localized to the stromal compartment (Kobayashi et al., 1994a), although more recent studies by Webb et al. (1995) and Walden et al. (1998) indicate that ETB receptors are the predominant subtype present in the stromal cells.
High levels of immunoreactive endothelin-1 have been shown to be present in human prostatic secretory epithelium (Langenstroer et al., 1993), suggesting that the prostate may contribute to the bulk of immunoreactive endothelin-1 found in the human seminal fluid (Casey et al., 1992). Exogenous application of endothelin-1 has been shown to potently contract human isolated prostate (Langenstroer et al., 1993; Kobayashi et al., 1994a; Webb et al., 1995). In our hands, the endothelin receptor agonists endothelins-1, -2 and -3, sarafotoxins S6b and S6c and BQ 3020 were without direct effect on the contractility of guinea-pig prostatic smooth muscle. This, together with the lack of effect of BQ-123 and BQ-788 on the magnitude of field stimulation-induced contractions of the guinea-pig prostatic smooth muscle suggests that endogenous endothelins are normally not involved in neurotransmission to the smooth muscle of this tissue. However, endothelin-1, endothelin-2 and sarafotoxin S6b produced concentration-dependent potentiation of the field stimulation-induced contractions of these preparations. These three endothelin agonists were equally efficacious and potent in facilitating neurotransmission. In contrast the endothelin ETB receptor-preferring agonist endothelin-3 was much less effective, and the endothelin ETB receptor-selective agonists sarafotoxin S6c and BQ-3020 were completely inactive up to 1 μM. This series of experiments using agonists indicates that endothelins modulate neuromuscular transmission to the smooth muscle of the prostate from the guinea-pig through activation of ETA receptors.
The involvement of ETA receptors in facilitating neurotransmission to the prostate is further substantiated by findings that the endothelin ETA receptor-selective antagonist BQ-123 markedly attenuated the endothelin-induced facilitation. The finding that the endothelin ETB receptor-selective antagonist BQ-788, at 1 μM, reduced responses to endothelin-1 and to sarafotoxin S6b, might be attributable to an action at ETA receptors since it has been reported to have an IC50 within the micromolar range at this subtype, while its KD value at ETB receptors is in the low nanomolar range (see review by Opgenorth et al., 1995).
Neurotransmission to the guinea-pig prostatic smooth muscle is predominantly sympathetic and noradrenergic (Ohkawa, 1983; Lau & Pennefather, 1995; Najbar-Kaszkiel et al., 1997; Lau et al., 1998). Since endothelin-1 was without appreciable influence on noradrenaline-induced contractions of the guinea-pig prostatic smooth muscle, it seems unlikely that endothelin-induced facilitation is due to a postjunctional interaction with noradrenaline. It is, however, possible that endothelins may interact postjunctionally with excitatory transmitters other than noradrenaline. Indeed, endothelin-1 has been shown to facilitate stimulation-induced contractions of the rat vas deferens (Donoso et al., 1992; Lau et al., 1995) and bladder (Donoso et al., 1994) by modulating the purinergic component of neurotransmission. In the guinea-pig prostate, acetylcholine does not contract stromal smooth muscle (Lau, 1998) and neither ATP nor its stable analogue α, β-methylene ATP, produce consistent effects (Lau, unpublished observations). Therefore postjunctional interactions of endothelins with these substances were not investigated. Nevertheless, the mechanism/s involved in the facilitation by endothelins on neurotransmission to the guinea-pig prostatic smooth muscle requires further investigation.
In conclusion, the present study has described the first pharmacological characterization of endothelin action on neurotransmission to the guinea-pig prostate gland. Both endothelin ETA and ETB binding sites have been shown to be present in this tissue. While the absence of an effect of endothelin antagonists on neurotransmission to the prostate indicates that endogenous endothelins are not normally involved in neurotransmission to the smooth muscle within this tissue, their ability to facilitate neurotransmission mainly via activation of ETA receptors may be of potential physiological and pathophysiological significance.
Acknowledgments
This study was supported by a grant from the Monash Research Fund. We are grateful to Dr I McCance for advice on the statistical analysis of the functional data.
Abbreviations
- BQ-123
cyclo-D-Asp-L-Pro-D-Val-Leu-D-Trp
- BQ-788
N-cis-2,6-dimethylpiperidinocarbonyl-L-γ-methyl-Leu-D-Trp[1-CO2CH3-D-Nle-ONa
- BQ-3020
Ac-[Ala11,15]-endothelin-1 (6-21)
- ECE
endothelin converting enzyme
References
- BATTISTINI B., CHAILLER P., D'ORLEANS-JUSTE P., BRIERE N., SIROIS P. Growth regulatory properties of endothelins. Peptides. 1993;14:395–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- BAX W.A., SAXENA P.R. The current endothelin receptor classification: time for reconsideration. Trends Pharmacol. Sci. 1994;15:379–386. doi: 10.1016/0165-6147(94)90159-7. [DOI] [PubMed] [Google Scholar]
- BUCHAN K.W., ALLDUS C., CHRISTODOULOU C., CLARK K.L., DYKES C.W., SUMNER M.J., WALLACE D.M., WHITE D.G., WATTS I.S. Characterization of three non-peptide endothelin receptor ligands using human cloned ETA and ETB receptors. Br. J. Pharmacol. 1994;112:1251–1257. doi: 10.1111/j.1476-5381.1994.tb13218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASEY M.L., BYRD W., MACDONALD P.C. Massive amounts of immunoreactive endothelin in human seminal fluid. J. Clin. Endocrinol. Metabolism. 1992;74:223–225. doi: 10.1210/jcem.74.1.1727824. [DOI] [PubMed] [Google Scholar]
- DE LEAN A., HANCOCK A.A., LEFKOWITZ R.J. Validation and statistical analysis of a computer modelling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol. Pharmacol. 1982;21:5–16. [PubMed] [Google Scholar]
- DONOSO M.V., MONTES C.G., LEWIN J., FOURNIER A., CALIXTO J.B., HUIDOBRO-TORO J.P. Endothelin-1 (ET)-induced mobilization of intracellular Ca2+ stores from the smooth muscle facilitates sympathetic cotransmission by potentiation of adenosine 5′-triphosphate (ATP) motor activity: studies in the rat vas deferens. Peptides. 1992;13:831–840. doi: 10.1016/0196-9781(92)90193-7. [DOI] [PubMed] [Google Scholar]
- DONOSO M.V., SALAS C., SEPULVEDA G., LEWIN J., FOURNIER A., HUIDOBRO-TORO J.P. Involvement of ETA receptors in the facilitation by endothelin-1 of non-adrenergic non-cholinergic transmission in the rat urinary bladder. Br. J. Pharmacol. 1994;111:473–482. doi: 10.1111/j.1476-5381.1994.tb14761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDES L.B., HENRY P.J., RIGBY P.J., GOLDIE R.G. EndothelinB (ETB) receptor-activated potentiation of cholinergic nerve-mediated contraction in human bronchus. Br. J. Pharmacol. 1996;118:1873–1874. doi: 10.1111/j.1476-5381.1996.tb15617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARA M., TOZAWA F., ITAZAKI K., MIHARA S-I, FUJIMOTO M. Endothelin ETB receptors show different binding profiles in intact cells and cell membrane preparations. Eur. J. Pharmacol. 1998;345:339–342. doi: 10.1016/s0014-2999(98)00016-8. [DOI] [PubMed] [Google Scholar]
- HAY D.W.P.Chapter 1: Endothelins Airways smooth muscle: peptide receptors, ion channels and signal transduction 1995Basel/Switzerland: Birkhăuser Verlag; 1–50.eds Raeburn, D & Giembycz, M.A. pp [Google Scholar]
- HAY D.W.P., LUTTMANN M.A., PULLEN M.A., NAMBI P. Functional and binding characterization of endothelin receptors in human bronchus: evidence for a novel endothelin B receptor subtype. J. Pharmacol. Exp. Ther. 1998;284:669–677. [PubMed] [Google Scholar]
- HENRY P.J., GOLDIE R.G. Potentiation of endothelin-1 of cholinergic nerve-mediated contractions in mouse trachea via activation of ETB receptors. Br. J. Pharmacol. 1995;114:563–569. doi: 10.1111/j.1476-5381.1995.tb17176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORSFALL D.J., MAYNE K., RICCIARDELLI C., RAO M., SKINNER J.M., HENDERSON D.W., MARSHALL V.R., TILLEY W.D. Age-related changes in guinea-pig prostatic stroma. Lab. Invest. 1994;70:753–763. [PubMed] [Google Scholar]
- HUGGINS J.P., PELTON J.T., MILLER R.C. The structure and specificity of endothelin receptors: their importance in physiology and medicine. Pharmac. Ther. 1993;59:55–123. doi: 10.1016/0163-7258(93)90041-b. [DOI] [PubMed] [Google Scholar]
- IHARA M., NOGUCHI K., SAEKI T., FUKURODA T., TSUCHIDA S., KIMURA S., FUKAMI T., ISHIKAWA K., NISHIKIBE M., YANO M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992a;50:247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- IHARA M., SAEKI T., FUKURODA T., KIMURA S., FUKAMI T., OZAKI S., PATEL A.C., YANO M. A novel radioligand [125I]BQ-3020 selective for endothelin (ETB) receptors. Life Sci. 1992b;51:47–52. doi: 10.1016/0024-3205(92)90418-o. [DOI] [PubMed] [Google Scholar]
- IMAJO C., WALDEN P.D., SHAPIRO E., DOHERTY A.M., LEPOR H. Evaluation of the effect of endothelin-1 and characterization of the selective endothelin A receptor antagonist PD155080 in the prostate. J. Urol. 1997;158:253–257. doi: 10.1097/00005392-199707000-00081. [DOI] [PubMed] [Google Scholar]
- INOUE A., YANAGISAWA M., KIMURA S., KASUYA Y., MIYAUCHI T., GOTO K., MASAKI T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA K., IHARA M., NOGUCHI K., MASE T., MINO N., SAEKI T., FUKURODA T., FUKAMI T., OZAKI S., NAGASE T., NISHIKIBE M., YANO M. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI S., TANG R., WANG B., OPGENORTH T., LANGENSTROER P., SHAPIRO E., LEPOR H. Binding and functional properties of endothelin receptor subtypes in the human prostate. Mol. Pharmacol. 1994a;45:306–311. [PubMed] [Google Scholar]
- KOBAYASHI S., TANG R., WANG B., OPGENORTH T., STEIN E., SHAPIRO E., LEPOR H. Localization of endothelin receptors in the human prostate. J. Urol. 1994b;151:763–766. doi: 10.1016/s0022-5347(17)35083-8. [DOI] [PubMed] [Google Scholar]
- KONDO S., MORITA T., TASHIMA Y. Benign prostatic hypertrophy affects the endothelin receptor density in the human urinary bladder and prostate. Urol. Int. 1995;54:198–203. doi: 10.1159/000282723. [DOI] [PubMed] [Google Scholar]
- LANGENSTROER P., TANG R., SHAPIRO E., DIVISH B., OPGENORTH T.J., LEPOR H. Endothelin-1 in the human prostate: tissue levels, source of production and isometric tension studies. J. Urol. 1993;149:495–499. doi: 10.1016/s0022-5347(17)35534-9. [DOI] [PubMed] [Google Scholar]
- LATIFPOUR J., FUKUMOTO Y., WEISS R.M. Regional differences in the density and subtype specificity of endothelin receptors in rabbit urinary tract. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:459–468. doi: 10.1007/BF00169378. [DOI] [PubMed] [Google Scholar]
- LAU W.A.K.Neurotransmission to the prostate: role of acetylcholine 1998. Masters of Science thesis, Monash University, Australia
- LAU W.A.K., COX S.L., PENNEFATHER J.N., MITCHELSON F.J. Endothelin receptor subtypes in the guinea-pig prostate gland. Proc. Aust. Neuroscience Soc. 1999;10:163. doi: 10.1038/sj.bjp.0702644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAU W.A.K., PENNEFATHER J.N. Neurotransmission to the smooth muscle of the prostate gland from the guinea-pig. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 1995;2:89. [Google Scholar]
- LAU W.A.K., VENTURA S., JIANG Q., PENNEFATHER J.N. Endothelin-induced facilitation of sympathetic neurotransmission to the rat vas deferens: effects of suramin. Eur. J. Pharmacol. 1995;272:31–38. doi: 10.1016/0014-2999(94)00624-g. [DOI] [PubMed] [Google Scholar]
- LAU W.A.K., VENTURA S., PENNEFATHER J.N. Pharmacology of neurotransmission to the smooth muscle of the rat and the guinea-pig prostate glands. J. Auton. Pharmacol. 1998;18:349–356. doi: 10.1046/j.1365-2680.1998.1860349.x. [DOI] [PubMed] [Google Scholar]
- LE BRUN G., MOLDOVAN F., AUBIN P., ROPIQUET F., CUSSENOT O., FIET J. Identification of endothelin receptors in normal and hyperplasic human prostate tissues. The Prostate. 1996;28:379–384. doi: 10.1002/(SICI)1097-0045(199606)28:6<379::AID-PROS7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAAS J., D'ORLEANS-JUSTE P., YANO M., RAE G.A. Evidence for atypical endothelin receptors and for presence of endothelin-converting enzyme activity in the mouse isolated vas deferens. Eur. J. Pharmacol. 1995;276:113–121. doi: 10.1016/0014-2999(95)00020-l. [DOI] [PubMed] [Google Scholar]
- MASAKI T., VANE J.R., VANHOUTTE P.M. V. International Union of Pharmacology Nomenclature of endothelin receptors. Pharmacol. Rev. 1994;46:137–142. [PubMed] [Google Scholar]
- MACPHERSON G.A. Analysis of radioligand binding experiment. A collection of computer programs for the IBM PC. J. Pharmacol. Methods. 1985;14:213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- MORIYAMA N., KURIMOTO S., MIYATA N., YAMAURA H., YAMAZAKI R., SUDOH K., INAGAKI O., TAKENAKA T., KAWABE K. Decreased contractile effect of endothelin-1 on hyperplastic prostate. Gen. Pharmacol. 1996;27:1061–1065. doi: 10.1016/0306-3623(95)00117-4. [DOI] [PubMed] [Google Scholar]
- NAJBAR-KASZKIEL A.T., DI IULIO J.L., LI C.G., RAND M.J. Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea-pigs, rabbits and pigs. Eur. J. Pharmacol. 1997;337:251–258. doi: 10.1016/s0014-2999(97)01270-3. [DOI] [PubMed] [Google Scholar]
- NELSON J.B., CHAN-TACK K., HEDICAN S.P., MAGNUSON S.R., OPGENORTH T.J., BOVA G.S., SIMONS J.W. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- OHKAWA H. Sympathetic neuromuscular transmission in the smooth muscle of guinea-pig prostate gland. Int. J. Fertil. 1983;28:68–77. [PubMed] [Google Scholar]
- OPGENORTH T.J. Endothelin receptor antagonism. Advances Pharmacol. 1995;33:1–65. doi: 10.1016/s1054-3589(08)60665-1. [DOI] [PubMed] [Google Scholar]
- PENNEFATHER J.N., COX S., LAU W.A.K., MCCANCE I., MITCHELSON F. Endothelin-induced facilitation of neurotransmission to the smooth muscle of the guinea-pig prostate. Proc. Aust. Neuroscience Soc. 1998;9:85. [Google Scholar]
- PRAYER-GALETTI T., ROSSI G.P., BELLONI A.S., ALBERTIN G., BATTANELLO W., PIOVAN V., GARDIMAN M., PAGANO F. Gene expression and autoradiographic localization of endothelin-1 and its receptors A and B in the different zones of the normal human prostate. J. Urol. 1997;157:2334–2339. [PubMed] [Google Scholar]
- RICCIARDELLI C., HORSFALL D.J., SKINNER J.M., HENDERSON D.W., MARSHALL V.R., TILLEY W.D. Development and characterization of primary cultures of smooth muscle cells from the fibromuscular stroma of the guinea-pig prostate. In Vitro Cell Dev. Biol. 1989;25:1016–1024. doi: 10.1007/BF02624135. [DOI] [PubMed] [Google Scholar]
- ROSSI G.P., ALBERTIN G., FRANCHIN E., SACCHETTO A., CESARI M., PALU G., PESSINA A.C. Expression of the endothelin-converting enzyme gene in human tissues. Biochem. Biophys. Res. Commun. 1995;211:249–253. doi: 10.1006/bbrc.1995.1803. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., POLOKOFF M.A. Endothelins: molecular biology, biochemistry, pharmacology, physiology and pathophysiology. Pharmacol. Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- SAENZ DE TEJADA I., MUELLER J.D., DE LAS MORENAS A., MACHADO M., MORELAND R.B., KRANE R.J., WOLFE H.J., TRAISH A.M. Endothelin in the urinary bladder. I. Synthesis of endothelin-1 by epithelia, smooth muscle and fibroblasts suggests autocrine and paracrine cellular regulation. J. Urol. 1992;148:1290–1298. doi: 10.1016/s0022-5347(17)36895-7. [DOI] [PubMed] [Google Scholar]
- SAITA Y., KOIZUMI T., YAZAWA H., MORITA T., TAKENAKA T., HONDA K. Endothelin receptors and their cellular signal transduction mechanism in human cultured prostatic smooth muscle cells. Br. J. Pharmacol. 1997;121:687–694. doi: 10.1038/sj.bjp.0701179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITA Y., YAZAWA H., KOIZUMI T., MORITA T., TAMURA T., TAKENAKA T., HONDA K. Mitogenic activity of endothelin on human cultured prostatic smooth muscle cells. Eur. J. Pharmacol. 1998;349:123–128. doi: 10.1016/s0014-2999(98)00183-6. [DOI] [PubMed] [Google Scholar]
- SOKOLOVSKY M. Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmac. Ther. 1995;68:435–471. doi: 10.1016/0163-7258(95)02015-2. [DOI] [PubMed] [Google Scholar]
- TAKIMOTO M., INUI T., OKADA T., URADE Y. Contraction of smooth muscle by activation of endothelin receptors on autonomic neurons. F.E.B.S. Lett. 1993;324:277–282. doi: 10.1016/0014-5793(93)80134-g. [DOI] [PubMed] [Google Scholar]
- WALDEN P.D., ITTMANN M., MONACO M.E., LEPOR H. Endothelin-1 production and agonist activities in cultured prostate-derived cells: implications for regulation of endothelin bioactivity and bioavailability in prostatic hyperplasia. The Prostate. 1998;34:241–250. doi: 10.1002/(sici)1097-0045(19980301)34:4<241::aid-pros1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- WEBB M.L., CHAO C.-C., RIZZO M., SHAPIRO R.A., NEUBAUER M., LIU E.C.K., AVERSA C.R., BRITTAIN R.J., TREIGER B. Cloning and expression of an endothelin receptor subtype B from human prostate that mediates contraction. Mol. Pharmacol. 1995;47:730–737. [PubMed] [Google Scholar]
- WIKLUND N.P., OHLEN A., WIKLUND C.U., CEDERQVIST B., HEDQVIST P., GUSTAFSSON L.E. Neuromuscular actions of endothelin on smooth, cardiac and skeletal muscle from guinea-pig, rat and rabbit. Acta Physiol. Scand. 1989;137:399–407. doi: 10.1111/j.1748-1716.1989.tb08770.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS D.L., JONES K.L., ALVES K., CHAN C.P., HOLLIS G.F., TUNG J.-S. Characterization of cloned human endothelin receptors. Life Sci. 1993;53:407–414. doi: 10.1016/0024-3205(93)90644-i. [DOI] [PubMed] [Google Scholar]
- WILLIAMS D.L., JONES K.L., PETTIBONE D.J., LIS E.V., CLINESCHMIDT B.V. Sarafotoxin S6c: an agonist which distinguishes between endothelin receptor subtypes. Biochem. Biophys. Res. Commun. 1991;175:556–561. doi: 10.1016/0006-291x(91)91601-8. [DOI] [PubMed] [Google Scholar]
- WU-WONG J.R., CHIOU W.J., DICKINSON R., OPGENORTH T.J. Endothelin attenuates apoptosis in human smooth muscle cells. Biochem. J. 1997a;328:733–737. doi: 10.1042/bj3280733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU-WONG J.R., DIXON D.B., CHIOU W.J., OPGENORTH T.J. Endothelin receptor antagonists: effects of serum albumin on potency and comparison of pharmacological characteristics. J. Pharmacol. Exp. Ther. 1997b;281:791–798. [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


